Abstract
Ubiquitin modification at double strand breaks (DSB) sites is an essential regulator of signaling and repair. γH2AX extends from DSB sites and provides a platform for subsequent recruitment and amplification of DNA repair proteins and signaling factors. Here, we found that RNF8/RNF168 ubiquitylates γH2AX. We identified that USP11 is a unique deubiquitylation enzyme for γH2AX. USP11 deubiquitylates γH2AX both in vivo and in vitro but not the canonical (ub)-K119-H2A and (ub)-K120-H2B in vitro, and USP11 ablation enhances the levels of γH2AX ubiquitylation. We also found that USP11 interacts with γH2AX both in vivo and in vitro. We found that 53BP1 and ubiquitin-conjugated proteins are misregulated to be retained longer and stronger at DSB sites after knockdown of USP11. We further found that cells are hypersensitive to γ-irradiation after ablation of USP11. Together, our findings elucidate deeply and extensively the mechanism of RNF8/RNF168 and USP11 to maintain the proper status of ubiquitylation γH2AX to repair DSB.
Keywords: deubiquitylation (deubiquitination); E3 ubiquitin-protein ligase RNF8 (RNF8); H2A histone family, member X (H2AFX); ubiquitin-dependent protease; ubiquitylation (ubiquitination)
Introduction
DNA damage response is a critical step to maintain genome integrity. Defects in DNA damage response causes genome instability, which is associated with cancer, stem cell exhaustion, developmental defects, infertility, immune deficiency, neurodegenerative disease, and premature aging (1, 2). Double strand breaks (DSBs)2 are the most cytotoxic lesion of all DNA damage lesions. A DSB triggers a chain of response of post-translational modifications, including phosphorylation, acetylation, and ubiquitylation (3).
Ubiquitin modification at DSB sites is an essential regulator of signaling and repair, by the orchestrated recruitment of proteins such as 53BP1 and BRCA1 onto chromatins surrounding DSB sites (3). In response to DSB, ATM is first recruited to DSB sites, where it phosphorylates H2AX at Ser-139 (γH2AX). γH2AX extends for up to a megabase from DSB sites in mammalian cells, providing a platform for subsequent DNA repair proteins recruitment and amplification at DSB sites. γH2AX is recognized by MDC1 (4). MDC1 is then phosphorylated by ATM, whose phospho-sites are recognized by RNF8 (5–7). RNF8 ubiquitylates proteins at DSB sites, and RNF8 activity is promoted by interactions with HERC2 (8). RNF168 is then recruited by its ubiquitin binding domains to recognize products of RNF8 and its own (9). RNF8/RNF168-dependent ubiquitylation orchestrates recruitment of DNA repair and signaling factors on DSB sites, which include 53BP1, RAD18, BRCA1, the RAP80 complex, HERC2, BMI1, RIF1, RNF169, NPM1, FAAP20, and NIPBL (3). 53BP1 and BRCA1 are the two main effectors of the RNF8 pathway. 53BP1 promotes DSB repair by NHEJ and opposes DNA end resection (10), whereas BRCA1 promotes HR and is linked to initiation of end resection (11).
RNF8/RNF168-dependent ubiquitylation plays a key role in the chain of recruitment of DNA repair and signaling factors on DSB sites (3, 5–9). RNF8/RNF168 ubiquitylate H2A-type histones (5–9) at K13/15-H2A (12, 13), which is different from the canonical K119-H2A. Histone ubiquitylation stimulated by DSB is not limited to K13/K15-H2A. RNF20 and RNF40 also ubiquitylate H2B at Lys-120 (14–16), which is recruited to DSB sites (14–16).
Deubiquitylation enzymes counteract RNF8/RNF168-dependent ubiquitylation in maintaining the status of the ubiquitylated proteins at DSB sites. Deubiquitylation enzymes (DUBs) including USP3, USP11, USP16, USP44, BRCC36, PSMD14, and OTUB1 have been identified as negative regulators to the RNF8/RNF168 pathway (17–32). USP44 is identified to deubiquitylate H2A (17). USP3 and USP16 are first identified to regulate RNF8 pathway through their deubiquitylation activity to H2A (18–22). Recently, USP3 was found to deubiquitylate (ub)-k13/15-H2A and (ub)-k13/15-γH2AX, to counteract RNF168-dependent ubiquitylation (19). PSMD14 negatively regulates the RNF8 pathway (23). BRCC36 is a component of the BRCA1-RAP80 complex (24–28). OTUB1 regulates RNF168-dependent ubiquitylation (29–31). USP11 is identified to participate in HR repair at DSB sites (32).
However, it is not clear whether γH2AX is ubiquitylated by RNF8/RNF168 and whether DUB deubiquitylates γH2AX and counteracts RNF8/RFN168-dependent ubiquitylation at DSB sites. In this study, we found that γH2AX is ubiquitylated by RNF8/RNF168 and deubiquitylated by USP11. Both RNF8/RNF168 and USP11 are essential to maintain properly the status of ubiquitylation γH2AX to recruit and amplify the DNA repair proteins and signaling factors at DSB sites.
Experimental Procedures
Plasmids, Antibodies, and Cell Culture
The plasmids of Flag-DUBs were cloned as described previously (33). The individual DUB cDNA obtained from either Marathon-ready cDNA (Clontech) or commercially available cDNA clones (Open Biosystem, RZPD) were amplified by PCR and cloned into TOPO TA cloning vector (Invitrogen). Each DUB cDNA was then subcloned into Flag-tagged expression vector, and its sequence was proofed by DNA sequencing. The expression of each DUB plasmid was confirmed by Western blot analysis using antibody against Flag with the cell extracts from transiently transfected 293 cells.
The plasmid of Flag-USP11 C318A was mutated from Flag-USP11 according to the manufacturer's protocol (Stratagene). The plasmid of HA-Flag-RNF8 was requested from Addgene, and HA-RNF8 was made based on HA-Flag-RNF8. The full-length USP11 was amplified by PCR and subcloned into pGEX (GST) vector for expressing in bacteria. Cells were transfected with plasmid DNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
The antibodies used in Western blot analysis were β-actin (A15), GFP, and Flag M2 from Sigma; HA (3F10) from Roche Applied Science; H2AX, γH2AX, ub-K119-H2A, and ub-K120-H2B from Millipore; USP11 (BL3984), RNF8 and ubiquitin from Bethyl; and conjugated ubiquitin (FK2) from Santa Cruz.
293, U2OS, H1299, and HeLa cells were cultured in DMEM (Cellgro) supplemented with 10% fetal bovine serum (Gibco). The Flag-HA-USP11/U2OS stable cell lines or Flag-HA-H2AX/H1299 stable cell lines were established by transfecting U2OS cells with plasmid of pCin4-Flag-HA-USP11 or by transfecting H1299 cells with plasmid of pCin4-Flag-HA-H2AX, respectively, and selecting with 1 mg/ml G418 (EMD Biosciences).
Cell-based Ubiquitylation Assay
The ubiquitylation assay was performed as previously described (34) with some modification. 293 cells were transfected with plasmids of Flag-H2AX, HA-RNF8, His-Ub, DUB, and GFP. After 48 h, partial of cells were lysed with radioimmune precipitation assay buffer + 0.5% SDS buffer and sonicated to get the whole cell extracts. The rest of the cells were lysed with phosphate/guanidine buffer (6 m guanidine HCl, 0.1 m Na2HPO4, 6.8 mm Na2H2PO4, 10 mm Tris-HCl, pH 8.0, 0.2% Triton X-100, freshly added with 10 mm β-mercaptoethanol and 5 mm imidazole), sonicated, and subjected to Ni-NTA (Qiagen) pulldown overnight at 4 °C. The Ni-NTA resin-bound proteins were washed with wash buffer 1 (8 m urea, 0.1 m Na2HPO4, 6.8 mm Na2H2PO4, 10 mm Tris-HCl, pH 8.0, 0.2% Triton X-100, freshly added with 10 mm β-mercaptoethanol and 5 mm imidazole) once and further washed with wash buffer 2 (8 m urea, 18 mm Na2HPO4, 80 mm Na2H2PO4, 10 mm Tris-HCl, pH 6.3, 0.2% Triton X-100, freshly added with 10 mm β-mercaptoethanol and 5 mm imidazole) three times. The bound proteins were eluted with elution buffer (0.5 m imidazole, 0.125 m DTT) and resolved by SDS-PAGE.
In Vitro Ubiquitylation Assay
The in vitro ubiquitylation assay was performed with γH2AX as substrate. γH2AX was purified with M2 beads from 293T cells after transfected with Flag-H2AX and treated with doxorubicin; then the endogenous ubiquitylated γH2AX was depleted with ubiquitin antibody. The enzyme RNF8 was purified from 293T cells transfected with HA-RNF8 plasmid. E1, E2, and ubiquitin were purchased from Boston Biochemical. For the reaction, 20 ng of γH2AX was mixed with 10 ng of E1, 20–100 ng of E2, 300 ng of RNF8, and 5 μg of ubiquitin in 30 μl of reaction buffer (40 mm Tris-HCl, pH 7.6, 5 mm MgCl2, 2 mm ATP, 2 mm DTT, and 100 ng/μl BSA). Each reaction was stopped after 1 h at 37 °C by the addition of SDS loading buffer and subsequently resolved by 12% SDS-PAGE gels for Western analysis with γH2AX antibody.
In Vitro Deubiquitylation Assay
The in vitro deubiquitylation assay was performed as previously described (33).
Immunoprecipitation
The cell nuclear pellet extracts were subjected to immunoprecipitation. Cells were lysed in Flag lysis buffer (50 mm Tris-HCl, pH 7.3, 137 mm NaCl, 10 mm NaF, 1 mm EDTA, 1% Triton, 0.2% sarkosyl, 20% glycerol, protease inhibitors, and phosphatase inhibitors), and the supernatants were removed after spin down. The pellet was washed once with Flag lysis buffer, resolved in the Flag lysis buffer containing 1/10 volumes of 3 m ammonium sulfate, sonicated, and spun down. The supernatant was diluted with 5× Flag lysis buffer and was subjected to do immunoprecipition. The nuclear pellet extracts of U2OS cells, which were treated with 10 μm doxorubicin for 6 h, were incubated with A/G Plus-agarose beads for 2 h at 4 °C to preclean. The extracts were incubated with mouse IgG or γH2AX-specific antibody, rabbit IgG, or USP11 specific antibody overnight at 4 °C and then were incubated with A/G Plus-agarose beads for 4 h at 4 °C. The beads bound proteins were washed five times with BC100 buffer. The bound proteins were eluted by boiling in 1× SDS sample buffer. The nuclear extracts from the Flag-HA-USP11/U2OS stable cell lines or Flag-HA-H2AX/H1299 stable cell lines were subjected to purify the protein complex by M2-agarose beads.
GST Pulldown
GST and GST-USP11 were purified from BL21 bacterial cells. 1 μg of GST or 4 μg of GST-USP11 proteins were incubated with nuclear pellet extract of HeLa cells that were treated with 10 μm doxorubicin for 6 h at 4 °C overnight. Glutathione-Sepharose beads were added and incubated for 4 h. The beads bound proteins were washed with BC100 buffer. The beads bound proteins were eluted by reduced glutathione, resolved on SDS-PAGE, and assayed by Western blot analysis using antibody against γH2AX.
Immunofluorescent Staining
Cells were fixed with 4% paraformaldehyde for 20 min, rehydrated for 5 min in serum-free DMEM, and permeabilized with 0.2% Triton X-100 for 10 min. Cells were incubated with 1% BSA/PBS for 30 min. Cells were incubated with primary antibodies (as indicated) diluted in 1% BSA/PBS for 45 min at room temperature. After washing with 1% BSA/PBS, cells were incubated with second antibodies for 30 min at room temperature. Finally, cells were counterstained with DAPI to visualize the nuclei.
Colony Formation Assay
U2OS cells were transfected three times with USP11 #1 siRNA, USP11 #2 siRNA, or control siRNA. Twenty-four hours later after the last transfection, cells were spread with the same amount of cells to the new plates and cultured for 1–5 days, or 24 h later, after the last transfected with siRNA, another batch of cells were treated with 0, 1, 2, or 5 grays γ-irradiation and recovered for 12 h. Cells were then spread at different dilution with the same amount of cells to the new plates and cultured for 7–10 days.
Cells were washed three times with cold PBS and stained with 2% of methylene blue (Sigma) in 50% of ethanol solution for 15 min at room temperature. The plate was gently washed with distilled water 10 times and left dry. Stained cells were extracted with 1% SDS and were subjected to spectrophotometer to read A640 nm for quantifying surviving cells.
USP11 Knockdown
Cells were transfected three times with siRNA oligonucleotides using HiPerfect transfection reagent (Qiagen) according to the manufacturer's protocol. siRNAs targeting RNF8, USP2, USP5, USP11, USP13, USP15, USP21, USP26, USP29, USP36, USP52, OTUD6A, OTUB1, VCPIP1, COPS6, EIF3H, MPND, PSMD7 UCHL1, UCHL5, and RNF8 were purchased from Dharmacon (SiGenome Smartpool). Sequences of USP11 #1 siRNA and #2 siRNA oligonucleotide are 5′-GCG UCG GGU ACG UGA UGA A-3′ and 5′-CGA UUC UAU UGG CCU AGU A-3′, respectively. They were purchased from Qiagen's HP Validated siRNA.
Results
RNF8/RNF168 Ubiquitylate γH2AX
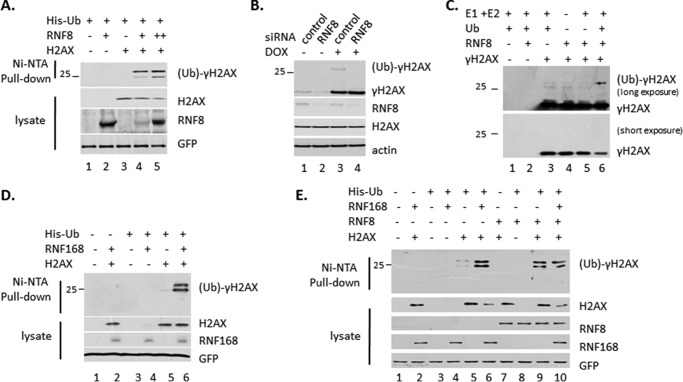
To examine whether RNF8/RNF168 can ubiquitylate γH2AX, we first performed in vivo ubiquitylation assay. 293 cells were transiently transfected with plasmids expressing His-Ub, Flag-H2AX and RNF8, or RNF168. Western blot analysis showed that ubiquitin-conjugated γH2AX bound Ni-NTA resin (Fig. 1, A, D, and E). We confirmed this result with endogenous system. U2OS cells were transfected with siRNA of RNF8 and then treated with doxorubicin for DNA damage. Western blot analysis with the whole cell extracts showed that the levels of ubiquitin-conjugated γH2AX were easily detected in cells with endogenous RNF8. In contrast, when RNF8 was down-regulated by siRNA, the ubiquitin-conjugated γH2AX was no longer detectable (Fig. 1B). We further performed in vitro ubiquitylation assay to show that RNF8 ubiquitylates γH2AX (Fig. 1C). These results demonstrated that RNF8/RNF168 ubiquitinate γH2AX.
FIGURE 1.
RNF8/RNF168 ubiquitylates γH2AX. A, ubiquitylated γH2AX was pulled down in an overexpression system. 293 cells were transiently transfected with plasmid DNA expressing Flag-H2AX, HA-RNF8, and His-UB. The whole cell extracts and elution of Ni-NTA-agarose bead pulldown with guanidine buffer were assayed by Western blot analysis using antibodies against γH2AX, H2AX, and HA. GFP was transfect control. B, the level of ubiquitylated γH2AX decrease after ablation of RNF8. U2OS cells were transfected with the control siRNA oligonucleotides and RNF8 specific siRNA oligonucleotides and treated with 10 μm of doxorubicin for 6 h to damage DNA. The whole cell extracts were assayed by Western blot analysis using antibodies against γH2AX, RNF8, and actin. C, RNF8 ubiquitylates γH2AX in vitro. γH2AX was purified with M2 beads from 293 cells after transfected with Flag-H2AX and treated with doxorubicin; then the endogenous ubiquitylated γH2AX was depleted with ubiquitin antibody. The enzyme RNF8 was purified from 293T cells transfected with HA-RNF8 plasmid. D and E, RNF168 increases the level of the ubiquitylated γH2AX in overexpression system as in A. ctl, control; DOX, doxorubicin.
USP11 Is a Unique Deubiquitylation Enzyme for γH2AX
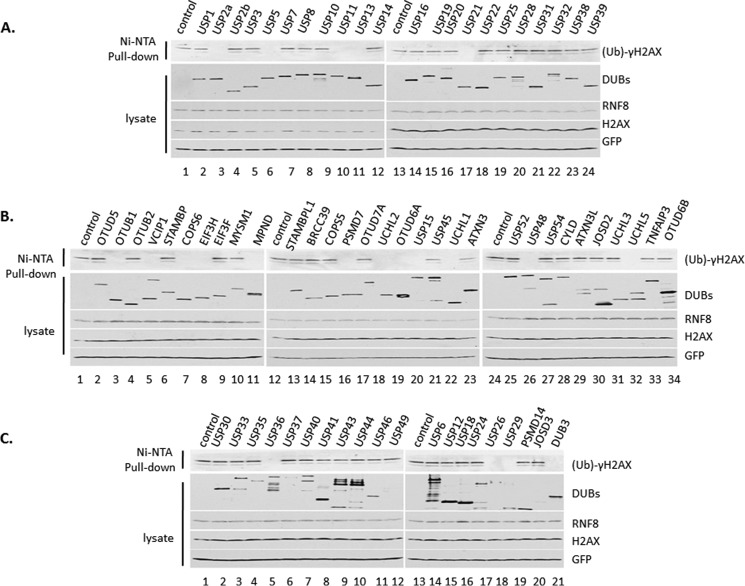
To identify the deubiquitylation enzyme for γH2AX, we start from screening the DUB library. First, we performed in vivo deubiquitylation assay. 293 cells were transiently transfected with plasmids expressing His-Ub, Flag-H2AX, RNF8, and different Flag-DUB. Cell extracts were subjected to Ni-NTA-agarose bead pulldown with guanidine buffer. Western blot analysis showed that ubiquitin-conjugated γH2AX was readily detected with many DUBs; however, it was undetectable with several DUBs, which may have deubiquitylation activity to γH2AX (Fig. 2, A–C). We have screened total of 72 DUBs for γH2AX, in which 20 DUBs may have deubiquitylation activity to γH2AX, including USP2, USP5, USP11, USP13, USP15, USP21, USP26, USP29, USP36, USP52, OTUD6A, OTUB1, VCPIP1, COPS6, EIF3H, MPND, PSMD7, UCHL1, UCHL5, and DUB3.
FIGURE 2.
Deubiquitin enzyme library was screened for γH2AX by in vivo deubiquitylation assay. A–C, 293 cells were transiently transfected with plasmids DNA expressing Flag-H2AX, HA-RNF8, His-UB, and different Flag-DUB. The whole cell extracts and the elution of Ni-NTA-agarose bead pulldown were assayed by Western blot analysis using antibodies against γH2AX, H2AX, Flag, and HA.
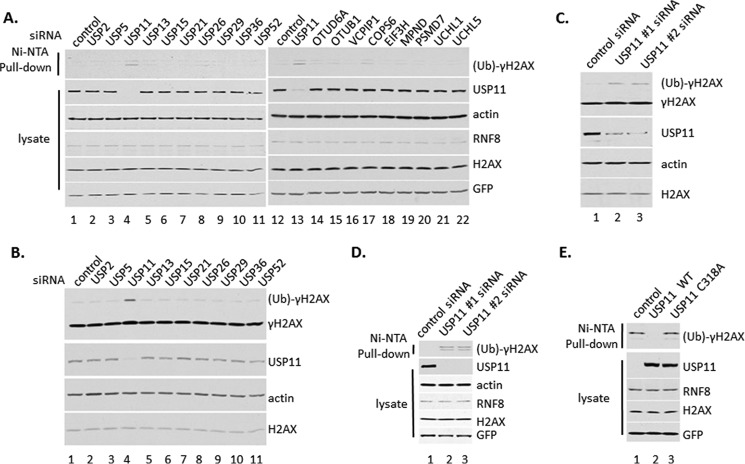
Second, we performed deubiquitylation assay in HeLa cells after ablation of 19 DUBs (except DUB3, of which we were unable to design siRNA oligonucleotide) based on the DUB library screen (Fig. 3A). HeLa cells were transfected three times with a different Smartpool of siRNA of 19 individual DUBs, respectively, which are the mixtures of the four different siRNA oligonucleotides to be sure to knock down enough DUBs. Then cells were transfected with plasmid DNA expressing His-Ub, Flag-H2AX, and RNF8. Cell extracts were subjected to Ni-NTA-agarose bead pulldown. Ubiquitylated γH2AX was most clearly detected in cells with ablation of USP11 (Fig. 3A).
FIGURE 3.
USP11 is a unique deubiquitin enzyme for γH2AX. A, HeLa cells were transfected with pool of siRNA oligonucleotides of the positive DUBs by the assay in Fig. 2. Then HeLa cells were transiently transfected with plasmids DNA expressing Flag-H2AX, HA-RNF8, and His-UB. The whole cell extracts and the elution of Ni-NTA-agarose bead pulldown were assayed by Western blot analysis using antibodies against γH2AX, H2AX, Flag, and HA. B, HeLa cells were transfected with a pool of siRNA oligonucleotides of DUBs, treated with 12 grays γ-irradiation, and recovered for 30 min. The whole cell extracts were assayed by Western blot analysis using antibodies against γH2AX, H2AX, USP11, and actin. C, HeLa cells were transfected with different siRNA oligonucleotides of USP11 and then treated with 12 grays γ-irradiation and recovered for 30 min. The whole cell extracts were assayed by Western blot analysis using antibodies against γH2AX, H2AX, USP11, and actin. D, HeLa cells were transfected with different siRNA oligonucleotides of USP11 and then transiently transfected with plasmids DNA expressing Flag-H2AX, HA-RNF8, and His-UB. The whole cell extracts and the elution of Ni-NTA-agarose bead pulldown were assayed by Western blot analysis using antibodies against γH2AX, H2AX, Flag, and HA. E, 293 cells were transiently transfected with plasmids DNA expressing Flag-H2AX, HA-RNF8, His-UB, and Flag-USP11 wild type or Flag-USP11 C318A inactive mutant. The whole cell extracts and elution of Ni-NTA-agarose bead pulldown were assayed by Western blot analysis using antibodies against γH2AX, H2AX, Flag, and HA.
Third, we further checked the level of ubiquitin-conjugated γH2AX in HeLa cells treated with γ-irradiation after inactivation of 10 DUBs of USP subfamily that have activity during the screening the DUB library (Fig. 3B). The result showed that only after inactivation of USP11, the level of ubiquitylated γH2AX increased strikingly (Fig. 3B).
Fourth, we performed the above assays with two independent different siRNA against USP11 to reduce the side effects of a pool of siRNA oligonucleotides (Fig. 3, C and D). The level of ubiquitin-conjugated γH2AX clearly increased after ablation of USP11 by any one of siRNAs (Fig. 3, C and D).
Fifth, we performed deubiquitylation assay with USP11 wild type and USP11 C318A mutant, which lost its enzymatic activity because its conserved enzymatic active site cysteine was mutated to alanine. The result showed that ubiquitin-conjugated γH2AX was clearly detected in inactive USP11, not in wild type USP11 (Fig. 3E, lane 2 versus lane 3).
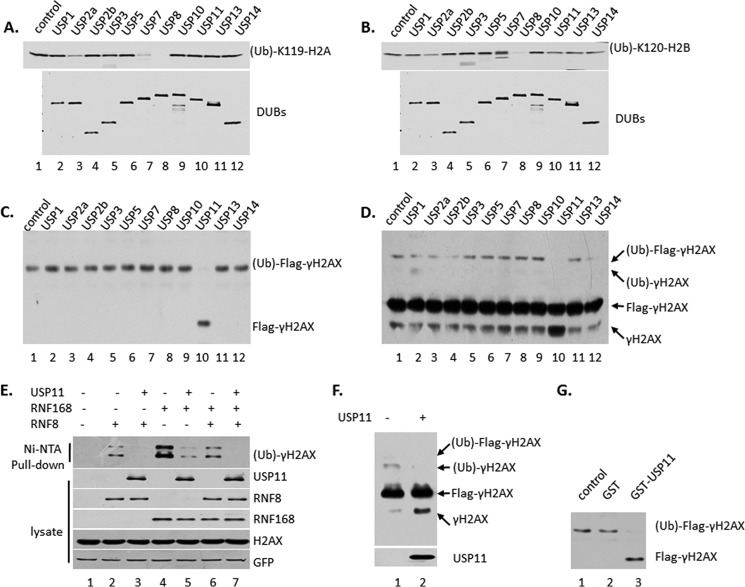
Sixth, we performed in vitro deubiquitylation assay with ubiquitylated γH2AX. The ubiquitin-conjugated γH2AX was purified from 293 cells transfected with plasmid DNA expressing Flag-H2AX, RNF8, and HA-Ub, by tandem immunoprecipitation with M2- and HA-agarose beads to remove the free γH2AX protein. USP11 is the only one of the batch of DUBs to deubiquitylate γH2AX in vitro (Fig. 4C). Then we purified ubiquitylated γH2AX from 293 cells transfected with plasmid DNA expressing Flag-H2AX, RNF8, by immunoprecipitation with M2-agarose beads. The result showed that only USP11 deubiquitylates γH2AX in vitro (Fig. 4D).
FIGURE 4.
USP11 deubiquitylates γH2AX but not H2A and H2B. A, USP11 does not deubiquitylate H2A in vitro. The ubiquitylated H2A were purified from 293 cells transfected with Flag-H2A and HA-Ub by tandem immunoprecipitation with M2- and HA-agarose beads and elution with Flag and HA peptides. The Flag-DUBs were purified from 293 cells transfected with plasmid expressing DUB by M2-agarose beads immunoprecipitation and elution with Flag peptide. The in vitro deubiquitylation reaction with different DUBs, respectively, was assayed by Western blot analysis using antibody against (ub)-K119-H2A. B, USP11 does not deubiquitylate H2B in vitro as in A. C, USP11 deubiquitylates γH2AX in vitro. The ubiquitylated γH2AX was purified from 293 cells transfected with Flag-H2AX, RNF8, and HA-UB by tandem IP with M2- and HA-agarose beads. The DUBs were the same batch used in A. D, USP11 deubiquitylates γH2AX in vitro. The ubiquitylated γH2AX was purified from 293 cells transfected with Flag-H2AX and RNF8 by IP with M2-agarose beads. The DUBs were the same batch used in A. E, USP11 deubiquitylates γH2AX that was catalyzed by RNF8 or RNF168 by in vivo deubiquitylation assay. 293 cells were transiently transfected with plasmids DNA expressing Flag-H2AX, His-UB, Flag-USP11, and HA-RNF8 or HA-RNF168. The whole cell extracts and the elution of Ni-NTA-agarose bead pulldown were assayed by Western blot analysis using antibodies against γH2AX, Flag, and HA. F, USP11 deubiquitylates γH2AX in vitro, which was catalyzed by RNF168. The ubiquitylated γH2AX was purified from 293 cells transfected with Flag-H2AX, RNF168, by IP with M2-agarose beads. G, GST-USP11 deubiquitylates γH2AX in vitro. The ubiquitylated γH2AX was purified as in C. GST-USP11 was purified from Escherichia coli.
Seventh, we compared USP11 activity to γH2AX, which catalyzed RNF8 or RNF168 by in vivo and in vitro deubiquitylation assay (Fig. 4, E and F). USP11 can deubiquitylate any kind of γH2AX (Fig. 4, E and F).
Eighth, we performed the in vitro deubiquitylation assay by GST-USP11, which was purified from bacteria, to reduce the possibility of contamination of other mammalian proteins during the purification process (Fig. 4G). The results showed that GST-USP11 deubiquitylates γH2AX in vitro.
Ninth, we performed in vitro deubiquitylation assay with ubiquitylated H2A and H2B to further confirm that USP11 is a unique deubiquitin enzyme for γH2AX (Fig. 4, A and B). The ubiquitylated H2A or H2B was purified from 293 cells transfected with plasmid DNA expressing Flag-H2A or Flag-H2B and HA-Ub, by tandem immunoprecipitation with M2- and HA-agarose beads and eluted with Flag and HA peptides. The enzymes were the same batch of DUBs as in γH2AX. The in vitro deubiquitylation reaction with different DUBs was assayed by Western blot analysis using antibody against (ub)-K119-H2A (Fig. 4A) or (ub)-K120-H2B (Fig. 4B). The results showed that USP11 could not deubiquitylate the canonical (ub)-K119-H2A or (ub)-K120-H2B (Fig. 4, A and B). Collectively, those data demonstrated that USP11 is a unique deubiquitylation enzyme for γH2AX.
USP11 Associates with the γH2AX Protein Complex
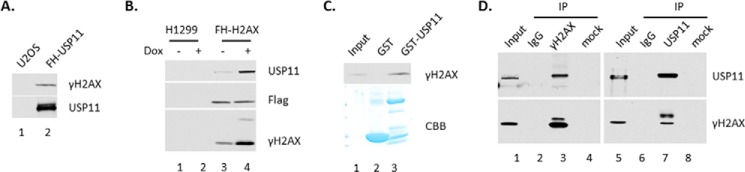
To investigate the relationship of USP11 and γH2AX in vivo, we examined the interaction between these two proteins. Flag-HA-USP11/U2OS stable cell lines were treated with 10 μm of doxorubicin for 6 h. The nuclear extracts were subjected to immunoprecipitate with M2-agarose beads. USP11 protein complex were assayed by Western blot analysis using antibodies against γH2AX. The result showed that γH2AX is clearly detected in USP11-associated protein complex (Fig. 5A, lane 2).
FIGURE 5.
USP11 interacts with γH2AX. A, USP11 interacts with endogenous γH2AX in the USP11 protein complex purified from Flag-HA-USP11/U2OS stable cell lines. B, phosphorylated H2AX increases the interaction with endogenous USP11. The Flag-HA-H2AX protein complex was purified from the Flag-HA-H2AX/H1299 stable cell lines as in A. C, USP11 interacts with γH2AX in vitro. The full-length GST-USP11 fusion protein or GST alone were used in the GST pulldown assay with nuclear pellet extract of HeLa cells treated with 10 μm doxorubicin for 6 h. D, endogenous USP11 co-immunoprecipitates with γH2AX in U2OS cells. CBB, Coomassie Brilliant Blue; Dox, doxorubicin; IP, immunoprecipitation.
We also isolated H2AX-associated protein complex from Flag-HA-H2AX/H1299 stable cell line. The cells were treated with 10 μm of doxorubicin for 6 h. The nuclear extracts were subjected to immunoprecipitate with M2-agarose beads. The result showed that USP11 is clearly detected in γH2AX-associated protein complex (Fig. 5B) and that phosphorylated H2AX increases its interaction with USP11 strikingly (Fig. 5B, lane 4 versus lane 3).
To investigate the interaction between USP11 and γH2AX, we performed GST pulldown assay. The full-length GST-USP11 fusion protein or GST alone were incubated with nuclear pellet extracts from HeLa cells treated with 10 μm doxorubicin for 6 h. The data showed that USP11 interacts with γH2AX (Fig. 5C).
To investigate the interaction between endogenous USP11 and γH2AX proteins, the nuclear pellet extracts from U2OS cells treated with 10 μm of doxorubicin for 6 h were subjected to immunoprecipitate with a γH2AX-specific antibody or a control IgG or with a USP11-specific antibody or a control IgG. As expected, USP11 was clearly detected in the immunoprecipitates obtained with the γH2AX antibody but not the control IgG or mock immunoprecipitates (Fig. 5D, lane 3 versus lanes 2 and 4). Vice versa, γH2AX is clearly detected in the USP11 antibody immunoprecipitates not the control IgG or mock immunoprecipitates (Fig. 5D, lane 7 versus lanes 6 and 8). Those results confirmed that endogenous USP11 and γH2AX associate in the same of the protein complex in cells.
USP11 Plays an Important Role at DSB Sites for Repair
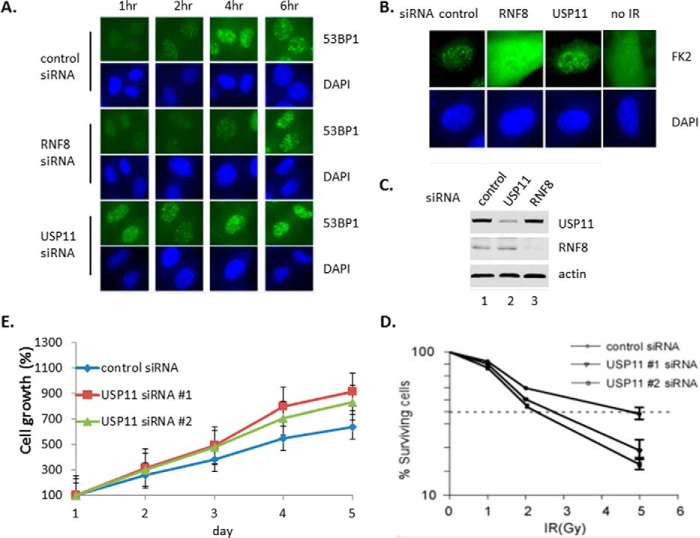
To investigate the role of γH2AX deubiquitylation by USP11, we examined whether ablation of USP11 has effect on DSB sites. HeLa cells were transfected with the siRNAs of control, RNF8 and USP11, respectively. The efficacy of siRNAs knock down was examined (Fig. 6C). Cells were treated with γ-irradiation and recovered for 1, 2, 4, and 6 h before they were checked the 53BP1 accumulation at DSB sites by immunofluorescent staining (Fig. 6A). The results showed that the 53BP1 accumulated at DSB sites rise to the highest levels when cells recovered for 4 h and then decreased, in the control siRNA. As expected, in the RNF8 knockdown cells, the levels of 53BP1 accumulation at DSB sites were delayed because RNF8 ubiquitylation activity in DSB sites was required for 53BP1 recruitment. However, in USP11 knockdown cells, the 53BP1 accumulation in DSB sites rose to the highest levels even when cells recovered for 1 h and extended the highest levels till 6 h (Fig. 6A).
FIGURE 6.
The results demonstrated that the recruitment of 53BP1 and ubiquitin-conjugated proteins to repair foci is misregulated after ablation of USP11 and RNF8. A, 53BP1 is misregulated at the DSB sites after inactivation of USP11. The pictures were taken with the same exposure time. B, ubiquitin-conjugated protein accumulation misregulated at the DSB sites after inactivation. The picture of RNF8 siRNA was taken with 5-fold longer exposure time than others especially to show no FK2 foci. C, cell extracts were assayed by Western blot analysis to show the knockdown of USP11 and RNF8. D, USP11 is required for cell survival following ionizing irradiation. USP11 depleted U2OS cells display increased radiation sensitivity as determined by colony formation assay. The values were obtained from three independent experiments with each performed in triplicate. Error bars indicate standard deviation. E, U2OS cells displayed growth fast after USP11 was depleted. The values were obtained from three independent experiments with each performed in triplicate and normalized to day 1 as 100%. The error bars indicate standard deviation.
We further examined whether ablation of USP11 has effect on the levels of ubiquitin-conjugated proteins in DSB sites. HeLa cells were transfected with the siRNAs of control, RNF8 and USP11, respectively. Cells were treated with γ-irradiation and recovered. The levels of ubiquitin-conjugated proteins at DSB sites were assayed by immunostaining with antibody against the conjugated-ubiquitin (FK2). The results showed that in control cells, ubiquitin-conjugated proteins accumulated at DSB sites; in RNF8 knock down cells, ubiquitin-conjugated proteins spread evenly in whole cells rather than accumulating at DSB sites, whereas in USP11 knockdown cells, ubiquitin-conjugated proteins accumulate at DSB sites stronger than in control (Fig. 6B). The results demonstrated that the recruitment of 53BP1 and ubiquitin-conjugated proteins to repair foci is misregulated after ablation of USP11 and RNF8.
USP11 Is Required for Cell Survival following Ionizing Irradiation
We further examined whether ablation of USP11 has effect on cell survival after γ-irradiation. U2OS cells were transfected with USP11 or control siRNA and treated with γ-irradiation. They were then cultured for 7–10 days before quantifying surviving cells. The results showed that after ablation of USP11, cells are more sensitive to γ-irradiation. The survival cells are less than control treatment with the same dose of IR, or 50% of survival cells need light dosage of IR (Fig. 6D). Those data confirmed that USP11 is required for cell survival following ionizing irradiation. As control, we examined whether ablation of USP11 has effect on cell growth. The results showed that after ablation of USP11, cells grow faster (Fig. 6E).
Discussion
Ubiquitin modification at DSB sites is an essential regulator of signaling and repair, by the orchestrated recruitment of DNA repair proteins and signaling factors such as 53BP1 and BRCA1 onto chromatins surrounding DSB sites (3). Histone ubiquitylation by RNF8/RNF168 was observed in the previous study (5). RNF8/RNF168 ubiquitylate H2A-type histones (5) on K13/15-H2A, which is different from the canonical K119-H2A site (12–13). γH2AX is the marker of DSB foci, providing the platform for subsequent recruitment and amplification of DNA repair proteins and signaling factors at DSB sites (3–7). Recent study found that RNF168 ubiquitylates γH2AX at Lys-13/15 (19). Here, we found that RNF8/RNF168 ubiquitylates γH2AX, which may further enhance the cascade of recruitment and amplification of DNA repair proteins and signaling factors at DSB sites (Fig. 1). The level of ubiquitylated γH2AX increases when RNF8 is overexpressed (Fig. 1A), whereas the ubiquitylation γH2AX level decreases with RNF8 inactivation (Fig. 1B). We found that RNF8 ubiquitylates γH2AX in vitro (Fig. 1C). The modification sites on γH2AX by RNF8 need further studies.
DUBs have been identified function at DSB sites (17–31). USP44 regulates RNF8/RNF168-dependent ubiquitylation at DSB sites (17). USP3 deubiquitylates H2A to regulate RNF168 pathway, and overexpression of USP3 can block RNF168 accumulation at DSB sites (18). USP3 deubiquitylates (ub)-K13/15-H2A and (ub)-k13/15-γH2AX and counteracts RNF168-dependent ubiquitylation (19). USP16 opposes the RNF8/RNF168-mediated DSB-induced transcriptional silencing (20), interacts with HERC2 (21), and regulates embryonic stem cell gene expression and lineage commitment (22). PSMD14 negatively regulates the RNF8 pathway (23). Conversely, USP3, USP16, USP44, and PSMD14 did not show activity to γH2AX in our deubiquitylation assay (Fig. 2, A–C). The question about the inconsistency needs further study to be answered. It may be because those DUBs show lower activity in our assay.
BRCC36 and OTUB1 were also identified function at DSB sites (24–31). BRCC36 is a component of the BRCA1-RAP80 complex (24–28). Unfortunately, our DUB library does not include BRCC36. OTUB1 regulates RNF168-dependent ubiquitylation (29–31). Notably, OTUB1 has shown activity to γH2AX not only in our overexpression deubiquitylation assay (Fig. 2B) but also in in vivo knockdown assay (Fig. 3A). Until now, we cannot expect that OTUB1 has no activity to γH2AX, which needs further work to be answered. This is different from the previous studies (29–31), in which OTUB1 negatively regulates RNF168-dependent ubiquitylation through independent OTUB1 deubiquitylation activity.
USP11 was identified to participate in HR repair at DSB sites in the previous study (32). After ablation of USP11, cells show spontaneously DNA damage response; hypersensitivity to PARP inhibition, ionizing radiation, and other genotoxic stress agents; HR repair pathway defects; and the recruitment of RAD51 and 53BP1 to DSB sites misregulated (32). However, the mechanism of USP11 function at DSB sites is not fully understood. In our study, we identified that USP11 is a unique deubiquitylation enzyme to γH2AX (Fig. 2 and 3). USP11 interacts with γH2AX in vivo and in vitro (Fig. 5). Overexpression of USP11 decreases the levels of ubiquitylated γH2AX (Fig. 2); ablation of USP11 increases the ubiquitylation γH2AX levels (Fig. 3, A–D). USP11 has no activity to (ub)-K119-H2A and (ub)-K120-H2B in in vitro deubiquitylation assay (Fig. 4, A and B), whereas USP11 has activity to (ub)-γH2AX in vitro (Fig. 4, C–F). We found that after knockdown of USP11, 53BP1 and ubiquitin-conjugated proteins are misregulated, retaining longer and stronger at DSB sites (Fig. 6, A–C), and we found that, similar with the previous study (32), after ablation of USP11, cells are also hypersensitive to γ-irradiation (Fig. 6D).
Ubiquitin modification γH2AX adds the layers of regulation fundamentally at DSB sites. γH2AX is the initial protein in the cascade of recruitment and amplification of DNA repair proteins and signaling factors at DSB sites, which extends from the DSB sites and provides the platform for subsequent recruitment and amplification (3–7). We found that RNF8/RNF168 ubiquitylates γH2AX, and USP11 deubiquitylates γH2AX. RNF8/RNF168 and USP11 function together to regulate the levels of ubiquitylation γH2AX. Our findings deeply and extensively elucidate the mechanism of USP11 and RNF8/RNF168 to keep the proper status of ubiquitylation γH2AX to repair DSB.
Author Contributions
M. Y. and K. L. executed the experiments. W. G. and W. Z. designed, executed, analyzed, and supervised the experiments. W. Z. wrote the manuscript. Z. M. and J. L. analyzed the experiments. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank members of Dr. Gu's lab for critical discussion and excellent technical assistance. We are grateful to Drs. Weiguo Zhu and Ying Zhao for providing RNF168 plasmid. We thank Drs. Baiya Liu and Minghui Liu in Dr. Luo's lab for technical assistance.
The authors declare that they have no conflicts of interest with the contents of this article.
- DSB
- double strand break
- DUB
- deubiquitylation enzyme
- Ni-NTA
- nickel-nitrilotriacetic acid
- ub
- Ub, or UB, ubiquitin or ubiquitylation.
References
- 1.Jackson S. P., and Bartek J. (2009) The DNA-damage response in human biology and disease. Nature 461, 1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson S. P., and Durocher D. (2013) Regulation of DNA damage responses by ubiquitin and sumo. Molecular Cell 49, 795–807 [DOI] [PubMed] [Google Scholar]
- 3.Lukas J., Lukas C., and Bartek J. (2011) More than just a focus: the chromatin response to DNA damage and its role in genome integrity maintenance. Nat. Cell Biol. 13, 1161–1169 [DOI] [PubMed] [Google Scholar]
- 4.Stucki M., Clapperton J. A., Mohammad D., Yaffe M. B., Smerdon S. J., and Jackson S. P. (2005) MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 123, 1213–1226 [DOI] [PubMed] [Google Scholar]
- 5.Huen M. S., Grant R., Manke I., Minn K., Yu X., Yaffe M. B., and Chen J. (2007) RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131, 901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolas N. K., Chapman J. R., Nakada S., Ylanko J., Chahwan R., Sweeney F. D., Panier S., Mendez M., Wildenhain J., Thomson T. M., Pelletier L., Jackson S. P., and Durocher D. (2007) Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 318, 1637–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mailand N., Bekker-Jensen S., Faustrup H., Melander F., Bartek J., Lukas C., and Lukas J. (2007) RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131, 887–900 [DOI] [PubMed] [Google Scholar]
- 8.Bekker-Jensen S., Rendtlew Danielsen J., Fugger K., Gromova I., Nerstedt A., Lukas C., Bartek J., Lukas J., and Mailand N. (2010) HERC2 coordinates ubiquitin-dependent assembly of DNA repair factors on damaged chromosomes. Nat. Cell Biol. 12, 80–86 [DOI] [PubMed] [Google Scholar]
- 9.Doil C., Mailand N., Bekker-Jensen S., Menard P., Larsen D. H., Pepperkok R., Ellenberg J., Panier S., Durocher D., Bartek J., Lukas J., and Lukas C. (2009) RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell 136, 435–446 [DOI] [PubMed] [Google Scholar]
- 10.Noon A. T., and Goodarzi A. A. (2011) 53BP1-mediated DNA double strand break repair: insert bad pun here. DNA Repair 10, 1071–1076 [DOI] [PubMed] [Google Scholar]
- 11.Li M. L., and Greenberg R. A. (2012) Links between genome integrity and BRCA1 tumor suppression. Trends Biochem. Sci. 37, 418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatti M., Pinato S., Maspero E., Soffientini P., Polo S., and Penengo L. (2012) A novel ubiquitin mark at the N-terminal tail of histone H2As targeted by RNF168 ubiquitin ligase. Cell Cycle 11, 2538–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattiroli F., Vissers J. H., van Dijk W. J., Ikpa P., Citterio E., Vermeulen W., Marteijn J. A., and Sixma T. K. (2012) RNF168 ubiquitinates K13–15 on H2A/H2AX to drive DNA damage signaling. Cell 150, 1182–1195 [DOI] [PubMed] [Google Scholar]
- 14.Zhu B., Zheng Y., Pham A. D., Mandal S. S., Erdjument-Bromage H., Tempst P., and Reinberg D. (2005) Monoubiquitination of human histone H2B: the factors involved and their roles in HOX gene regulation. Mol. Cell 20, 601–611 [DOI] [PubMed] [Google Scholar]
- 15.Moyal L., Lerenthal Y., Gana-Weisz M., Mass G., So S., Wang S. Y., Eppink B., Chung Y. M., Shalev G., Shema E., Shkedy D., Smorodinsky N. I., van Vliet N., Kuster B., Mann M., Ciechanover A., Dahm-Daphi J., Kanaar R., Hu M. C., Chen D. J., Oren M., and Shiloh Y. (2011) Requirement of ATM-dependent monoubiquitylation of histone H2B for timely repair of DNA double-strand breaks. Mol. Cell 41, 529–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura K., Kato A., Kobayashi J., Yanagihara H., Sakamoto S., Oliveira D. V., Shimada M., Tauchi H., Suzuki H., Tashiro S., Zou L., and Komatsu K. (2011) Regulation of homologous recombination by RNF20-dependent H2B ubiquitination. Mol. Cell 41, 515–528 [DOI] [PubMed] [Google Scholar]
- 17.Mosbech A., Lukas C., Bekker-Jensen S., and Mailand N. (2013) The deubiquitylating enzyme USP44 counteracts the DNA double-strand break response mediated by the RNF8 and RNF168 ubiquitin ligases. J. Biol. Chem. 288, 16579–16587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicassio F., Corrado N., Vissers J. H., Areces L. B., Bergink S., Marteijn J. A., Geverts B., Houtsmuller A. B., Vermeulen W., Di Fiore P. P., and Citterio E. (2007) Human USP3 is a chromatin modifier required for S phase progression and genome stability. Curr. Biol. 17, 1972–1977 [DOI] [PubMed] [Google Scholar]
- 19.Sharma N., Zhu Q., Wani G., He J., Wang Q. E., and Wani A. A. (2014) USP3 counteracts RNF168 via deubiquitinating H2A and γH2AX at lysine 13 and 15. Cell Cycle 13, 106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanbhag N. M., Rafalska-Metcalf I. U., Balane-Bolivar C., Janicki S. M., and Greenberg R. A. (2010) ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell 141, 970–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z., Yang H., and Wang H. (2014) The histone H2A deubiquitinase USP16 interacts with HERC2 and fine-tunes cellular response to DNA damage. J. Biol. Chem. 289, 32883–32894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang W., Lee Y. H., Jones A. E., Woolnough J. L., Zhou D., Dai Q., Wu Q., Giles K. E., Townes T. M., and Wang H. (2014) The histone H2A deubiquitinase Usp16 regulates embryonic stem cell gene expression and lineage commitment. Nat. Commun. 5, 3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler L. R., Densham R. M., Jia J., Garvin A. J., Stone H. R., Shah V., Weekes D., Festy F., Beesley J., and Morris J. R. (2012) The proteasomal de-ubiquitinating enzyme POH1 promotes the double-strand DNA break response. EMBO J. 31, 3918–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper E. M., Cutcliffe C., Kristiansen T. Z., Pandey A., Pickart C. M., and Cohen R. E. (2009) K63-specific deubiquitination by two JAMM/MPN+ complexes: BRISC-associated Brcc36 and proteasomal Poh1. EMBO J. 28, 621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Y., Hakimi M. A., Chen X., Kumaraswamy E., Cooch N. S., Godwin A. K., and Shiekhattar R. (2003) Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair. Mol. Cell 12, 1087–1099 [DOI] [PubMed] [Google Scholar]
- 26.Shao G., Lilli D. R., Patterson-Fortin J., Coleman K. A., Morrissey D. E., and Greenberg R. A. (2009) The Rap80-BRCC36 de-ubiquitinating enzyme complex antagonizes RNF8-Ubc13-dependent ubiquitination events at DNA double strand breaks. Proc. Natl. Acad. Sci. U.S.A. 106, 3166–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobhian B., Shao G., Lilli D. R., Culhane A. C., Moreau L. A., Xia B., Livingston D. M., and Greenberg R. A. (2007) RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science 316, 1198–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B., and Elledge S. J. (2007) Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc. Natl. Acad. Sci. U. S.A. 104, 20759–20763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakada S., Tai I., Panier S., Al-Hakim A., Iemura S., Juang Y. C., O'Donnell L., Kumakubo A., Munro M., Sicheri F., Gingras A. C., Natsume T., Suda T., and Durocher D. (2010) Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature 466, 941–946 [DOI] [PubMed] [Google Scholar]
- 30.Juang Y. C., Landry M. C., Sanches M., Vittal V., Leung C. C., Ceccarelli D. F., Mateo A. R., Pruneda J. N., Mao D. Y., Szilard R. K., Orlicky S., Munro M., Brzovic P. S., Klevit R. E., Sicheri F., and Durocher D. (2012) OTUB1 co-opts Lys48-linked ubiquitin recognition to suppress E2 enzyme function. Mol. Cell 45, 384–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiener R., Zhang X., Wang T., and Wolberger C. (2012) The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature 483, 618–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiltshire T. D., Lovejoy C. A., Wang T., Xia F., O'Connor M. J., and Cortez D. (2010) Sensitivity to poly(ADP-ribose) polymerase (PARP) inhibition identifies ubiquitin-specific peptidase 11 (USP11) as a regulator of DNA double-strand break repair. J. Biol. Chem. 285, 14565–14571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shan J., Zhao W., and Gu W. (2009) Supression of cancer cell growth by promoting cyclin D1 degradation. Mol. Cell 36, 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M., Brooks C. L., Wu-Baer F., Chen D., Baer R., and Gu W. (2003) Mono-versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302, 1972–1975 [DOI] [PubMed] [Google Scholar]