Abstract
Cell-cell interaction via the gap junction regulates cell growth and differentiation, leading to formation of organs of appropriate size and quality. To determine the role of connexin43 in salivary gland development, we analyzed its expression in developing submandibular glands (SMGs). Connexin43 (Cx43) was found to be expressed in salivary gland epithelium. In ex vivo organ cultures of SMGs, addition of the gap junctional inhibitors 18α-glycyrrhetinic acid (18α-GA) and oleamide inhibited SMG branching morphogenesis, suggesting that gap junctional communication contributes to salivary gland development. In Cx43−/− salivary glands, submandibular and sublingual gland size was reduced as compared with those from heterozygotes. The expression of Pdgfa, Pdgfb, Fgf7, and Fgf10, which induced branching of SMGs in Cx43−/− samples, were not changed as compared with those from heterozygotes. Furthermore, the blocking peptide for the hemichannel and gap junction channel showed inhibition of terminal bud branching. FGF10 induced branching morphogenesis, while it did not rescue the Cx43−/− phenotype, thus Cx43 may regulate FGF10 signaling during salivary gland development. FGF10 is expressed in salivary gland mesenchyme and regulates epithelial proliferation, and was shown to induce ERK1/2 phosphorylation in salivary epithelial cells, while ERK1/2 phosphorylation in HSY cells was dramatically inhibited by 18α-GA, a Cx43 peptide or siRNA. On the other hand, PDGF-AA and PDGF-BB separately induced ERK1/2 phosphorylation in primary cultured salivary mesenchymal cells regardless of the presence of 18α-GA. Together, our results suggest that Cx43 regulates FGF10-induced ERK1/2 phosphorylation in salivary epithelium but not in mesenchyme during the process of SMG branching morphogenesis.
Keywords: connexin, extracellular-signal-regulated kinase (ERK), fibroblast growth factor (FGF), gap junction, phosphorylation, branching morphogenesis, salivary gland
Introduction
Gap junctions are specialized membrane channels that allow for transfer of small molecules, ions, metabolites, and secondary messengers via the cytoplasm of connected cells (1). Gap junctions are produced as the result of oligomerization of 6 different connexin (Cx)2 proteins, which form a connexon that is delivered to the plasma membrane (2). A connexon exists either as a single unit within the plasma membrane, known as a hemichannel, or is associated with a connexon from an opposing cell to form a gap junction channel (2). A mutation in the gene encoding the nearly ubiquitously expressed connexin43 results in development of oculodentodigital dysplasia (ODDD), a predominantly autosomal dominant disease that is characterized by several common clinical phenotypes, such as syndactyly of the digits, microdontia, enamel hypoplasia, ophthalmic defects, and craniofacial abnormalities (3, 4).
Cx43 plays an important role in skeletal development, as well as survival of osteoblasts and osteocytes (5). In particular, germline ablation of Gja1, the gene encoding Cx43, results in delayed endochondral and intramembranous ossification, osteoblast dysfunction, and craniofacial abnormalities (6). Because of the perinatal lethality of the germline mutation, which results in heart and lung abnormalities, a method for tissue-specific Gja1 ablation has been developed. Conditional ablation of the Gja1 gene regulated by the Col1A1 promoter, which is expressed in committed osteoblasts, results in accrual of a low peak bone mass and attenuates response to the anabolic effects of parathyroid hormone, the consequence of an osteoblast defect (7). Further proof of the critical role of Cx43 has been provided by identification of Gja1 mutations as the cause of ODDD. Two Gja1 mouse mutations were previously described with skeletal phenotypes closely resembling the human form of the disease, such as syndactyly and craniofacial malformations including amelogenesis imperfecta (8, 9). This evidence suggests that Cx43 is important for development of hard tissues, including bone and tooth.
Epithelial and mesenchymal interactions are important for development of a variety of organs, including teeth, salivary glands, mammary glands, lungs, and kidneys. Representative features of Cx43 knock-out mice are severe tooth abnormalities and pulmonary outflow obstruction (6). In addition, those mice show decreased expression of aquaporin-5, surfactant protein C, and α-smooth muscle action, indicating that Cx43 is important for alveolar and vascular formation during lung development (10). The process of branching morphogenesis, which is utilized in all animals to form complex ordered structures, involves dynamic and reciprocal interactions between different tissue types, often through the use of conserved molecular programs (11, 12). In vivo, SMG development is initiated by thickening of oral epithelium on about embryonic day 12 (E12), when the initial epithelium bud on a stalk grows into condensing neural crest-derived mesenchyme. Clefts in the epithelium result in the appearance of 3–5 epithelial buds by E13.5, then branching morphogenesis occurs with continued proliferation, along with successive rounds of cleft formation, duct elongation, and lumen formation, resulting in a highly branched gland by E14 (13). Branching morphogenesis by mouse SMGs is regulated by multiple growth factors, which has been shown in ex vivo experiments to involve FGFR signaling (14). The expression and distribution of connexins have been investigated in salivary glands of mature rats, with high levels of Cx32 and Cx26 expression, but none of Cx43, observed in rat salivary glands (15). In contrast, Cx32 was found to be located exclusively between acinar cells (16). However, Cx43 expression in the early embryonic stage has not been clearly identified.
The role of gap junctions in salivary gland morphogenesis has also not been fully elucidated. In this study, we investigated the role of Cx43 in salivary gland branching morphogenesis, and our findings showed that Cx43 is necessary for branching morphogenesis and salivary epithelium proliferation via FGF10-induced ERK1/2 phosphorylation in salivary gland epithelium.
Experimental Procedures
Preparation of Tissue Sections and Immunostaining
ICR mouse heads were dissected on E13.5, E16, or postnatal day 1 (P1) and fixed with 4% PFA in phosphate-buffered saline (PBS) overnight at 4 °C. The tissues were embedded in OCT compound (Sakura Finetechnical Co.) for frozen sectioning, then cut into 8-μm sections with a cryostat (2800 FRIGOCUT; Leica). Submandibular and sublingual salivary gland rudiments (referred to as SMGs in this study) were dissected from E16.5 connexin 43 heterozygote (Cx43+/−) and homozygote (Cx43−/−) specimens, and fixed for 20 min at room temperature in 4% PFA in phosphate-buffered saline (PBS). Fixed submandibular glands were washed three times in PBS, then dehydrated using alcohol and embedded in paraffin. Sections were cut at a thickness of 10 μm and stained with hematoxylin-eosin. For immunostaining, sections were incubated in 1% BSA/PBS for 1 h before incubation with the primary antibody. Primary antibodies specific for connexin 43 (SC-9059: Santa Cruz Biotechnology) were detected using Alexa594-conjugated secondary antibodies (Invitrogen). Nuclei were stained with DAPI (Vector Laboratories). A fluorescence microscope (BZ-8000, KEYENCE Co, Osaka, Japan) was used for imaging analysis. Images were prepared using a BZ analyzer (KEYENCE) and Photoshop (Adobe Systems, Inc.).
Ex Vivo Submandibular Organ Cultures
SMGs were dissected from E13 ICR mice, then Cx43+/− and Cx43−/− specimens were cultured on PET track-etched membranes (BD Falcon) with a pore size of 0.4 μm at the air/medium interface. The filters were floated on 2 ml of DMEM/F12 in 6-well culture plates (BD Falcon). The medium was supplemented with 100 units/ml of penicillin, 100 mg/ml of streptomycin, and 150 mg/ml of vitamin C, with or without 5 μg/ml 18α-glycyrrhetinic acid (18α-GA: Sigma-Aldrich) or oleamide (Sigma-Aldrich). Five SMGs were cultured on each filter at 37 °C in an atmosphere of humidified 5% CO2/95% air. When an exogenous platelet-derived growth factor (PDGF) was used, either 10 ng/ml of PDGF-AA (Upstate), 1 ng/ml of PDGF-BB (R&D Systems), 100 ng/ml of FGF7 (R&D Systems), 500 ng/ml of FGF10 (R&D Systems), or Cx43 peptide (Cx2605-P-5: Alpha Diagnostic International) (17) was added to the culture medium. The SMGs were photographed after 2, 24, and 48 h, with the end buds counted at each time point. Each experiment was repeated at least five times.
RNA Isolation and PCR
Total RNA was isolated using TRIzol (Invitrogen) from E13.5, E16, and P1 submandibular glands, and first-strand cDNA was synthesized at 50 °C for 60 min using oligo(dT) or random primers with the SuperScript III First-strand Synthesis System (Invitrogen). PCR was performed with Takara Ex Taq (Takara) for semi-quantitative RT-PCR or using SYBR green PCR reagent (Applied Biosystems) for real-time PCR. Primers for PDGF-A (Pdgfa), PDGF-B (Pdgfb), PGF receptor α (pdgfrα), PDGF receptor β (Pdgfrβ), FGF7, FGF10, FGF receptor 1b (Fgfr1b), FGF receptor 2b (Fgfr2b), and Hprt were prepared as previously described (18). Primers forconnexin 43 (forward 5′-GAGTCAGCTTGGGGTGATGAACAG-3′, reverse 5′-AGCAGGAAGGCCACCTCGAAGACAGAC-3′), Bmp2 (forward 5′-GGGACCCGCTGTCTTCTAGT-3′, reverse 5′-TCAACTCAAATTCGCTGAGGAC-3′), and Bmp4 (forward 5′-ACTGCCGCAGCTTCTCTGAG-3′, reverse 5′-TTCTCCAGATGTTCTTCGTG-3′) were used for real-time PCR. PCR amplicons were separated and visualized on 1.5% agarose gels after staining with SYBR Green using the ImageQuant LAS 4000 Mini image analysis system (GE Healthcare Life Science). Statistical analysis of gene expression was performed using Student's t test.
BrdU Incorporation Assay
After treatment with FGF10 with or without 18α-GA or MEK inhibitor U0126 (Cell Signaling), BrdU was added to the plates (10 μm) for 30 min, then HSY cells from a human salivary cell line were fixed with cold methanol for 10 min, rehydrated in PBS, and incubated for 30 min in 1.5 m HCl. After washing three times in PBS, the organs were incubated with a 1:50 dilution of fluorescein-isothiocyanate (FITC)-conjugated anti-BrdUrd antibody (Roche, Manheim, Germany) for 30 min at room temperature. Finally, the cells were washed 3 times in PBS and the nuclei stained with propidium iodide (Vector laboratories). BrdU-positive cells were examined under a microscope (Biozero-8000; Keyence, Japan), as previously described.
Western Blotting
HSY cells or primary cultured E14.5 SMG mesenchymal cells were grown in DMEM/F-12 supplemented with 10% FBS with or without 5 μm 18α-GA, 1 μm Cx43 peptide, and siRNA for Cx43, after stimulation with 500 ng/ml FGF10 at 37 °C in a humidified incubator in an atmosphere containing 5% CO2. Cells were washed twice with 1 mm ice-cold sodium orthovanadate (Sigma) in PBS, lysed with Nonidet P-40 buffer supplemented with a proteinase inhibitor mixture (Sigma), and centrifuged, then the supernatants were transferred to fresh tubes. Lysates were separated using 4–12% SDS-PAGE (NuPAGE: Invitrogen) and analyzed by Western blotting. The blotted membranes were incubated with antibodies for ERK1/2 or phosho-ERK1/2 (Cell Signaling), then detected with an ECL kit (Amersham Biosciences) and visualized using the ImageQuant LAS 4000 Mini image analysis system (GE Healthcare Life Science).
[Ca2+]i Measurements
HSY cells were grown in 96-well plates for 3 days and loaded with 5 μm Fura-2AM (Invitrogen) prepared in HBSS for 45 min at 37 °C. The Ca2+ transients were recorded as the 340/380 nm ratio (R) of the resulting 510-nm emissions using a plate reader (Mithras LB 940; Berthold Technologies). For stimulation, 500 ng/ml FGF10 was automatically injected into the cells using the Mithras 940. For inhibition experiments, cells were incubated for 30 min before analysis with 1 μm Cx43 peptide for blocking the hemichannel and gap junction channel, or 100 μm 2-APB for blocking IP3R. [Ca2+]i levels were calculated as previously described (19).
Results
Expression of Connexin 43 in Submandibular Gland Development
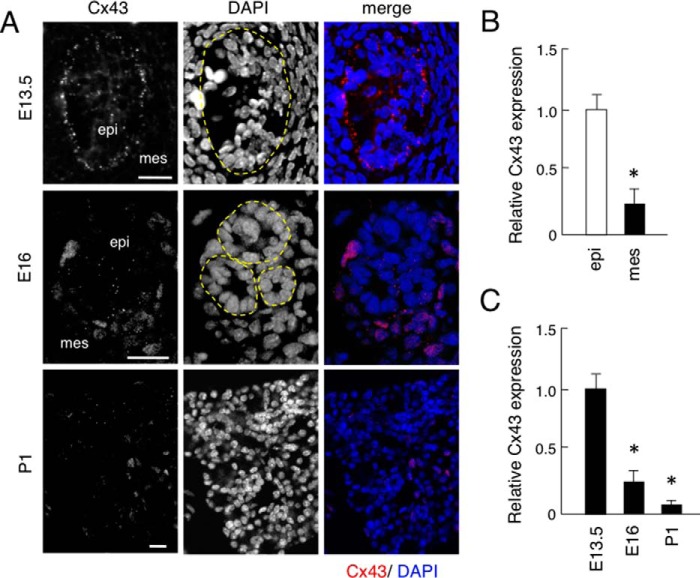
Connexin 43 (Cx43) is a gap junctional protein, though its expression in salivary glands has not been identified. To examine the expression of Cx43 in salivary gland development, we performed immunostaining using an antibody for mouse Cx43 and found that it was expressed in salivary gland epithelium, but not in neural crest-derived mesenchyme tissues obtained on E13.5 (Fig. 1A). In E16 tissues, Cx43 was shown to be expressed in salivary gland epithelium with nonspecific staining in blood cells, though the level of expression was reduced as compared with that on E13.5. In P1 tissues, Cx43 was weakly expressed (Fig. 1, A and C). Real-time PCR assays of micro-dissected samples revealed that Cx43 was highly expressed in salivary gland epithelium, but not in mesenchyme on E13.5 (Fig. 1B). Thus, Cx43 may regulate the proliferation and differentiation of salivary gland epithelium.
FIGURE 1.
Expression of Cx43 in mouse salivary glands. A, immunofluorescence analysis of Cx43 expression using anti-Cx43 antibody (red) in frontal sections obtained on embryonic day 13.5 (E13.5), E16, and postnatal day 1 (P1). Nuclei were stained with DAPI (blue). B, real-time PCR was used to analyze the expression of Cx43 in E13.5 salivary gland epithelium and mesenchyme, with the expression level of Cx43 in E13.5 salivary gland epithelium set at a value of 1 for comparison. C, real-time PCR was used to analyze the expression of Cx43 in E13.5, E16, and P1 salivary glands, with the expression level of Cx43 in E13.5 salivary glands set at a value of 1 for comparison. Abbreviations: epi, salivary gland epithelium; mes, salivary gland mesenchyme. Statistical analysis was performed using ANOVA (n = 5 per group; *, p < 0.01).
Gap Junctional Inhibitors 18α-GA and Oleamide Inhibited Branching of SMGs
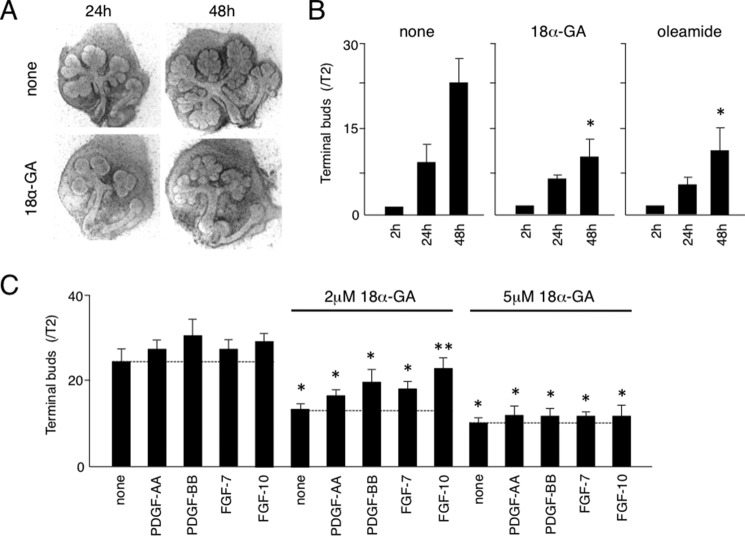
To examine the role of Cx43 in salivary gland development, especially branching morphogenesis, we performed SMG organ cultures with or without the gap junctional inhibitors 18α-GA and oleamide. Both inhibited branching morphogenesis in SMG organ cultures at 24 and 48 h (Fig. 2, A and B). Previously, we showed that exogenously added PDGF-AA and PDGF-BB in organ cultures induced the expression of FGF-7 and FGF-10 in salivary gland mesenchyme (18), while FGF7 and FGF10 have been shown to induce epithelial proliferation and branching morphogenesis of SMGs (14). In the presence of 2 and 5 μm of 18α-GA, the numbers of terminal buds in SMG organ cultures were dramatically decreased, while PDGF-AA, PDGF-BB, FGF7, and FGF10 partially rescued the branching of SMGs inhibited by 2 μm, but not that inhibited by 5 μm of 18α-GA (Fig. 2C). These findings indicate that gap junctional communication may regulate branching morphogenesis induced by PDGF or FGF signaling.
FIGURE 2.
Gap junctional inhibitors 18a-GA and oleamide inhibited SMG branching morphogenesis. A, phase contrast image of SMG organ cultures with and without 18α-GA. B, quantification of data shown in A, with the numbers of terminal buds at 24 and 48 h compared with those at 2 h. C, quantification of number of terminal buds in presence of PDGF-AA, PDGF-BB, FGF-7, or FGF-10 with or without 18α-GA (2–5 μm). Statistical analysis was performed using ANOVA (n = 6 per group; *, p < 0.01; **, p < 0.05).
Reduced Terminal Bud Number and Salivary Gland Size in Cx43 Knock-out Mice
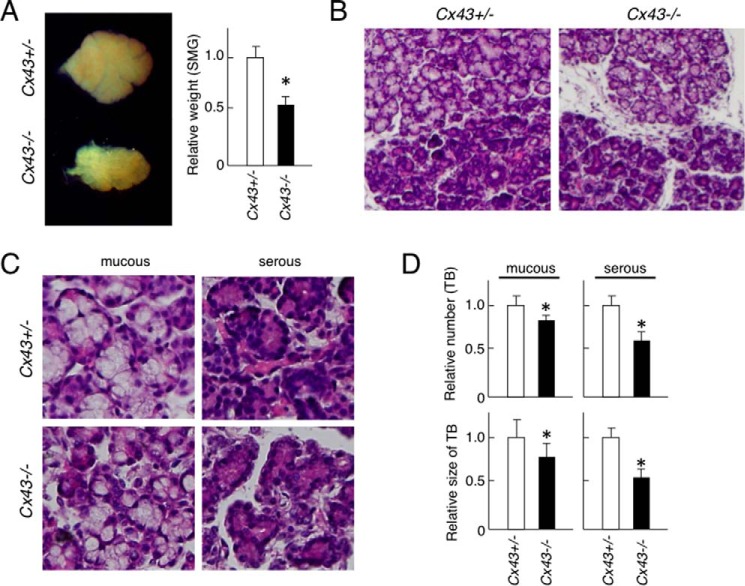
Since 18α-GA and oleamide are general gap junctional inhibitors, they inhibit not only Cx43 but also other gap junctional proteins. To identify the role of Cx43 in salivary gland development, we examined the phenotype of Cx43-disrupted mice. Cx43−/− mice on P1 showed smaller sized SMGs as compared with those from Cx43+/− mice, while the SMG weight in Cx43−/− mice was reduced by ∼50% (Fig. 3A). Also, atrophic terminal buds with mesenchymal tissues were observed in the Cx43−/− tissues (Fig. 3B), while the numbers and size of terminal buds were reduced in both mucous and serous glands of Cx43−/− mice (Fig. 3, C and D). Together, these results indicate that Cx43 is necessary for salivary gland development.
FIGURE 3.
Phenotype of SMG in Cx43−/− mice. A, stereomicroscope image of SMGs from P1 C43+/− and Cx43−/− mice. The graph shows relative weights of the SMGs, with the weight of the Cx43± specimens set at a value of 1.0 for comparison. B, hematoxylin-eosin staining of SMGs from C43+/− and Cx43−/− mice. C, high magnification image of mucous and serious glands from SMGs of each mouse strain. D, relative numbers of terminal buds (TB) in mucous and serous glands in SMGs of C43+/− and Cx43−/− mice, with the number found in the Cx43+/− SMG specimens set at a value of 1.0 for comparison. Statistical analysis was performed using ANOVA (n = 5 per group; *, p < 0.01).
Expression of PDGF, FGF, and Their Receptors in Cx43-disrupted Salivary Glands
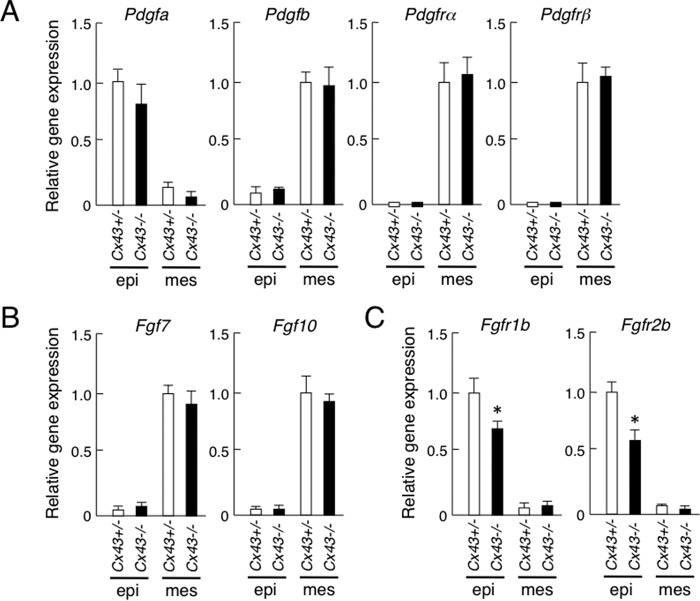
Our previous study revealed that PDGF-AA and PDGF-BB induce FGF7 and FGF10 in salivary gland mesenchymal cells, while FGF7 and FGF10 induce epithelial cell proliferation, indicating that both PDGF and FGF signaling are important for salivary gland branching morphogenesis. In Cx43−/− salivary glands, the expression of Pdgfa, Pdgfb, Pdgfrα, Pdgfrβ, Fgf7, and Fgf10 mRNA were not different as compared with those from the heterozygotes (Fig. 4, A and B). In contrast, the expression of Fgfr1b and Fgfr2b in Cx43−/− salivary gland epithelium was decreased as compared with the heterozygotes (Fig. 4C). Reduced expression of Fgfr1b and Fgfr2b may affect branching morphogenesis in Cx43−/− salivary glands. In addition, BMP2 and BMP4 are known to be inhibitory factors for salivary gland branching morphogenesis (14, 20). In the Cx43−/− specimens, Bmp2 expression was not changed. However, Bmp4 was reduced as compared with the heterozygotes (Fig. 5), indicating that BMP signaling in salivary glands may not affect the phenotype of SMG in Cx43−/−.
FIGURE 4.
PDGFs, FGFs, and expression of their receptors in SMGs from C43+/− and Cx43−/−mice. A–C, real-time PCR was used to analyze the expression of pdgfa, pdgfb, pdgfra, and pdgfrb (A), Fgf7 and Fgf10 (B), and Fgfr1b and Fgfr2b (C). The expression of Cx43+/− in SMGs was set at a value of 1.0 for comparison. Statistical analysis was performed using ANOVA (n = 4 per group; *, p < 0.01).
FIGURE 5.
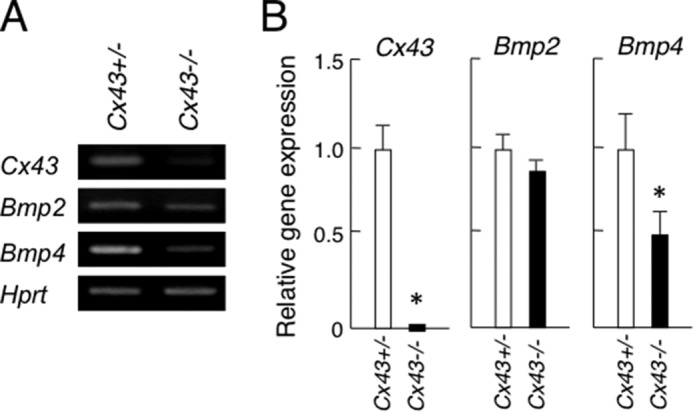
Expression of Cx43, Bmp2, and Bmp4 in SMGs from C43+/− and Cx43−/−mice. A, mRNA expression of Cx43, Bmp2, and Bmp4 were analyzed by RT-PCR. B, expression level of each gene was quantified using bands of the PCR products and compensated using hypoxanthine phosphoribosyltransferase (Hprt) as the internal control. Statistical analysis was performed using ANOVA (n = 3 per group; *, p < 0.01).
Reduced Branching Morphogenesis in Cx43-disrupted Salivary Gland Organ Cultures
To evaluate the branching morphogenesis of Cx43−/− salivary gland, we performed ex vivo organ cultures. Branching of terminal buds was dramatically inhibited in the cultures of Cx43−/− salivary glands at 48 h, similar to the histology of Cx43−/− (Fig. 6). FGF10-enhanced branching in control samples has been reported (21). However, addition of FGF10 did not affect the Cx43−/− salivary gland organ cultures in our study, suggesting that FGF10 signaling in salivary gland epithelium is disturbed in Cx43−/− mice (Fig. 6). To confirm the role of Cx43 in salivary gland morphogenesis, a Cx43 peptide that inhibits gap junction function was added to the organ cultures. Peptides homologous to the first extracellular loop motifs of Cx43 reversibly abolish rhythmic contractile activity in arteries (22). In our experiment, addition of a Cx43 peptide inhibited salivary gland branching. On the other hand, the control peptide did not have an effect on branching morphogenesis (Fig. 7).
FIGURE 6.
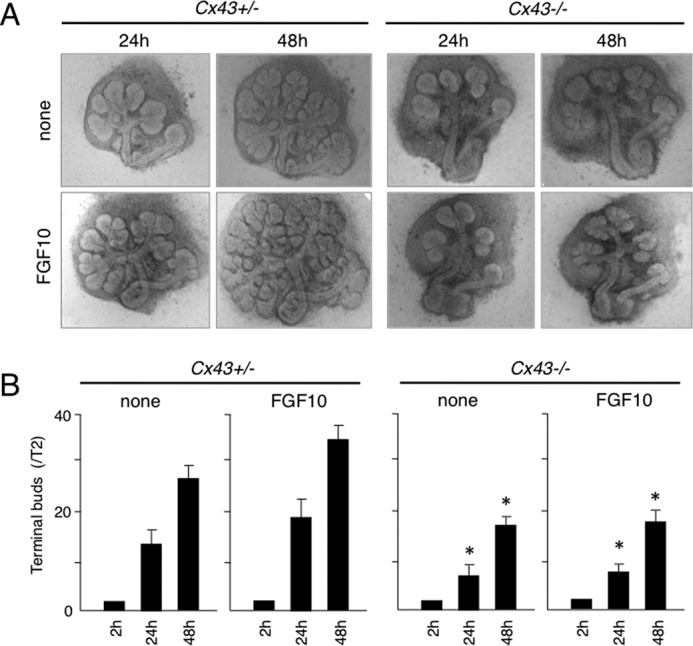
FGF10 induced branching morphogenesis in SMGs in Cx43+/− but not Cx43−/− mice. A, phase-contrast images of organ cultures of SMGs obtained from Cx43+/− and Cx43−/− mice on E13.5 with and without FGF10. B, quantitation of findings shown in A. The numbers of terminal buds at 24 and 48 h were compared with the number at 2 h. Statistical analysis was performed using ANOVA (n = 6 per group; *, p < 0.01).
FIGURE 7.
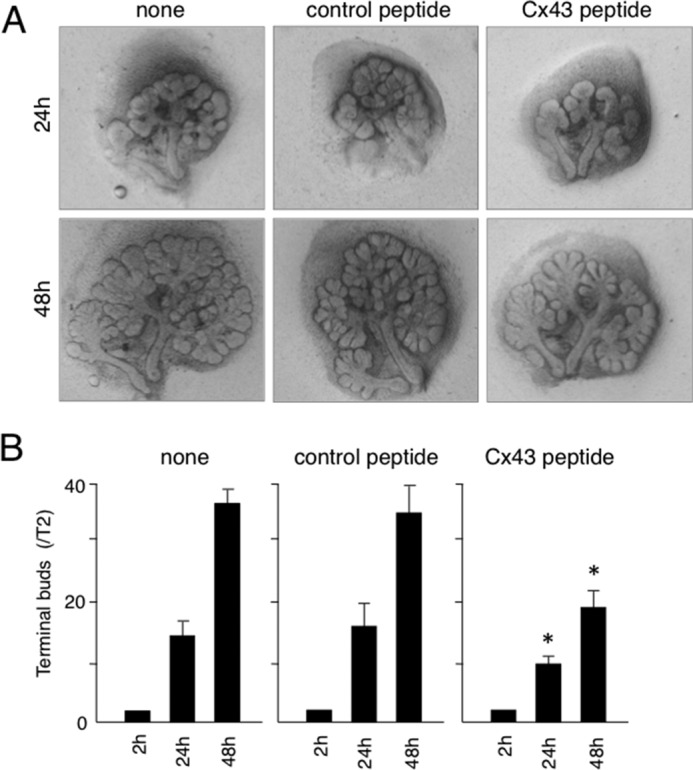
Cx43 peptide inhibited SMG branching morphogenesis. A, phase-contrast images of organ cultures of SMGs obtained on E13.5 with or without Cx43 peptide. B, quantitation of findings shown in A. The numbers of terminal buds at 24 and 48 h were compared with the number at 2 h. Statistical analysis was performed using ANOVA (n = 6 per group; *, p < 0.01).
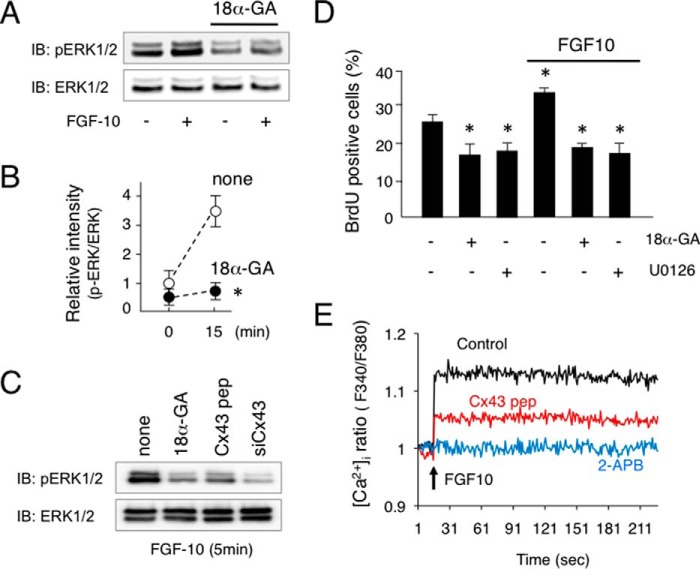
Inhibition of FGF10-induced ERK1/2 Phosphorylation in Salivary Gland Epithelial Cells by 18α-GA
In our experiments with ex vivo organ cultures, epithelial signaling, especially FGF10 signaling, was found to be disturbed in the Cx43−/− samples. To evaluate the role of Cx43 in salivary gland epithelium development, we examined FGF10 signaling using HSY cells from human salivary epithelium. FGF10 induced the phosphorylation of ERK1/2 in HSY cells after 5 min, while the gap junction inhibitor 18α-GA inhibited FGF10-induced ERK1/2 phosphorylation (Fig. 8, A and B). Furthermore, a Cx43 peptide and siRNA for Cx43 inhibited FGF10-induced ERK1/2 phosphorylation in a manner similar to 18α-GA (Fig. 8C). FGF10 also induced HSY cell proliferation, whereas 18α-GA and the MEK inhibitor U0126 inhibited FGF10-induced cell proliferation (Fig. 8D). Cx43 functions not only in cell-to-cell gap junctional communication but also as a hemichannel-regulating signaling molecule to moderate intracellular calcium level. To examine the role of Cx43 in intracellular calcium level regulation, we performed a [Ca2+]i measurement assay using Fura-2AM. FGF10 increased the level of [Ca2+]i in salivary gland epithelial cells, while 2-APB, an IP3R inhibitor, inhibited Ca2+ release from endoplasmic reticulum and the increase of [Ca2+]i induced by FGF10 (Fig. 8E). Furthermore, the Cx43 peptide partially inhibited increased [Ca2+]i, indicating that Cx43 may be important for the initial increase of [Ca2+]i following stimulation by FGF10 via the hemichannel. PDGF-AA and PDGF-BB were previously found to induce FGF7 and FGF10 expression in salivary gland epithelium, and then promote epithelial cell proliferation (18). To examine the role of Cx43 in salivary gland mesenchymal cells, we examined PDGF signaling using primary cultures of E14 mouse salivary gland epithelium specimens. PDGF-AA and PDGF-BB were separately added to cultures of salivary gland mesenchymal cells, and both induced ERK1/2 phosphorylation. In contrast, 18α-GA did not affect the phosphorylation of ERK1/2 (Fig. 9A) or proliferation of salivary gland mesenchymal cells (Fig. 9B), indicating that Cx43 regulates growth factor signaling in salivary gland epithelial cells, but not in mesenchymal cells.
FIGURE 8.
FGF10 induced ERK1/2 phosphorylation in salivary gland epithelial cell line HSY. A, ERK1/2 phosphorylation in human salivary epithelial cell line HSY after stimulation with FGF10 with or without 18α-GA was analyzed by western immunoblotting. B, quantitation of findings shown in A. C, phosphorylation of ERK1/2 in HSY cells after stimulation with FGF10 for 5 min with 18α-GA, Cx43 peptide (Cx43 pep), or siRNA for Cx43 (siCx43) was analyzed by Western immunoblotting. D, BrdU incorporation in HSY cells after stimulation with FGF10 with or without 18α-GA or MEK inhibitor U0126. E, intracellular calcium level [Ca2+]i was measured after stimulation of FGF10 with Cx43 peptide or 2-APB. Statistical analysis was performed using ANOVA (n = 5 per group; *, p < 0.01).
FIGURE 9.
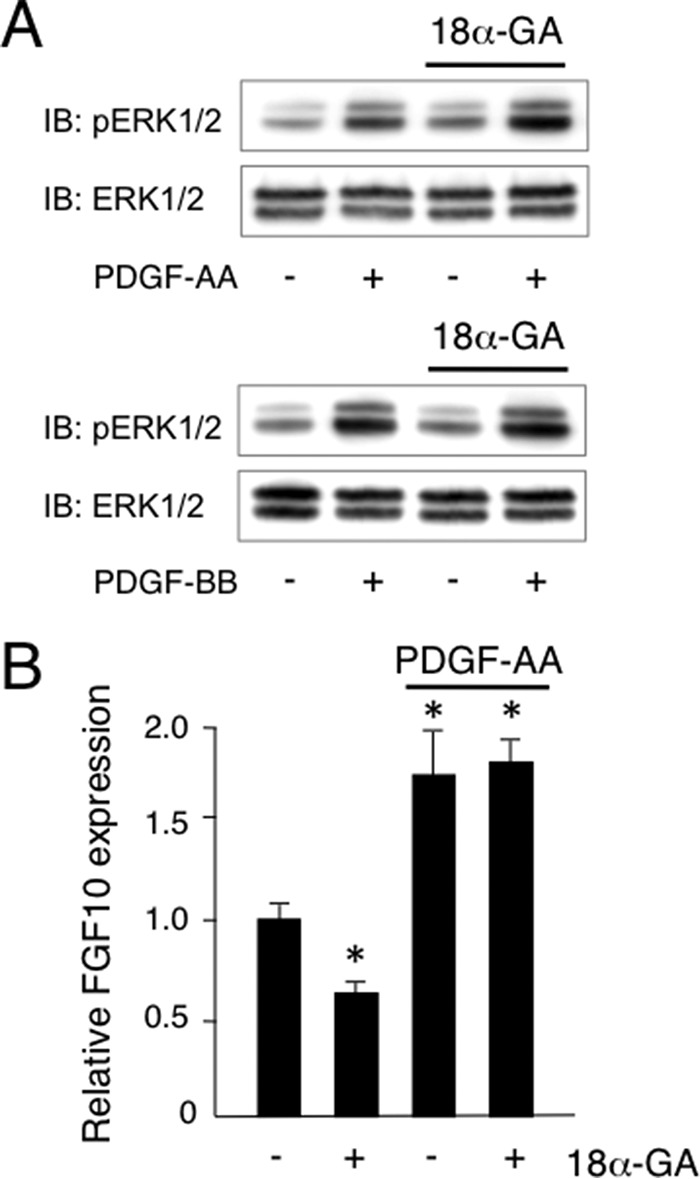
PDGF-AA and PDGF-BB each induced ERK1/2 phosphorylation in primary cultures of mouse salivary gland mesenchymal cells. A, phosphorylation of ERK1/2 in primary cultures of SMG mesenchymal cells after stimulation with PDGF-AA or PDGF-BB with or without 18α-GA was analyzed by Western immunoblotting. B, expression of FGF10 in primary cultures of SMG mesenchymal cells after stimulation with PDGF-AA. Statistical analysis was performed using ANOVA (n = 5 per group; *, p < 0.01).
Discussion
The present study revealed that Cx43 plays key roles in salivary gland development, particularly branching morphogenesis (Fig. 10). It was previously reported that Cx43 is associated with osteogenesis and odontogenesis (6). Furthermore, the pattern of Cx43 expression in developing salivary glands has been elucidated, though the role of Cx43 in salivary gland development remains unclear. The involvement of transmission by electrical stimulation through gap junctions was shown to occur in the same manner as in skeletal muscles due to Cx43 expression in myoepithelial cells from salivary glands (23). In the present study, we demonstrated the crucial role of Cx43 in the process of branching structure formation of salivary glands, as abnormalities were observed in our analysis of Cx43 knock-out mice.
FIGURE 10.
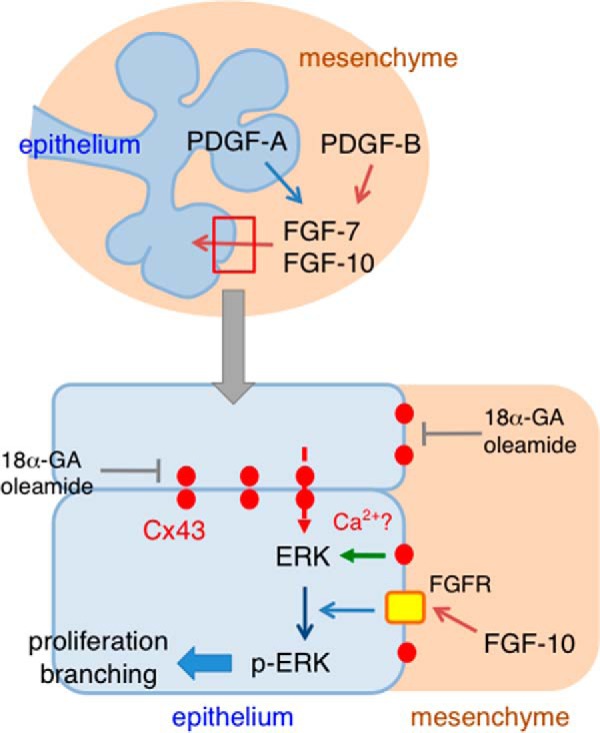
Schema of FGF-10-induced ERK1/2 phosphorylation regulated by Cx43. PDGF-A and PDGF-B induce the expression of FGF7 and FGF10 in SMG mesenchymal cells. Cx43 does not affect the expression of FGF10 induced by PDGFs, because of the present findings of no expression in SMG mesenchyme. Gap junctional communication or the Cx43 hemichannel has an effect on ERK1/2 phosphorylation induced by FGF10 in SMG epithelium, indicating that Cx43 is necessary for FGF, but not PDGF-induced branching morphogenesis of SMG tissues.
In the initial stage of salivary gland development, the branching structure is known to be facilitated by growth factors including fibroblast growth factor (FGF) (14, 21). Another report found that clefts are formed in acini during the early stage of branch initiation, with Fibronectin, an extracellular matrix component, involved in that formation (24). PDGF, which is produced by salivary epithelial cells, induces FGF expression in salivary gland mesenchymal tissue (18). In the present study, we found evidence of epithelial-mesenchymal interaction, which induced FGF activity in salivary gland epithelium (Fig. 10). During this process, Cx43 is not involved in Fgf7 or Fgf10 expression in mesenchymal tissue induced by PDGF-AA and PDGF-BB, indicating that Cx43 is not expressed in salivary gland mesenchymal tissue. Furthermore, ERK1/2 phosphorylation in salivary gland mesenchymal cells induced by PDGF-AA or PDGF-BB was not changed in the presence of the gap junctional inhibitor. However, Cx43 was found to be involved in phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) in salivary epithelial cells stimulated by FGF10. It has been reported that epithelial cell proliferation is controlled by FGF7 and FGF10 in the process of salivary gland epithelium branching structure formation (14, 21). At that time, ERK1/2 is phosphorylated by FGF10 in salivary epithelial cells. The inhibitor of MEK signaling, which is present in the upper signaling cascade of ERK1/2, blocks phosphorylation of ERK1/2, while branching morphogenesis of the salivary gland is also inhibited. Therefore, ERK1/2 phosphorylation by FGF10 is thought to be a crucial signaling factor in branching formation. ERK1/2 phosphorylation induced by FGF10 is likely blocked due to a decrease in Cx43 expression or just prior to Cx43 expression, because Cx43 in salivary epithelial cells significantly induced ERK1/2 phosphorylation. In other words, our results clearly showed that a gap junction was appropriately formed in salivary epithelial cells and branching morphogenesis was controlled by growth factor stimulation. Another report showed that Cx43 expression is important for ERK1/2 phosphorylation in osteoblast differentiation (26), thus a similar mechanism may be involved in differentiation of salivary gland epithelium. Osteoblasts are tightly coupled by gap junctions formed by Cx43. Disruption of gap junctional communication using pharmacological inhibitors leads to reduce activation of Raf, ERK, and Akt. The result of attenuated gap junction-dependent signal cascade activation is a decrease in phosphorylation of the transcription factor Sp1 by ERK, resulting in a decrease in Sp1 recruitment and inhibition of gene transcription associated with osteoblast differentiation (26). Together, these results indicate that Gap junctional communication provided by Cx43 is essential for optimal activation of signal transduction via ERK.
Cx43 forms gap junctions with a neighboring cell on the cell surface. However, it has not been clearly demonstrated how ERK1/2 phosphorylation is controlled by growth factors through this gap junction in terms epithelial-mesenchymal interaction in such tissues as teeth and salivary glands. Gap junctions are involved in intercellular transport of Ca2+, IP3, and other factors, while intracellular Ca2+ concentration is also involved in activation of Ras, as part of the upper signaling cascade for ERK1/2 (27). IP3 binds to the IP3 receptor found in the endoplasmic reticulum (ER) membrane and facilitates Ca2+ release from the ER (28, 29). These findings suggest that Cx43 helps to accumulate intracellular Ca2+ and, as a result, growth factors that promote ERK1/2 phosphorylation. Therefore, affected cells may not be able to differentiate, because they are not able to adequately react due to growth factor stimulation from extracellular space when gap junctions are not fully formed. Epithelial-mesenchymal interaction via growth factors is crucial for proper tissue formation, while it has also become evident that close communication among epithelial cells is important. However, the direct mechanistic regulation of branching morphogenesis by Cx43 remains unclear.
Cx43 is a post-translational product modified by phosphorylation, and has been reported to be involved in gap junction and hemichannel activities, protein assembly, trafficking, and turnover (30). Several protein kinases including protein kinase A, protein kinase C, ERK1/2, and casein kinase 1 phosphorylate the C-terminal region of Cx43, while Cx43 phosphorylation regulates gap junction assembly (31). Furthermore, activation of PI3K-Akt signaling induced by fluid flow shear stress plays an essential role in activating the Cx43 hemichannel (32). Regulation of opening and closure of the hemichannel is important for membrane depolarization, as well as disruption of electrical and chemical gradients. Cx43 is involved in not only gap junctional communication but also activates signaling via the hemichannel. Thus, clarification of the role of the Cx43 hemichannel is necessary to better understand the molecular mechanism of salivary gland branching morphogenesis. The direct relationship between the Cx43 hemichannel and ERK1/2 phosphorylation in salivary glands should be examined in future experiments.
ODDD is a genetic disorder that leads to abnormalities in osteogenesis and odontogenesis (3). Gja1Jrt/+ mice, used as an ODDD model, display early and progressive bone marrow atrophy, with a significant increase in bone marrow adiposity as compared with wild-type littermates, though without an increase in adipose tissue (33), indicating that Cx43 may regulate the cell fate of osteogenic precursor cells. Conditional abbreviation of Cx43 under the control of an osterix promoter shows that cell communication mediated by gap junctions is indispensable for normal differentiation of osteoblasts induced by BMP-2 (34). Although Cx43 is the disease-causing gene in ODDD, abnormalities in salivary glands have not been reported in affected patients, while severe dental caries formation is known to occur. The cause of such severe caries is thought to be enamel hypoplasia (35), though its onset may be related to abnormalities of the salivary glands associated with dysplasia and decreased saliva secretion. Abnormal salivation also causes dental caries formation (36) and influences eating function, thus further analysis is required in a future study. Lung malformation can be observed in ODDD model mice and abnormal expression of molecular markers for alveoli of the lung have been reported (10), though the molecular mechanisms remain unclear. The salivary glands and lungs have a similar process of embryonic development, thus lung malformation may also be caused by growth factor signaling defects during the process of differentiation.
This is the first known report of the role of Cx43 in salivary gland branching morphogenesis. We believe that our results provide basic findings for development of gland regeneration technology including salivary glands, in addition to novel solutions for human disease phenotypes such as ODDD, such as diagnosis and treatment strategies.
Author Contributions
A. Y., E. F., and S. F. designed the study and wrote the paper. A. Y., M. F., K. S., and K. Y. performed the salivary gland organ cultures. A. Y., M. A., R. H., Y. S., M. N., and K. M. analyzed Cx43 expression in salivary gland development, and the Cx43 KO phenotype. M. F. and T. N. examined the phosphorylation of ERK. M. I. measured intracellular calcium levels. All authors analyzed the results and approved the final version of the manuscript.
This work was supported in part by Grants-in-aid from the Ministry of Education, Science, and Culture of Japan (20679006 and 26670880, to S. F.) and the NEXT program (LS010, to S. F.), a Grant-in-Aid for Scientific Research (24390460, to A. Y.), a Grant-in-Aid for Scientific Research (15H05032 and 15K15752, to T. N.), and a Grant-in-Aid for Young Scientists (24792294, to K. S.). The authors declare that they have no conflicts of interest with the contents of this article.
- Cx
- connexin
- ODDD
- oculodentodigital dysplasia
- SMG
- submandibular gland
- 18α-GA
- 18α-glycyrrhetinic acid
- ERK
- extracellular-signal-regulated kinase
- FGF
- fibroblast growth factor.
References
- 1.Alexander D. B., and Goldberg G. S. (2003) Transfer of biologically important molecules between cells through gap junction channels. Curr. Med. Chem. 10, 2045–2058 [DOI] [PubMed] [Google Scholar]
- 2.Goodenough D. A., and Paul D. L. (2003) Beyond the gap: functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 4, 285–294 [DOI] [PubMed] [Google Scholar]
- 3.Paznekas W. A., Boyadjiev S. A., Shapiro R. E., Daniels O., Wollnik B., Keegan C. E., Innis J. W., Dinulos M. B., Christian C., Hannibal M. C., and Jabs E. W. (2003) Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 72, 408–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paznekas W. A., Karczeski B., Vermeer S., Lowry R. B., Delatycki M., Laurence F., Koivisto P. A., Van Maldergem L., Boyadjiev S. A., Bodurtha J. N., and Jabs E. W. (2009) GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum. Mutat. 30, 724–733 [DOI] [PubMed] [Google Scholar]
- 5.Stains J. P., and Civitelli R. (2005) Gap junctions in skeletal development and function. Biochim. Biophys. Acta 1719, 69–81 [DOI] [PubMed] [Google Scholar]
- 6.Lecanda F., Warlow P. M., Sheikh S., Furlan F., Steinberg T. H., and Civitelli R. (2000) Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J. Cell Biol. 151, 931–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung D. J., Castro C. H., Watkins M., Stains J. P., Chung M. Y., Szejnfeld V. L., Willecke K., Theis M., and Civitelli R. (2006) Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J. Cell Sci. 119, 4187–4198 [DOI] [PubMed] [Google Scholar]
- 8.Flenniken A. M., Osborne L. R., Anderson N., Ciliberti N., Fleming C., Gittens J. E., Gong X. Q., Kelsey L. B., Lounsbury C., Moreno L., Nieman B. J., Peterson K., Qu D., Roscoe W., Shao Q., Tong D., Veitch G. I., Voronina I., Vukobradovic I., Wood G. A., Zhu Y., Zirngibl R. A., Aubin J. E., Bai D., Bruneau B. G., Grynpas M., Henderson J. E., Henkelman R. M., McKerlie C., Sled J. G., Stanford W. L., Laird D. W., Kidder G. M., Adamson S. L., and Rossant J. (2005) A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development 132, 4375–4386 [DOI] [PubMed] [Google Scholar]
- 9.Dobrowolski R., Sasse P., Schrickel J. W., Watkins M., Kim J. S., Rackauskas M., Troatz C., Ghanem A., Tiemann K., Degen J., Bukauskas F. F., Civitelli R., Lewalter T., Fleischmann B. K., and Willecke K. (2008) The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum. Mol. Genet. 17, 539–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata K., Masumoto K., Esumi G., Teshiba R., Yoshizaki K., Fukumoto S., Nonaka K., and Taguchi T. (2009) Connexin43 plays an important role in lung development. J. Pediatr. Surg. 44, 2296–2301 [DOI] [PubMed] [Google Scholar]
- 11.Hogan B. L. (1999) Morphogenesis. Cell 96, 225–233 [DOI] [PubMed] [Google Scholar]
- 12.Metzger R. J., and Krasnow M. A. (1999) Genetic control of branching morphogenesis. Science 284, 1635–1639 [DOI] [PubMed] [Google Scholar]
- 13.Patel V. N., Rebustini I. T., and Hoffman M. P. (2006) Salivary gland branching morphogenesis. Differentiation 74, 349–364 [DOI] [PubMed] [Google Scholar]
- 14.Hoffman M. P., Kidder B. L., Steinberg Z. L., Lakhani S., Ho S., Kleinman H. K., and Larsen M. (2002) Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development 129, 5767–5778 [DOI] [PubMed] [Google Scholar]
- 15.Meda P., Pepper M. S., Traub O., Willecke K., Gros D., Beyer E., Nicholson B., Paul D., and Orci L. (1993) Differential expression of gap junction connexins in endocrine and exocrine glands. Endocrinology 133, 2371–2378 [DOI] [PubMed] [Google Scholar]
- 16.Muramatsu T., Hashimoto S., and Shimono M. (1996) Differential expression of gap junction proteins connexin32 and 43 in rat submandibular and sublingual glands. J. Histochem. Cytochem. 44, 49–56 [DOI] [PubMed] [Google Scholar]
- 17.Desplantez T., Verma V., Leybaert L., Evans W. H., and Weingart R. (2012) Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels. Pharmacol. Res. 65, 546–552 [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto S., Fukumoto E., Yoshizaki K., Iwamoto T., Yamada A., Tanaka K., Suzuki H., Aizawa S., Arakaki M., Yuasa K., Oka K., Chai Y., Nonaka K., and Fukumoto S. (2008) Platelet-derived growth factor receptor regulates salivary gland morphogenesis via fibroblast growth factor expression. J. Biol. Chem. 283, 23139–23149 [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa M., Iwamoto T., Fukumoto S., and Yamada Y. (2014) Pannexin 3 inhibits proliferation of osteoprogenitor cells by regulating Wnt and p21 signaling. J. Biol. Chem. 289, 2839–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niswander L., and Martin G. R. (1993) FGF-4 and BMP-2 have opposite effects on limb growth. Nature 361, 68–71 [DOI] [PubMed] [Google Scholar]
- 21.Steinberg Z., Myers C., Heim V. M., Lathrop C. A., Rebustini I. T., Stewart J. S., Larsen M., and Hoffman M. P. (2005) FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development 132, 1223–1234 [DOI] [PubMed] [Google Scholar]
- 22.Chaytor A. T., Evans W. H., and Griffith T. M. (1997) Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J. Physiol. 503, 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ihara A., Muramatsu T., and Shimono M. (2000) Expression of connexin 32 and 43 in developing rat submandibular salivary glands. Arch. Oral Biol. 45, 227–235 [DOI] [PubMed] [Google Scholar]
- 24.Sakai T., Larsen M., and Yamada K. M. (2003) Fibronectin requirement in branching morphogenesis. Nature 423, 876–881 [DOI] [PubMed] [Google Scholar]
- 25.Deleted in proof
- 26.Stains J. P., and Civitelli R. (2005) Gap junctions regulate extracellular signal-regulated kinase signaling to affect gene transcription. Mol. Biol. Cell 16, 64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cullen P. J., and Lockyer P. J. (2002) Integration of calcium and Ras signalling. Nat. Rev. Mol. Cell Biol. 3, 339–348 [DOI] [PubMed] [Google Scholar]
- 28.Mikoshiba K. (2007) IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J. Neurochem. 102, 1426–1446 [DOI] [PubMed] [Google Scholar]
- 29.Mikoshiba K. (2007) The IP3 receptor/Ca2+ channel and its cellular function. Biochem. Soc. Symp. 74, 9–22 [DOI] [PubMed] [Google Scholar]
- 30.Solan J. L., and Lampe P. D. (2009) Connexin43 phosphorylation: structural changes and biological effects. Biochem. J. 419, 261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solan J. L., and Lampe P. D. (2014) Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 588, 1423–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batra N., Riquelme M. A., Burra S., Kar R., Gu S., and Jiang J. X. (2014) Direct regulation of osteocytic connexin 43 hemichannels through AKT kinase activated by mechanical stimulation. J. Biol. Chem. 289, 10582–10591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zappitelli T., Chen F., and Aubin J. E. (2015) Up-regulation of BMP2/4 signaling increases both osteoblast-specific marker expression and bone marrow adipogenesis in Gja1Jrt/+ stromal cell cultures. Mol. Biol. Cell 26, 832–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashida Y., Nakahama K., Shimizu K., Akiyama M., Harada K., and Morita I. (2014) Communication-dependent mineralization of osteoblasts via gap junctions. Bone 61, 19–26 [DOI] [PubMed] [Google Scholar]
- 35.Caufield P. W., Li Y., and Bromage T. G. (2012) Hypoplasia-associated severe early childhood caries–a proposed definition. J. Dent. Res. 91, 544–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephen M. (1997) The role of diet, fluoride and saliva in caries prevention. J. Indian Soc. Pedod. Prev. Dent. 15, 109–113 [PubMed] [Google Scholar]