Abstract
Src phosphorylates Runx1 on one central and four C-terminal tyrosines. We find that activated Src synergizes with Runx1 to activate a Runx1 luciferase reporter. Mutation of the four Runx1 C-terminal tyrosines to aspartate or glutamate to mimic phosphorylation increases trans-activation of the reporter in 293T cells and allows induction of Cebpa or Pu.1 mRNAs in 32Dcl3 myeloid cells, whereas mutation of these residues to phenylalanine to prevent phosphorylation obviates these effects. Three mechanisms contribute to increased Runx1 activity upon tyrosine modification as follows: increased stability, reduced histone deacetylase (HDAC) interaction, and increased DNA binding. Mutation of the five modified Runx1 tyrosines to aspartate markedly reduced co-immunoprecipitation with HDAC1 and HDAC3, markedly increased stability in cycloheximide or in the presence of co-expressed Cdh1, an E3 ubiquitin ligase coactivator, with reduced ubiquitination, and allowed DNA-binding in gel shift assay similar to wild-type Runx1. In contrast, mutation of these residues to phenylalanine modestly increased HDAC interaction, modestly reduced stability, and markedly reduced DNA binding in gel shift assays and as assessed by chromatin immunoprecipitation with the −14-kb Pu.1 or +37-kb Cebpa enhancers after stable expression in 32Dcl3 cells. Affinity for CBFβ, the Runx1 DNA-binding partner, was not affected by these tyrosine modifications, and in vitro translated CBFβ markedly increased DNA affinity of both the translated phenylalanine and aspartate Runx1 variants. Finally, further supporting a positive role for Runx1 tyrosine phosphorylation during granulopoiesis, mutation of the five Src-modified residues to aspartate but not phenylalanine allows Runx1 to increase Cebpa and granulocyte colony formation by Runx1-deleted murine marrow.
Keywords: hematopoiesis, histone deacetylase (HDAC), myeloid cell, Src, transcription factor, Runx1, tyrosine kinase
Introduction
Runx1/AML1 directs the emergence of long term adult hematopoietic stem cells from hemogenic endothelium during development and subsequently contributes to lymphoid, myeloid, and megakaryocyte lineage development (1–4). Runx1 contributes to myeloid lineage specification in part via induction of Pu.1 via its −14-kb enhancer and of Cebpa via its +37-kb enhancer (4–7). Runx1 gene deletion in adult mice reduces both Pu.1 and Cebpa RNA levels in marrow myeloid progenitors, with consequent reduced granulopoiesis and increased monopoiesis (4). In addition to directing differentiation, Runx1 favors G1 to S cell cycle progression, in part via induction of cdk4 and cyclin D3, and inhibits apoptosis, in part due to suppression of p53 induction and stimulation of Bcl-2 expression (8–11).
Inactivating RUNX1 point mutations or expression of dominant-inhibitory RUNX1 fusion proteins are common in human acute myeloid leukemia and myelodysplastic syndrome (12, 13). Consequent reduction in Pu.1 and Cebpa transcription likely contributes to impaired differentiation and myeloid transformation (14). RUNX1 alterations are also present in solid tumors, including gene mutations in breast cancer and esophageal adenocarcinoma and increased expression in squamous cell carcinomas (15).
Runx1 binds DNA at 5′-PuACCPuCA-3′ consensus sites via its N-terminal Runt domain, and interaction of the Runt domain with CBFβ/PEPB2β markedly increases Runx1 DNA affinity (16, 17), in part reflecting relief of C-terminal auto-inhibition of Runx1 DNA interaction (18, 19). CBFβ also stabilizes Runx1 by inhibiting its ubiquitin-mediated proteolysis (20). Once bound to DNA, Runx1 activates or represses transcription dependent on gene and cellular context, in part mediated by interaction of a central trans-activation domain with the p300/CBF co-activators and of a C-terminal repression domain with HDAC1 or HDAC3 (21–23).
Several post-translational modifications have been found to regulate Runx1 activity (15). For example, Ser-249/Ser-266 phosphorylation by ERK or Lys-206/Lys-210 methylation by PRMT1 reduces interaction with mSin3a to increase trans-activation (24, 25), and Ser-21/Ser-249/Ser-266/Ser-397 phosphorylation by cyclin-dependent kinase increases Runx1 trans-activation potency by reducing HDAC2 interaction and prevents ubiquitin-mediated Runx1 degradation (23, 26, 27).
In addition, Src phosphorylates Runx1(Tyr-260), and a cluster of C-terminal tyrosines, Tyr-375, Tyr-378, Tyr-379, and Tyr-386; Runx1(5F), in which these five tyrosines are changed to phenylalanine, preventing phosphorylation, induces megakaryopoiesis from wild-type marrow and rescues CD8 T cell development from Runx1-deleted marrow more potently than Runx1(5D), in which these tyrosines are changed to aspartic acid to mimic phosphorylation or wild-type Runx1; and SHP2 interacts with Runx1 and reduces its tyrosine phosphorylation (28).
Neither the effect of Runx1 tyrosine modification on intrinsic Runx1 trans-activation potency nor the role of Runx1 tyrosine phosphorylation during normal granulopoiesis has been previously investigated. We find that activated Src strongly increases Runx1 activity in 293T cells and that conversion of the five Runx1 tyrosines modified by Src to phenylalanine to generate Runx1(5F) markedly reduces activation of a model reporter in 293T cells, and Runx1(4F) induces Pu.1 or Cebpa less effectively than wild-type Runx1 in 32Dcl3 myeloid cells. Potentially accounting for these findings, Runx1 tyrosine modification on these residues markedly reduces HDAC1 and HDAC3 interaction and increases steady-state Runx1 expression and stability, although lack of phosphorylation reduced DNA affinity in gel shift or myeloid chromatin immunoprecipitation (ChIP) assays. In addition, Runx1(5D) rescues formation of granulocyte progenitors from Runx1-deleted marrow more potently than does Runx1(5F).
Experimental Procedures
Cell Culture
293T cells (ATCC) were cultured in Dulbecco's modified Eagle's medium with 10% heat-inactivated fetal bovine serum (HI-FBS, Hyclone) and transiently transfected using Lipofectamine 2000 (Invitrogen). Luciferase and β-galactosidase assays were conducted for 48 h as described (26). Cycloheximide was provided at 50 μg/ml. 32Dcl3 cells (29) cultured in Iscove's modified Dulbecco's medium (IMDM) with 1 ng/ml murine interleukin 3 (IL-3, Cell Signaling) and penicillin/streptomycin were subjected to retroviral transduction as described (4). Subclones were obtained by limiting dilution. 4-Hydroxytamoxifen (4HT) was utilized at 200 nm. 32Dcl3 cells were washed twice with phosphate-buffered saline (PBS) prior to transfer to IMDM with 10% HI-FBS and 20 ng/ml G-CSF (Amgen). PP2 (Millipore) in DMSO was provided at 20 μm final concentration from a 1000× stock.
Plasmids
(Runx1)4TKLUC, CMV-βGal, pCEFL-Src(E381G), pBabePuro-Runx1-ER(T), CMV-CBFβ, CMV-FLAG-HDAC1, CMV-FLAG-HDAC3, CMV-HA-Cdc20, CMV-HA-Cdh1, pMIG, and pMIGC were previously described (4, 16, 23, 26, 27, 30, 31). CMV-HA-ubiquitin was obtained commercially (Addgene). The cDNA encoding the Runx1b isoform of murine Runx1 and its 5F, 5D, and 2F variants (28) were transferred to pcDNA3. The 4F, 4D, 1F, and 1D variants were then generated by subcloning taking advantage of a BamHI site separating Tyr-260 and the C-terminal tyrosine cluster (Fig. 1A). The 2F*, 2E, and 2E* variants were generated by synthesis (Blue Heron) and ligation of 584-bp BamHI/EcoRI fragments. pEF1α-FLAG-BioRunx1 and its mutant variants and pEF1α-birA were described (28). pBabePuro-Runx1(4F)-ER(T) and Runx1(4E)-ER(T) were generated from pBabePuro-Runx1-ER(T) by synthesis and ligation of 589-bp BamHI/MluI fragments. Runx1 and its variants were inserted into pMIGC as XhoI/EcoRI fragments.
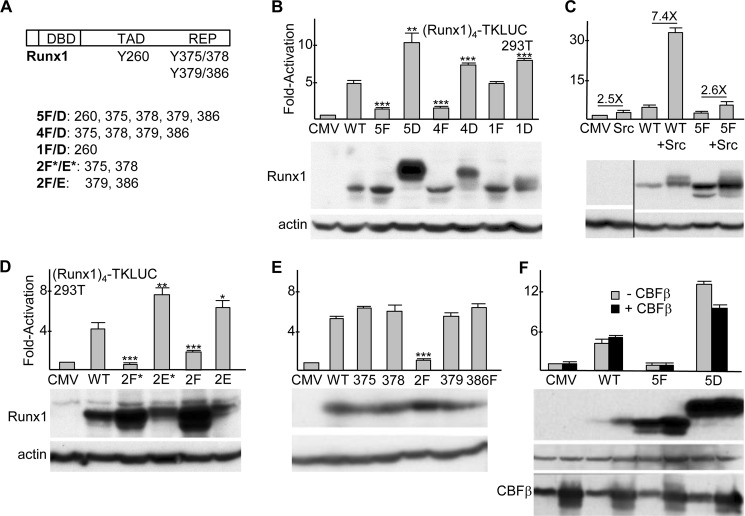
FIGURE 1.
A, diagram of murine Runx1b, with locations of the DNA-binding domain (DBD), trans-activation domain (TAD), C-terminal repression domain (REP), and five tyrosine residues shown previously to be modified by Src kinase (28) indicated (top). Tyrosine residues altered in Runx1 variants are also listed (bottom). B, 150 ng of (Runx1)4TKLUC was co-transfected into sub-confluent 293T cells in a 24-well plate with 0.8 ng of CMV-βGal and 5 ng of either pcDNA3 (CMV) or pcDNA3 vectors expressing wild-type Runx1 (WT) or its indicated tyrosine variants. The ratio of luciferase/β-galactosidase activity was assessed at 48 h, and the values relative to CMV, which was set on average to 1.0, are shown (mean and S.E. from three determinations). *, p < 0.05; **, p < 0.01; ***, p < 0.001 (top). 293T cells in 6-well plates were transfected with 1 μg of CMV or CMV-Runx1 expression vectors, and total cellular proteins were assessed at 48 h for Runx1 and β-actin expression by Western blotting (bottom). C, 293T cells were transfected with (Runx1)4TKLUC, CMV-βGal, and CMV, CMV-Runx1, or CMV-5F, alone or with 40 ng of CEFL-Src. Activation relative to CMV alone is shown (mean and S.E. from three determinations). The fold effect of Src expression on the average activity of CMV, Runx1, or 5F is also indicated (top). 293T cells in 6-well dishes were transfected with 250 ng of CMV, CMV-Runx1, or CMV-5F, alone or with 1 μg of CEFL-Src, and total cellular proteins were assessed at 48 h for Runx1 and β-actin expression (bottom). D, activation of the Runx1 reporter and protein expression was assessed as in A for WT Runx1 and its indicated variants (mean and S.E. from three determinations). E, activation by FLAG-BioRunx1 or its indicated variants and their expression in 293T cells is shown (mean and S.E. from three determinations). F, 293T cells were transfected with (Runx1)4TKLUC, CMV-βGal, and CMV, CMV-Runx1, or CMV-5F, alone or with 5 ng of CMV-CBFβ. Activation relative to CMV alone is shown (mean and S.E. from three determinations). 293T cells in 6-well dishes were transfected with 1 μg of CMV, CMV-Runx1, or CMV-5F, alone or with 1 μg of CMV-CBFβ, and total cellular proteins were assessed at 48 h for Runx1, β-actin, and CBFβ expression by Western blotting (bottom).
Protein and RNA Expression
Total cellular proteins were generated in Laemmli sample buffer and subjected to Western blotting as described (23). Antibodies used were as follows: C/EBPα (14AA), PU.1 (D-19), HA (Y-11), ERα (MC-20), or lamin B (M-20; Santa Cruz Biotechnology); Runx1 (Active Motif); Tyr(P) (4G10; Millipore); HDAC1 (Ab7028) or HDAC3 (3G6; Abcam); HA (16B12; Biolegend); FLAG (M2) or β-actin (AC-15; Sigma); GAPDH (14C10; Cell Signaling); or CBFβ (32). Total cellular RNA was prepared with the NucleoSpin RNA II kit, including use of RNase-free DNase (Machery-Nagel). First-strand cDNA was prepared with avian myeloblastosis virus reverse transcriptase (Promega) and oligo(dT) primer at 42 °C for 1 h. Quantitative PCR was performed with 5 ng of each cDNA with the use of iQ SYBR Green (Bio-Rad). Oligonucleotides used were Cebpa-F, Cebpa-R, Pu.1-F, Pu.1-R, Runx1-F, Runx1-R, Actin-F, Actin-R, mS16-F, and mS16-R (4).
DNA Binding Analysis
Nuclear extracts were generated from transfected 293T cells and subjected to gel shift analysis as described using a radiolabeled Runx1-binding site from the myeloperoxidase promoter (4). Bradford assay was used to quantify and equalize protein amounts. Proteins generated by coupled in vitro transcription and translation using rabbit reticulocyte lysate (Promega) were analyzed similarly. ChIP was conducted using 1E6 32Dcl3 cells and 2 μg ERα antiserum or normal rabbit IgG as described (4), followed by quantitative amplification of input or precipitated genomic DNAs using CEBPA-enhR3-F plus CEBP-enhR4-R, CEBPA-2500F plus CEBPA-2500R, actin-1500F and actin-1500R (4), or PU.1enhF (CTGGTGGCAAGAGCGTTTC) and PU.1enhR (CCACATCGGCAGCAGCAAG) primers.
Protein Immunoprecipitation
Co-immunoprecipitation (co-IP) of Runx1 or its variants with FLAG-HDAC1 or FLAG-HDAC3, or of Runx1 variants with HA-ubiquitin, in 293T cells was assessed in the presence of 20 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm DTT, 0.2% Nonidet P-40, 0.2% Triton X-100, 0.2% deoxycholate, 10% (w/v) glycerol, 1 mm NaF, 1 mm sodium orthovanadate, 1 mm PMSF, and a protease inhibitor mixture (Sigma) as described (23). For analysis of Runx1-ER(T) tyrosine phosphorylation in 32Dcl3 cells, Western blotting for Tyr(P) was conducted after similar IP using ERα antiserum, after the 32Dcl3 cells were pretreated with 1.25 mm sodium orthovanadate for 15 min to inhibit endogenous tyrosine phosphatases. To analyze interaction of FLAG-BioRunx1 with CBFβ, these proteins were co-expressed with birA in 293T cells, followed by agarose-streptavidin pulldown and CBFβ Western blotting, as described (28).
Marrow Transduction
Retroviral vectors were packaged by transient transfection into 293T cells with pkat2ecopac, and supernatants were filtered and used to transduce marrow isolated from 14- to 16-week-old Runx1(f/f) mice that had been subjected to one 5-fluorouracil injection 6 days prior to marrow harvest, as described (4). Four days after initiating transduction, cells were lineage-depleted, and GFP+ cells were isolated by flow cytometry and plated at 1E3/ml in methylcellulose with IMDM, 10% HI-FBS (Methocult 3231, Stem Cell Technology) supplemented with 10 ng/ml murine IL-3, 10 ng/ml murine IL-6, and 10 ng/ml murine stem cell factor (PeproTech). Hematopoietic colonies were enumerated 8 days later based on colony structure.
Statistics
Means and standard errors are shown. The Student's t test was used for statistical comparisons. Band intensities were quantified with ImageJ software from National Institutes of Health.
Results
Runx1 Tyrosine Phosphorylation Increases Trans-activation
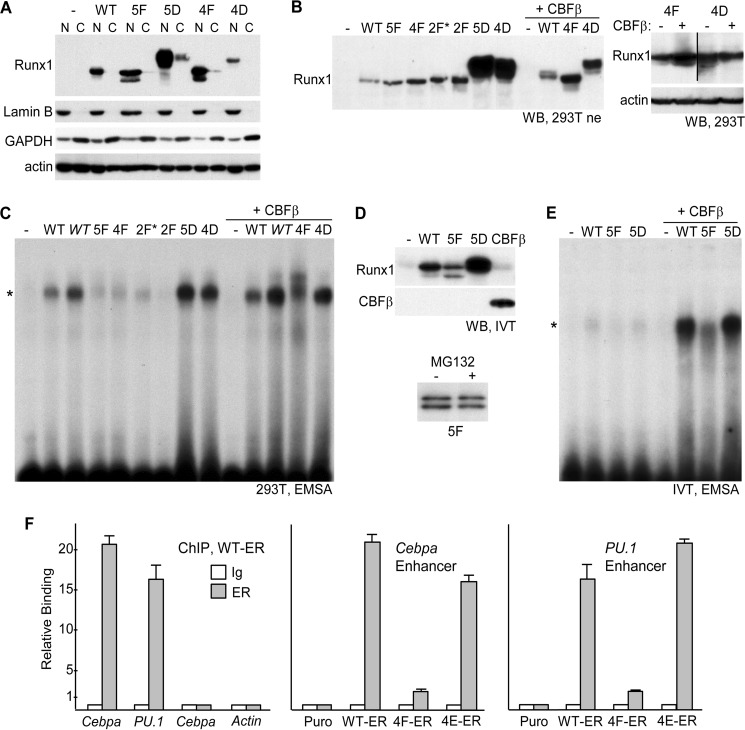
The locations of the five Runx1 tyrosines modified by Src are diagrammed, and tyrosine clusters changed to aspartate (Asp), glutamate (Glu), or phenylalanine (Phe) are listed (Fig. 1A). (Runx1)4TKLUC, containing four Runx1 consensus sites positioned upstream of a minimal thymidine kinase promoter and luciferase cDNA, was co-transfected into 293T cells with CMV expression vectors for wild-type Runx1 (WT) or its 5F, 5D, 4F, 4D, 1F, or 1D variants (Fig. 1B, top). Relative to empty CMV vector, Runx1 activated the reporter 5-fold, on average, and 5F and 4F showed minimal activation, whereas 1F had activity similar to WT. 5D was twice as active as WT, and 4D or 1D showed mildly increased activity. Total cellular proteins obtained after transfection of equal quantities of each expression vector were assessed for Runx1 and actin expression by Western blotting (Fig. 1B, bottom). Despite having markedly reduced activity, 5F and 4F were expressed at levels equal to or greater than WT. 4D was expressed at mildly increased levels and 5D at markedly increased levels, potentially accounting in part for their increased activity. 4D and 5D also manifested slower mobility, likely reflecting their increased negative charge.
We then assessed the effect of co-expressed activated Src on the activities of Runx1 or its 5F variant (Fig. 1C, top). Src generated 2.5-fold, Runx1 4.2-fold, and their combination 31-fold activation, displaying marked synergy, whereas Src only increased activation by 5F 2.6-fold, essentially identical to the activity of Src alone. Western blot analysis demonstrated that Src also increased the expression of Runx1, including acquisition of a more slowly migrating band, with these effects also evident to a lesser extent with 5F (Fig. 1C, bottom). During granulopoiesis, G-CSF receptor signaling not only activates Src kinases but also JAK kinases, and Fes tyrosine kinase is preferentially active in myeloid cells (33); however, neither activated Fes, JAK2, nor JAK2(V617F) increased activation of (Runx1)4TKLUC by Runx1 (data not shown).
As the 4F mutation largely obviated Runx1 activity, we generated the 2F*, 2E*, 2F, and 2E variants, mutating Tyr-375/378 or Tyr-379/386 to Phe or Glu. We chose Glu rather than Asp as Glu has an extra methyl group and so might better mimic a bulky tyrosine residue. Runx1(2F*) was inactive, and Runx1(2F) had reduced activity, despite expression above WT, whereas the 2E* and 2E variants demonstrated increased activity, with expression similar to or less than WT (Fig. 1D). We also took advantage of the availability of single residue tyrosine variants and 2F in the context of FLAG-BioRunx1 to compare their activities (Fig. 1E). 2F again manifested reduced activity, whereas each of the single residue variants had full activity, with expression of each variant similar to WT on Western blot analysis. Thus, dephosphorylation of at least two Runx1 C-terminal tyrosine residues is required to reduce trans-activation in 293T cells.
The CBFβ subunit increases the DNA affinity of Runx1 and protects Runx1 from ubiquitination and proteasomal degradation. We co-transfected the (Runx1)4TKLUC reporter with WT, 5F, or 5D with or without CMV-CBFβ (Fig. 1F). 293T cells manifest endogenous CBFβ, and increasing this level severalfold via exogenous transfection did not increase WT, 5F, or 5D activation of the reporter. The ability of exogenous CBFβ to increase WT and 5F levels indicates that basal CBFβ is limiting relative to WT or 5F expression. Also of note, whereas exogenous CBFβ increased the level of WT and 5F, the already markedly elevated level of 5D was not further increased.
C-terminal Runx1 Tyrosine Phosphorylation Enables Myeloid Gene Induction
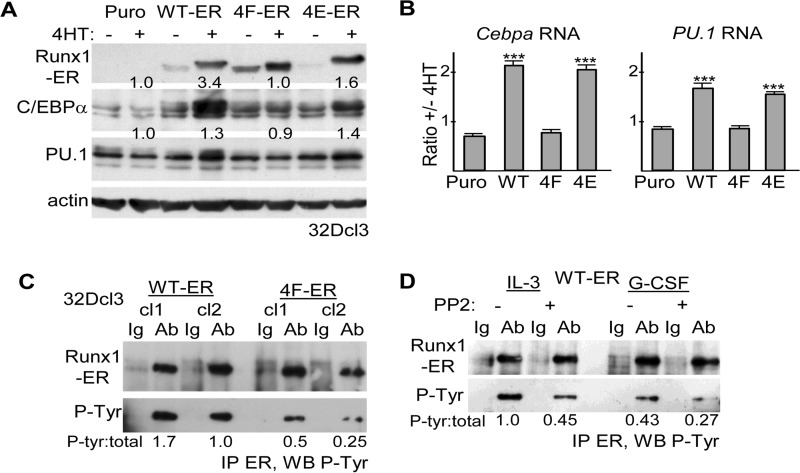
To determine whether Runx1 tyrosine phosphorylation is also needed for optimal activity in myeloid cells, 32Dcl3 cells were stably transduced with Runx1-ER(T) (WT-ER) or its 4F or 4E variants (4F-ER and 4E-ER) or with the empty pBABE Puro retroviral vector. Total cellular proteins prepared from pooled transductants were then analyzed for expression of the Runx1-ER fusion proteins and for the protein products of the Cebpa and PU.1 Runx1 target genes, in the absence or presence of 4HT (Fig. 2A). WT-ER and to a lesser extent 4E-ER increased expression of endogenous C/EBPα or PU.1 proteins at 24 h, whereas 4F-ER was ineffective, as confirmed by densitometry. Of note, WT-ER, 4F-ER, and 4E-ER proteins were expressed at similar levels in the presence of 4HT. As a more direct measure of Cebpa and PU.1 gene induction, total cellular RNAs from these same cultures were analyzed by quantitative RT-PCR (Fig. 2B). Again, WT-ER and 4E-ER, but not 4F-ER, induced Cebpa or PU.1 mRNA expression. To determine whether the four C-terminal Runx1 tyrosines are phosphorylated in 32Dcl3 cells, protein extracts from two WT-ER or 4F-ER subclones with similar expression of total Runx1-ER proteins were subjected to IP with ERα antiserum followed by Western blotting for phosphotyrosine (Fig. 2C). Both 4F-ER subclones manifested a reduced phosphorylated/total Runx1/ER ratio, on average >3-fold. Finally, similar analysis was done using a pool of WT-ER cells cultured for 8 h with PP2, a pan-Src family kinase inhibitor, or DMSO vehicle (Fig. 2D). Src inhibition reduced WT-ER tyrosine phosphorylation in either IL-3 or G-CSF.
FIGURE 2.
A, total cellular proteins from equal numbers (2E5) of 32Dcl3 cells stably transduced with pBABE Puro (Puro), Runx1-ER(T) (WT-ER), or its 4F-ER or 4E-ER variants and cultured ±4HT for 24 h were subjected to Western blotting using ERα, C/EBPα, PU. 1, or β-actin antibodies. Fold-increases in C/EBPα or PU.1 induced by 4HT, normalized to β-actin, are shown. B, total cellular RNAs from these same cultures were subjected to quantitative RT-PCR analysis for Cebpa and PU.1 and for actin as internal control. The expression of Cebpa or PU.1, normalized to actin, is shown as a ratio of expression ±4HT (mean and S.E. from three determinations). C, total cellular proteins from two WT-ER or 4F-ER 32Dcl3 subclones were subjected to ERα immunoprecipitation followed by Western blotting (WB) for phosphotyrosine. The ratios of phosphorylated/total WT-ER or 4F-ER are shown. D, pool of WT-ER-expressing 32Dcl3 cells was exposed to 20 μm PP2 or DMSO control for 8 h, either in IL-3 or 24 h after transfer to G-CSF. Total cellular proteins were then subjected to ERα immunoprecipitation followed by Western blotting for phosphotyrosine. The ratios of phosphorylated/total WT-ER are shown. Ab, antibody.
Tyrosine Modification Increases Runx1 Stability
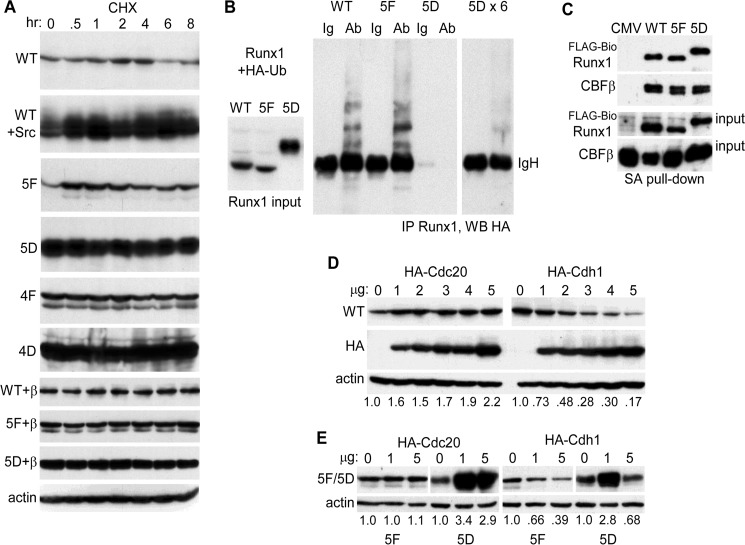
The markedly increased expression of Runx1(5D) compared with WT or 5F and the increased expression of Runx1 in the presence of activated Src suggests increased Runx1 stability in response to tyrosine phosphorylation. To evaluate this further, Runx1 was expressed alone or with activated Src in 293T cells, followed by addition of cycloheximide (CHX). Protein expression was then evaluated over an 8-h period (Fig. 3A, panels 1 and 2). Src again induced markedly increased Runx1 expression at time 0, and this level was maintained for 8 h, whereas levels of Runx1 alone, although stable for 4 h, decreased markedly between 4 and 6 h post-CHX addition. Expression and stability of the 5F, 5D, 4F, and 4D variants were also evaluated in CHX, with data from a representative experiment from two repetitions again shown (Fig. 3A, panels 3–6). At time 0, 5D and 4D were expressed at much higher levels than WT, 5F, or 4F, and while 5D and 4D levels were maintained through 8 h, the level of 5F or 4F decreased between 2 and 4 h; during this interval WT diminished 1.08-fold, 5F 1.37-fold, and 1.33-fold, as assessed by densitometry. The ability of Src to dramatically increase Runx1 levels indicates that in the absence of exogenous Src, the large majority of exogenous Runx1 lacks the full extent of tyrosine phosphorylation achieved upon Src expression. Overall, these data indicate markedly increased stability of 5D, 4D, or WT Runx1 + Src compared with WT, 5F, or 4F. In addition, 5F and 4F appear to be mildly destabilized. Increased Runx1 stability and thus expression in response to tyrosine phosphorylation may contribute to increased Runx1 trans-activation potency.
FIGURE 3.
A, 293T cells in 6-well dishes were transfected with 1 μg of CMV-Runx1 (WT), 0.25 μg of WT + 1 μg of CMV-Src; 1 μg of CMV-5F, CMV-5D, CMV-4F, or CMV-4D; 1 μg of CMV-WT + 1 μg of CMV-CBFβ, 5F + CBFβ, or 5D + CBFβ. CHX was added for 0.5–8 h, and total cellular proteins from equal numbers of cells were then evaluated for Runx1 isoform expression by Western blotting. Actin expression was assessed on similarly cultured 293T cells exposed to CHX. B, 293T cells in 10-cm dishes were transfected with 2 μg of CMV-WT, 1 μg of CMV-5F, or 0.4 μg of CMV-5D with 3 μg of CMV-HA-ubiquitin (HA-Ub). Extracts prepared 2 days later were subjected to Runx1 Western blotting, with 1/12th volume of the 5D extract compared with the WT or 5F extracts loaded to equalize expression (left). In addition 0.5 mg of total cell lysates were subjected to rabbit anti-Runx1 immunoprecipitation followed by anti-HA (16B12) Western blotting, with 1/12th (center) or 1/2 (right) of the 5D immunoprecipitate loaded relative to WT or 5F. The position of immunoglobulin heavy chain (IgH) is shown. Ab, antibody. C, total cellular proteins from 293T cells in 10-cm dishes transfected with 1.5 μg of CMV-CBFβ, 1.5 μg of EF1α-birA, and 3 μg of EF1α-FLAG-BioWT, 3 μg of EF1α-FLAG-Bio5F, or 10 ng of EF1α-FLAG-Bio5D were subjected to streptavidin-agarose pulldown followed by Western blotting of 2.5% input or pulldown samples using Runx1 and CBFβ antisera. D, 293T cells in 6-well dishes were transfected with 2 μg of Runx1 and 0–5 μg of HA-Cdc20 or HA-Cdh1 expression vectors. Total cellular proteins were then analyzed by Western blotting using Runx1, HA (Y-11), and β-actin antibodies. The relative intensities of the Runx1 bands, normalized to β-actin expression, are shown below each lane. E, 293T cells were transfected with 1 μg of 5F or 0.5 μg of 5D along with 0, 1, or 5 μg of HA-Cdc20 or HA-Cdh1 expression vectors, followed by Western blot analysis using Runx1 or β-actin antibodies.
We also evaluated whether co-expression of CBFβ above basal levels present in 293T cells would increase the stability of WT Runx1 or 5F (Fig. 3A, panels 7–9). As described previously (20), CBFβ co-expression stabilized WT Runx1, which now remained constant from 0 to 8 h; 5F and 5D levels also remained constant throughout the 8-h period. Actin expression was not affected by CHX (Fig. 3A, panel 10).
As a further assessment of relative stability, we co-expressed WT, 5F, or 5D with HA-ubiquitin in 293T cells. Extract volumes were then adjusted in an effort to equalize Runx1 input levels, although 5D levels remained about 2-fold above WT (Fig. 3B, left), followed by Runx1 IP and HA Western blotting (Fig. 3B, center). 5F manifested increased and 5D markedly decreased ubiquitination. Repeating this analysis with a 6-fold increased amount of 5D extract and obtaining a 3-fold longer autoradiographic exposure still led to a ubiquitination signal below WT (Fig. 3B, right). Of note, the molecular mass of Runx1, 53 kDa, is similar to that of the strong IgH band evident in these blots.
As CBFβ protects Runx1 from ubiquitination, we directly compared the ability of FLAG-Bio-tagged WT, 5F, and 5D to interact with CBFβ by expressing these proteins together in 293T cells followed by streptavidin-agarose bead pulldown and CBFβ Western blotting (Fig. 3C). Streptavidin pulldown rather than Runx1 antiserum IP was utilized because of concern that the Runx1 antiserum might interfere with the Runx1-CBFβ interaction. Using a markedly reduced amount of FLAG-Bio5D expression vector, 80-fold less than FLAG-BioWT, and 2.5-fold increased FLAG-Bio5F expression vector, approximately equal amounts of WT, 5F, and 5D were present in the pulldown extract (Fig. 3C, panel 1), and a similar amount of CBFβ interacted with each of these (Fig. 3C, panel 2). Thus, altered CBFβ interaction does not account for reduced 5F and increased 5D stability compared with WT Runx1.
Potentially accounting for cell cycle regulation of Runx1 expression (34), prior work demonstrated that the anaphase-promoting complex ubiquitin ligase promotes Runx1 degradation in cooperation with the Cdh1 and Cdc20 E3 co-activator proteins, with Cdh1 active against total Runx1 and Cdc20 selective for Ser-303-phosphorylated Runx1 (27). We therefore also evaluated the ability of Cdh1 or Cdc20 to induce Runx1 degradation in comparison with its 5F or 5D variants. As seen previously, Cdc20 did not induce degradation of total Runx1, whereas Cdh1 reduced Runx1 protein expression in a dose-dependent manner, with data shown representative of four determinations (Fig. 3D). The ability of 1 or 5 μg of Cdc20 or Cdh1 to reduce expression of 5F or 5D was then assessed (Fig. 3E). Cdc20 did not affect 5F expression, and in contrast with its effect on phospho-Runx1(Ser-303), co-transfection of Cdc20 paradoxically increased 5D expression as seen in the experiment shown and in a second independent experiment. Cdh1 induced 5F degradation in a dose-dependent manner similar to its effect on WT Runx1, as was also seen in a second experiment (data not shown). 1 μg of Cdh1 reproducibly increased 5D expression, whereas 5 μg of Cdh1 reproducibly reduced 5D expression, albeit <2-fold. These data indicate that the markedly increased stability of Runx1(5D) in part reflects its resistance to degradation by the anaphase-promoting complex.
Runx1 Tyrosine Phosphorylation Reduces HDAC Interaction
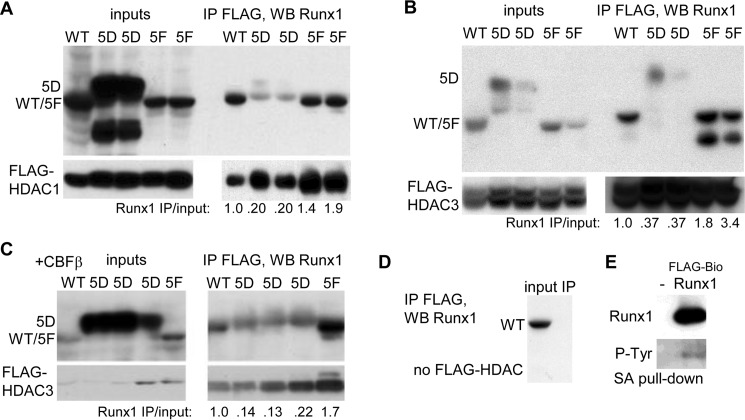
We previously demonstrated reduced Runx1c interaction with HDAC1 and HDAC3 in response to cyclin-dependent kinase modification of Ser-48, Ser-303, and Ser-424, potentially contributing to increased Runx1 activity when these serines are changed to aspartate, individually and additively in combination (23, 26). HDAC1 and HDAC3 are the predominant HDACs that interact with Runx1, and they do so at least in part via the Runx1 C-terminal region that includes Runx1b Tyr-375/378/379/386 and Runx1c Ser-424, which corresponds to Runx1b Ser-396 (35). Runx1 WT, 5F, or 5D were co-transfected with FLAG-HDAC1 or FLAG-HDAC3 in 293T cells, and total cellular extracts were subjected to IP with FLAG antibody-conjugated beads followed by Western blotting for Runx1 or FLAG (Fig. 4, A and B). Relative to Runx1 isoform input, FLAG-HDAC1 had 5-fold reduced affinity for 5D and modestly increased affinity for 5F, and FLAG-HDAC3 had 3-fold reduced affinity for 5D and ∼2-fold increased affinity for 5F, compared with WT. Co-expression of CBFβ above basal levels in 293T cells with FLAG-HDAC3 did not alter the relative affinities of HDAC3 for WT, 5D, or 5F (Fig. 4C). As a control WT Runx1 was transfected alone, followed by FLAG IP (Fig. 4D). No nonspecific interaction of Runx1 with the FLAG-agarose beads was evident. In addition, birA was expressed alone or with FLAG-BioWT Runx1, followed by streptavidin pulldown and Western blotting for Runx1 and phosphotyrosine (Fig. 4E). Tyrosine phosphorylation was evident, potentially accounting for reduced affinity of WT compared with 5F for HDAC1 and HDAC3. These findings indicate that reduced interaction with HDAC1 and HDAC3 may further contribute to increased Runx1 trans-activation potency upon its Src-mediated tyrosine modification.
FIGURE 4.
A, 293T cells in 10-cm dishes were transfected with 5 μg of CMV-WT, 0.4 or 0.2 μg of CMV-5D, or 1 or 0.5 μg of CMV-5F, together with 3 μg of CMV-FLAG-HDAC1, and total cell extracts were prepared 2 days later. After FLAG IP, pulldown, or 10% of input, samples were subjected to Western blotting (WB) with Runx1 or FLAG antibodies. The ratio of IP/input Runx1 band intensity for each reaction is shown. B, similar experiment was conducted substituting CMV-FLAG-HDAC3. C, 293T cells were transfected with 1 μg of CMV-WT, 0.5, 0.25, or 0.1 μg of CMV-5D, or 0.5 μg of CMV-5F together with 2 μg of CMV-FLAG-HDAC3 and 2 μg of CMV-CBFβ, followed by FLAG IP and Runx1 or FLAG Western blot analysis. D, 293T cells were transfected with 5 μg of CMV-WT, followed by FLAG IP and Runx1 Western blot analysis. E, 293T cells were transfected with 3 μg of EF1α-birA, alone or with 3 μg of EF1α-FLAG-BioRunx1, followed by streptavidin pulldown and Western blotting for Runx1 and phosphotyrosine.
Lack of Runx1 Tyrosine Phosphorylation Reduces DNA Affinity
To determine the effect of Runx1 tyrosine phosphorylation on its interaction with DNA, we first expressed WT Runx1 and its 5F, 4F, 2F*, 2F, 5D, or 4D variants in 293T cells and generated nuclear extracts. Predominant nuclear expression of WT, 5F, 5D, 4F, and 4D was verified by Western blotting of these extracts or cytoplasmic extracts from equivalent cell numbers for Runx1, lamin B (a nuclear protein), GAPDH (a cytoplasmic protein), or β-actin (Fig. 5A). As CBFβ increases Runx1 DNA affinity, we also generated extracts from cells expressing WT, 4F, or 4D with exogenous CBFβ. Equal amounts of each extract was analyzed for Runx1 expression by Western blotting (Fig. 5B, left). 5D and 4D again demonstrated markedly increased expression. Co-expression of CBFβ increased WT and 4F expression, reflecting its ability to stabilize Runx1, but 4D expression was reduced. To confirm this phenomenon, in a separate experiment, total cellular proteins isolated from 293T cells expressing 4F or 4D alone or with CBFβ were analyzed similarly (Fig. 5B, right); again CBFβ increased 4F but reduced 4D expression.
FIGURE 5.
A, 293T cells in 10-cm dishes were transfected with 6 μg of CMV (−) or CMV vectors expressing WT Runx1 or its 5F, 5D, 4F, or 4D variants. Nuclear (N) and cytoplasmic (C) extracts from equal cell numbers, prepared 48 h later, were subjected to Western blotting for Runx1, lamin B, GAPDH, or β-actin. B, 293T cells in 10-cm dishes were transfected with 6 μg of CMV (−) or CMV vectors expressing WT Runx1 or its 5F, 4F, 2F*, 2F, 5D, or 4D variants alone, or 3 μg of CMV or WT, 4F, or 4D with 3 μg of CMV-CBFβ. 20 μg of nuclear extracts prepared 48 h later were subjected to Western blotting (WB) using Runx1 antiserum (left). 293T cells in 6-well dishes were transfected with 1 μg of 4F or 4D, alone or with 1 μg of CBFβ, and total cellular proteins prepared 48 h later were subjected to Western blotting using Runx1 and β-actin antibodies (right). C, 6 μg of each nuclear extract were subjected to electrophoretic mobility shift analysis (EMSA) using a radiolabeled 32-bp double-stranded oligonucleotide containing a central consensus Runx1-binding site derived from the myeloperoxidase promoter. In addition, lanes with 12 μg of the WT or WT+CBFβ extracts (WT) were included. *indicates location of the specific gel shift band. D, Runx1 WT, 5F, or 5D, as well as CBFβ, were generated by in vitro transcription-translation (IVT) using 1 μg of linearized pcDNA3 expression vectors in a 50-μl reaction. Equal volumes, together with control reticulocyte lysate (−), were subjected to Western blotting for Runx1 and CBFβ (top). In addition, expression of 5F generated with or without 25 μm MG132 was analyzed similarly (bottom). E, after equalizing WT, 5F, and 5D protein based on densitometry, these samples as well as control lysate were analyzed for DNA binding by EMSA, alone or with addition of 7 μl of CBFβ lysate. F, 1E6 32Dcl3 Puro, WT-ER, 4F-ER, or 4E-ER cells cultured for 24 h in 4HT were subjected to ChIP assay using 2 μg of rabbit IgG or rabbit-anti-ERα (ER) antiserum followed by quantitative PCR using genomic primers specific for the +37-kb Cebpa enhancer, the −14 kb-PU.1 enhancer, the −2.5-kb Cebpa promoter region, or the β-actin promoter. Relative binding of WT-ER to each of these elements (left) or of WT-ER, 4F-ER, and 4E-ER to the Cebpa enhancer (center) or the PU.1 enhancer (right) is shown, with signal obtained with IgG in Puro cells set to 1.0 for each primer pair (mean and S.E. from three determinations).
These nuclear extracts were then subjected to the electrophoretic mobility shift assay (EMSA) using a radiolabeled Runx1-binding site (Fig. 5C). As expression of WT Runx1 was reduced compared with 5F or 4F, two levels of the Runx1 extract were utilized, one equal in amount to the other extracts (WT) and one twice the amount (Fig. 5C, WT). Efforts to reduce the volume of the 5D or 4D extracts to equalize total Runx1 protein led to complete absence of DNA binding, potentially reflecting dilution of CBFβ or another cooperating factor (data not shown). In the absence of exogenous CBFβ and despite the presence of endogenous CBFβ, 5F, 4F, 2F*, and 2F had markedly reduced DNA affinity compared with WT, 4D, or 5D. The reduced affinity of 4F relative to WT or 4D was retained even in the presence of exogenous CBFβ; of note, in this setting 4F and 4D were expressed at similar levels.
C-terminal truncation increases Runx1 DNA affinity, indicating the presence of a negative regulatory domain that could potentially be regulated by tyrosine modification (18, 19). To determine whether Runx1 5D manifests increased DNA binding in the complete absence of CBFβ, WT, 5F, 5D, and CBFβ were expressed separately using in vitro transcription translation. Interestingly, Western blot analysis of equal volumes of translated proteins demonstrated reduced amounts of 5F and increased amount of 5D, relative to WT (Fig. 5D, top). The abundant hemin present in reticulocyte lysates is thought to inhibit proteasome activity; nevertheless, we also determined whether inclusion of the proteasomal inhibitor MG132 would increase 5F expression (Fig. 5D, bottom); no increase was evident. Gel shift analysis was then conducted with in vitro transcription translation WT, 5F, and 5D, with their volumes adjusted based on densitometry to equalize total Runx1 protein alone or with CBFβ (Fig. 5E). In the absence of CBFβ, minimal DNA binding was evident, although affinity of 5F appears to be less than WT or 5D. Upon addition of CBFβ, DNA affinity of each Runx1 variant increased markedly, with 5F again showing reduced affinity. Thus, tyrosine modification of Runx1 does not relieve its dependence upon CBFβ for high DNA affinity.
Finally, to determine whether the ability of Runx1(WT)-ER(T) and Runx1(4E)-ER(T) but not Runx1(4F)-ER to induce endogenous Runx1 target gene expression in 32Dcl3 myeloid cells in part reflects their differential ability to bind relevant regulatory DNA elements, we conducted ChIP assays using pooled 32Dcl3 cell lines expressing these transgenes. WT-ER demonstrated strong binding to the Cebpa +37-kb and the PU.1 −14-kb enhancers, which contain functional Runx1-binding sites, but not to −2.5 kb within the Cebpa locus or the β-actin promoter, which lack such elements (Fig. 5F, left). 4E-ER occupancy of these enhancers was similar to WT-ER, whereas 4F-ER occupancy was reduced 5–8-fold (Fig. 5F, center and right). Thus, consistent with the gel shift data, WT and 4E manifest markedly increased affinity for two enhancers harboring known Runx1-binding sites.
Runx1 Tyrosine Phosphorylation Facilitates Granulopoiesis
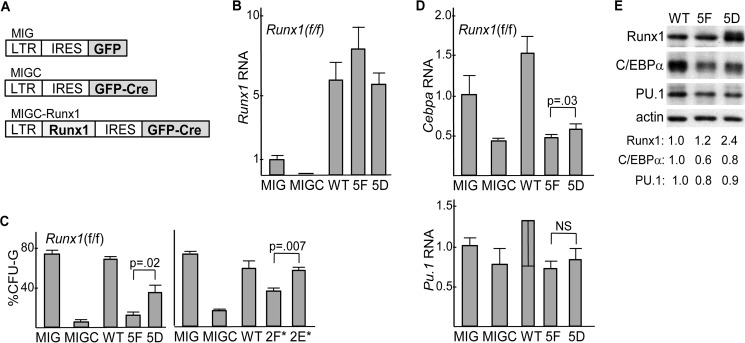
We previously demonstrated that transduction of Runx1(f/f) marrow with Cre impairs in vitro formation of granulocyte colony forming units (CFU-G) and that exogenous Runx1 rescues granulopoiesis upon Runx1 gene deletion (4). To compare Runx1, 5F, and 5D in this regard, their cDNAs were introduced into the MIGC retroviral vector in which the MSCV LTR directs expression of a Runx1-IRES-GFP-Cre mRNA that will simultaneously express Runx1 or its variants together with a GFP-Cre fusion protein. MIG, which only expresses GFP, serves as a control (Fig. 6A). Runx1(f/f) marrow, isolated from mice exposed 6 days earlier to 5-fluorouracil to draw stem and progenitor cells into a cycle, was transduced with MIG, MIGC, or MIGC expressing WT, 5F, or 5D. Four days later, transduced marrow was lineage-depleted, and GFP+ cells were isolated, and total cellular RNA was analyzed for Runx1 expression (Fig. 6B). Endogenous Runx1 mRNA was reduced 10-fold by MIGC compared with MIG, indicative of efficient Runx1 gene deletion. Exogenous WT, 5F, and 5D were each expressed at similar levels, about 6-fold higher than endogenous Runx1. Equal numbers of flow-sorted, Lin−GFP+ cells were plated in methylcellulose with IL-3, IL-6, and stem cell factor, and myeloid CFUs were enumerated 8 days later (Fig. 6C, left). As seen previously, MIGC-mediated Runx1 gene deletion markedly reduced the proportion of CFU-G and WT Runx1 fully rescued granulopoiesis. In addition, 5D rescued CFU-G formation more effectively than 5F, albeit less effectively than WT. Reduced efficacy of 5D compared with WT may reflect the fact that an aspartate residue does not perfectly mimic phosphorylated tyrosine. The abilities of WT Runx1, 2F*, and 2E* to rescue granulopoiesis were compared in a separate set of experiments (Fig. 6C, right). 2E* increased CFU-G formation to a level similar to WT, whereas 2F* was less effective. Thus, Runx1 5D or 2E* induced granulopoiesis more effectively than 5F or 2F*.
FIGURE 6.
A, diagram of MIG, MIGC, and MIGC-Runx1 retroviral vectors. LTR, long-terminal repeat; IRES, internal ribosome entry site; GFP, green fluorescent protein; GFP-CRE, fusion protein linking GFP and CRE. B, expression of Runx1 RNA, relative to mS16 ribosomal subunit RNA, in lineage-depleted (Lin−), GFP+ cells isolated 4 days after transduction of Runx1(f/f) marrow cells with MIG, MIGC, MIGC-WT, MIGC-5F, or MIGC-5D. Average Runx1 expression after MIG transduction was set to 1.0 (mean and S.E. of three determinations). C, percentage of CFU-G relative to CFU-G + CFU-M obtained from equal numbers of lineage-depleted GFP+ cells plated in methylcellulose culture at 1E3/ml with HI-FBS and IL-3, IL-6, and stem cell factor after similar transduction which is shown (left, mean and S.E. from three determinations). A related set of experiments was conducted using MIG, MIGC, MIGC-WT, MIGC-2F*, and MIGC-2E* (right, mean and S.E. from three determinations). D, expression of Cebpa or Pu.1 RNAs, relative to mS16 ribosomal subunit RNA, in Lin−GFP+ cells isolated 4 days after transduction of Runx1(f/f) marrow cells with MIG, MIGC, MIGC-WT, MIGC-5F, or MIGC-5D. Average Cebpa or Pu.1 expression after MIG transduction was set to 1.0 (mean and S.E. of three determinations). E, total cellular proteins from equal numbers of Lin−GFP+ cells isolated 4 days after transduction of Runx1(f/f) marrow cells with MIGC-WT, MIGC-5F, or MIGC-5D were subjected to Western blotting for Runx1, C/EBPα, PU.1, or β-actin. Band intensities, normalized to that of β-actin and set to 1.0 after WT transduction, are also shown.
RNAs isolated from transduced Lin−GFP+ cells were also analyzed for Cebpa or Pu.1 (Fig. 6D). As seen previously (4), Runx1 gene deletion reduced Cebpa more effectively than Pu.1; exogenous WT Runx1 rescued Cebpa expression, and transduction of 5D, but not 5F, also increased Cebpa, albeit mildly. Finally, total cellular proteins from Lin−GFP+ cells transduced with WT, 5F, or 5D were subjected to Western blotting for Runx1, C/EBPα, PU.1, and β-actin (Fig. 6E). Levels of WT Runx1 and its 5F variant were similar, whereas 5D was expressed at a 2.4-fold increased level, consistent with increased stability of Runx1(5D) also in immature marrow myeloid cells. Also consistent with the RNA data, 5D increased C/EBPα 1.3-fold compared with 5F.
Discussion
This study demonstrates that Runx1 tyrosine phosphorylation, by Src or potentially other kinases, increases its trans-activation potency in 293T and in 32Dcl3 myeloid cells. Potentially accounting for increased Runx1 trans-activation potency, we find that tyrosine phosphorylation increases Runx1 stability, reduces Runx1 interaction with HDAC1 or HDAC3, and increases its DNA affinity. In addition, Runx1 tyrosine phosphorylation facilitates rescue of granulopoiesis in the absence of endogenous Runx1.
Whether Runx1 tyrosine phosphorylation leads to increased trans-activation potency in other lineages remains to be determined; variations could, for example, reflect different levels of HDAC or anaphase-promoting complex component expression. In contrast to our findings during granulopoiesis, Runx1(5F) but not 5D rescues CD8 T cell development from Runx1-deleted marrow and induces megakaryopoiesis from wild-type marrow (28). Runx1 represses CD4 expression in CD4−CD8− immature thymocytes, and Runx3 represses CD4 in CD4−CD8+ T cells (36). In addition, Runx proteins repress the gene encoding Th-POK, a key mediator of CD4 T cell formation (37). If Runx1(5F) lacks trans-activation activity in these T cell subsets as in 293T cells, perhaps it dominantly represses the CD4 and Th-POK enhancers to restore CD8 T cell formation when introduced into Runx1-deleted marrow cells. Whether Runx1 contributes to megakaryopoiesis predominantly through gene activation or gene repression remains to be elucidated. Of note, during megakaryopoiesis Runx1 binds and represses the Klf1 promoter, associated with reduced histone acetylation, to suppress the erythroid program (38).
Src phosphorylates Runx1 Tyr-260 and a cluster of four neighboring tyrosines, Tyr-375, Tyr-378, Tyr-379, and Tyr-386. Mutation of the C-terminal cluster but not Tyr-260 to phenylalanine impaired Runx1 trans-activation, with mutation of Tyr-375/378 or Tyr-379/386 sufficient to reduce activation, whereas mutation of either of these tyrosine pairs to glutamate had the opposite effect. In contrast, mutation of individual tyrosines to phenylalanine did not impair Runx1 activity. Determination of the precise set of tyrosines modified by Src in vivo and whether additional tyrosine kinases can modify any or all of these tyrosines remain to be determined, although we found both activated Fes and activated JAK2 incapable of stimulating Runx1 activity, in sharp contrast to the strong synergy seen between Src and Runx1. Of note, mice lacking the Src family kinases Lyn, Hck, and Fgr have normal neutrophils and CFU-G numbers, PP2, a pan-Src inhibitor, did not impair in vitro granulopoiesis in response to G-CSF (39), and our finding that PP2 reduces Ruinx1-ER phosphorylation in 32Dcl3 cells must be interpreted with the understanding that PP2 likely has off-target effects; thus, Src kinases may not be the only or even the most relevant tyrosine kinases regulating Runx1 during granulopoiesis. In contrast to their role during normal myeloid development, reduced activity of Src or other tyrosine kinases capable of modifying Runx1 or increased SHP2 activity could contribute to myeloid transformation by reducing Runx1 activity, as commonly occurs due to chromosomal translocations or point mutations directly affecting the RUNX1 gene (12, 13).
The C terminus of Runx1 binds HDAC1 and HDAC3, and this binding is modulated by Ser-424 phosphorylation by cyclin-dependent kinases (23, 35). Herein, we demonstrate that mutation of the C-terminal tyrosine cluster to aspartate markedly reduces this interaction, with mutation to phenylalanine conversely reducing HDAC binding. Besides HDACs, Runx1 interacts with multiple additional co-repressors or co-activators whose affinity for Runx1 might be modulated by its tyrosine phosphorylation, including p300, CBP, MOZ, TAZ, MLL, PMRT1, YAP1, Sin3A, SUV39H1, Sin3A, SMRT, and TLE (40). We focused here on HDACs as among these they contact Runx1 most specifically in the vicinity of the Tyr-375/378/379/386 cluster, but it will be important in future studies to examine the effect of Runx1 tyrosine modification on its interaction with additional cofactors.
Runx1(5D) manifested markedly increased steady-state expression and stability in CHX, had markedly reduced steady-state ubiquitination, and was severalfold more resistant to HA-Cdh1-mediated degradation, compared with WT Runx1. Also, Runx1(5F) manifested mildly reduced half-life in CHX and increased steady-state ubiquitination compared with WT Runx1. Reduced 5F stability and its increased HDAC affinity compared with WT might reflect partial tyrosine modification of WT in 293T cells.
Finally, Runx1(4F) or 5F demonstrated reduced DNA binding in EMSA, compared with WT, 4D, or 5D. Consistent with reduced DNA affinity in the absence of tyrosine modification, 4F-ER had markedly reduced contact with the Cebpa or PU.1 enhancers in 32Dcl3 myeloid cells, compared with WT-ER or 4E-ER, as assessed by the ChIP assay.
CBFβ increases Runx1 stability and DNA binding (16, 17, 20). Reduced reporter trans-activation by Runx1(5F) was not rescued by exogenous CBFβ, although both WT and 5F were stabilized by CBFβ in the presence of CHX. Similarly, co-expressed CBFβ did not modify the reduced affinity of 5D or the increased affinity of 5F for HDAC3 or the reduced affinity of 4F or 5F for DNA. In addition, WT, 5F, and 5D manifested similar binding to CBFβ as assessed by co-IP, and despite the fact that C-terminal deletion of Runx1 markedly increases its DNA affinity (18, 19), Runx1(5D) only manifested minimal DNA binding in EMSA when expressed in vitro in the absence of CBFβ, indicating that tyrosine phosphorylation of Runx1 does not substitute for its interaction with CBFβ to relieve auto-inhibition of DNA binding, and absence of Runx1 tyrosine modification does not limit access of CBFβ to the DNA-binding Runt domain. Perhaps the absence of C-terminal Runx1 tyrosine phosphorylation allosterically modifies the conformation of the Runt domain even when bound to CBFβ.
In summary, Runx1 tyrosine phosphorylation increases its activity in 293T and 32Dcl3 myeloid cells and perhaps in other lineages by increasing Runx1 stability and DNA affinity and by reducing its interaction with HDACs, independent of effects on Runx1 interaction with CBFβ. Induction of Cebpa or PU.1 by tyrosine-phosphorylated Runx1, mediated by Src family and/or other kinases, may contribute to granulopoiesis.
Author Contributions
W. Y. L. designed, performed, and analyzed the experiments shown in Figs. 1, C–F, 2, 3, and 5. H. G. designed, performed, and analyzed the experiments shown in Figs. 4 and 6. O. M. designed, performed, and analyzed the experiment shown in Fig. 1B and constructed several Runx1 variant expression vectors. H. H. constructed the majority of the Runx1 variant expression vectors. A. B. C. and A. D. F. conceived and coordinated the study, and A. D. F. wrote the paper. All authors analyzed the results and approved the final version of the manuscript.
This work was supported by National Institutes of Health Grants U01 HL099775 (to A. D. F.) and R01 HL082952 (to A. B. C.), National Institutes of Health Cancer Center Core Grant P30 CA006973, a grant from the Gabrielle's Angel Foundation (to A. B. C.), and by the Giant Food Children's Cancer Research Fund. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- HDAC
- histone deacetylase
- 4HT
- 4-hydroxytamoxifen
- CHX
- cycloheximide
- ER
- estradiol receptor
- IP
- immunoprecipitation
- HI-FBS
- heat-inactivated fetal bovine serum
- IMDM
- Iscove's modified Dulbecco's medium.
References
- 1.Swiers G., de Bruijn M., and Speck N. A. (2010) Hematopoietic stem cell emergence in the conceptus and the role of Runx1. Int. J. Dev. Biol. 54, 1151–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ichikawa M., Asai T., Saito T., Seo S., Yamazaki I., Yamagata T., Mitani K., Chiba S., Ogawa S., Kurokawa M., and Hirai H. (2004) AML-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nat. Med. 10, 299–304 [DOI] [PubMed] [Google Scholar]
- 3.Growney J. D., Shigematsu H., Li Z., Lee B. H., Adelsperger J., Rowan R., Curley D. P., Kutok J. L., Akashi K., Williams I. R., Speck N. A., and Gilliland D. G. (2005) Loss of Runx1 perturbs adult hematopoiesis and is associated with a myeloproliferative phenotype. Blood 106, 494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo H., Ma O., Speck N. A., and Friedman A. D. (2012) Runx1 deletion or dominant inhibition reduces Cebpa transcription via conserved promoter and distal enhancer sites to favor monopoiesis over granulopoiesis. Blood 119, 4408–4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang G., Zhang P., Hirai H., Elf S., Yan X., Chen Z., Koschmieder S., Okuno Y., Dayaram T., Growney J. D., Shivdasani R. A., Gilliland D. G., Speck N. A., Nimer S. D., and Tenen D. G. (2008) PU.1 is a major downstream target of AML1 (RUNX1) in adult mouse hematopoiesis. Nat. Genet. 40, 51–60 [DOI] [PubMed] [Google Scholar]
- 6.Guo H., Ma O., and Friedman A. D. (2014) The Cebpa +37-kb enhancer directs transgene expression to myeloid progenitors and to long-term hematopoietic stem cells. J. Leukoc. Biol. 96, 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper S., Guo H., and Friedman A. D. (2015) The +37-kb Cebpa enhancer is critical for Cebpa myeloid gene expression and contains functional sites that bind SCL, GATA2, C/EBPα, PU.1, and additional Ets factors. PLoS ONE 10, e0126385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman A. D. (2009) Cell cycle and developmental control of hematopoiesis by Runx1. J. Cell. Physiol. 219, 520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britos-Bray M., Ramirez M., Cao W., Wang X., Liu P. P., Civin C. I., and Friedman A. D. (1998) CBFβ-SMMHC, expressed in M4eo acute myeloid leukemia, reduces p53 induction and slows apoptosis in hematopoietic cells exposed to DNA-damaging agents. Blood 92, 4344–4352 [PubMed] [Google Scholar]
- 10.Cai X., Gaudet J. J., Mangan J. K., Chen M. J., De Obaldia M. E., Oo Z., Ernst P., and Speck N. A. (2011) Runx1 loss minimally impacts long-term hematopoietic stem cells. PLoS ONE 6, e28430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyama S., Schibler J., Cunningham L., Zhang Y., Rao Y., Nishimoto N., Nakagawa M., Olsson A., Wunderlich M., Link K. A., Mizukawa B., Grimes H. L., Kurokawa M., Liu P. P., Huang G., and Mulloy J. C. (2013) Transcription factor RUNX1 promotes survival of acute myeloid leukemia cells. J. Clin. Invest. 123, 3876–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman A. D. (1999) Leukemogenesis by CBF oncoproteins. Leukemia 13, 1932–1942 [DOI] [PubMed] [Google Scholar]
- 13.Tang J. L., Hou H. A., Chen C. Y., Liu C. Y., Chou W. C., Tseng M. H., Huang C. F., Lee F. Y., Liu M. C., Yao M., Huang S. Y., Ko B. S., Hsu S. C., Wu S. J., Tsay W., et al. (2009) AML1/RUNX1 mutations in 470 adult patients with de novo acute myeloid leukemia: prognostic implication and interaction with other gene alterations. Blood 114, 5352–5361 [DOI] [PubMed] [Google Scholar]
- 14.Friedman A. D. (2015) C/EBPα in normal and malignant myelopoiesis. Int. J. Hematol. 101, 330–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyama S., Huang G., Kurokawa M., and Mulloy J. C. (2015) Posttranslational modifications of RUNX1 as potential anticancer targets. Oncogene 34, 3483–3492 [DOI] [PubMed] [Google Scholar]
- 16.Ogawa E., Inuzuka M., Maruyama M., Satake M., Naito-Fujimoto M., Ito Y., and Shigesada K. (1993) Molecular cloning and characterization of PEBP2β, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2α. Virology 194, 314–331 [DOI] [PubMed] [Google Scholar]
- 17.Wang S., Wang Q., Crute B. E., Melnikova I. N., Keller S. R., and Speck N. A. (1993) Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol. Cell. Biol. 13, 3324–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanno T., Kanno Y., Chen L. F., Ogawa E., Kim W. Y., and Ito Y. (1998) Intrinsic transcriptional activation-inhibition domains of the polyomavirus enhancer binding protein 2/core binding factor α subunit revealed in the presence of the β subunit. Mol. Cell. Biol. 18, 2444–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu T. L., Goetz T. L., Graves B. J., and Speck N. A. (2000) Auto-inhibition and partner proteins, core-binding factor β (CBFβ) and Ets-1, modulate DNA binding by CBFα2 (AML1). Mol. Cell. Biol. 20, 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang G., Shigesada K., Ito K., Wee H. J., Yokomizo T., and Ito Y. (2001) Dimerization with PEBP2β protects RUNX1/AML1 from ubiquitin-mediated degradation. EMBO J. 20, 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitabayashi I., Yokoyama A., Shimizu K., and Ohki M. (1998) Interaction and functional cooperation of the leukemia-associated factors AML1 and p300 in myeloid cell differentiation. EMBO J. 17, 2994–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durst K. L., Lutterbach B., Kummalue T., Friedman A. D., and Hiebert S. W. (2003) the inv(16) fusion protein associates with corepressors via a smooth muscle myosin-heavy chain domain. Mol. Cell. Biol. 23, 607–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H., and Friedman A. D. (2011) Phosphorylation of RUNX1 by cyclin-dependent kinase reduces direct interaction of with HDAC1 and HDAC3. J. Biol. Chem. 286, 208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai Y., Kurokawa M., Yamaguchi Y., Izutsu K., Nitta E., Mitani K., Satake M., Noda T., Ito Y., and Hirai H. (2004) The corepressor mSin3A regulates phosphorylation-induced activation, intranuclear location, and stability of AML1. Mol. Cell. Biol. 24, 1033–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao X., Jankovic V., Gural A., Huang G., Pardanani A., Menendez S., Zhang J., Dunne R., Xiao A., Erdjument-Bromage H., Allis C. D., Tempst P., and Nimer S. D. (2008) Methylation of RUNX1 by PRMT1 abrogates SIN3A binding and potentiates its transcriptional activity. Genes Dev. 22, 640–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L., Fried F. B., Guo H., and Friedman A. D. (2008) Cyclin-dependent kinase phosphorylation of RUNX1/AML1 on three sites increases trans-activation potency and stimulates cell proliferation. Blood 111, 1193–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biggs J. R., Peterson L. F., Zhang Y., Kraft A. S., and Zhang D. E. (2006) AML1/RUNX1 phosphorylation by cyclin-dependent kinases regulates the degradation of AML1/RUNX1 by the anaphase-promoting complex. Mol. Cell. Biol. 26, 7420–7429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H., Woo A. J., Waldon Z., Schindler Y., Moran T. B., Zhu H. H., Feng G. S., Steen H., and Cantor A. B. (2012) A Src family kinase-Shp2 axis controls RUNX1 activity in megakaryocyte and T-lymphocyte differentiation. Genes Dev. 26, 1587–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valtieri M., Tweardy D. J., Caracciolo D., Johnson K., Mavilio F., Altmann S., Santoli D., and Rovera G. (1987) Cytokine-dependent granulocytic differentiation, regulation of proliferative and differentiative responses in a murine progenitor cell line. J. Immunol. 138, 3829–3835 [PubMed] [Google Scholar]
- 30.Bjorge J. D., Bellagamba C., Cheng H. C., Tanaka A., Wang J. H., and Fujita D. J. (1995) Characterization of two activated mutants of human pp60c-src that escape c-Src kinase regulation by distinct mechanisms. J. Biol. Chem. 270, 24222–24228 [DOI] [PubMed] [Google Scholar]
- 31.Chan G., Cheung L. S., Yang W., Milyavsky M., Sanders A. D., Gu S., Hong W. X., Liu A. X., Wang X., Barbara M., Sharma T., Gavin J., Kutok J. L., Iscove N. N., Shannon K. M., et al. (2011) Essential role of Ptpn11 in survival of hematopoietic stem and progenitor cells. Blood 117, 4253–4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao W., Britos-Bray M., Claxton D. F., Kelley C. A., Speck N. A., Liu P. P., and Friedman A. D. (1997) CBFβ-SMMHC, expressed in M4eo AML, reduced CBF DNA-binding and inhibited the G1 to S cell cycle transition at the restriction point in myeloid and lymphoid cells. Oncogene 15, 1315–1327 [DOI] [PubMed] [Google Scholar]
- 33.Kim J., Ogata Y., Ali H., and Feldman R. A. (2004) The Fes tyrosine kinase: a signal transducer that regulates myeloid-specific gene expression through transcriptional activation. Blood Cells Mol. Dis. 32, 302–308 [DOI] [PubMed] [Google Scholar]
- 34.Bernardin-Fried F., Kummalue T., Leijen S., Collector M. I., Ravid K., and Friedman A. D. (2004) AML1/RUNX1 increases during G1 to S cell cycle progression independent of cytokine-dependent phosphorylation and induces cyclin D3 gene expression. J. Biol. Chem. 279, 15678–15687 [DOI] [PubMed] [Google Scholar]
- 35.Reed-Inderbitzin E., Moreno-Miralles I., Vanden-Eynden S. K., Xie J., Lutterbach B., Durst-Goodwin K. L., Luce K. S., Irvin B. J., Cleary M. L., Brandt S. J., and Hiebert S. W. (2006) Runx1 associates with histone deacetylases and SUV39H1 to repress transcription. Oncogene 25, 5777–5786 [DOI] [PubMed] [Google Scholar]
- 36.Taniuchi I., Osato M., Egawa T., Sunshine M. J., Bae S. C., Komori T., Ito Y., and Littman D. R. (2002) Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell 111, 621–633 [DOI] [PubMed] [Google Scholar]
- 37.Setoguchi R., Tachibana M., Naoe Y., Muroi S., Akiyama K., Tezuka C., Okuda T., and Taniuchi I. (2008) Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science 319, 822–825 [DOI] [PubMed] [Google Scholar]
- 38.Kuvardina O. N., Herglotz J., Kolodziej S., Kohrs N., Herkt S., Wojcik B., Oellerich T., Corso J., Behrens K., Kumar A., Hussong H., Urlaub H., Koch J., Serve H., Bonig H., et al. (2015) RUNX1 represses the erythroid gene expression program during megakaryocytic differentiation. Blood 125, 3570–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mermel C. H., McLemore M. L., Liu F., Pereira S., Woloszynek J., Lowell C. A., and Link D. C. (2006) Src family kinases are important negative regulators of G-CSF-dependent granulopoiesis. Blood 108, 2562–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chuang L. S., Ito K., and Ito Y. (2013) RUNX family: regulation and diversification of roles through interacting proteins. Int. J. Cancer 132, 1260–1271 [DOI] [PubMed] [Google Scholar]