Abstract
The synthesis of eukaryotic ribosomes is a complex, energetically demanding process requiring the aid of numerous non-ribosomal factors, such as the PeBoW complex. The mammalian PeBoW complex, composed of Pes1, Bop1, and WDR12, is essential for the processing of the 32S preribosomal RNA. Previous work in Saccharomyces cerevisiae has shown that release of the homologous proteins in this complex (Nop7, Erb1, and Ytm1, respectively) from preribosomal particles requires Rea1 (midasin or MDN1 in humans), a large dynein-like protein. Midasin contains a C-terminal metal ion-dependent adhesion site (MIDAS) domain that interacts with the N-terminal ubiquitin-like (UBL) domain of Ytm1/WDR12 as well as the UBL domain of Rsa4/Nle1 in a later step in the ribosome maturation pathway. Here we present the crystal structure of the UBL domain of the WDR12 homologue from S. cerevisiae at 1.7 Å resolution and demonstrate that human midasin binds to WDR12 as well as Nle1 through their respective UBL domains. Midasin contains a well conserved extension region upstream of the MIDAS domain required for binding WDR12 and Nle1, and the interaction is dependent upon metal ion coordination because removal of the metal or mutation of residues that coordinate the metal ion diminishes the interaction. Mammalian WDR12 displays prominent nucleolar localization that is dependent upon active ribosomal RNA transcription. Based upon these results, we propose that release of the PeBoW complex and subsequent release of Nle1 by midasin is a well conserved step in the ribosome maturation pathway in both yeast and mammalian cells.
Keywords: ATPases associated with diverse cellular activities (AAA), crystallography, metal ion-protein interaction, ribosomal RNA processing (rRNA processing), ribosome assembly
Introduction
Ribosomes are large macromolecular machines composed of four pieces of ribosomal RNA (rRNA)2 and 80 (79 in yeast) associated ribosomal proteins (1, 2). Ribosomes are responsible for carrying out the synthesis of all proteins within a cell, and the eukaryotic ribosome is composed of two subunits known as the small subunit (40S) and the large subunit (60S). The assembly of ribosomes begins in the nucleolus with the transcription of the rRNAs. Three of the rRNAs are transcribed as a single polycistronic precursor (18S, 5.8S, and 25S), which must then be modified, folded, processed, and exported to the cytoplasm in a carefully orchestrated manner (3, 4). Ribosome biogenesis in eukaryotic cells is an incredibly complex and energetically demanding process that requires more than 200 essential non-ribosomal assembly factors (3–6).
Defects in the mammalian ribosome biogenesis pathway are linked to a group of human diseases that are collectively called ribosomopathies. These are all congenital, inherited disorders with a broad clinical spectrum that have perplexed researchers for years because they cause tissue-specific effects although ribosomes are essential in all cell types (7). Ribosome biogenesis has also been emerging as a new target for cancer therapy. Recent studies have shown that several important oncogenes, including cMYC, RAS, and PI3K, play key roles in promoting hyperactive ribosome biogenesis, and deregulated ribosomal DNA transcription is a requirement for the transformed phenotype (8). Moreover, studies have also shown that the nucleolus is a key element in regulating the cellular stress response and is directly involved in regulating the activity of the tumor-suppressor p53 in response to stress (8–12). Furthering our understanding of the ribosome biogenesis pathway is essential for the development of new cancer therapeutics and treatments for ribosomopathies.
The majority of what is known about eukaryotic ribosome biogenesis is based on extensive studies in the budding yeast Saccharomyces cerevisiae (recently reviewed in Ref. 4). Although the overall process is thought to be well conserved among eukaryotes, much less is known about ribosome biogenesis in higher organisms (14). One complex that has been well characterized in both yeast and mammalian cells is the PeBoW complex (Nop7 complex in S. cerevisiae). The PeBoW complex was named for the mammalian assembly proteins Pes1 (Pescadillo), Bop1 (block of proliferation), and WDR12 (WD repeat domain 12), whereas the yeast homologues are Nop7, Erb1, and Ytm1, respectively. Knockdown of any of the proteins within the mammalian PeBoW complex blocks processing of the large subunit 32S pre-RNA and triggers p53-dependent cell cycle arrest (15, 16). Similarly in yeast, Nop7, Erb1, and Ytm1 are all essential proteins required for the processing of the 27SA3 pre-rRNA (17, 18). All components of the PeBoW/Nop7 complex are thought to be multifunctional proteins and have been linked with roles in various cellular processes, including DNA replication, cell cycle regulation, and cardiac function in addition to ribosome biogenesis (19, 20). The PeBoW/Nop7 complex is thought to primarily localize to the nucleolus. The complex is not found on ribosomal particles within the nucleoplasm or cytoplasm, indicating that the PeBoW complex must be released from preribosomal particles before their transport out of the nucleolus (16, 18, 21).
One way in which ribosome maturation factors are released from preribosomal particles is through the aid of three different AAA-ATPases, Rea1/midasin, Rix7, and Drg1/Afg2, which all utilize both nucleotide binding and hydrolysis to drive release of distinct ribosome maturation factors (reviewed in Ref. 22). Rea1 is responsible for driving release of both the Nop7 complex and the maturation factor Rsa4 from pre-60S particles in S. cerevisiae. Rea1 is the largest protein in S. cerevisiae and contains an N-terminal domain, six concatenated ATPase domains, a 260-kDa linker domain, and a C-terminal MIDAS domain that is well conserved across all eukaryotes (23). Electron microscopy studies reveal that Rea1 has a large AAA motor domain connected to a flexible tail-like structure, which contains the MIDAS domain at the end (24). Electron microscopy studies also indicate that Rea1 contacts preribosomal particles adjacent to the Rix1-Ipi1-Ipi3 subcomplex with the tail being able to reach the preribosomal factor Rsa4 (24). Further studies in S. cerevisiae demonstrated that Rea1 and Rsa4 interact with one another in vivo and in vitro through the MIDAS domain of Rea1 and the conserved N-terminal UBL domain of Rsa4, which was previously referred to as the MIDO (MIDAS-interacting) domain (24). Interaction between Rea1 and Rsa4 coupled with ATP hydrolysis triggers release of Rsa4 from preribosomal particles, probably through a large scale mechanical conformational change (22, 24). The mammalian homologue of Rsa4 is called Notchless (Nle1) and was so named because it is a direct regulator of the Notch signaling pathway in Drosophila melanogaster in addition to its role in ribosome maturation (25).
In addition to driving release of Rsa4, Rea1 has also been shown to drive release of the Nop7 complex from nucleolar preribosomal particles in S. cerevisiae. Bioinformatic analysis revealed that the N-terminal UBL domain of Rsa4 is homologous to the N-terminal UBL domain of Ytm1, suggesting that Rea1 could interact with Ytm1 through its MIDAS domain (21). Earlier work in mice also suggested that the N-terminal domain of WDR12 showed similarity to the N-terminal domain of Nle1, and it was originally referred to in the literature as the Nle1 domain (26). Subsequent experiments in S. cerevisiae demonstrated that Ytm1 interacts with Rea1 through its UBL domain. This interaction is essential for an earlier step in the 60S maturation pathway, and coupled with ATP hydrolysis, it drives release of the Nop7 complex from preribosomal particles (21). Rea1 is therefore able to act on two different substrates at two different stages of assembly of the large ribosomal subunit, including the nucleolar Nop7 complex and the nucleoplasmic Rsa4 (22).
Here we present the crystal structure of the UBL domain of the S. cerevisiae homologue of WDR12 (Ytm1), which revealed that it is structurally homologous with the UBL domain of Rsa4/Nle1. We demonstrate that human midasin and WDR12 as well as midasin and Nle1 interact through their MIDAS and UBL domains, respectively. The interaction between the MIDAS and UBL domains is reminiscent of integrin receptor ligand binding and is dependent upon coordination of a metal ion. However, the MIDAS domain of midasin also contains a well conserved extension region that we show is required for binding WDR12 and Nle1. We also demonstrate that nucleolar localization of WDR12 in U2OS cells is dependent upon active rRNA transcription and that midasin primarily localizes to the nucleoplasm. Taken together, our data reveal the first piece of structural information for WDR12 and suggest that interactions between midasin with the UBL domains of WDR12 and Nle1 are important evolutionarily conserved interactions in the ribosome assembly pathway.
Experimental Procedures
Reagents
Normal rabbit IgG antibodies against midasin and WDR12 for use in immunohistochemistry were obtained from Sigma. Normal mouse antibodies conjugated with horseradish peroxidase against GST (B-14) and His (H3) for use in Western blots were obtained from Santa Cruz Biotechnology. Monoclonal mouse antibody against the Strep-Tag peptide and normal rabbit antibody against actin for use as primary antibodies in Western blots were obtained from Novagen and Sigma-Aldrich, respectively. Goat anti-rabbit IgG and anti-mouse IgG antibodies conjugated with horseradish peroxidase for use as secondary antibodies in Western blots were obtained from Sigma-Aldrich (anti-rabbit) and Millipore (anti-mouse). Goat anti-rabbit IgG antibodies with Alexa Fluor 633 conjugate for fluorescence was obtained from Molecular Probes, Inc. Normal goat serum was obtained from Santa Cruz Biotechnology, Inc. Actinomycin D, paraformaldehyde, and bovine serum albumin were obtained from Sigma-Aldrich. 4′,6-Diamidino-2-phenylindole (DAPI) was purchased from Life Technologies, Inc.
Cloning, Protein Expression, and Protein Purification of the Ytm1 UBL Domain
Residues 1–94 of S. cerevisiae Ytm1 were PCR-amplified from genomic DNA and inserted into the pGST2 parallel vector between the BamHI and NotI restriction sites (27). The Ytm1-UBL plasmid was transformed into BL21(DE3) star cells (Life Technologies). Cells were grown in the presence of 100 μg/ml ampicillin in LB broth at 37 °C to an A600 of 0.6, and then protein expression was induced with 0.9 mm isopropyl-β-d-thiogalactopyranoside for 3 h at 37 °C. Cells were harvested and then frozen at −20 °C until further use. Cells were resuspended in lysis buffer (50 mm Tris, pH 8.0, 500 mm NaCl, 10 mm MgCl2, 0.01% Triton X-100) containing one EDTA-free protease inhibitor tablet (Roche Applied Science) and lysed by sonication at 4 °C. Clarified lysate was applied to a gravity flow column containing 5 ml of GST resin (GE Healthcare) and incubated for 1 h at 4 °C. Unbound protein was removed by successive washes with lysis buffer, and the protein was eluted with 20 ml of elution buffer (50 mm glutathione, 50 mm Tris, pH 8, 200 mm NaCl). The sample was then concentrated and run over a Superdex 75 16/600 size exclusion column (GE Healthcare) pre-equilibrated with 20 mm Tris, pH 8, 200 mm NaCl, 0.1 mm tris(2-carboxyethyl)phosphine. Fractions containing the GST-tagged UBL domain were pooled, and then the GST tag was cleaved overnight at 4 °C with tobacco etch virus protease. The cleaved sample was then run back over the Superdex 75 16/600 size exclusion column to remove GST and any uncleaved protein. Fractions containing the UBL domain were concentrated to 14.4 mg/ml and used immediately for crystallization. Selenomethionyl (SeMet) incorporation was achieved by expression of the UBL domain in BL21 (DE3) star cells grown in M9 minimal medium that was supplemented with SeMet. The SeMet UBL domain was purified as described above, and incorporation of SeMet was verified by mass spectrometry.
Crystallization of the UBL Domain
The Ytm1 UBL domain was crystallized by hanging drop vapor diffusion using equal volumes of protein and well solution containing 0.1 m sodium cacodylate, pH 6.2–6.8, and 0.9–1.2 m sodium citrate. Crystals grew as plate clusters in 2–3 days. Crystal quality was improved by several rounds of microseeding with seed beads (Hampton Research) to yield single plate crystals with dimensions up to 200 × 200 × 50 μm. Crystals were cryoprotected by the stepwise addition of ethylene glycol to a final concentration of 35% (v/v) and then flash-frozen in liquid nitrogen.
Data Collection and Structure Determination
Native x-ray diffraction data were collected at the Advanced Photon Source on the SER-CAT 22-ID beamline (Chicago, IL), and SeMet diffraction data were collected at the Advanced Photon Source on the SER-CAT 22-BM beamline (Chicago, IL). The crystals are in the P1 space group with two copies in the asymmetric unit. The structure was initially solved by molecular replacement using a homology model derived from the structure of the UBL domain of Rsa4 (Protein Data Bank entry 4WJS) in the program MRage (28). Bucanneer (29) was used to build the model, followed by several rounds of manual model building with COOT and refinement with Phenix (28). To confirm that the assignment of the backbone was correct, we used the molecular replacement phases to calculate an anomalous difference map from the SeMet data set, which confirmed the correct placement of the two Met residues. The structure was refined to a final Rwork/Rfree of 17.5%/23.2%.
Yeast Growth Assays
The S. cerevisiae strains used in the study are listed in Table 2. The tetO7 parental strains were obtained from Open Biosystems. Ytm1 together with its endogenous promoter were PCR-amplified from S. cerevisiae genomic DNA and cloned into the centromeric plasmid YCplac111 (30). Mutations to Ytm1 were generated using QuikChange mutagenesis, and all mutations were verified by sequencing. To carry out the dilution plating assays, cells were grown in YPD and then diluted to an A600 of 0.05. 1:10 serial dilutions were then plated on YPD plates with and without 10 μg/ml doxycycline and incubated at 20, 30, and 37 °C for 3 days.
TABLE 2.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| tetO7-Ytm1 | URA::CMV-tTA Ytm1:: kanR-tetO7TATA MATa his3-1 leu2-0 met15-0 | Open Biosystems |
| tetO7-Ytm1 + Ytm1-ycplac111 | URA::CMV-tTA Ytm1:: kanR-tetO7TATA MATa his3-1 leu2-0 met15-0; ycplac111-Ytm1 | This study |
| tetO7-Ytm1 + ycplac111 | URA::CMV-tTA Ytm1:: kanR-tetO7TATA MATa his3-1 leu2-0 met15-0; ycplac111 | This study |
| tetO7-Ytm1 + Ytm1E80A | URA::CMV-tTA Ytm1:: kanR-tetO7TATA MATa his3-1 leu2-0 met15-0; ycplac111-Ytm1E80A | This study |
| tetO7-Ytm1 + Ytm1E86A | URA::CMV-tTA Ytm1:: kanR-tetO7TATA MATa his3-1 leu2-0 met15-0; ycplac111-Ytm1E86A | This study |
| tetO7-Ytm1 + Ytm1R65A | URA::CMV-tTA Ytm1:: kanR-tetO7TATA MATa his3-1 leu2-0 met15-0; ycplac111-Ytm1R65A | This study |
| tetO7-Ytm1 + Ytm1L58S | URA::CMV-tTA Ytm1:: kanR-tetO7TATA MATa his3-1 leu2-0 met15-0; ycplac111-Ytm1 L58S | This study |
| tetO7-Ytm1 + Ytm1F57S | URA::CMV-tTA Ytm1:: kanR-tetO7TATA MATa his3-1 leu2-0 met15-0; ycplac111-Ytm1 F57S | This study |
| tetO7-Ytm1 + Ytm1F57S L58S | URA::CMV-tTA Ytm1:: kanR-tetO7TATA MATa his3-1 leu2-0 met15-0; ycplac111-Ytm1 F57S L58S | This study |
Mammalian Transfections and GST Pull-down Assays
Full-length human Bop1 and Pes1 were amplified from cDNAs (Open Biosystems) and cloned into the pLexM vector (31) containing either an N-terminal His12 tag or N-terminal GST tag. The DNA encoding for residues 5287–5596, which includes the MIDAS domain of human midasin, was codon-optimized for expression in HEK293 cells and cloned into the pLexM vector encoding an N-terminal GST tag and the pCAG-OSF vector (32) encoding an N-terminal OSF (One-Strep-FLAG) tag. Full-length human WDR12 and Nle1 were cloned into the pCAG-OSF vector encoding an N-terminal OSF tag. To create WDR12ΔUBL, residues 84–423 of WDR12 were PCR-amplified from cDNA encoding WDR12 and cloned into the pCAG-OSF vector. To create Nle1ΔUBL, residues 100–485 of Nle1 were PCR-amplified from cDNA encoding Nle1 and cloned into the pCAG-OSF vector. Mutations were introduced using QuikChange mutagenesis (Stratagene). 40-ml cultures of HEK293 cells adapted to grow in suspension were transfected with 1 μg/ml purified plasmid DNA and 2 μg/ml PEI. Cells were harvested 72 h after transfection, washed with PBS, and frozen at −80 °C until further use. Cells were lysed by resuspension in lysis buffer (50 mm Tris, pH 7.4, 500 mm NaCl, 10 mm MgCl2, 10% (v/v) glycerol, 1% (v/v) Triton X-100, EDTA-free protease inhibitors (Roche Applied Science), and 10 units of Benzonase (Sigma)) with gentle rocking at 4 °C for 1 h. Lysates were clarified by centrifugation and then incubated with 100 μl of glutathione resin (GE Healthcare) for 1 h at 4 °C. Unbound protein was removed by three 200-μl washes with lysis buffer, followed by two 200-μl washes with lysis buffer, including 5 mm ATP, and followed by a final 200-μl wash with lysis buffer. Protein that was retained on the resin was analyzed by both SDS-PAGE and Western blotting.
Western Blots
Proteins were separated on 4–15% Mini-PROTEAN TGX Stain-Free gels (Bio-Rad) and transferred to polyvinylidene difluoride membranes (Bio-Rad). Following transfer, membranes were blocked for several hours in 5% (w/v) nonfat milk in Tris-buffered saline, 0.1% Tween 20 (TBST). Membranes were then incubated overnight at 4 °C with either anti-GST (B-14) HRP-conjugated antibody (1:200 dilution), anti-His (H3) HRP-conjugated antibody (1:50 dilution), anti-Strep-Tag primary antibody (1:1000 dilution), or anti-actin primary antibody (1:100 dilution) in 5% (w/v) nonfat milk and 1% (w/v) bovine serum albumin in TBST. Following this overnight incubation, membranes were vigorously washed three times with TBST. Membranes requiring a secondary antibody for visualization were then incubated with either goat anti-mouse IgG (1:10,000 dilution)- or goat anti-rabbit IgG (1:80,000 dilution)-conjugated HRP antibodies in 5% (w/v) nonfat milk and 1% (w/v) bovine serum albumin in TBST and incubated for at least 1 h at room temperature. Subsequent to this incubation, membranes were again vigorously washed three times with TBST. Protein bands were visualized with ECL Plus Western blotting detection reagent (GE Healthcare).
Cell Cultures
U2OS cells were cultured in Dulbecco's modified Eagle's medium high glucose supplemented with 10% (v/v) fetal bovine serum, antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin), and 2 mm glutamine. Cells were incubated at 37 °C in a CO2 incubator.
Immunohistochemistry/Fluorescence
U2OS cells were cultured on 35-mm glass bottom culture dishes (MatTek Corp.) until 80–100% confluence was reached. Medium was discarded; cells were rinsed with 1× PBS, pH 7.4; and cells were fixed in 3–4% paraformaldehyde solubilized in 1× PBS for 1 h. Paraformaldehyde solution was removed, the cells were rinsed with 1× PBS, and cells were permeablized with 0.5% Triton X-100, 1× PBS (Sigma-Aldrich) for 1.5 h. Cells were then washed three times (10 min each wash) with 1× PBS. Cells were then incubated for a minimum of 1.5 h with blocking buffer (1% bovine serum albumin, 4% normal goat serum, and 0.4% Triton X-100 in 1× PBS). Blocking buffer was then removed, and cells were incubated overnight at 4 °C with a primary antibody from Sigma at the manufacturer's recommended dilutions. Cells were then washed three times (10 min each wash) with 1× PBS and then protected from light and incubated with the Alexa Fluor secondary antibodies for 1 h at the manufacturer's recommended dilutions. Cells were then washed again with 1× PBS, as described previously. Cells were finally incubated with 300 nm DAPI, washed once with 1× PBS, and then covered with 1× PBS and visualized with a Zeiss Axio Observer Z1 Epifluorescence Microscope with a Zeiss EC Plan-Neofluar 40×/0.9 objective and aperture lens. The Alexa Fluor 633 conjugate fluorochrome was imaged at room temperature on the Zeiss AxioCam MRm camera with Zen Pro 2012 (Blue Edition) software and processed with ImageJ.
Results
Structure of the UBL Domain of S. cerevisiae Ytm1
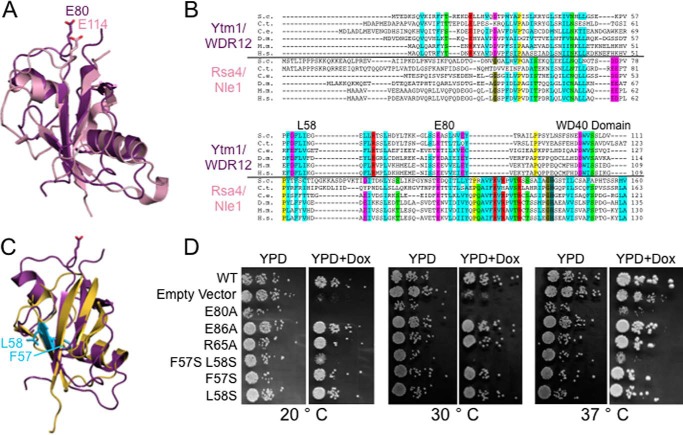
To further our structural knowledge of Ytm1/WDR12 (Fig. 1A), we determined a crystal structure of the UBL domain (density was visible for residues 5–91) of Ytm1 from S. cerevisiae at 1.7 Å resolution (Fig. 1B). The structure was solved by molecular replacement with the recently published UBL domain of Rsa4 (Protein Data Bank entry 4WJS (33)) as a search model, and the backbone assignment was confirmed by calculation of an anomalous difference map from a selenium methionine derivative. The structure was refined to an Rwork/Rfree of 17.5%/23.2% (see Fig. 1C for a representative section of unbiased electron density; for further data collection and crystallography statistics, see Table 1). There are two nearly identical copies of the domain in the asymmetric unit, but there is no evidence to suggest that the domain forms a dimer in solution or in vivo. The structure revealed that the UBL domain of Ytm1 has a classic, compact ubiquitin β-grasp fold, composed of four β-strands that form a central β-sheet and two α-helices located on one side of the β-sheet (Fig. 1B).
FIGURE 1.
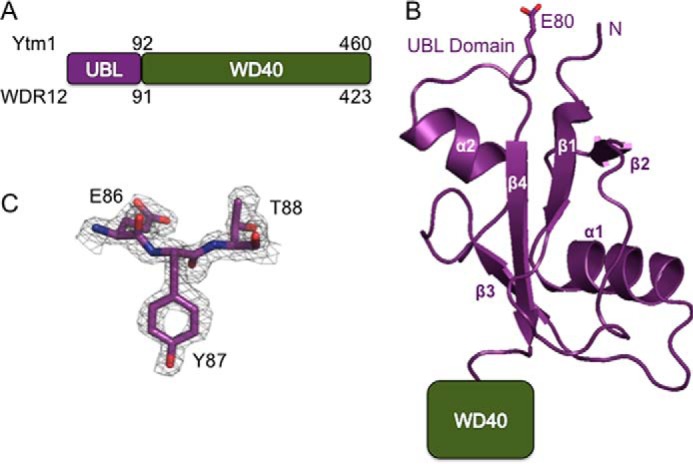
The crystal structure of the UBL domain from S. cerevisiae Ytm1. A, domain diagram of Ytm1 and WDR12. The numbers at the top correspond to the amino acid residues from S. cerevisiae Ytm1, and numbers at the bottom correspond to the amino acid residues from Homo sapiens WDR12. B, ribbon diagram of the UBL domain of S. cerevisiae Ytm1. The N terminus of the protein is labeled as well as the C terminus of the domain that leads to the WD40 domain. Residue Glu80, which is thought to coordinate the metal ion with the MIDAS domain of Rea1, is shown as a stick representation. C, composite omit map showing representative electron density of the structure at 1.7 Å resolution between residues Glu86 and Thr88. The map was contoured at 1.7 σ.
TABLE 1.
Data collection and refinement statistics
Statistics for the high resolution shell are shown in parentheses.
| UBL native | UBL SeMet | |
|---|---|---|
| Data collection | ||
| Beamline | SER-CAT 22ID | SET-CAT 22BM |
| Wavelength (Å) | 1.0000 | 0.9649 |
| Resolution range (Å) | 50–1.70 (1.76–1.70) | 50.00–1.91 (1.98–1.91) |
| Space group | P1 | P1 |
| Cell dimensions | ||
| a, b, c (Å) | 35.299, 37.742, 41.135 | 37.525, 35.067, 41.012 |
| α, β, γ (degrees) | 82.542, 64.309, 69.619 | 64.395, 82.480, 69.558 |
| Total reflections | 47,152 (4135) | 40,477 (3359) |
| Unique reflections | 18,667 (1654) | 12,086 (1244) |
| Completeness (%) | 95.3 (85.4) | 87.5 (88.5) |
| Mean Ι/σ | 8.2 (2.5) | 14.2 (2.7) |
| Multiplicity | 2.5 (2.0) | 3.3 (2.7) |
| Rmerge (%) | 9.1 (34.4) | 9.0 (35.4) |
| Refinement | ||
| Resolution range (Å) | 50–1.70 | |
| Rwork/Rfree (%) | 17.5/23.2 | |
| Root mean square deviation | ||
| Bond lengths (Å) | 0.0058 | |
| Bond angles (Å) | 0.93 | |
| Ramachandran plot | ||
| Most favored (%) | 96.5 | |
| Additional allowed (%) | 2.94 | |
| Disallowed (%) | 0.6 | |
| Wilson B factor (Å2) | 28.96 | |
| Protein Data Bank entry | 5DTC | |
Despite little sequence homology between the UBL domains of Rsa4 and Ytm1 (Fig. 2B), the structures of the two domains superimpose well with one another with a root mean square deviation of 4.5 Å over 80 Cα residues (Fig. 2A). Both Rsa4 and Ytm1 contain well conserved glutamate residues that are thought to coordinate the metal ion with the Rea1 MIDAS domain in S. cerevisiae (21, 24). The conserved glutamate residue (residue Glu80 in S. cerevisiae) in Ytm1, important for binding the Rea1 MIDAS domain lies in an extended loop before the final β-strand in the same structural location as the conserved glutamate in Rsa4 (Figs. 1B and 2A). A search of the Dali server revealed that the Ytm1 UBL domain is most similar to the autophagy-associated ubiquitin-like protein, Atg12, and the E3 ubiquitin-protein ligase, RING2, highlighting the range of diversity of biological activity of UBL domains. The Ytm1 UBL domain also superimposes reasonably well with ubiquitin (Protein Data Bank entry 1UBQ) with a root mean square deviation of 5.5 Å over 71 Cα residues (Fig. 2C). Overall, given the structural similarity between Rsa4 and Ytm1 along with the overlapping glutamate residues, Rea1 undoubtedly interacts with both proteins in an analogous fashion.
FIGURE 2.
Comparison of the UBL domains from Ytm1/WDR12 and Rsa4/Nle1. A, alignment of the crystal structures of the UBL domains from S. cerevisiae Ytm1 (purple) and C. thermophilum Rsa4 (pink; Protein Data Bank entry 4WJS). B, multiple sequence alignment from the UBL domains of Ytm1/WDR12 and Rsa4/Nle1 from different homologues of Homo sapiens (H.s.), Mus musculus (M.m.), D. melanogaster (D.m.), Caenorhabditis elegans (C.e.), C. thermophilum (C.t.), and S. cerevisiae (S.c.). C, alignment of the crystal structure of the UBL domain from S. cerevisiae Ytm1 (purple) with ubiquitin (yellow; Protein Data Bank entry 1UBQ). Residues Phe57 and Leu58 of Ytm1 are shown as cyan stick representations. D, yeast growth assay of Ytm1 UBL mutants. Transformants were spotted in 10-fold serial dilutions onto YPD plates in the presence or absence of 10 μg/ml doxycycline to deplete the expression of endogenous Ytm1 and were grown at 20, 30, and 37 °C for 3 days.
Mutations in the UBL Domain of Ytm1 Cause Growth Defects in Yeast
The UBL domain of Ytm1 is essential for viability in S. cerevisiae and contains many well conserved residues (Fig. 2B). It has been previously shown in S. cerevisiae that truncation of the UBL domain or mutation of residue E80A in yeast is lethal (18, 21). However, there are many other well conserved residues within the UBL domain. To determine which of these residues were essential for cell viability, we made mutations to the UBL domain and tested the effects on growth. We transformed plasmids encoding wild type or mutant Ytm1 into cells harboring endogenous Ytm1 under a Tet promoter (Hughes Tet-Promoter Collection, Open Biosystems) and assayed for growth in the presence and absence of 10 μg of doxycycline at 20, 30, and 37 °C (see Table 2 for a list of yeast strains used in this study). The addition of doxycycline turns off expression of endogenous Ytm1. The E80A mutation was used as a positive control, and as expected, both depletion of endogenous Ytm1 and expression of the E80A mutant conferred a severe growth defect relative to growth of the plasmid expressing wild type Ytm1 (Fig. 2D). Surprisingly, mutation of the well conserved residues Arg65 and Glu86 to alanine had no effect on growth of yeast, indicating that they are not essential for function (Fig. 2D).
Given the high structural similarity between the UBL domain of Ytm1 and ubiquitin, we hypothesized that Ytm1 may contain an essential hydrophobic patch required for function. It has been well established in ubiquitin that residue Ile44 forms part of an essential hydrophobic patch along with residues Leu8 and Val70 that is important for maintaining interactions with α-helical ubiquitin-associated domains, such as S5a of the 26S proteasome (34). There are two well conserved hydrophobic residues in Ytm1, Phe57 and Leu58, that superimpose near Ile44 in ubiquitin (Fig. 2C). Individual mutation of Phe57 or Leu58 to serine was not lethal in yeast, but the double mutant of F57S/L58S conferred a severe growth defect in yeast that is comparable with depletion of the endogenous protein (Fig. 2D), suggesting that retention of this hydrophobic patch is critical for function. Phe57-Leu58 in Ytm1 could be essential for interacting with another portion of midasin and/or the Rix1 complex, which is known to be associated with midasin preribosomal particles (24, 35).
Human Midasin Binds WDR12 through Its UBL Domain
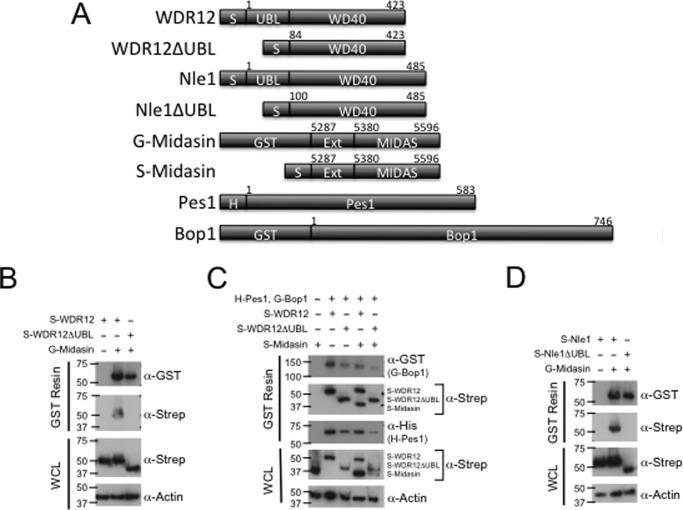
After solving the crystal structure of the UBL domain of Ytm1, we probed the function of the UBL domain of WDR12 in mammalian cells and demonstrated that it is required for binding midasin. Previous work has shown that WDR12ΔUBL inhibits cell proliferation, triggers accumulation of p53, and disrupts rRNA processing, indicating that the UBL domain of WDR12 is essential for mammalian ribosome biogenesis (15). Given the importance of the UBL domain in mammalian cells, we hypothesized that WDR12 binds to midasin through its UBL domain like its yeast counterpart. To determine whether midasin and WDR12 are binding partners, HEK293 cells were transfected with plasmids containing human midasin and human WDR12 and assayed for binding by GST pull-downs (Fig. 3A). Our starting construct for the midasin MIDAS domain was based on equivalent residues used to carry out in vitro pull-downs with the yeast homologues (21). GST-tagged residues 5287–5596 of midasin, which included the full MIDAS domain and about 100 residues upstream of the MIDAS domain, were able to pull down full-length WDR12 but not WDR12ΔUBL (Fig. 3B). Expression of both WDR12 and WDR12ΔUBL was confirmed by Western blot analysis, and full-length WDR12 does not interact with GST-bound glutathione resin in the absence of midasin (Fig. 3B). Therefore, the WDR12 UBL domain is necessary for interaction with midasin.
FIGURE 3.
Human midasin binds WDR12 and Nle1 through their UBL domains. A, schematic diagram of the WDR12, Nle1, midasin, Pes1, and Bop1 constructs used for GST pull-down analysis. H, His tag; S, One-Strep-FLAG tag; Ext, Midas extension region. B, the UBL domain of WDR12 is required to bind midasin. HEK293 cells were transfected with plasmids harboring GST-midasin-MIDAS and OSF-WDR12 with and without the UBL domain. GST pull-downs were analyzed by Western blot. C, the UBL domain of WDR12 is not required to form the PeBoW complex. HEK293 cells were transfected with plasmids harboring GST-Bop1, His-Pes1, OSF-WDR12 with and without the UBL domain, and OSF-midasin. D, the UBL domain of Nle1 is required for binding midasin. HEK293 cells were transfected with plasmids harboring GST-midasin MIDAS and OSF-Nle1 with and without the UBL domain. WCL, whole cell lysate.
Midasin Binds the PeBoW Complex through the UBL Domain of WDR12
After demonstrating that WDR12 binds to midasin through its UBL domain, we wanted to determine how midasin interacts with the PeBoW complex. First we transfected HEK293 cells with plasmids harboring full-length GST-tagged Bop1 as bait, full-length Pes1, and full-length WDR12 or WDR12ΔUBL, to determine what effect the UBL domain of WDR12 has on formation of the PeBoW complex. We assayed for binding by GST pull-down followed by Western blot analysis, which revealed that the UBL domain of WDR12 is not required for formation of the PeBoW complex (Fig. 3C). We do observe differences in the amounts of WDR12 that is pulled down. However, if we normalize everything to the amount of Bop1, which was used as bait, the levels of Pes1 and WDR12 are fairly uniform. All of our pull-downs were carried out in cells that were transiently transfected with multiple plasmids, so we attribute these differences to levels of transfection efficiency.
After we confirmed that the UBL domain of WDR12 is not required for formation of the PeBoW complex, we looked at binding to midasin. HEK293 cells were transfected with plasmids harboring full-length GST-tagged Bop1 as bait, full-length Pes1, full-length WDR12 or WDR12ΔUBL, and residues 5287–5596 of midasin. GST-tagged Bop1 was able to pull down the midasin MIDAS domain only in the presence of full-length WDR12 (Fig. 3C). Therefore, midasin can interact with WDR12 alone and within the PeBoW complex; however, both interactions are dependent upon the UBL domain of WDR12.
Human Midasin Binds Nle1 through Its UBL Domains
We further demonstrated that human Nle1 also binds to midasin through its UBL domain. Because we showed that the UBL domains of the yeast homologues of Nle1 (Rsa4) and WDR12 (Ytm1) are structurally homologous (Fig. 2A), we assumed that Nle1 would also bind midasin though its UBL domain in mammalian cells. Nle1 has previously been shown to bind to midasin/Rea1 in several organisms, including S. cerevisiae and Solanum chacoense (24, 36). To determine whether mammalian midasin and Nle1 are binding partners, we transfected HEK293 cells with plasmids encoding residues 5287–5596 of midasin and Nle1 with or without the UBL domain and assayed for binding by GST pull-downs. GST-tagged residues 5287–5596 of midasin were able to pull down full-length Nle1 but not Nle1ΔUBL (Fig. 3D). Expression of both Nle1 and Nle1ΔUBL was confirmed by Western blot analysis, and full-length Nle1 does not interact with GST-bound glutathione resin in the absence of midasin (Fig. 3B). Our results confirm that, as in yeast, mammalian midasin is able to bind both WDR12 and Nle1 through their respective UBL domains.
The Extension Region of the Midasin MIDAS Domain Is Required to Bind WDR12 and Nle1
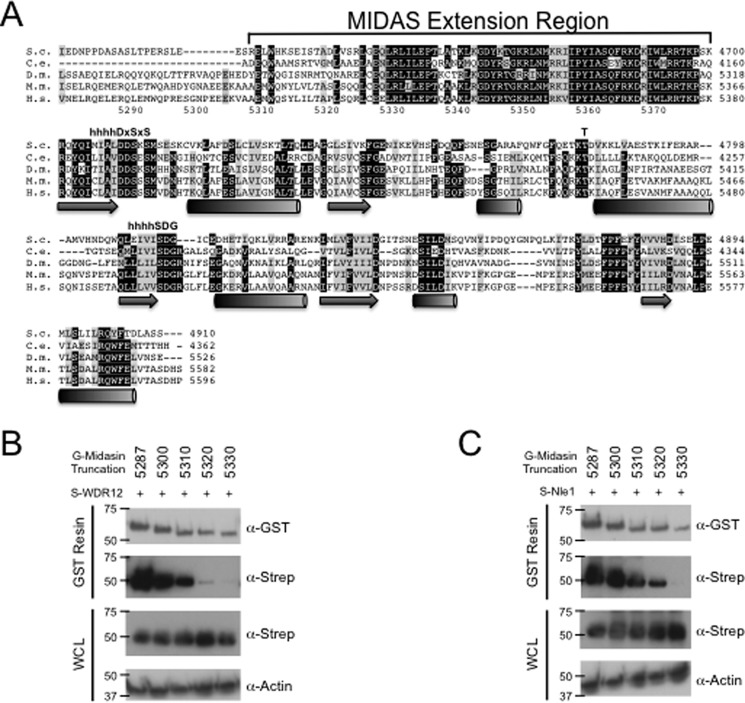
After confirming that midasin binds to both WDR12 and Nle1, we mapped the binding interface between the proteins and found that midasin contains a well conserved region required for binding WDR12 and Nle1. Just upstream of the MIDAS consensus motif, there is a region that is highly conserved (46% invariant) in midasin (Fig. 4A) and is predicted to be α-helical but shares no significant homology with other known proteins (23). This will be referred to as the MIDAS extension region for the remainder of this paper. We hypothesized that, given its high sequence conservation, the MIDAS extension region must play an important role in midasin function and may be important for binding WDR12 and Nle1. To test this hypothesis, a series of N-terminal truncations to the MIDAS extension region were created and then assayed for binding to WDR12 and Nle1 by GST pull-down. Starting with the midasin truncation 5310–5596, we see a diminished capacity for WDR12 binding to midasin (Fig. 4B). N-terminal truncations past residue 5320 were unable to pull down WDR12, indicating that the entire MIDAS extension region is required for binding WDR12 (Fig. 4B). Given that the extension region is not part of the canonical MIDAS consensus sequence, the interaction between midasin and WDR12 must require more than the typical MIDAS/ligand metal ion interface. We repeated the same experiment with Nle1 as well and obtained similar results (Fig. 4C), indicating the importance of the midasin MIDAS extension region for binding both Nle1 and WDR12.
FIGURE 4.
The MIDAS extension region of midasin is required to bind WDR12. A, multiple sequence alignment of the MIDAS domain and ∼100 residues upstream of the MIDAS domain of midasin from different homologues of H. sapiens (H.s.), M. musculus (M.m.), D. melanogaster (D.m.), C. elegans (C.e.), and S. cerevisiae (S.c.). The predicted secondary structure of the MIDAS domain based on secondary structure alignments in HHPRED is shown below the sequence, and the numbers at the bottom of the first row correspond to the H. sapiens midasin sequence. The metal ion consensus sequence in MIDAS domains is composed of the following: hhhhDXSXS, followed by a conserved threonine in ∼70 residues and an hhhh(S/T)DG in ∼30 residues, where h is any hydrophobic and X is any residue (23, 37). MIDAS consensus sequence residues are indicated above the sequence. B, Western blot analysis of GST pull-downs of midasin MIDAS N-terminal truncations with WDR12. The constructs used contained the following residues of midasin fused to an N-terminal GST tag: 5287 (residues 5287–5596), 5300 (residues 5300–5596), 5310 (residues 5310–5596), 5320 (residues 5320–5596), and 5530 (residues 5530–5596). C, Western blot analysis of GST pull-downs of midasin MIDAS truncations with Nle1. WCL, whole cell lysate.
Glu78 of WDR12 Is Required for Binding Midasin
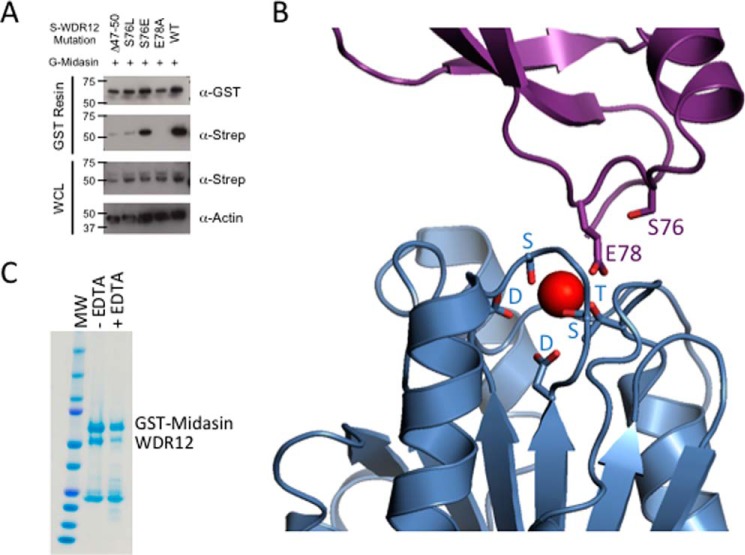
We mapped the interface between midasin and WDR12 and confirmed that the conserved glutamate, residue Glu78 of WDR12 (Glu80 in S. cerevisiae Ytm1), is required for binding midasin. Mutation of either the conserved aspartic acid residues in the MIDAS consensus motif or the conserved glutamate in the UBL domains of Ytm1 and Rsa4 abolishes the interactions between midasin and Ytm1/Rsa4 in yeast (21, 24). To determine whether this also holds true in mammalian cells, we mutated the corresponding glutamate in WDR12 (Glu78) to alanine and found that the E78A mutation was unable to bind the midasin MIDAS domain (Fig. 5A). Mutation of S78L in yeast Ytm1 has been shown to be synthetic lethal when combined with a mutant of Rea1 (21). This serine residue is conserved in mammalian WDR12 (Fig. 2B), and mutation of Ser76 to leucine significantly reduced binding to the midasin MIDAS domain (Fig. 5A). Mutation of Ser76 to a glutamate residue, however, did not affect binding to midasin (Fig. 5A). Sequence alignments of WDR12 across numerous species (Fig. 2B) revealed that higher organisms have a small insertion in the loop following the first α-helix, and deletion of residues 47–50 of mammalian WDR12 also significantly reduced binding to the midasin MIDAS domain (Fig. 5A). We also mutated the conserved aspartic acid residues within the MIDAS consensus motif in midasin but were unable to determine whether they abolish WDR12 binding because we could not detect expression of the midasin-MIDAS double aspartic acid mutant when transfected in HEK293 cells.
FIGURE 5.
Metal ion is required for WDR12 and midasin binding. A, Western blot analysis of GST pull-downs with WDR12 UBL mutations. B, model of the interaction between midasin (blue) and the UBL domain of WDR12 (purple). The homology model of midasin was generated from a crystal structure of the αLβ2 integrin (Protein Data Bank entry 1T0P). Residues important for coordinating the metal ion (red) are shown as stick representations. C, GST pull-downs of midasin and WDR12 with and without EDTA. Cells were lysed in the presence or absence of EDTA, and the binding was assayed by GST pull-downs followed by SDS-PAGE analysis (Simply Blue Stain (Thermo)). WCL, whole cell lysate.
Metal Ion Is Required for Midasin-MIDAS and WDR12 Binding
After verifying that the residues thought to be important for coordinating the metal ion between midasin and WDR12 are important for binding, we demonstrated that complex formation is dependent upon the presence of a metal ion. MIDAS domains have been extensively studied in integrins, where they play important roles in mediating ligand binding through coordination of a metal ion that is essential for ligand binding (22, 37, 38). We generated a homology model for the WDR12 UBL domain and midasin MIDAS domain interaction using our Ytm1 UBL structure for WDR12 and the αLβ2 integrin (Protein Data Bank entry 1T0P) MIDAS domain structure for midasin (Fig. 5B).
In the crystal structure of the αLβ2 integrin bound to its ICAM ligand, the metal bound between the two proteins is a magnesium ion. The midasin MIDAS domain contains the full canonical MIDAS consensus sequence (hhhhDXSXS, ∼70 residues T, and an hhhh(S/T)DG in ∼30 residues, where h is any hydrophobic residue and X is any residue (Fig. 4A)), as is found in the αLβ2 integrin (39). WDR12 and Nle1 also contain the conserved glutamic acid residue like the ICAM ligand. Thus, all six residues that coordinate the metal ion are conserved, so we believe that the metal ion coordination will be the same as the αLβ2 integrin bound to its ICAM ligand. Missing from our homology model is the MIDAS extension region because we have no structural information for this region. To determine the importance of the metal ion for WDR12 binding to the midasin MIDAS domain, we transfected HEK293 cells with plasmids containing human midasin MIDAS and human WDR12. 72 h after transfection, the cells were harvested, split in half, and then lysed in the presence or absence of 10 mm EDTA to ablate metal coordination. EDTA greatly diminishes the amount of WDR12 that can be pulled down by GST-tagged midasin-MIDAS (Fig. 5C). This result confirms that a metal ion is required to promote complex formation between the MIDAS domain of midasin and WDR12.
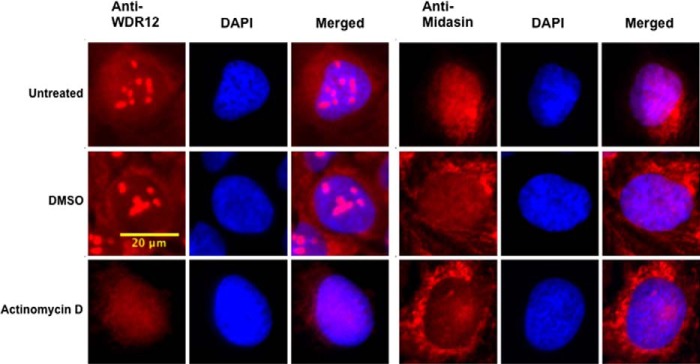
WDR12 but Not Midasin Localizes to the Nucleolus in U2OS Cells
To further characterize mammalian WDR12 and midasin, we carried out immunofluorescence experiments in U2OS osteoscarcoma cells with specific antibodies for WDR12 and midasin. Endogenous WDR12 showed prominent nucleolar localization, as expected (15), whereas endogenous midasin localized to both the nucleoplasm and the cytoplasm (Fig. 6). Interestingly, midasin also appears to have prominent localization in a distinct location in the cytoplasm, suggesting that it may have additional non-ribosomal roles in the cell (Fig. 6). The localization of endogenous WDR12 to the nucleolus is consistent with it playing a role in the early stages of ribosome biogenesis that take place in the nucleolus, whereas the localization of midasin in the nucleoplasm is consistent with its role in the later stages of ribosome biogenesis (40).
FIGURE 6.
WDR12 requires RNA polymerase I transcription for nucleolar localization. U2OS cells were fixed and immunostained with an anti-WDR12 antibody (red) or anti-midasin antibody (red), and DNA was visualized by staining with DAPI (blue). Cells were treated with DMSO or actinomycin D for 2 h prior to fixing. The scale bar is representative of all panels.
Next, we wanted to determine whether the localization of WDR12 and midasin was affected by cellular stress and rRNA transcription. Disruption of rRNA transcription by various cellular stressors can cause a relocalization of proteins involved in ribosome biogenesis (41). For example, Pes1, one of the other proteins associated with WDR12 in the PeBoW complex, relocalizes from the nucleolus to the nucleoplasm upon induction of cellular stress (42). To mimic cellular stress, we treated U2OS cells with a low concentration (10 μm) of actinomycin D to inhibit rRNA transcription by RNA polymerase I, and we repeated our immunofluorescence experiments using DMSO as a control. WDR12 loses its nucleolar localization upon treatment with actinomycin D but not DMSO alone. It is instead found in the nucleoplasm, confirming that cellular stress affects the localization of WDR12 (Fig. 6). In contrast, the nucleoplasmic localization of midasin did not change upon treatment of cells with actinomycin D or DMSO (Fig. 6).
Discussion
The assembly of eukaryotic ribosomes requires two essential proteins, Ytm1/WDR12 and Rsa4/Nle1, that both harbor UBL domains followed by C-terminal WD40 β-propeller domains. Using x-ray crystallography, we determined the crystal structure of the UBL domain from the yeast homologue of Ytm1/WDR12. UBL domains mimic the properties of ubiquitin through incorporation of the ubiquitin β-grasp fold within their protein coding regions. They are used in a wide variety of cellular processes, such as protein degradation, DNA repair, cell division, and autophagy, and are often part of protein-protein interaction motifs (34, 43, 44). WDR12 and Nle1 are the only proteins known to contain a UBL domain in the ribosome assembly pathway. The function of this domain is to recruit the tail of the large motor protein midasin and drive release of both the PeBoW complex and Nle1 from preribosomal particles, presumably through factor-relay mechanisms that cause conformation changes within the pre-rRNA (33). The recent crystal structure of the yeast homologue of Rsa4/Nle1 contained two copies of the protein in the asymmetric unit, and interestingly, the UBL domain in each had a different orientation to the β-propeller, suggesting that the rotational flexibility of the UBL domain could be important for function (33). During the revision of this manuscript, another group published the crystal structure of Chaetomium thermophilium Ytm1 bound to the C-terminal end of Erb1. As was observed for Rsa4, they also found that the UBL domain of Ytm1 was found in different orientations, emphasizing the flexible orientation of this domain with respect to the β-propeller domain (45). Given the structural similarity of the UBL domain of Ytm1/WDR12 presented here and Rsa4/Nle1 (Fig. 2A), including the overlapping glutamate residues, the interactions between the midasin MIDAS domain and the UBL domains of WDR12 and Nle1 should be very similar. This is reminiscent of integrins, which can interact with a range of different ligands through their MIDAS domains (46).
Whereas WDR12 and Nle1 are well conserved, little is known about their roles in mammalian ribosome biogenesis. In this study, we present the first evidence that interactions with midasin and WDR12 as well as midasin and Nle1 are conserved in higher organisms. As in yeast, the UBL domain of WDR12 is required for binding midasin but not for formation of the PeBoW complex along with Pes1 and Bop1 (Fig. 3C). The UBL domain of Nle1 is also required for binding midasin (Fig. 3D). These results suggest that release of the nucleolar PeBoW complex and the nuclecoplasmic Nle1 by the large motor protein, midasin, is a well conserved step in the eukaryotic ribosome maturation pathway.
Roles of WDR12 and midasin in mammalian ribosome biogenesis are also further supported by their endogenous localization. WDR12 displays prominent nucleolar localization; however, cellular stress induced by treatment of cells with actinomycin D halts rRNA transcription and leads to a relocalization of WDR12 in the nucleoplasm (Fig. 6). Human midasin did not display nucleolar localization but rather was found in the nucleoplasm and cytoplasm under both normal and stress conditions (Fig. 6). The localization of human midasin is similar to that of its yeast homologue, which is not found in the nucleolus except in the presence of the E80A glutamate mutant of Ytm1 that halts ribosome maturation (21). How midasin gets recruited to preribosomal particles in the nucleolus is still unknown. Previous work has suggested that WDR12 and the Rix complex are the two most likely candidates for recruitment, although other trans-acting factors could be involved (21). Collectively, these results suggest that midasin is only transiently associated with the nucleolus to drive release of the PeBoW complex.
We also identified that the MIDAS extension region in mammalian midasin is required for binding WDR12 and Nle1 (Fig. 4). The extension region is not part of the canonical MIDAS domain but is highly conserved and essential for binding WDR12 and Nle1, suggesting that it may also play an important role in metal ion coordination. Integrins are dependent upon metal ions for their function, and their dependence upon metal ions has been well studied (38). Some integrin domains contain metal ion-binding sites in addition to the MIDAS domain. For example, the βI integrin domain is made up of three interlinking metal ion-binding sites, including the MIDAS site, adjacent to MIDAS (AMIDAS) site, and synergistic metal ion-binding site (SyMBS). The exact in vivo roles of all three sites are unclear, but in vitro all three sites appear to play important roles in mediating ligand binding (38). There is no sequence similarity between the MIDAS extension region and the SyMBS or AMIDAS metal ion-binding sites, but the MIDAS extension region could represent a new type of metal ion coordination site. Bioinformatic analysis suggested that there is a weak similarity between the MIDAS extension region and the D subunit of magnesium chelatases based upon the high density of basic and hydrophobic residues just upstream of the MIDAS domain (23). Magnesium chelatases, which play an important role in chlorophyll biosynthesis by inserting Mg2+ into protoporphyrin, also contain C-terminal MIDAS domains and two N-terminal AAA domains (47). In addition to magnesium chelatases and midasin, there is only one other known protein, VWA8 (only found in higher organisms; function is unknown), that contains the combination of AAA and MIDAS domains (37). Further work will be needed to determine the exact role of the MIDAS extension region in midasin, but it will be interesting to see whether there is a functional relationship between the AAA domains and the MIDAS domain because this combination also occurs in two other proteins.
In this paper, we present the first high resolution structure of the UBL domain of the yeast homologue of Ytm1/WDR12 and demonstrate that mammalian midasin binds to both WDR12 and Nle1, two proteins that have interesting implications for human health. Recent work has demonstrated that overexpression of WDR12 is induced by cardiac overload, and genome association studies have associated the WDR12 gene with early onset myocardial infarction and coronary artery disease (20, 48). Up-regulation of WDR12 has also been shown to lead to activation of the p38 MAPK pathway, an increase in levels of Bop1, and ultimately myocardial dysfunction, suggesting that WDR12 could be a novel therapeutic target for patients with failing hearts (20). Moreover, elevated levels of the proteins associated with the PeBoW complex have been found in many different types of human cancers, including neuroblastoma, hepatocellular carcinoma, and breast, colon, and ovarian cancers (42, 49–55). Nle1 was also recently shown to be a crucial factor for intestinal homeostasis and a requirement for organogenesis, especially in axial skeleton formation in mice (13, 56). Further investigations into the roles of WDR12 and Nle1 are needed to determine the mechanisms by which they are involved in cardiac function and intestinal homeostasis, but our work identifies that the interactions between the UBL domains of WDR12 and Nle1 with midasin are both evolutionarily conserved interactions important for the synthesis of eukaryotic ribosomes.
Author Contributions
E. M. R. purified and crystallized the UBL domain of Ytm1 and solved the structure. E. M. R. designed and constructed vectors for pull-downs and characterized the complex interaction in mammalian cells. M. S. carried out the immunofluorescence experiments and all Western blot analysis. R. E. S. designed the study and conceived the experiments. E. M. R., M. S., and R. E. S. analyzed data, prepared figures, and wrote the paper.
Acknowledgments
We thank Traci M. Tanaka Hall and Michael J. Ragusa for critical reading of the manuscript. We also thank the NIEHS, National Institutes of Health, Protein Expression Core for help with mammalian cell culture and the NIEHS X-ray Crystallography Core for help with data collection. Diffraction data were collected at the Southeast Regional Collaborative Access Team (SER-CAT) 22-ID and 22-BM beamlines at the Advanced Photon Source, Argonne National Laboratory. We also thank the NIEHS Fluorescence Microscopy and Imaging Center for help with the immunofluorescence experiments.
This work was supported in whole or in part by the Intramural Research Program of NIEHS, National Institutes of Health. Use of the Advanced Photon Source was supported by the United States Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract W-31-109-Eng-38. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The atomic coordinates and structure factors (code 5DTC) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- rRNA
- ribosomal RNA
- MIDAS
- metal ion-dependent adhesion site
- AMIDAS
- adjacent to MIDAS
- SyMBS
- synergistic metal ion-binding site
- UBL
- ubiquitin-like
- ICAM
- intercellular adhesion molecule.
References
- 1.Jenner L., Melnikov S., Garreau de Loubresse N., Ben-Shem A., Iskakova M., Urzhumtsev A., Meskauskas A., Dinman J., Yusupova G., and Yusupov M. (2012) Crystal structure of the 80S yeast ribosome. Curr. Opin. Struct. Biol. 22, 759–767 [DOI] [PubMed] [Google Scholar]
- 2.Wilson D. N., and Doudna Cate J. H. (2012) The structure and function of the eukaryotic ribosome. Cold Spring Harb. Perspect. Biol. 10.1101/cshperspect.a011536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson E., Ferreira-Cerca S., and Hurt E. (2013) Eukaryotic ribosome biogenesis at a glance. J. Cell Sci. 126, 4815–4821 [DOI] [PubMed] [Google Scholar]
- 4.Woolford J. L. Jr., and Baserga S. J. (2013) Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics 195, 643–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kressler D., Hurt E., and Bassler J. (2010) Driving ribosome assembly. Biochim. Biophys. Acta 1803, 673–683 [DOI] [PubMed] [Google Scholar]
- 6.Tafforeau L., Zorbas C., Langhendries J. L., Mullineux S. T., Stamatopoulou V., Mullier R., Wacheul L., and Lafontaine D. L. (2013) The complexity of human ribosome biogenesis revealed by systematic nucleolar screening of pre-rRNA processing factors. Mol. Cell 51, 539–551 [DOI] [PubMed] [Google Scholar]
- 7.McCann K. L., and Baserga S. J. (2013) Genetics: mysterious ribosomopathies. Science 341, 849–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods S. J., Hannan K. M., Pearson R. B., and Hannan R. D. (2015) The nucleolus as a fundamental regulator of the p53 response and a new target for cancer therapy. Biochim. Biophys. Acta 1849, 821–829 [DOI] [PubMed] [Google Scholar]
- 9.Bursac S., Brdovcak M. C., Donati G., and Volarevic S. (2014) Activation of the tumor suppressor p53 upon impairment of ribosome biogenesis. Biochim. Biophys. Acta 1842, 817–830 [DOI] [PubMed] [Google Scholar]
- 10.Golomb L., Volarevic S., and Oren M. (2014) p53 and ribosome biogenesis stress: the essentials. FEBS Lett. 588, 2571–2579 [DOI] [PubMed] [Google Scholar]
- 11.James A., Wang Y., Raje H., Rosby R., and DiMario P. (2014) Nucleolar stress with and without p53. Nucleus 5, 402–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quin J. E., Devlin J. R., Cameron D., Hannan K. M., Pearson R. B., and Hannan R. D. (2014) Targeting the nucleolus for cancer intervention. Biochim. Biophys. Acta 1842, 802–816 [DOI] [PubMed] [Google Scholar]
- 13.Stedman A., Beck-Cormier S., Le Bouteiller M., Raveux A., Vandormael-Pournin S., Coqueran S., Lejour V., Jarzebowski L., Toledo F., Robine S., and Cohen-Tannoudji M. (2015) Ribosome biogenesis dysfunction leads to p53-mediated apoptosis and goblet cell differentiation of mouse intestinal stem/progenitor cells. Cell Death Differ. 22, 1865–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullineux S. T., and Lafontaine D. L. (2012) Mapping the cleavage sites on mammalian pre-rRNAs: where do we stand? Biochimie 94, 1521–1532 [DOI] [PubMed] [Google Scholar]
- 15.Hölzel M., Rohrmoser M., Schlee M., Grimm T., Harasim T., Malamoussi A., Gruber-Eber A., Kremmer E., Hiddemann W., Bornkamm G. W., and Eick D. (2005) Mammalian WDR12 is a novel member of the Pes1-Bop1 complex and is required for ribosome biogenesis and cell proliferation. J. Cell Biol. 170, 367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohrmoser M., Hölzel M., Grimm T., Malamoussi A., Harasim T., Orban M., Pfisterer I., Gruber-Eber A., Kremmer E., and Eick D. (2007) Interdependence of Pes1, Bop1, and WDR12 controls nucleolar localization and assembly of the PeBoW complex required for maturation of the 60S ribosomal subunit. Mol. Cell. Biol. 27, 3682–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miles T. D., Jakovljevic J., Horsey E. W., Harnpicharnchai P., Tang L., and Woolford J. L. Jr. (2005) Ytm1, Nop7, and Erb1 form a complex necessary for maturation of yeast 66S preribosomes. Mol. Cell. Biol. 25, 10419–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang L., Sahasranaman A., Jakovljevic J., Schleifman E., and Woolford J. L. Jr. (2008) Interactions among Ytm1, Erb1, and Nop7 required for assembly of the Nop7-subcomplex in yeast preribosomes. Mol. Biol. Cell 19, 2844–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Y. C., and Stillman B. (2002) Yph1p, an ORC-interacting protein: potential links between cell proliferation control, DNA replication, and ribosome biogenesis. Cell 109, 835–848 [DOI] [PubMed] [Google Scholar]
- 20.Moilanen A. M., Rysä J., Kaikkonen L., Karvonen T., Mustonen E., Serpi R., Szabó Z., Tenhunen O., Bagyura Z., Näpänkangas J., Ohukainen P., Tavi P., Kerkelä R., Leósdóttir M., Wahlstrand B., Hedner T., Melander O., and Ruskoaho H. (2015) WDR12, a member of nucleolar PeBoW-complex, is up-regulated in failing hearts and causes deterioration of cardiac function. PLoS One 10, e0124907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassler J., Kallas M., Pertschy B., Ulbrich C., Thoms M., and Hurt E. (2010) The AAA-ATPase Rea1 drives removal of biogenesis factors during multiple stages of 60S ribosome assembly. Mol. Cell 38, 712–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kressler D., Hurt E., Bergler H., and Bassler J. (2012) The power of AAA-ATPases on the road of pre-60S ribosome maturation: molecular machines that strip pre-ribosomal particles. Biochim. Biophys. Acta 1823, 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garbarino J. E., and Gibbons I. R. (2002) Expression and genomic analysis of midasin, a novel and highly conserved AAA protein distantly related to dynein. BMC Genomics 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulbrich C., Diepholz M., Bassler J., Kressler D., Pertschy B., Galani K., Böttcher B., and Hurt E. (2009) Mechanochemical removal of ribosome biogenesis factors from nascent 60S ribosomal subunits. Cell 138, 911–922 [DOI] [PubMed] [Google Scholar]
- 25.Royet J., Bouwmeester T., and Cohen S. M. (1998) Notchless encodes a novel WD40-repeat-containing protein that modulates Notch signaling activity. EMBO J. 17, 7351–7360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nal B., Mohr E., Silva M. I., Tagett R., Navarro C., Carroll P., Depetris D., Verthuy C., Jordan B. R., and Ferrier P. (2002) Wdr12, a mouse gene encoding a novel WD-repeat protein with a notchless-like amino-terminal domain. Genomics 79, 77–86 [DOI] [PubMed] [Google Scholar]
- 27.Sheffield P., Garrard S., and Derewenda Z. (1999) Overcoming expression and purification problems of RhoGDI using a family of “parallel” expression vectors. Protein Expr. Purif. 15, 34–39 [DOI] [PubMed] [Google Scholar]
- 28.Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., and Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winn M. D., Ballard C. C., Cowtan K. D., Dodson E. J., Emsley P., Evans P. R., Keegan R. M., Krissinel E. B., Leslie A. G., McCoy A., McNicholas S. J., Murshudov G. N., Pannu N. S., Potterton E. A., Powell H. R., Read R. J., Vagin A., and Wilson K. S. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gietz R. D., and Sugino A. (1988) New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74, 527–534 [DOI] [PubMed] [Google Scholar]
- 31.Aricescu A. R., Lu W., and Jones E. Y. (2006) A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D Biol. Crystallogr. 62, 1243–1250 [DOI] [PubMed] [Google Scholar]
- 32.Morita E., Sandrin V., Alam S. L., Eckert D. M., Gygi S. P., and Sundquist W. I. (2007) Identification of human MVB12 proteins as ESCRT-I subunits that function in HIV budding. Cell Host Microbe 2, 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassler J., Paternoga H., Holdermann I., Thoms M., Granneman S., Barrio-Garcia C., Nyarko A., Stier G., Clark S. A., Schraivogel D., Kallas M., Beckmann R., Tollervey D., Barbar E., Sinning I., and Hurt E. (2014) A network of assembly factors is involved in remodeling rRNA elements during preribosome maturation. J. Cell Biol. 207, 481–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurley J. H., Lee S., and Prag G. (2006) Ubiquitin-binding domains. Biochem. J. 399, 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galani K., Nissan T. A., Petfalski E., Tollervey D., and Hurt E. (2004) Rea1, a dynein-related nuclear AAA-ATPase, is involved in late rRNA processing and nuclear export of 60 S subunits. J. Biol. Chem. 279, 55411–55418 [DOI] [PubMed] [Google Scholar]
- 36.Chantha S. C., and Matton D. P. (2007) Underexpression of the plant NOTCHLESS gene, encoding a WD-repeat protein, causes pleitropic phenotype during plant development. Planta 225, 1107–1120 [DOI] [PubMed] [Google Scholar]
- 37.Whittaker C. A., and Hynes R. O. (2002) Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell 13, 3369–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang K., and Chen J. (2012) The regulation of integrin function by divalent cations. Cell Adh. Migr. 6, 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song G., Yang Y., Liu J. H., Casasnovas J. M., Shimaoka M., Springer T. A., and Wang J. H. (2005) An atomic resolution view of ICAM recognition in a complex between the binding domains of ICAM-3 and integrin αLβ2. Proc. Natl. Acad. Sci. U.S.A. 102, 3366–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez-Verdun D., Roussel P., Thiry M., Sirri V., and Lafontaine D. L. (2010) The nucleolus: structure/function relationship in RNA metabolism. Wiley Interdiscip. Rev. RNA 1, 415–431 [DOI] [PubMed] [Google Scholar]
- 41.Castle C. D., Cassimere E. K., and Denicourt C. (2012) LAS1L interacts with the mammalian Rix1 complex to regulate ribosome biogenesis. Mol. Biol. Cell 23, 716–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J., Yang Y., and Wu J. (2009) B23 interacts with PES1 and is involved in nucleolar localization of PES1. Acta Biochim. Biophys. Sin. 41, 991–997 [DOI] [PubMed] [Google Scholar]
- 43.Grabbe C., and Dikic I. (2009) Functional roles of ubiquitin-like domain (ULD) and ubiquitin-binding domain (UBD) containing proteins. Chem. Rev. 109, 1481–1494 [DOI] [PubMed] [Google Scholar]
- 44.Su V., and Lau A. F. (2009) Ubiquitin-like and ubiquitin-associated domain proteins: significance in proteasomal degradation. Cell Mol. Life Sci. 66, 2819–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wegrecki M., Rodriguez-Galan O., de la Cruz J., and Bravo J. (2015) The structure of Erb1-Ytm1 complex reveals the functional importance of a high-affinity binding between two β-propellers during the assembly of large ribosomal subunits in eukaryotes. Nucleic Acids Res., 10.1093/nar/gkv1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humphries J. D., Byron A., and Humphries M. J. (2006) Integrin ligands at a glance. J. Cell Sci. 119, 3901–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams N. B., and Reid J. D. (2013) The allosteric role of the AAA+ domain of ChlD protein from the magnesium chelatase of synechocystis species PCC 6803. J. Biol. Chem. 288, 28727–28732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zabalza M., Subirana I., Lluis-Ganella C., Sayols-Baixeras S., de Groot E., Arnold R., Cenarro A., Ramos R., Marrugat J., and Elosua R. (2015) Association between coronary artery disease genetic variants and subclinical atherosclerosis: an association study and meta-analysis. Rev. Esp. Cardiol. 68, 869–877 [DOI] [PubMed] [Google Scholar]
- 49.Killian A., Sarafan-Vasseur N., Sesboüé R., Le Pessot F., Blanchard F., Lamy A., Laurent M., Flaman J. M., and Frébourg T. (2006) Contribution of the BOP1 gene, located on 8q24, to colorectal tumorigenesis. Genes Chromosomes Cancer 45, 874–881 [DOI] [PubMed] [Google Scholar]
- 50.Chung K. Y., Cheng I. K., Ching A. K., Chu J. H., Lai P. B., and Wong N. (2011) Block of proliferation 1 (BOP1) plays an oncogenic role in hepatocellular carcinoma by promoting epithelial-to-mesenchymal transition. Hepatology 54, 307–318 [DOI] [PubMed] [Google Scholar]
- 51.Cheng L., Li J., Han Y., Lin J., Niu C., Zhou Z., Yuan B., Huang K., Li J., Jiang K., Zhang H., Ding L., Xu X., and Ye Q. (2012) PES1 promotes breast cancer by differentially regulating ERα and ERβ. J. Clin. Invest. 122, 2857–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie W., Feng Q., Su Y., Dong B., Wu J., Meng L., Qu L., and Shou C. (2012) Transcriptional regulation of PES1 expression by c-Jun in colon cancer. PLoS One 7, e42253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Zhuang Q., Lan X., Zeng G., Jiang X., and Huang Z. (2013) PES1 differentially regulates the expression of ERα and ERβ in ovarian cancer. IUBMB Life 65, 1017–1025 [DOI] [PubMed] [Google Scholar]
- 54.Xie W., Qu L., Meng L., Liu C., Wu J., and Shou C. (2013) PES1 regulates sensitivity of colorectal cancer cells to anticancer drugs. Biochem. Biophys. Res. Commun. 431, 460–465 [DOI] [PubMed] [Google Scholar]
- 55.Nakaguro M., Kiyonari S., Kishida S., Cao D., Murakami-Tonami Y., Ichikawa H., Takeuchi I., Nakamura S., and Kadomatsu K. (2015) Nucleolar protein PES1 is a marker of neuroblastoma outcome and is associated with neuroblastoma differentiation. Cancer Sci. 106, 237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beck-Cormier S., Escande M., Souilhol C., Vandormael-Pournin S., Sourice S., Pilet P., Babinet C., and Cohen-Tannoudji M. (2014) Notchless is required for axial skeleton formation in mice. PLoS One 9, e98507. [DOI] [PMC free article] [PubMed] [Google Scholar]