Abstract
Recent studies have recognized G protein-coupled receptors as important regulators of oligodendrocyte development. GPR17, in particular, is an orphan G protein-coupled receptor that has been identified as oligodendroglial maturation inhibitor because its stimulation arrests primary mouse oligodendrocytes at a less differentiated stage. However, the intracellular signaling effectors transducing its activation remain poorly understood. Here, we use Oli-neu cells, an immortalized cell line derived from primary murine oligodendrocytes, and primary rat oligodendrocyte cultures as model systems to identify molecular targets that link cell surface GPR17 to oligodendrocyte maturation blockade. We demonstrate that stimulation of GPR17 by the small molecule agonist MDL29,951 (2-carboxy-4,6-dichloro-1H-indole-3-propionic acid) decreases myelin basic protein expression levels mainly by triggering the Gαi/o signaling pathway, which in turn leads to reduced activity of the downstream cascade adenylyl cyclase-cAMP-PKA-cAMP response element-binding protein (CREB). In addition, we show that GPR17 activation also diminishes myelin basic protein abundance by lessening stimulation of the exchange protein directly activated by cAMP (EPAC), thus uncovering a previously unrecognized role for EPAC to regulate oligodendrocyte differentiation. Together, our data establish PKA and EPAC as key downstream effectors of GPR17 that inhibit oligodendrocyte maturation. We envisage that treatments augmenting PKA and/or EPAC activity represent a beneficial approach for therapeutic enhancement of remyelination in those demyelinating diseases where GPR17 is highly expressed, such as multiple sclerosis.
Keywords: cAMP response element-binding protein (CREB), cell signaling, G protein, G protein-coupled receptor (GPCR), multiple sclerosis, oligodendrocyte, protein kinase A (PKA), GPR17, EPAC, label-free dynamic mass redistribution
Introduction
Myelination is a central nervous system (CNS) process where oligodendrocytes establish myelin sheaths that stabilize, protect, and electrically insulate neuronal axons. During postnatal development, oligodendrocytes progress through a sequence of differentiation steps from oligodendrocyte precursor cells (OPCs)5 to myelinating mature oligodendrocytes (1). Failure during this process leads to impaired myelination and, consequently, to slow conduction of signals along nerves, which results in debilitating symptoms such as paralysis and cognitive impairments. Likewise, in many severe neurological disorders, among others multiple sclerosis (MS), loss of oligodendrocytes and destruction of CNS myelin precedes the serious neurological deficits. Although OPCs are abundant in chronic MS lesion sites, no remyelination occurs, which suggests that oligodendrocyte differentiation does not take place due to either the absence of pro-myelinating signals or presence of myelination inhibitors in the MS lesion (2, 3).
During the previous years, an increasing number of oligodendroglial differentiation inhibitors have been identified (for review see Ref. 4). Genetic evidence from transgenic mice supports the notion that the orphan G protein-coupled receptor GPR17 (5) negatively regulates oligodendrocyte maturation and myelination (6). Notably, GPR17 is highly abundant within active white matter plaques of MS patients as well as in animal models of this disease (6), suggesting that this membrane protein may play a crucial role in MS by impairing the remyelination process.
MDL29,951 (2-carboxy-4,6-dichloro-1H-indole-3-propionicacid) is a small molecule recently reported to specifically activate GPR17 both in heterologous cell expression systems and in primary rat oligodendrocyte cultures (7). In addition, MDL29,951 arrests primary wild-type but not GPR17-deficient mouse oligodendrocytes at a less differentiated stage, resulting in a pronounced loss of myelin basic protein (MBP)-positive cells (7), thus confirming the selective linkage of GPR17 activation by MDL29,951 with blockade of OPC maturation. However, the underlying mechanism connecting GPR17 to oligodendroglial maturation impairment is elusive at present. Different signaling cascades downstream of activated G protein-coupled receptors have been identified as modulators of myelination. For instance, inhibition of protein kinase C (PKC), a ubiquitously expressed effector of heterotrimeric Gαq proteins, enhances oligodendrocyte maturation in the presence of inhibitory CNS myelin debris (8). A similar result was observed upon down-regulation of Rho-associated, coiled-coil containing protein kinase 2 (ROCK2) (8). Conversely, sustained activation of either PKC or ROCK2 is associated with less efficient differentiation. Indeed, the adhesion receptor GPR56 has recently been shown to inhibit oligodendrocyte myelination in zebrafish via activation of the Gα12/13-RhoA-ROCK pathway (9). Furthermore, down-regulation of the adenylyl cyclase-cyclic-adenosine 3′,5′-monophosphate (cAMP)-PKA-CREB cascade also impairs oligodendrocyte differentiation (10–12).
The present study sets out to elucidate which members of the heterotrimeric G protein family are triggered by GPR17 during oligodendroglia differentiation. We utilized Oli-neu cells, an immortalized cell line derived from primary murine oligodendrocytes, and primary rat oligodendrocyte cultures as model systems in conjunction with the GPR17 agonist MDL29,951 to provide a comprehensive map of the downstream effector molecules linking cell surface GPR17 to lessening of MBP expression. Within this framework, we uncover a previously unrecognized role for the exchange protein directly activated by cAMP (EPAC) to regulate oligodendrocyte differentiation. Knowledge on the molecular details of how GPR17 transduces differentiation-inhibiting signals from the plasma membrane to the nucleus will be valuable for development of pharmacological agents targeting this receptor to treat demyelinating diseases such as MS.
Experimental Procedures
Materials and Reagents
Tissue culture media and reagents were purchased from Invitrogen. MDL29,951 was from Maybridge. We obtained from BIOLOG the EPAC activator (8-CPT-2′-O-Me-cAMP), the protein kinase A (PKA) activator (Sp-6-Bnz-cAMPS), the EPAC inhibitor (ESI-05), and the PKA inhibitor (Rp-8-CPT-cAMPS). The cAMP-analog, 8-CPT-cyclic AMP (sodium salt), was obtained from Cayman Chemical. Ciliary neurotrophic factor, platelet-derived growth factor AA (PDGF-AA), and basic fibroblast growth factor were purchased from PeproTech. Antibodies to detect ERK, p-ERK, CREB, p-CREB, and EPAC were from Cell signaling Technology. The MBP antibody was from LifeSpan BioSciences, the GPR17 antibody was from Cayman, and the Gαs, Gαq, Gαo, and Gαi antibodies were from Santa Cruz Biotechnology. All other laboratory reagents were obtained from Sigma if not stated otherwise.
Cell Culture
Tissue culture media and reagents were purchased from Invitrogen. Oli-neu cells were kindly provided by Prof. J. Trotter (University of Mainz) and cultured in Sato medium containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 1% (v/v) horse serum, insulin (5 μg/ml), penicillin (100 units/ml), streptomycin (0.1 mg/ml), 1% (v/v) N2 supplement, sodium selenite (190 nm), gentamicin (25 μg/ml), triiodothyronine (400 nm), and l-thyroxine (520 nm). Cells were kept in a humidified atmosphere with 5% CO2 at 37 °C. Differentiation was induced by adding 1 μm PD174265 (Calbiochem) to the medium.
Primary rat OPCs were isolated from the forebrains of Wistar rat pups at P0-P2 with a differential detachment method as previously described (7, 13). Briefly, cerebra were mechanically dissociated with a syringe and two different hollow needles (first 1.2 × 40 and then 0.60 × 30). Clump-free cell suspension was filtered through a 70-μm cell strainer (BD Biosciences) and plated into poly-d-lysine-coated 75-cm2 culture flasks in DMEM supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS), penicillin (100 units/ml), and streptomycin (0.1 mg/ml) with medium exchanged every second day. After 8–11 days, mixed cultures were shaken (240 rpm) for 14–24 h to detach OPCs from astrocytes and microglia. To further enrich for OPCs, the suspension was plated onto uncoated Petri dishes (Corning) for 45 min. Then, OPCs were seeded into poly-l-ornithine-coated plates and maintained in proliferating Neurobasal medium supplemented with 2% (v/v) B27, 2 mm GlutaMAX, 100 units/ml penicillin, 0.1 mg/ml streptomycin, 10 ng/ml PDGF-AA, and 10 ng/ml basic FGF for 3–4 days (37 °C, 5% CO2), changing the medium every second day. Thereafter, medium was switched to growth factor-free Neurobasal medium to induce spontaneous in vitro differentiation and GPR17 protein expression. For terminal differentiation and quantification analyses of protein expression, after 24 h the growth factor-free medium was supplemented with 20 ng/ml triiodothyronine (T3) and 10 ng/ml ciliary neurotrophic factor together with the analyzed compounds for additional 48 h.
RNA Interference
Cells were transfected using the transfection reagent Lipofectamine RNAiMAX (Life Technologies), with 50–100 nm scrambled siRNA (AllStars Negative Control siRNA, Qiagen) or a siRNA designed to silence mouse and rat GPR17 (5′-CCCGGTTGGTTTATCACTTCT-3′, Qiagen) according to the manufacturer's instructions. Before transfection, Oli-neu cells were seeded and incubated for 24 h in the presence of 1 μm PD174265, whereas primary rat OPCs were cultured for 3 days in proliferating medium. After transfection, OPCs were switched to medium lacking growth factors to induce differentiation. GPR17 knockdown in both oligodendroglial cells was analyzed after 24–48 h by Western blotting, label-free dynamic mass redistribution assay, or Ca2+ flux assay.
Western Blotting
OPCs were seeded in 6- or 12-well poly-l-ornithine-coated tissue culture plates (12,000–20,000 cells per cm2). Oli-neu cells were seeded at a density of 300,000 cells per well into 12-well poly-d-lysine-coated tissue culture plates in Sato medium and grown overnight; afterward, cells were incubated with indicated compounds and 1 μm PD174265.
At specified times cells were washed twice with ice-cold PBS and lysed in ice-cold lysis buffer (25 mm Tris, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 1% IGEPAL) supplemented with protease inhibitor mixture (Sigma). Lysates were rotated 20 min at 4 °C and centrifuged at 15,000 × g at 4 °C for 10 min. Protein concentration was determined using the Pierce BCA Protein Assay (Thermo Scientific) according to manufacturer's instructions.
7.5–15 μg of protein were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (HybondTM-C Extra, GE Healthcare) by electroblotting. After washing, membranes were blocked with Roti-Block (1×; Carl Roth) for 1 h at room temperature and incubated overnight at 4 °C in Roti-Block with antibodies specific for MBP (1:5000), GPR17 (1:5000), ERK (1:2500), p-ERK (1:2500), CREB (1:2500), p-CREB (1:1000), Gαs (1:3000), Gαq (1:3000), Gαo (1:5000), or Gαi (1:5000). Membranes were washed 3 times with PBS containing 0.1% Tween and then incubated for 1 h at room temperature with a horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit IgG Antibody HRP (ABIN) or goat anti-mouse IgG antibody HRP (Sigma)) in Roti-Block. The immunoreactive proteins were visualized by chemiluminescence using Amersham Biosciences ECL Prime Western blotting detection reagent (GE Healthcare) and quantified by densitometry using Gelscan software (Bioscitec, Frankfurt, Germany). To normalize for equal loading and protein transfer, membranes were stripped and reprobed with an antibody against β-actin (1:2500; BioLegend).
Ca2+ Mobilization Assays
Intracellular Ca2+ mobilization was quantified with the Calcium 5 Assay kit and the FlexStation 3 Multimode Microplate Reader (Molecular Devices). Oli-neu cells were seeded at a density of 70,000 cells per well into black 96-well poly-d-lysine-coated tissue culture plates with clear bottoms. Cells were cultured for 24–48 h in the presence or absence of 1 μm PD174265. Pertussis toxin (PTX, 100 ng/ml; BIOTREND Chemikalien GmbH) was added 16 h before the assay. Cells were loaded with the Calcium 5 indicator dye for 30 min and processed according to manufacturer's instructions.
cAMP Accumulation Assays
Inhibition of the intracellular second messenger cAMP in Oli-neu cells was analyzed using the HTRF-cAMP dynamic 2 kit according to the manufacturer's instructions (Cisbio International), and fluorescence was quantified by use of Mithras LB 940 reader (Berthold Technologies). In brief, cells were seeded in the presence of 1 μm PD174265 at a density of 30,000 cells per well into 48-well poly-d-lysine-coated tissue culture plates. After 48 h cells were preincubated for 20 min in assay buffer (Hanks' buffered salt solution supplemented with 20 mm HEPES and 1 mm isobutylmethylxanthine) and then stimulated with MDL29,951 in the presence of 3 μm forskolin (AppliChem) for 20 min at 37 °C. The reactions were stopped by the addition of 40 μl of HTRF assay lysate buffer, and samples were frozen overnight. After transfer of 10 μl of lysate to a 384-well plate, HTRF reagents were added and incubated for 60 min at room temperature before time-resolved FRET signals were measured (excitation at 320 nm). Data analysis was made based on the fluorescence ratio emitted by d2 (acceptor fluorophore)-labeled cAMP (665 nm) over the light emitted by Eu3+-Cryptate-labeled anti-cAMP (620 nm). Levels of cAMP were normalized to the values obtained with 3 μm forskolin.
ERK1/2 Phosphorylation Assays
Quantification of phosphorylated ERK1/2 levels was performed with the HTRF Cellul'erk kit (Cisbio International) following the manufacturer's instructions (two plate assay protocol) and Mithras LB 940 reader (Berthold Technologies) as described previously (14). OPCs were seeded in proliferating media at a density of 5000 cells per well and Oli-neu cells at 40,000 cells per well in the presence of 1 μm PD174265 into clear 96-well poly-l-ornithine- and poly-d-lysine-coated plates, respectively. After differentiation, cells were washed with Hanks' buffered salt solution supplemented with 20 mm HEPES and starved for 4 h in serum-free medium followed by incubation for different time points with MDL29,951 at 37 °C. Then the agonist was removed, and cells were lysed in 50 μl of the supplemented lysis buffer. Plates were incubated while shaking for 30 min at room temperature and then frozen overnight. After transfer of 16 μl of lysate to a white 384-well plate, d2-labeled anti-phospho-ERK1/2 (2 μl) and Eu3+-Cryptate-labeled anti-ERK1/2 (2 μl) were added and incubated in the dark for 2 h at room temperature. Time-resolved FRET signals were measured after excitation at 320 nm. Data analysis was based on the fluorescence ratio emitted by the d2-labeled anti-phospho-ERK1/2 (665 nm) over the light emitted by the Eu3+-Cryptate-labeled anti-ERK1/2 (620 nm). Levels of phosphorylated ERK1/2 were normalized to ERK-phosphorylation values obtained with 10 μm MDL29,951.
Label-free Dynamic Mass Redistribution (DMR) Assays
DMR was recorded as previously described (15, 16) using the PerkinElmer Ensight or the Corning Epic system (Corning) in conjunction with the Cybi-SELMA semi-automated electronic pipetting system (Analytik Jena AG). In brief, OPCs were seeded in proliferation medium at a density of 3000 cells per well on 384-well fibronectin-coated biosensor plates. Oli-neu cells were seeded at a density of 18,000 cells per well in the presence of 1 μm PD174265. After differentiation, cells were washed twice with Hanks' buffered salt solution containing 20 mm HEPES and incubated for 1 h in the DMR reader at 28 °C. The sensor plate was scanned to record a baseline optical signature, and then compound solutions were transferred into the biosensor plate to monitor DMR for 3200 s. PTX (100 ng/ml) was added 16 h before the measurement.
Cell Viability Assays
Cell viability was assessed using a fluorimetric detection of resorufin formation (CellTiter-Blue Cell Viability Assay, Promega). Oli-neu cells were seeded at a density of 25,000 cells per well into black 96-well poly-d-lysine-coated plates with clear bottoms. After 12–24 h, cells were treated with 0.1% dimethyl sulfoxide (DMSO) or indicated compounds diluted in medium containing 1 μm PD174265 for 48 h. To detect cell viability, CellTiter-Blue reagent (20 μl) was added, and cells were incubated for 3 h at 37 °C according to the manufacturer's instructions. Fluorescence (excitation 560 nm, emission 590 nm) was measured using a FlexStation 3 Multimode Microplate Reader (Molecular Devices), and data were expressed as the percentage of cell viability relative to DMSO control.
Data and Statistical Analysis
Data are presented as the means ± S.E., and calculations were performed using GraphPad Prism 6 (GraphPad Software). Comparison between two experimental groups was performed with a two-tailed Student's t test. p value significance thresholds were p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***).
Results
Activation of GPR17 by MDL29,951 Triggers Gαq and Gαi/o Signaling Pathways in Differentiating Oli-neu Cells
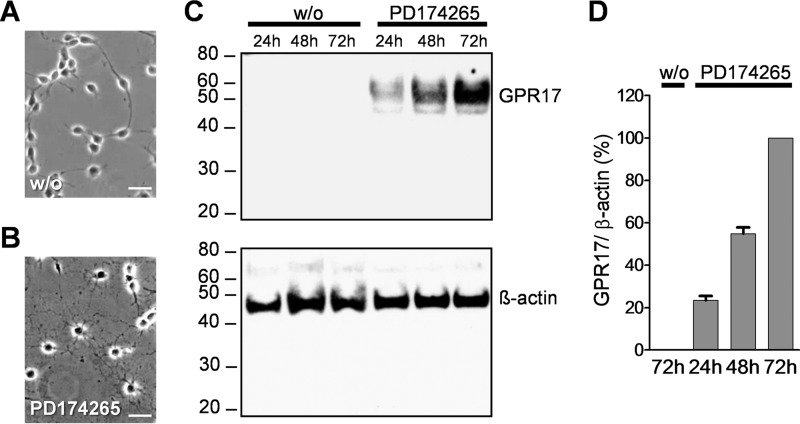
To assess which intracellular pathways are utilized by GPR17 to regulate oligodendrocyte maturation, we selected as cellular models primary rat oligodendrocyte cultures and the murine oligodendroglial precursor cell line, Oli-neu, which undergoes differentiation into MBP-expressing cells upon induction by different chemical treatments (17). PD174265 is a selective inhibitor of the EGF receptor that has been previously shown as the differentiating agent in Oli-neu cells (18). Therefore, we initially investigated whether PD174265 might also serve to induce GPR17 expression during Oli-neu differentiation. As expected, PD174265 successfully stopped cell proliferation and increased dendrocyte outgrowth (Fig. 1, A and B). In agreement with previous findings (19), Western blotting analysis of cell lysates revealed a specific 47-kDa GPR17 immunoreactive band that was initially detected after 24 h incubation with PD174265 and subsequently increased during the following days in culture, in contrast to the lack of GPR17 in Oli-neu cells incubated under control conditions without the EGF receptor inhibitor (Fig. 1, C and D).
FIGURE 1.
PD174265 induced GPR17 expression in Oli-neu cells. Cells were cultured in Sato medium either untreated (A) or in the presence of 1 μm PD174265 (B) for 48 h. Note the induced formation of oligodendrocytic processes in treated Oli-neu cells. Scale bar = 20 μm. C, lysates from Oli-neu cells cultivated in the absence or presence of 1 μm PD174265 after 24, 48, and 72 h were analyzed by Western blotting using the GPR17 antibody (upper panel) and, after stripping, reprobed with the β-actin antibody (lower panel). D, quantitative analysis of GPR17 immunoreactive band corrected by β-actin from three independent experiments.
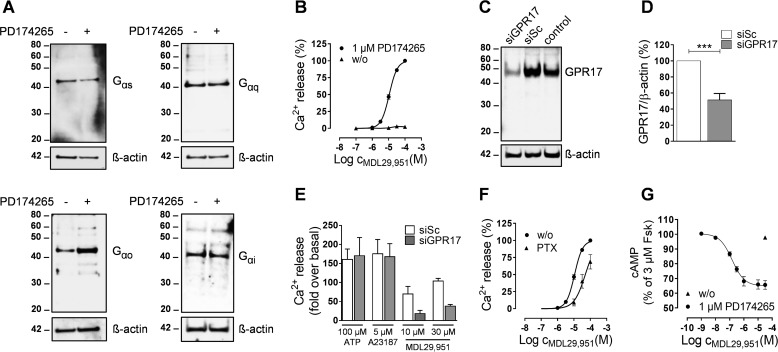
To confirm that the small molecule MDL29,951 also acts as GPR17 agonist in differentiating Oli-neu cells, we next performed a series of classical second messenger assays. Similarly to primary rat oligodendrocytes (20), Oli-neu cells express Gαs, Gαq, Gαo, and Gαi during both proliferation and PD174265-mediated differentiation (Fig. 2A), thus assuring that G protein signaling pathways linked to second messenger production subsist in this cell line.
FIGURE 2.
MDL29,951 specifically activated GPR17 in differentiating Oli-neu cells. A, lysates were prepared from Oli-neu cells cultured in the absence or presence of 1 μm PD174265 for 48 h and then analyzed by Western blotting using antibodies against Gαs, Gαq, Gαo, and Gαi; the blots were stripped and reprobed for β-actin. B, concentration-effect profile of the intracellular Ca2+ release triggered by MDL29,951 in Oli-neu cells previously cultured for 24 h in the absence (n = 4 independent experiments) or presence (n = 8) of 1 μm PD174265. Levels of Ca2+ were normalized for each experiment as percentage of the values obtained with 100 μm MDL29,951 in differentiating Oli-neu cells (-fold over basal at 100 μm MDL29,951 = 120.86 ± 18.44). C, a representative Western blot showing effect of scrambled siRNA (siSc) and GPR17-siRNA (siGPR17) on GPR17 abundance compared with untransfected control sample. D, quantitative analysis of GPR17 expression levels detected in Western blots upon GPR17 knockdown from five independent experiments. ***, p < 0.001. E, intracellular Ca2+ mobilization assay in differentiating Oli-neu cells comparing the effect of GPR17-siRNA knockdown on ATP-, A23187-, and MDL29,951-mediated Ca2+ flux (n = 4). F, MDL29,951-stimulated Ca2+ release in the absence (n = 8) and presence (n = 3) of the Gαi/o inhibitor PTX in differentiating Oli-neu cells (-fold over basal at 100 μm MDL29,951 = 120.86 ± 18.44). G, effect of MDL29,951 on forskolin-stimulated cAMP production in Oli-neu cells previously cultured for 48 h in the absence (n = 3) or presence (n = 6) of PD174265.
MDL29,951 led to a robust and concentration-dependent release of Ca2+ from intracellular stores (Fig. 2B). No significant alteration of Ca2+ mobilization upon MDL29,951 exposure was observed when Oli-neu cells were maintained in the absence of PD174265 (Fig. 2B), thus demonstrating that GPR17 expression was required for the functional effect. To further confirm agonist specificity, differentiating Oli-neu cells were transfected with a small interfering (si)RNA to specifically lower GPR17 abundance. Western blotting displayed a reduction in the intensity of the GPR17 immunoreactive band upon GPR17 knockdown compared with cells transfected with a nonspecific (scrambled) siRNA (Fig. 2, C and D). Consistent with this result, MDL29,951-mediated signaling was largely diminished in GPR17-siRNA-transfected cells (Fig. 2E). RNA interference did not affect the viability of Oli-neu cells as Ca2+ flux induced by either the non-receptor stimulus calcium ionophore (A23187) or adenosine 5′-triphosphate (ATP) through activation of endogenous P2Y receptors was unaltered (Fig. 2E). Pretreatment of the cells with PTX, which inhibits Gαi/o signaling, diminished but did not abolish MDL29,951-induced Ca2+ flux (Fig. 2F), suggesting that both Gαi/o- and Gαq-dependent mechanisms account for the observed Ca2+ release.
MDL29,951 also decreased the amount of forskolin-stimulated intracellular cAMP in a concentration-dependent manner, thus indicating a reduction of adenylyl cyclase activity by Gαi/o proteins (Fig. 2G). Accumulation of cAMP at higher concentrations of the GPR17 agonist was not apparent, suggesting that the Gαs signaling pathway is not operative by GPR17 in this cellular background (Fig. 2G) despite Gαs presence (Fig. 2A). Taken together, these data are in keeping with our previous observations in primary rat oligodendrocyte cultures (7), which also in Oli-neu cells MDL29,951 specifically activates GPR17 and subsequently triggers downstream signaling effectors via Gαi/o and Gαq, thus corroborating Oli-neu cells as a suitable cellular model system to study GPR17-associated signaling cascades.
Activation of GPR17 Largely Elicits Gαi/o Signaling and Inhibits Oligodendrocyte Differentiation
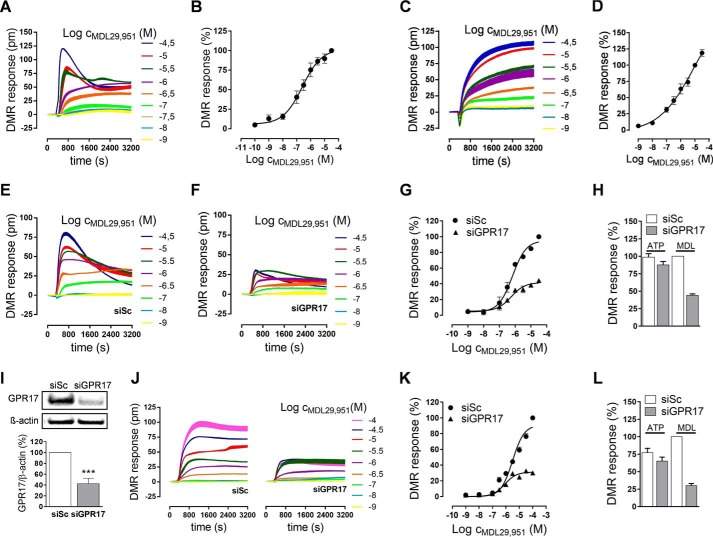
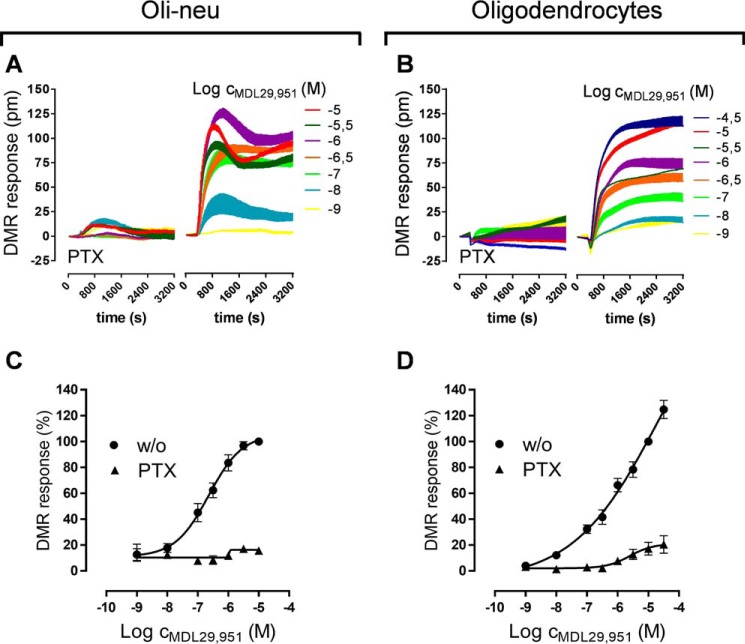
Because GPR17 triggers different pathways linked to second messenger production, we sought to determine whether the downstream Gαi/o and Gαq signal machineries play equivalent roles in modifying oligodendroglial cell activity upon GPR17 stimulation. Therefore, we analyzed global activation of living cells with a non-invasive label-free biosensor assay based on detection of DMR that occurs within cells as a consequence of receptor stimulation, irrespective of the particular G protein signaling pathways involved (15, 16). Akin to previous observations in heterologous cell expression systems (7), during differentiation of both Oli-neu cells (Fig. 3, A and B) and primary rat oligodendrocytes (Fig. 3, C and D) treatment with MDL29,951 triggered robust whole-cell responses in DMR assays. Importantly, when compared with Oli-neu cells transfected with scrambled siRNA as control (Fig. 3E), receptor knockdown by GPR17-specific siRNA diminished DMR recordings (Fig. 3, F and G). This is in contrast to ATP-induced signaling via endogenous P2Y receptors, which was unaffected upon GPR17 knockdown (Fig. 3H). These data corroborate both Oli-neu cell integrity after siRNA transfection and GPR17 specificity of the MDL29,951 response. In agreement with these findings, transfection with GPR17 siRNA of primary rat oligodendrocytes effectively diminished receptor expression (Fig. 3I) and MDL29,951-induced DMR responses (Fig. 3, J and K), whereas no alterations were observed when ATP was applied as the stimulus for purinergic receptors (Fig. 3L). Notably, pretreatment of both oligodendroglial cells with PTX almost entirely abolished MDL29,951-mediated DMR recordings (Fig. 4, A–D). These results indicate that Gαi/o proteins and their downstream effectors are major contributors to global cellular activity in oligodendrocytes.
FIGURE 3.
GPR17 activation modified oligodendroglial cell global activity. A, representative traces of MDL29,951 determined by label-free DMR assays in Oli-neu cells pretreated for 24 h with 1 μm PD174265. B, concentration-effect curve derived from the DMR traces in A of seven independent experiments; DMR responses were normalized for each experiment as the percentage of the values obtained with 100 μm MDL29,951. C, characteristic DMR recordings of MDL29,951 in primary rat differentiating oligodendrocytes after culturing for 48 h in growth factor-free medium. D, DMR dose-effect curve in oligodendrocytes from the traces of six independent experiments; DMR responses were normalized for each experiment to the values obtained with 30 μm MDL29,951. E and F, representative DMR assays in Oli-neu cells after transfection with scrambled siRNA (E) and GPR17-siRNA (F); note the decrease in MDL29,951-induced traces upon GPR17 knockdown. G, concentration-response quantification of DMR responses in Oli-neu cells after GPR17 knockdown from five independent experiments. siSc, scrambled siRNA; siGPR17, GPR17-siRNA. H, GPR17-siRNA knockdown does not impair whole-cell DMR recordings triggered by 100 μm ATP in Oli-neu cells (n = 5); DMR responses were normalized for each experiment to the values obtained with 30 μm MDL29,951 (MDL) in Oli-neu cells after transfection with scrambled siRNA. I, illustrative Western blot (upper panel) and quantitative analysis from five independent experiments (lower panel) of GPR17 expression levels upon GPR17 knockdown in primary rat oligodendrocytes. ***, p < 0.001. J, representative DMR assays in rat oligodendrocytes after transfection with scrambled siRNA or GPR17-siRNA. K, dose-response quantification of DMR responses in oligodendrocytes after GPR17 knockdown from six different experiments. L, DMR responses triggered by 100 μm ATP are not affected by GPR17 knockdown in differentiating oligodendrocytes (n = 6); DMR responses were normalized for each experiment to the values obtained with 100 μm MDL29,951 (MDL) in rat oligodendrocytes after transfection with scrambled siRNA.
FIGURE 4.
Stimulation of GPR17 predominantly triggered Gαi/o signaling in oligodendroglial cells. Characteristic DMR traces triggered by MDL29,951 in Oli-neu cells pretreated for 24 h with 1 μm PD174265 (A) and primary rat differentiating oligodendrocytes after culturing for 48 h in growth factor-free medium (B) in the presence or absence of PTX. Shown are concentration-response curves generated from the traces of seven independent experiments in Oli-neu cells (C) and six different experiments in oligodendrocytes (D).
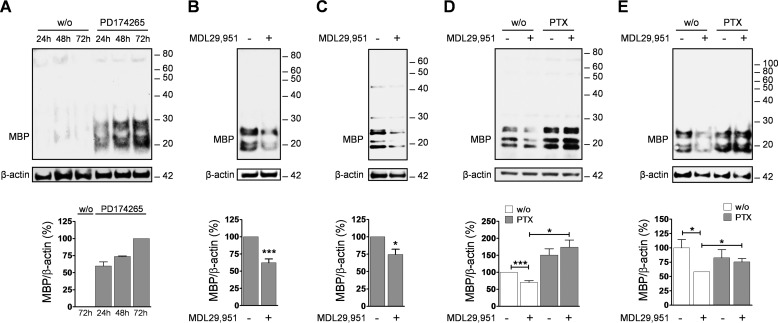
We, therefore, hypothesized that stimulation of the Gαi/o signaling cascade by GPR17 may largely be responsible for the reported delay in oligodendrocyte maturation. To test this assumption, we first incubated both oligodendroglial cells in the presence or absence of MDL29,951 and quantified expression of the typical myelination marker MBP (21). Western blotting and densitometric analysis showed that differentiating Oli-neu cells express high levels of MBP already after 48 h of incubation with PD174265, in contrast to untreated cells (Fig. 5A). In the presence of MDL29,951, MBP levels were markedly attenuated compared with control samples (Fig. 5B). A similar result was obtained in primary rat differentiating oligodendrocytes (Fig. 5C), where treatment with the GPR17 agonist overcame the MBP increase induced by the thyroid hormone triiodothyronine (T3), a known promoter of oligodendrocyte maturation. These data are congruent with previous findings in primary mouse oligodendroglial cells, in which MDL29,951-mediated GPR17 activation arrests oligodendrocytes at a less differentiated stage, resulting in a pronounced loss of MBP-positive cells (7). If Gαi/o proteins and their downstream effectors were responsible for the observed maturation arrest, Gαi/o inhibitor PTX should prevent the decrease of MBP expression induced by GPR17 agonist MDL29,951 in both cell types. Immunoblotting analyses showed that this was indeed the case in both Oli-neu (Fig. 5D) and rat oligodendrocytes (Fig. 5E). Taken together, our data provide evidence that in oligodendroglial cells GPR17 activation by MDL29,951 predominantly triggers the Gαi/o signaling pathway that, in turn, leads to arrest of the oligodendrocyte maturation program.
FIGURE 5.
Gαi/o signaling triggered by GPR17 decreased MBP expression levels. A, representative Western blot analysis of MBP levels in lysates from Oli-neu cells cultured for 24, 48, and 72 h in the absence and presence of 1 μm PD174265 (upper panel) and quantification of MBP levels in differentiating Oli-neu cells from three separate experiments (lower panel). B, illustrative Western blot of MBP abundance (upper panel) and quantitative analysis of MBP immunoreactive band from eight independent experiments (lower panel) showing that Oli-neu cells treated with 10 μm MDL29,951 for 48 h in the presence of 1 μm PD174265 expressed lower MBP levels compared with untreated cells. C, similar studies and MBP quantification analysis as in B performed in rat primary oligodendrocytes (n = 6) cultured in the presence of triiodothyronine (T3) and 30 μm MDL29,951 for 48 h. D, Oli-neu cells were treated with 1 μm PD174265 and 10 μm MDL29,951 in the absence and presence of PTX. Lysates were prepared and analyzed by Western blotting with antibodies against MBP and β-actin (upper panel); quantification of the effect of PTX on MBP expression levels in Oli-neu cells from 10 different experiments is shown in the lower panel. E, rat OPCs were treated with PTX for 16 h in growth factor-free medium, then T3 and 30 μm MDL29,951 were added to the cells and cultured for further 48 h. Lysates were analyzed by Western blotting for MBP abundance (upper panel); summary of MBP level quantification upon PTX treatment in differentiating oligodendrocytes from eight independent experiments (lower panel). *, p < 0.05; ***, p < 0.001.
Elevation of Intracellular cAMP Levels Induces Oligodendrocyte Differentiation in the Presence of GPR17 Agonist
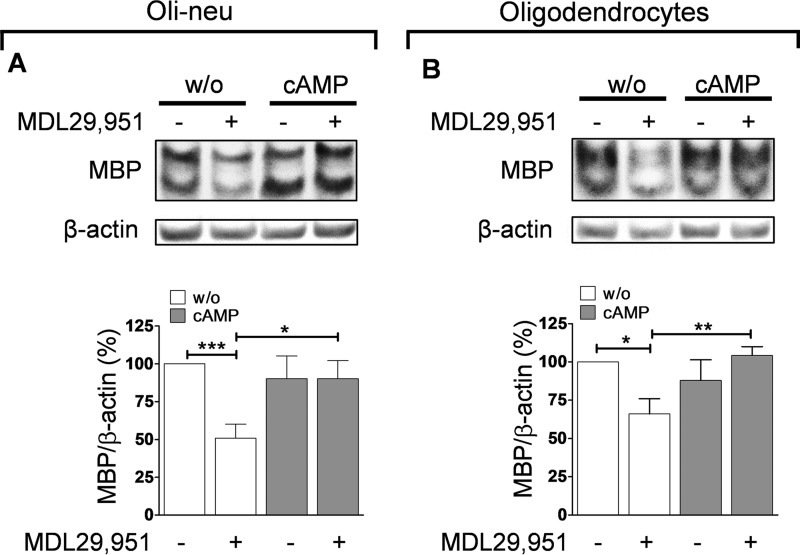
Gαi/o mainly diminishes the activity of the enzyme adenylyl cyclase, which converts ATP to cAMP and PPi (pyrophosphate). Elevated intracellular levels of cAMP have been reported to influence oligodendrocyte development, favoring their differentiation and inducing expression of myelin proteins (22–24). Because activation of Gαi/o proteins inhibits adenylyl cyclase and thereby reduces intracellular cAMP levels, we assumed that GPR17 transduces its effect on MBP expression by lowering oligodendrocyte cAMP concentration. To test this prediction, we treated both oligodendroglial cells with the nonhydrolyzable cAMP analog 8-(4-chlorophenylthio)-adenosine-3′,5′-cAMP (8-CPT-cAMP), which mimics an increase of intracellular cAMP in the presence or absence of MDL29,951. Western blot analyses revealed that 8-CPT-cAMP did not alter MBP levels in its own right but enhanced MBP expression in the presence of MDL29,951 to levels comparable with those of control-treated cells (Fig. 6, A and B). These results indicate that 8-CPT-cAMP is competent to counteract the MDL29,951-mediated differentiation blockade and restores oligodendrocyte maturation, thus supporting the notion that inhibition of adenylyl cyclase is the downstream target of Gαi/o upon GPR17 activation.
FIGURE 6.
Treatment with 8-CPT-cAMP promoted MBP expression in the presence of GPR17 agonist. A, upper panel, representative Western blot examining MBP levels in differentiating Oli-neu cells treated for 48 h with 10 μm MDL29,951 in the absence (w/o) and presence of 10 μm 8-CPT-cAMP (cAMP); lower panel, assessment of MBP abundance in Oli-neu cells from 10 independent experiments. B, upper panel, illustrative Western blotting analyzing MBP expression from primary rat oligodendrocytes cultured for 48 h with triiodothyronine (T3) and co-treated with 30 μm MDL29,951 in combination with 1 μm 8-CPT-cAMP; lower panel, summary of MBP quantification obtained in rat oligodendrocyte lysates from five different experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Effects of GPR17 Stimulation on Protein Kinase A Function and Phosphorylation of CREB
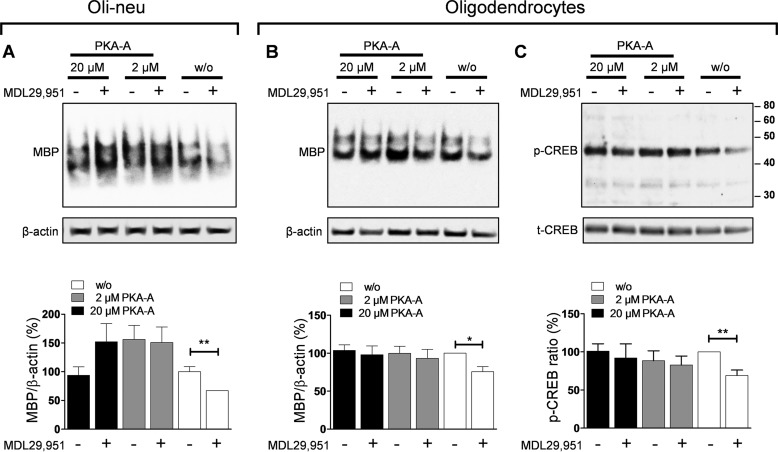
Intracellular levels of cAMP regulate complex cellular processes through the activation of a selected range of downstream effector proteins, such as the serine/threonine PKA. To test whether PKA activity is altered upon GPR17 stimulation, both Oli-neu cells and primary rat oligodendrocytes were treated with N6-benzoyladenosine-3,5-cyclic monophosphate (Sp-6-Bnz-cAMPS), a selective cell-permeable activator of PKA (25). Immunoblotting quantification analyses of MBP expression displayed that treatment with Sp-6-Bnz-cAMPS overcame the decrease of MBP expression induced by MDL29,951 (Fig. 7, A and B). These data suggest that depression of PKA activity is a downstream signaling consequence of GPR17 activation.
FIGURE 7.
Inhibition of oligodendrocyte differentiation by GPR17 was caused by down-regulation of PKA and CREB activities. A, upper panel, representative Western blot of MBP levels in differentiating Oli-neu cells treated for 48 h with 10 μm MDL29,951 in the absence and presence of different concentrations of the PKA activator Sp-6-Bnz-cAMPS (PKA-A); lower panel, assessment of MBP levels from eight separate experiments in Oli-neu cells showing that treatment with Sp-6-Bnz-cAMPS promotes MBP expression in the presence of the GPR17 agonist. B, upper panel, similar experiment as in A carried out in primary rat oligodendrocytes cultured for 48 h in medium with triiodothyronine (T3) and 30 μm MDL29,951; lower panel, bar graph showing quantification of MBP abundance in differentiating oligodendrocytes (n = 7). C, the upper panel displays an illustrative blot of phosphorylated CREB (p-CREB) and afterward reprobed for total CREB levels (t-CREB) in oligodendrocytes after 48 h incubation with T3 in the absence and presence of 30 μm MDL29,951 showing both the significant decrease of CREB phosphorylation upon GPR17 activation and promotion of CREB phosphorylation with different concentrations of Sp-6-Bnz-cAMPS in the presence of MDL29,951. Lower panel, evaluation of phosphorylated to total CREB ratios from seven independent experiments in oligodendrocytes differentiated for 48 h with T3. *, p < 0.05; **, p < 0.01.
PKA elicits changes in gene expression in cells by phosphorylating transcription factors within the nucleus. One of the best characterized PKA-activated transcription factors is CREB, which is known to stimulate MBP expression in oligodendrocytes (24, 26, 27). To examine whether activation of CREB in rat differentiating oligodendrocytes is altered upon GPR17 stimulation, we measured CREB phosphorylation in the presence of MDL29,951. We detected a significant decrease in CREB phosphorylation in cells pretreated with GPR17 agonist MDL29,951 but not in those exposed to solvent only (Fig. 7C). Notably, incubation with Sp-6-Bnz-cAMPS counteracted the reduction of CREB phosphorylation mediated by MDL29,951 (Fig. 7C). Altogether, our data point to a key role of GPR17 decreasing cAMP levels, which in turn depresses PKA function and CREB activation, thereby ultimately diminishing MBP expression.
GPR17 Agonist Does Not Induce Sustained Activation of ERK1/2 Phosphorylation
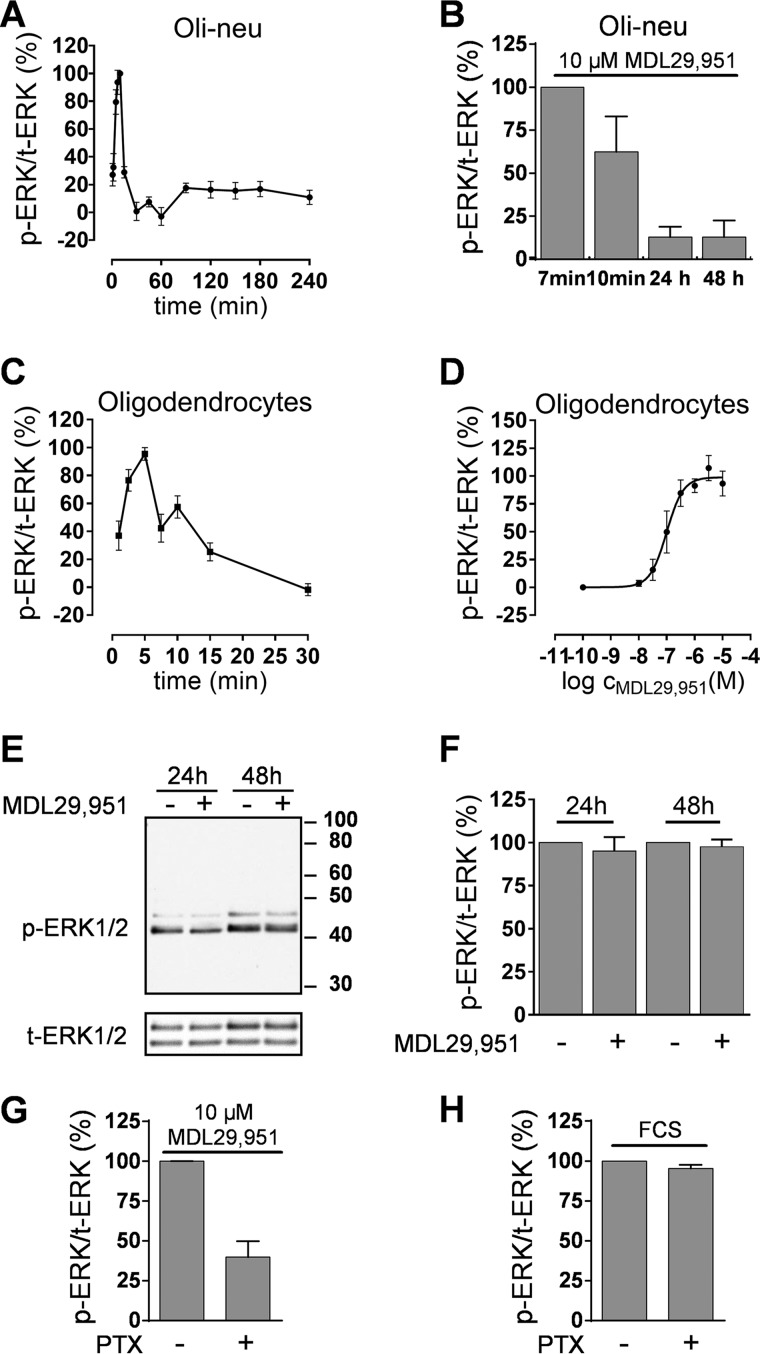
PKA is known to activate CREB through two downstream mechanisms; first, by direct translocation of activated PKA to the cell nucleus where it phosphorylates and activates CREB and, second, by activation of the mitogen-activated protein kinase (MAPK) ERK1/2 cascade, which in turn phosphorylates CREB (12, 28, 29). Because ERK1/2 long term activation is known to enhance CNS myelination (30, 31) and stimulation of GPR17 increases the levels of phosphorylated ERK1/2 (pERK1/2) in heterologous cell expression systems (7), we investigated whether activation of GPR17 in oligodendroglial cells was also followed by phosphorylation modifications of ERK1/2. Treatment of Oli-neu cells with MDL29,951 resulted in robust increases of pERK1/2 that were rapid and transient in nature, peaking within 7–10 min and returning to baseline after 30 min (Fig. 8A). Longer incubations with MDL29,951 were not associated with alterations of pERK1/2 (Fig. 8B), demonstrating active short term signaling of GPR17 through the ERK pathway, but no sustained activation that would be necessary for effective control of MBP expression. Similar to our observations in Oli-neu cells, primary rat differentiating oligodendrocytes also responded with an early and transient peak of pERK1/2 upon GPR17 stimulation (Fig. 8C), which was concentration-dependent (Fig. 8D). No evidence for sustained ERK phosphorylation was detected at later time points (Fig. 8, E and F). Gαi/o inhibitor PTX partly ablated the transient pERK1/2 increases (Fig. 8G) but was ineffective when FCS was applied as the activating agent (Fig. 8H). These data indicate that MDL29,951-stimulated GPR17 triggers phosphorylation of ERK1/2 only at short time points and in part via G proteins of the Gαi/o family but does not confer sustained ERK activation, which is considered key for efficient enhancement of MBP expression.
FIGURE 8.
GPR17 stimulation exclusively triggered short term ERK1/2 activation. A, kinetics of ERK1/2 phosphorylation in differentiating Oli-neu cells after treatment with 10 μm MDL29,951 (n = 6). B, phosphorylation ratio of ERK1/2 in Oli-neu cells after incubation with MDL29,951 for 10 min and 24 and 48 h compared with the phosphorylation peak detected upon 7 min treatment (n = 4). C, time-course of ERK1/2 phosphorylation triggered by 10 μm MDL29,951 in primary rat oligodendrocytes previously differentiated for 48 h in growth factor-free medium (n = 5). D, concentration-effect curve derived from the data (phosphorylation peak upon 5 min of treatment) of three separate experiments in oligodendrocytes. E, representative Western blot analysis of phosphorylated ERK1/2 (p-ERK1/2) reprobed for total ERK1/2 levels (t-ERK1/2) in differentiating primary rat oligodendrocytes after 24 and 48 h of incubation in the presence and absence of 10 μm MDL29,951. F, quantitative analysis of phosphorylated to total ERK1/2 ratios in oligodendrocytes from five independent experiments. G, MDL29,951-mediated short term phosphorylation (5 min) of ERK1/2 in oligodendrocytes is diminished after pretreatment with PTX (n = 5). H, PTX treatment does not affect phosphorylation of ERK1/2 triggered by 10% fetal calf serum (FCS) in oligodendrocytes (n = 3).
GPR17 Inhibits Oligodendrocyte Differentiation via EPAC
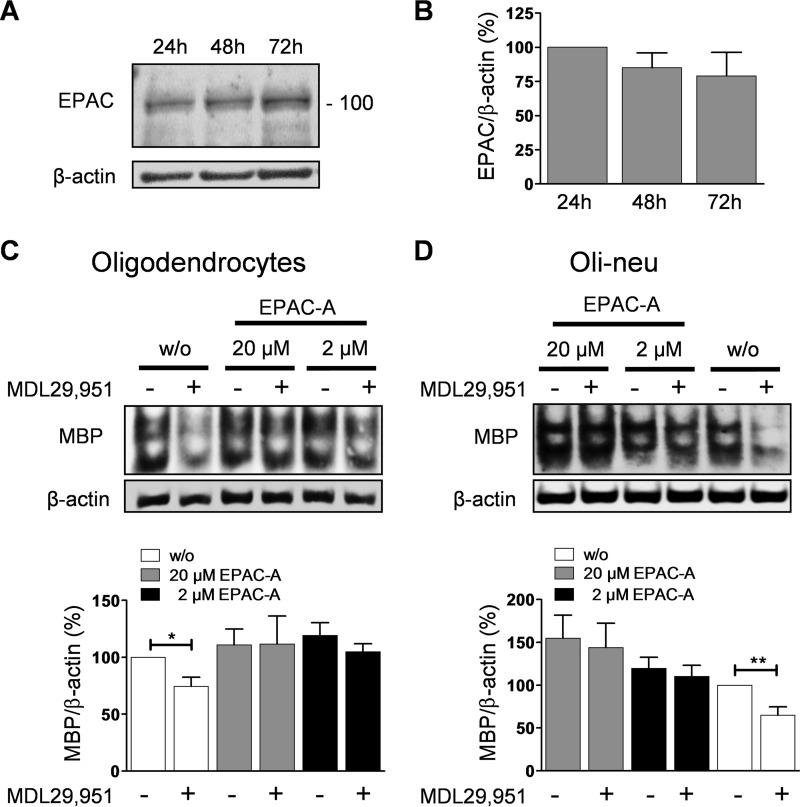
In addition to PKA, elevations of intracellular cAMP also lead to activation of a second type of effector protein, the EPAC (32). It has become apparent that PKA and EPAC coordinately regulate multiple processes within the same cell (33). Because the role of EPAC in oligodendrocytes is still elusive, we chose to also investigate contribution of EPAC as cAMP downstream effector to GPR17 signaling and function. We initially verified EPAC expression during oligodendrocyte differentiation by detecting its specific immunoreactive band in Western blot analysis (Fig. 9, A and B). Treatment of oligodendrocytes with increasing concentrations of the selective EPAC activator 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8-CPT-2′-O-Me-cAMP) (34, 35) did not affect expression levels of MBP in its own right but completely restored MDL29,951-induced MBP depression (Fig. 9C). Essentially similar findings were obtained in Oli-neu cells, in which incubation with the EPAC activator also overcame the GPR17-mediated lessening of MBP (Fig. 9D). Taken together, our data indicate that cAMP reduction triggered by MDL29,951 alters EPAC function as a downstream signaling consequence of GPR17 activation.
FIGURE 9.
Activation of EPAC by treatment with 8-CPT-2′-O-Me-cAMP promoted oligodendrocyte differentiation in the presence of GPR17 agonist. A, rat oligodendrocytes were differentiated in the presence of triiodothyronine (T3) for 24, 48, and 72 h; lysates were analyzed by Western blotting for EPAC expression with antibodies recognizing EPAC1/2 (EPAC). B, assessment of EPAC abundance during oligodendrocyte differentiation from four different experiments. C, upper panel, representative Western blotting demonstrating that co-treatment with the EPAC activator 8-CPT-2′-O-Me-cAMP (EPAC-A) increases MBP expression in the presence of 30 μm MDL29,951 in rat oligodendrocytes differentiated with T3 for 48 h. Lower panel, quantitative analysis from six independent experiments of MBP expression levels in primary rat oligodendrocytes. D, upper panel, illustrative Western blot displaying MBP expression levels in Oli-neu cells differentiated with PD174265 for 48 h treated with 10 μm MDL29,951 and 8-CPT-2′-O-Me-cAMP. Lower panel, bar graph showing quantification of MBP abundance in Oli-neu cells from eight different experiments. *, p < 0.05; **, p < 0.01.
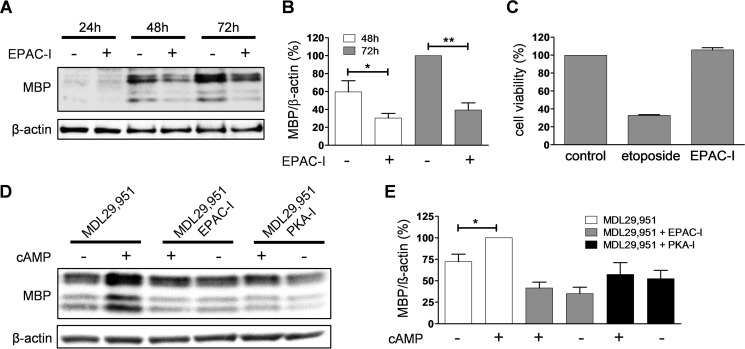
Our findings also suggest that EPAC activation could play a heretofore unappreciated role during oligodendrocyte differentiation. To further confirm the hypothesis that active signaling via EPAC is relevant for oligodendrocyte maturation, we incubated primary rat oligodendrocytes in the presence of the specific EPAC inhibitor ESI-05 (36). In line with our prediction, pharmacological silencing of EPAC resulted in a significant reduction of MBP immunoreactivity (Fig. 10, A and B), indicating arrest of oligodendrocyte differentiation. The decrease in MBP abundance is not due to alterations in the integrity of oligodendroglial cells as no change in cell viability was found after long term treatment with ESI-05 (Fig. 10C). Furthermore, treatment with ESI-05 also blocked the counteracting effect of 8-CPT-cAMP on MDL29,951-mediated MBP depression to a similar extent as 8-(4-chlorophenylthio)adenosine-3′,5′-cyclic monophosphorothioate, Rp isomer (Rp-8-CPT-cAMPS), a selective inhibitor of protein kinase A (37, 38), confirming the downstream involvement of both EPAC and PKA upon cAMP modulation mediated by GPR17 activation (Fig. 10, D and E). Therefore, our data identify signaling via EPAC as a novel determinant of oligodendrocyte differentiation.
FIGURE 10.
Inhibition of EPAC arrested oligodendrocyte differentiation. A, representative Western blot analysis of MBP abundance of lysates prepared from rat oligodendrocytes differentiated with triiodothyronine (T3) for 24, 48, and 72 h in the presence and absence of the EPAC inhibitor ESI-05 (EPAC-I). B, evaluation of MBP expression levels in rat oligodendrocytes (n = 5) after 48 h and 72 h differentiation with T3 treated with 10 μm ESI-05 (EPAC-I). C, quantification of cell viability analysis from three independent experiments showing that in contrast to the cytotoxic effect of the topoisomerase II inhibitor etoposide (50 μm), treatment with 10 μm ESI-05 did not affect cell metabolic capacity and survival after 48 h treatment in Oli-neu cells. D, illustrative Western blotting analyzing the effect of 10 μm ESI-05 (EPAC-I) and the PKA inhibitor Rp-8-CPT-cAMPS (PKA-I) on MBP expression in primary rat oligodendrocytes cultured for 48 h with triiodothyronine (T3) and 30 μm MDL29,951 in the absence and presence of 1 μm 8-CPT-cAMP (cAMP). E, quantitative analysis of MBP immunoreactive band corrected by β-actin from five independent experiments showed that treatment with inhibitors of either PKA (PKA-I) or EPAC (EPAC-I) impaired the counteracting effect of 8-CPT-cAMP (cAMP) on MDL29,951-mediated MBP reduction. *, p < 0.05; **, p < 0.01.
Discussion
The present study demonstrates that stimulation of GPR17 by the small molecule agonist MDL29,951 diminishes oligodendrocyte differentiation through the Gαi/o signaling pathway, reducing the activity of the downstream cAMP-specific effectors PKA and EPAC and finally leading to decreased cellular abundance of the myelination marker MBP. These findings further extend previous studies characterizing GPR17 as inhibitor of oligodendrocyte differentiation (6, 7) in that they delineate the cellular mechanisms employed by this receptor to control the oligodendrocyte maturation program.
In contrast to heterologous expression systems, where MDL29,951-activated GPR17 triggers the entire repertoire of pathways linked to second messenger production, i.e. Gαq, Gαi/o, and Gαs, GPR17 exclusively signals via Gαi/o and Gαq in primary rat differentiating oligodendrocytes (7). Here, we observed the same Gα protein preference in Oli-neu cells, an immortalized oligodendroglial cell line that expresses GPR17 during its differentiation upon induction by PD174265. Interestingly, both Gαi/o and Gαq cascades have been associated with impaired oligodendrocyte maturation (8, 12). We determine that diminishing of differentiation in Oli-neu cells and primary rat oligodendrocytes after GPR17 activation is largely mediated by the Gαi/o signaling pathway because the Gαi/o protein inhibitor PTX abolished the observed MDL29,951-mediated decrease of MBP. Accordingly, analyses of MDL29,951-triggered cell DMR also displayed a major contribution of Gαi/o signaling to the global cell response profiles recorded in both oligodendroglial cells. Together, these results uncover a predominant role for Gαi/o proteins as critical relays that functionally link GPR17 to oligodendroglial cellular activity and control of oligodendrocyte maturation.
Triggering of Gαi/o proteins by GPR17 led us to investigate whether reduction of cAMP levels may be crucial for MDL29,95-mediated inhibition of oligodendrocyte differentiation. Incubation with analogs of cAMP or sustained elevation of endogenous cAMP by either activation of adenylyl cyclase (10, 23, 26, 39, 40) or inhibition of the cAMP-hydrolyzing enzyme phosphodiesterase-7 (11) have been reported to increase oligodendrocyte differentiation, demonstrating that regulation of cAMP intracellular abundance is an efficient mechanism to control oligodendrocyte maturation. Accordingly, CNS myelin debris, a known inhibitor of oligodendroglial differentiation, partly exerts its action by attenuating cAMP levels in oligodendrocytes, which can be overcome by treatment with either dibutyryl-cAMP or rolipram, an inhibitor of phosphodiesterase-4 (12). Our study shows that impairment of oligodendroglial differentiation by MDL29,951 is also counteracted by incubation with 8-CPT-cAMP, suggesting that GPR17 operates via a similar inhibitory mechanism, i.e. effective reduction of cAMP and its associated downstream signaling.
The cAMP response element-binding protein CREB has been implicated in controlling myelin gene expression and oligodendrocyte maturation (41–43). Augmentation in CREB phosphorylation is observed during in vitro spontaneous oligodendrocyte differentiation (12). Treatments that induce a sustained elevation of cAMP levels in oligodendrocytes activate CREB and subsequently increase MBP protein levels by activating the MBP promoter (24, 26, 27, 44). Conversely, inactivation of CREB has been reported in the presence of inhibitors of oligodendrocyte maturation, such as GSK3β (45) or CNS myelin (12). In agreement with the role of GPR17 as oligodendroglial differentiation inhibitor, we observed a reduction in the phosphorylation level of CREB upon incubation with MDL29,951, which is reversed by treatment with Sp-6-Bnz-cAMPS, a specific activator of the CREB upstream kinase PKA. Consistent with this finding, incubation with Sp-6-Bnz-cAMPS also counteracted the decrease of MBP triggered by the GPR17 agonist. Furthermore, our data point to a direct activation of CREB by PKA rather than via the MAPK ERK1/2 cascade, as we could not detect long term variations of ERK1/2 phosphorylation upon GPR17 activation. Sustained but not short term ERK activation plays an important role in oligodendrocytes for timing of the progression of maturation (41, 46–49), exerting its action partly by modifying CREB phosphorylation (12, 41–43). Regardless, we only detected short term ERK phosphorylation when MDL29,951 was added to the cultures. From these data we conclude that a signal transduction module consisting of GPR17-Gαi/o-adenylyl cyclase-PKA-CREB controls expression of genes associated to oligodendroglia differentiation, such as MBP.
The finding that cAMP levels are down regulated upon GPR17 activation prompted us to investigate whether other known cAMP effector proteins are affected during oligodendrocyte maturation. In a variety of cellular contexts, EPAC mediates cAMP signaling independent of PKA, acting either synergistically or antagonistically even in the same cell (25, 33, 50–55). Our data reveal EPAC expression in oligodendrocytes, thus remaining as an alternative cAMP-mediated pathway for oligodendroglia differentiation. Similar to activation of PKA, stimulation of EPAC reverses the MBP decrease induced by GPR17, whereas EPAC inhibition attenuates MBP abundance. Therefore, both PKA and EPAC independently mediate cAMP-regulated differentiation in oligodendroglia.
Previous studies have indicated that the RhoA pathway and the PI3 kinase-AKT cascade regulate oligodendroglia maturation (9, 56, 57). Interestingly, EPAC is known to directly modulate both pathways. In vascular endothelium, EPAC controls vascular tone by down-regulation of RhoA activity (51, 58), whereas in primary cortical neurons EPAC acts as an important enhancer of AKT activity (59). Moreover, cAMP overcomes nitric oxide-induced apoptosis in neonatal rat cardiomyocytes through both cAMP-EPAC-AKT and cAMP-PKA-CREB pathways (60). Therefore, by employing both PKA and EPAC, cAMP may distinctly control discrete signaling pathways that enhance oligodendrocyte differentiation. Consequently, EPAC might provide a new attractive target for therapeutic enhancement of remyelination, potentially allowing other cellular functions mediated by the cAMP-PKA signaling module to continue undisturbed. Clearly, future studies will be required to dissect the exact contributions of EPAC and PKA to cAMP-dependent oligodendroglia maturation.
In summary, our findings suggest a model (Fig. 11) in which increasing concentrations of intracellular cAMP activate EPAC and PKA, which in turn activates CREB, leading to oligodendrocyte differentiation. Stimulation of GPR17 inhibits adenylyl cyclase, thus depressing cAMP levels, which ultimately leads to arrest of differentiation due to impaired activation of PKA and EPAC. The model identifies PKA and EPAC as key downstream effectors of GPR17 that inhibit oligodendrocyte maturation. Moreover, the model predicts that interventions that increase intracellular cAMP will overcome the GPR17-mediated blockade of oligodendrocyte differentiation. We, therefore, envisage that demyelinating pathologies where GPR17 is highly expressed, as is the case in multiple sclerosis (6), will benefit greatly from augmentation of intracellular cAMP to specifically foster the remyelination process.
FIGURE 11.
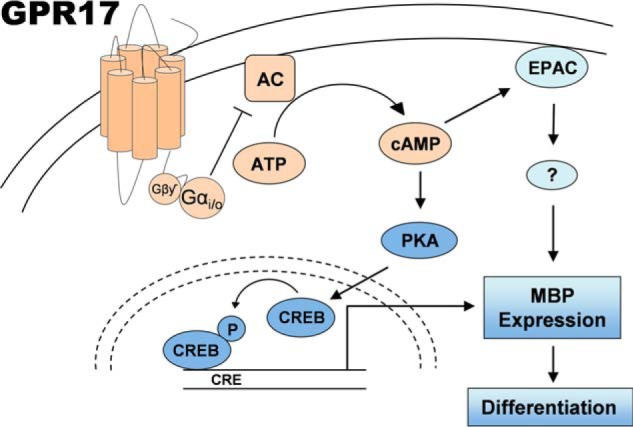
Proposed model of GPR17 function during oligodendrocyte differentiation. Stimulation of GPR17 triggers activation of Gαi/o to impair cAMP signaling by reducing the activity of adenylyl cyclase. Decreased levels of cAMP in the oligodendrocyte led to diminished PKA activity and lower levels of phosphorylated CREB, thus altering transcription of genes involved in maturation and myelination, such as MBP. In addition, reduced intracellular amount of cAMP also lessens EPAC activation, thereby affecting MBP abundance. The model predicts that treatments which lead to an increase of intracellular cAMP should promote oligodendrocyte differentiation in demyelinating pathologies where GPR17 is highly expressed, such as multiple sclerosis.
Author Contributions
K. S. and S. H. designed, performed, and analyzed the majority of functional and cell biology experiments. N. M. designed, performed, and analyzed functional cell biology, RNA interference, and cell viability experiments. S. B. designed, performed, and analyzed RNA interference assays. M. G. provided important ideas and assisted in project coordination. E. K. and J. G. designed the research, coordinated the project, and wrote the manuscript.
Acknowledgments
We thank Prof. M. Eckhardt for providing the Oli-neu cell line and advising on its culturing, M. Vasmer-Ehses for excellent technical assistance, and Corning Inc. and PerkinElmer for their support on label-free DMR (Corning Epic BT System and PerkinElmer EnSight).
This work was supported by a grant from the German Federal Ministry for Education and Research (BMBF) within the BioPharma Initiative “Neuroallianz” (Grant 1615609B; to E. K.). K. S. is a member of the German Research Foundation (DFG)-funded Research Training Group RTG1873. The authors declare that they have no conflicts of interest with the contents of this article.
- OPC
- oligodendrocyte precursor cell
- MS
- multiple sclerosis
- MBP
- myelin basic protein
- CREB
- cAMP-response element-binding protein
- EPAC
- exchange protein directly activated by cAMP
- MDL29,951
- 2-carboxy-4,6-dichloro-1H-indole-3-propionic acid
- Sp-6-Bnz-cAMPS
- N6-benzoyladenosine-3,5-cyclic monophosphate
- CPT
- chlorophenylthio-
- DMR
- dynamic mass redistribution
- PTX
- pertussis toxin.
References
- 1.Miller R. H. (2002) Regulation of oligodendrocyte development in the vertebrate CNS. Prog. Neurobiol. 67, 451–467 [DOI] [PubMed] [Google Scholar]
- 2.Franklin R. J., and Ffrench-Constant C. (2008) Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9, 839–855 [DOI] [PubMed] [Google Scholar]
- 3.Fancy S. P., Chan J. R., Baranzini S. E., Franklin R. J., and Rowitch D. H. (2011) Myelin regeneration: a recapitulation of development? Annu. Rev. Neurosci. 34, 21–43 [DOI] [PubMed] [Google Scholar]
- 4.Kremer D., Aktas O., Hartung H.-P., and Küry P. (2011) The complex world of oligodendroglial differentiation inhibitors. Ann. Neurol. 69, 602–618 [DOI] [PubMed] [Google Scholar]
- 5.Qi A.-D., Harden T. K., and Nicholas R. A. (2013) Is GPR17 a P2Y/leukotriene receptor? examination of uracil nucleotides, nucleotide sugars, and cysteinyl leukotrienes as agonists of GPR17. J. Pharmacol. Exp. Ther. 347, 38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., Wu H., Wang S., Koito H., Li J., Ye F., Hoang J., Escobar S. S., Gow A., Arnett H. A., Trapp B. D., Karandikar N. J., Hsieh J., and Lu Q. R. (2009) The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci. 12, 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hennen S., Wang H., Peters L., Merten N., Simon K., Spinrath A., Blättermann S., Akkari R., Schrage R., Schröder R., Schulz D., Vermeiren C., Zimmermann K., Kehraus S., Drewke C., Pfeifer A., König G. M., Mohr K., Gillard M., Müller C. E., Lu Q. R., Gomeza J., and Kostenis E. (2013) Decoding signaling and function of the orphan G protein-coupled receptor GPR17 with a small-molecule agonist. Sci. Signal. 6, ra93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baer A. S., Syed Y. A., Kang S. U., Mitteregger D., Vig R., Ffrench-Constant C., Franklin R. J., Altmann F., Lubec G., and Kotter M. R. (2009) Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain 132, 465–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackerman S. D., Garcia C., Piao X., Gutmann D. H., and Monk K. R. (2015) The adhesion GPCR Gpr56 regulates oligodendrocyte development via interactions with Gα12/13 and RhoA. Nat. Commun. 6, 6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiemelt A. P., Engleka M. J., Skorupa A. F., and McMorris F. A. (1997) Immunochemical visualization and quantitation of cyclic AMP in single cells. J. Biol. Chem. 272, 31489–31495 [DOI] [PubMed] [Google Scholar]
- 11.Medina-Rodríguez E. M., Arenzana F. J., Pastor J., Redondo M., Palomo V., García de Sola R., Gil C., Martínez A., Bribián A., and de Castro F. (2013) Inhibition of endogenous phosphodiesterase 7 promotes oligodendrocyte precursor differentiation and survival. Cell. Mol. Life Sci. 70, 3449–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syed Y. A., Baer A., Hofer M. P., González G. A., Rundle J., Myrta S., Huang J. K., Zhao C., Rossner M. J., Trotter M. W., Lubec G., Franklin R. J., and Kotter M. R. (2013) Inhibition of phosphodiesterase-4 promotes oligodendrocyte precursor cell differentiation and enhances CNS remyelination. EMBO Mol. Med. 5, 1918–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y., Balasubramaniyan V., Peng J., Hurlock E. C., Tallquist M., Li J., and Lu Q. R. (2007) Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat. Protoc. 2, 1044–1051 [DOI] [PubMed] [Google Scholar]
- 14.Bock A., Merten N., Schrage R., Dallanoce C., Bätz J., Klöckner J., Schmitz J., Matera C., Simon K., Kebig A., Peters L., Müller A., Schrobang-Ley J., Tränkle C., Hoffmann C., De Amici M., Holzgrabe U., Kostenis E., and Mohr K. (2012) The allosteric vestibule of a seven transmembrane helical receptor controls G-protein coupling. Nat. Commun. 3, 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schröder R., Janssen N., Schmidt J., Kebig A., Merten N., Hennen S., Müller A., Blättermann S., Mohr-Andrä M., Zahn S., Wenzel J., Smith N. J., Gomeza J., Drewke C., Milligan G., Mohr K., and Kostenis E. (2010) Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat. Biotechnol. 28, 943–949 [DOI] [PubMed] [Google Scholar]
- 16.Schröder R., Schmidt J., Blättermann S., Peters L., Janssen N., Grundmann M., Seemann W., Kaufel D., Merten N., Drewke C., Gomeza J., Milligan G., Mohr K., and Kostenis E. (2011) Applying label-free dynamic mass redistribution technology to frame signaling of G protein-coupled receptors noninvasively in living cells. Nat. Protoc. 6, 1748–1760 [DOI] [PubMed] [Google Scholar]
- 17.Jung M., Krämer E., Grzenkowski M., Tang K., Blakemore W., Aguzzi A., Khazaie K., Chlichlia K., von Blankenfeld G., and Kettenmann H. (1995) Lines of murine oligodendroglial precursor cells immortalized by an activated neu tyrosine kinase show distinct degrees of interaction with axons in vitro and in vivo. Eur. J. Neurosci. 7, 1245–1265 [DOI] [PubMed] [Google Scholar]
- 18.Gobert R. P., Joubert L., Curchod M.-L., Salvat C., Foucault I., Jorand-Lebrun C., Lamarine M., Peixoto H., Vignaud C., Frémaux C., Jomotte T., Françon B., Alliod C., Bernasconi L., Abderrahim H., Perrin D., Bombrun A., Zanoguera F., Rommel C., and Hooft van Huijsduijnen R. (2009) Convergent functional genomics of oligodendrocyte differentiation identifies multiple autoinhibitory signaling circuits. Mol. Cell. Biol. 29, 1538–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fratangeli A., Parmigiani E., Fumagalli M., Lecca D., Benfante R., Passafaro M., Buffo A., Abbracchio M. P., and Rosa P. (2013) The regulated expression, intracellular trafficking, and membrane recycling of the P2Y-like receptor GPR17 in Oli-neu oligodendroglial cells. J. Biol. Chem. 288, 5241–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mir F., and Le Breton G. C. (2008) A novel nuclear signaling pathway for thromboxane A2 receptors in oligodendrocytes: evidence for signaling compartmentalization during differentiation. Mol. Cell. Biol. 28, 6329–6341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vartanian T., Szuchet S., Dawson G., and Campagnoni A. T. (1986) Oligodendrocyte adhesion activates protein kinase C-mediated phosphorylation of myelin basic protein. Science 234, 1395–1398 [DOI] [PubMed] [Google Scholar]
- 22.Pleasure D., Parris J., Stern J., Grinspan J., and Kim S. U. (1986) Incorporation of tritiated galactose into galactocerebroside by cultured rat oligodendrocytes: effects of cyclic adenosine 3′,5′-monophosphate analogues. J. Neurochem. 46, 300–302 [DOI] [PubMed] [Google Scholar]
- 23.Raible D. W., and McMorris F. A. (1990) Induction of oligodendrocyte differentiation by activators of adenylate cyclase. J. Neurosci. Res. 27, 43–46 [DOI] [PubMed] [Google Scholar]
- 24.Pende M., Fisher T. L., Simpson P. B., Russell J. T., Blenis J., and Gallo V. (1997) Neurotransmitter- and growth factor-induced cAMP response element binding protein phosphorylation in glial cell progenitors: role of calcium ions, protein kinase C, and mitogen-activated protein kinase/ribosomal S6 kinase pathway. J. Neurosci. 17, 1291–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen A. E., Selheim F., de Rooij J., Dremier S., Schwede F., Dao K. K., Martinez A., Maenhaut C., Bos J. L., Genieser H.-G., and Døskeland S. O. (2003) cAMP analog mapping of Epac1 and cAMP kinase: discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J. Biol. Chem. 278, 35394–35402 [DOI] [PubMed] [Google Scholar]
- 26.Sato-Bigbee C., and DeVries G. H. (1996) Treatment of oligodendrocytes with antisense deoxyoligonucleotide directed against CREB mRNA: effect on the cyclic AMP-dependent induction of myelin basic protein expression. J. Neurosci. Res. 46, 98–107 [DOI] [PubMed] [Google Scholar]
- 27.Afshari F. S., Chu A. K., and Sato-Bigbee C. (2001) Effect of cyclic AMP on the expression of myelin basic protein species and myelin proteolipid protein in committed oligodendrocytes: differential involvement of the transcription factor CREB. J. Neurosci. Res. 66, 37–45 [DOI] [PubMed] [Google Scholar]
- 28.Costes S., Broca C., Bertrand G., Lajoix A.-D., Bataille D., Bockaert J., and Dalle S. (2006) ERK1/2 control phosphorylation and protein level of cAMP-responsive element-binding protein: a key role in glucose-mediated pancreatic beta-cell survival. Diabetes 55, 2220–2230 [DOI] [PubMed] [Google Scholar]
- 29.Smith F. D., Langeberg L. K., Cellurale C., Pawson T., Morrison D. K., Davis R. J., and Scott J. D. (2010) AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade. Nat. Cell Biol. 12, 1242–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubinfeld H., and Seger R. (2005) The ERK cascade: a prototype of MAPK signaling. Mol. Biotechnol. 31, 151–174 [DOI] [PubMed] [Google Scholar]
- 31.Xiao L., Guo D., Hu C., Shen W., Shan L., Li C., Liu X., Yang W., Zhang W., and He C. (2012) Diosgenin promotes oligodendrocyte progenitor cell differentiation through estrogen receptor-mediated ERK1/2 activation to accelerate remyelination. Glia 60, 1037–1052 [DOI] [PubMed] [Google Scholar]
- 32.de Rooij J., Zwartkruis F. J., Verheijen M. H., Cool R. H., Nijman S. M., Wittinghofer A., and Bos J. L. (1998) Epac is a Rap1 guanine nucleotide-exchange factor directly activated by cyclic AMP. Nature 396, 474–477 [DOI] [PubMed] [Google Scholar]
- 33.Schmidt M., Dekker F. J., and Maarsingh H. (2013) Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol. Rev. 65, 670–709 [DOI] [PubMed] [Google Scholar]
- 34.Enserink J. M., Christensen A. E., de Rooij J., van Triest M., Schwede F., Genieser H. G., Døskeland S. O., Blank J. L., and Bos J. L. (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4, 901–906 [DOI] [PubMed] [Google Scholar]
- 35.Holz G. G., Chepurny O. G., and Schwede F. (2008) Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell. Signal. 20, 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chepurny O. G., Bertinetti D., Diskar M., Leech C. A., Afshari P., Tsalkova T., Cheng X., Schwede F., Genieser H.-G., Herberg F. W., and Holz G. G. (2013) Stimulation of proglucagon gene expression by human GPR119 in enteroendocrine L-cell line GLUTag. Mol. Endocrinol. 27, 1267–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedegaard E. R., Nielsen B. D., Mogensen S., Rembold C. M., Frøbert O., and Simonsen U. (2014) Mechanisms involved in increased sensitivity to adenosine A(2A) receptor activation and hypoxia-induced vasodilatation in porcine coronary arteries. Eur. J. Pharmacol. 723, 216–226 [DOI] [PubMed] [Google Scholar]
- 38.Kankaanranta H., Parkkonen J., Ilmarinen-Salo P., Giembycz M. A., and Moilanen E. (2011) Salbutamol delays human eosinophil apoptosis via a cAMP-dependent mechanism. Pulm. Pharmacol. Ther. 24, 394–400 [DOI] [PubMed] [Google Scholar]
- 39.Jensen N. A., Smith G. M., Garvey J. S., Shine H. D., and Hood L. (1993) Cyclic AMP has a differentiative effect on an immortalized oligodendrocyte cell line. J. Neurosci. Res. 35, 288–296 [DOI] [PubMed] [Google Scholar]
- 40.Joubert L., Foucault I., Sagot Y., Bernasconi L., Duval F., Alliod C., Frossard M.-J., Pescini Gobert R., Curchod M.-L., Salvat C., Nichols A., Pouly S., Rommel C., Roach A., and van Hooft Huijsduijnen R. (2010) Chemical inducers and transcriptional markers of oligodendrocyte differentiation. J. Neurosci. Res. 88, 2546–2557 [DOI] [PubMed] [Google Scholar]
- 41.Baron W., de Jonge J. C., de Vries H., and Hoekstra D. (2000) Perturbation of myelination by activation of distinct signaling pathways: an in vitro study in a myelinating culture derived from fetal rat brain. J. Neurosci. Res. 59, 74–85 [PubMed] [Google Scholar]
- 42.Bhat N. R., Zhang P., and Mohanty S. B. (2007) p38 MAP kinase regulation of oligodendrocyte differentiation with CREB as a potential target. Neurochem. Res. 32, 293–302 [DOI] [PubMed] [Google Scholar]
- 43.McNulty S., Crouch M., Smart D., and Rumsby M. (2001) Differentiation of bipolar CG-4 line oligodendrocytes is associated with regulation of CREB, MAP kinase and PKC signalling pathways. Neurosci. Res. 41, 217–226 [DOI] [PubMed] [Google Scholar]
- 44.Meffre D., Massaad C., and Grenier J. (2015) Lithium chloride stimulates PLP and MBP expression in oligodendrocytes via Wnt/β-catenin and Akt/CREB pathways. Neuroscience 284, 962–971 [DOI] [PubMed] [Google Scholar]
- 45.Azim K., and Butt A. M. (2011) GSK3β negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia 59, 540–553 [DOI] [PubMed] [Google Scholar]
- 46.Bhat N. R., and Zhang P. (1996) Activation of mitogen-activated protein kinases in oligodendrocytes. J. Neurochem. 66, 1986–1994 [DOI] [PubMed] [Google Scholar]
- 47.Colognato H., Baron W., Avellana-Adalid V., Relvas J. B., Baron-Van Evercooren A., Georges-Labouesse E., and ffrench-Constant C. (2002) CNS integrins switch growth factor signalling to promote target-dependent survival. Nat. Cell Biol. 4, 833–841 [DOI] [PubMed] [Google Scholar]
- 48.Laursen L. S., and Ffrench-Constant C. (2007) Adhesion molecules in the regulation of CNS myelination. Neuron Glia Biol. 3, 367–375 [DOI] [PubMed] [Google Scholar]
- 49.Fyffe-Maricich S. L., Karlo J. C., Landreth G. E., and Miller R. H. (2011) The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J. Neurosci. 31, 843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts O. L., and Dart C. (2014) cAMP signalling in the vasculature: the role of Epac (exchange protein directly activated by cAMP). Biochem. Soc. Trans. 42, 89–97 [DOI] [PubMed] [Google Scholar]
- 51.Parnell E., Palmer T. M., and Yarwood S. J. (2015) The future of EPAC-targeted therapies: agonism versus antagonism. Trends Pharmacol. Sci. 36, 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray A. J., and Shewan D. A. (2008) Epac mediates cyclic AMP-dependent axon growth, guidance, and regeneration. Mol. Cell. Neurosci. 38, 578–588 [DOI] [PubMed] [Google Scholar]
- 53.Corredor R. G., Trakhtenberg E. F., Pita-Thomas W., Jin X., Hu Y., and Goldberg J. L. (2012) Soluble adenylyl cyclase activity is necessary for retinal ganglion cell survival and axon growth. J. Neurosci. 32, 7734–7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray A. J., Tucker S. J., and Shewan D. A. (2009) cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A. J. Neurosci. 29, 15434–15444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mironov S. L., and Skorova E. Y. (2011) Stimulation of bursting in pre-Bötzinger neurons by Epac through calcium release and modulation of TRPM4 and K-ATP channels. J. Neurochem. 117, 295–308 [DOI] [PubMed] [Google Scholar]
- 56.Flores A. I., Narayanan S. P., Morse E. N., Shick H. E., Yin X., Kidd G., Avila R. L., Kirschner D. A., and Macklin W. B. (2008) Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. 28, 7174–7183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goebbels S., Oltrogge J. H., Kemper R., Heilmann I., Bormuth I., Wolfer S., Wichert S. P., Möbius W., Liu X., Lappe-Siefke C., Rossner M. J., Groszer M., Suter U., Frahm J., Boretius S., and Nave K.-A. (2010) Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J. Neurosci. 30, 8953–8964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zieba B. J., Artamonov M. V., Jin L., Momotani K., Ho R., Franke A. S., Neppl R. L., Stevenson A. S., Khromov A. S., Chrzanowska-Wodnicka M., and Somlyo A. V. (2011) The cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by down-regulation of RhoA activity. J. Biol. Chem. 286, 16681–16692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nijholt I. M., Dolga A. M., Ostroveanu A., Luiten P. G., Schmidt M., and Eisel U. L. (2008) Neuronal AKAP150 coordinates PKA and Epac-mediated PKB/Akt phosphorylation. Cell. Signal. 20, 1715–1724 [DOI] [PubMed] [Google Scholar]
- 60.Kwak H.-J., Park K.-M., Choi H.-E., Chung K.-S., Lim H.-J., and Park H.-Y. (2008) PDE4 inhibitor, roflumilast protects cardiomyocytes against NO-induced apoptosis via activation of PKA and Epac dual pathways. Cell. Signal. 20, 803–814 [DOI] [PubMed] [Google Scholar]