Abstract
To translate the 13 mtDNA-encoded mRNAs involved in oxidative phosphorylation (OXPHOS), mammalian mitochondria contain a dedicated set of ribosomes comprising rRNAs encoded by the mitochondrial genome and mitochondrial ribosomal proteins (MRPs) that are encoded by nuclear genes and imported into the matrix. In addition to their role in the ribosome, several MRPs have auxiliary functions or have been implicated in other cellular processes like cell cycle regulation and apoptosis. For example, we have shown that human MRPL12 binds and activates mitochondrial RNA polymerase (POLRMT), and hence has distinct functions in the ribosome and mtDNA transcription. Here we provide concrete evidence that there are two mature forms of mammalian MRPL12 that are generated by a two-step cleavage during import, involving efficient cleavage by mitochondrial processing protease and a second inefficient or regulated cleavage by mitochondrial intermediate protease. We also show that knock-down of MRPL12 by RNAi results in instability of POLRMT, but not other primary mitochondrial transcription components, and a corresponding decrease in mitochondrial transcription rates. Knock-down of MRPL10, the binding partner of MRPL12 in the ribosome, results in selective degradation of the mature long form of MRPL12, but has no effect on POLRMT. We propose that the two forms of MRPL12 are involved in homeostatic regulation of mitochondrial transcription and ribosome biogenesis that likely contribute to cell cycle, growth regulation, and longevity pathways to which MRPL12 has been linked.
Keywords: mitochondria, proteolysis, ribosome, RNA polymerase, transcription, MIP, MRPL12, POLRMT, mitochondrial import, mtDNA
Introduction
Although mitochondria have long been appreciated for their role in metabolism, ATP production by the process of oxidative phosphorylation (OXPHOS),6 and generation of reactive oxygen species, they are also important signaling organelles, involved in controlling apoptosis, ion homeostasis, and immune responses (1–6). Because of their multifactorial nature, it is perhaps not surprising that mitochondria are implicated in many human diseases and age-related pathology (7–9). Critical to their function is the maternally inherited mitochondrial DNA (mtDNA) that resides in the matrix and encodes 13 essential OXPHOS subunits, as well as two rRNAs and 22 tRNAs needed for translation of the corresponding mRNAs by dedicated mitochondrial ribosomes (10, 11). The remaining ∼1,200 mitochondrial proteins are encoded by genes in the nucleus and imported into the organelle, including ∼70 additional OXPHOS proteins, and those required for mitochondrial gene expression (i.e. transcription, RNA processing, and translation) (12–14). An interesting subset of the latter is the mitochondrial ribosomal proteins (MRPs), which are imported into mitochondria to assemble with the mtDNA-encoded rRNAs to carry out mitochondrial translation. While mitochondrial ribosomes resemble their ancestral bacterial counterparts, they also possess unique proteins and features not found in bacterial or cytoplasmic ribosomes (15).
Several direct connections between mitochondrial ribosomes and the primary mitochondrial transcription machinery exist. For example, we showed MRPL12 activates transcription by binding directly to POLRMT, the single-subunit mtRNA polymerase, in complexes distinct from those with TFB2M involved in transcription initiation (16, 17). In addition, POLRMT has a transcription-independent role in mitochondrial ribosome biogenesis (18). However, the precise functional relationships between the mitochondrial transcription machinery and ribosome biogenesis are largely unknown.
The Escherichia coli ortholog of MRPL12 exists as two forms (originally called L7 and L12) that differ by the presence or absence of an N-terminal acetyl group and exist as a multimer in the ribosome bound to MRPL10 (19). In mammalian mitochondria, MRPL12 dimers are bound to the ribosome (20). We and others have observed two forms of mammalian MRPL12 in Western blots, which appear to differ in size by more that can be accounted for by the presence and absence of N-terminal acetylation (16, 21). In this report, we investigated how these two forms of human MRPL12 are generated and whether they have different functional properties.
Experimental Procedures
Expression Plasmids
The pINCY plasmid containing the cDNA sequence of MRPL12 was purchased from Open Biosystems. This was used as a template for PCR of the MRPL12 ORF using primers that introduced a consensus Kozak sequence upstream of the MRPL12 start codon. The product was ligated into pCIneo (Promega). HA-tagged POLRMT has been described previously (16).
Cell Culture, Transfections, and RNAi
Human HEK293 and HeLa cells, and mouse embryonic fibroblasts were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) (v/v) (Gibco). HEK293 cells were transfected with MRPL12 expression plasmids as follows. Cells (4 × 106 in 10 ml of DMEM) were plated in a 10-cm dish ∼3 h preceding the transfection. Plasmid DNA (7 μg, see above for plasmid details) was diluted in 1 ml of OptiMEM (Gibco) to which 21 μg of PEI was added and incubated for 15 min at room temperature. This mixture was added to the cells, which were then placed at 37 °C in a CO2 incubator for 24 h before harvesting the cells.
RNA interference was done in several ways. To achieve transient knockdown, a few hours preceding transfection, cells (4 × 105) were seeded in 2 ml of DMEM supplemented with 10% FBS per well in 6-well plate. Cells were transfected with the desired siRNA duplex in the presence of 5 μl of RNAimax Oligofectamine (Invitrogen) and OptiMEM to achieve a final concentration of 10 nm duplex in a total volume of 2.5 ml. The siRNA duplexes used were: MRPL10#1, 5′-GCUUAUCAACUACUCCAAGCUCCC-3′ and MRPL10#2, 5′-CCUCCAGACUGUCCGCUAUGGCUCC-3′; MRPL12#1, 5′-ACGUUGGAAGAUCCAGGAUGUCGGGC-3′ and MRPL12#2, 5′-UCAAGAACUACAUCCAAGGCAUCAA-3′; POLRMT, 5′-AGAUACUGGAGAAGGAUAAGCGGAC-3′; MIP#1, 5′-CUGGUAGAACUUGGAAUAAAUAATT-3′ and MIP#2, 5′-GCAAUGAUUAUCGAGUAGUUAACC-3′. For sucrose density-gradientfractionation experiments, the format was scaled up to a 10-cm dish. Cells were harvested 72 h after siRNA transfection. To achieve stable knockdown, two different MRPL12 target sequences (#1, 5′-GCCTCACTCTCTTGGAAAT-3′; and #2, 5′-TCAACGAGCTCCTGAAGAAA-3′) were cloned into pLKO.1 vector (Addgene), and lentiviral particles were packaged using 293FT cells (Invitrogen). Infected HeLa cells were selected with 0.5 μg/ml of puromycin and harvested after 7 days of treatment.
Protein Sequencing
A confluent culture of HEK293 cells transfected with the human HA-tagged MRPL12 was harvested and lysed in Nonidet P-40 lysis buffer (50 mm Tris, pH 7.5, 1% Nonidet P-40, 120 mm NaCl, 1 mm EDTA, 10% glycerol) containing protease inhibitor mixture (Roche). Cell lysate (2 mg) was subjected to immunoprecipitation using 20 μl of anti-HA antibody (slurry from Pierce) covalently bound to magnetic beads (Thermo Scientific) for 4 h. Postincubation, the beads were washed three times with 1× TBST buffer, then boiled in 40 μl of 2× Laemmli buffer at 100 °C for 5 min. The eluate was loaded onto a 15% SDS-PAGE gel capable of separating the mature long and short forms of MRPL12 and then transferred onto a PVDF membrane (Bio-Rad). The PVDF membrane was stained with Ponceau S and the band of interest (short or long form of MRPL12) was cut out and destained in water several times. The dried membrane was submitted to the Tufts University Core Facility for Edman N-terminal protein sequencing.
Western Blotting
For Western analysis, 20–80 μg of protein were loaded on a 12% Tris glycine SDS gel. After electrophoresis, proteins were transferred to a 0.45-μm PVDF membrane (Millipore). The following primary antibodies were used for immunodetection: rabbit anti-MRPL12 and anti-MRPS18B polyclonal (14795-1-AP and 16139-1-AP, Protein Tech), rabbit anti-MRPL10, anti-MRPL28, anti-MRPL45, and anti-MRPS10 polyclonal (HPA021234, HPA030594, HPA023373, and HPA029134, Sigma), rabbit anti-POLRMT, rabbit anti-HA (ab32988 and ab9110, Abcam), mouse anti-GAPDH monoclonal (AM4300, Ambion), and rabbit anti-TFAM polyclonal and anti-TFB2M polyclonal (provided by Dr. Craig Cameron). Secondary antibodies for ECL detection were peroxidase-conjugated anti-mouse or anti-rabbit IgGs (Jackson Laboratory). The signal was generated using Luminata Crescendo reagent (Millipore) and captured on x-ray film or chemiluminescence imaging. As documented by the manufacturer, the POLRMT antibody detects a nonspecific, cross-reacting band that migrates to a position above POLRMT in some of the blots (e.g. see Fig. 3C).
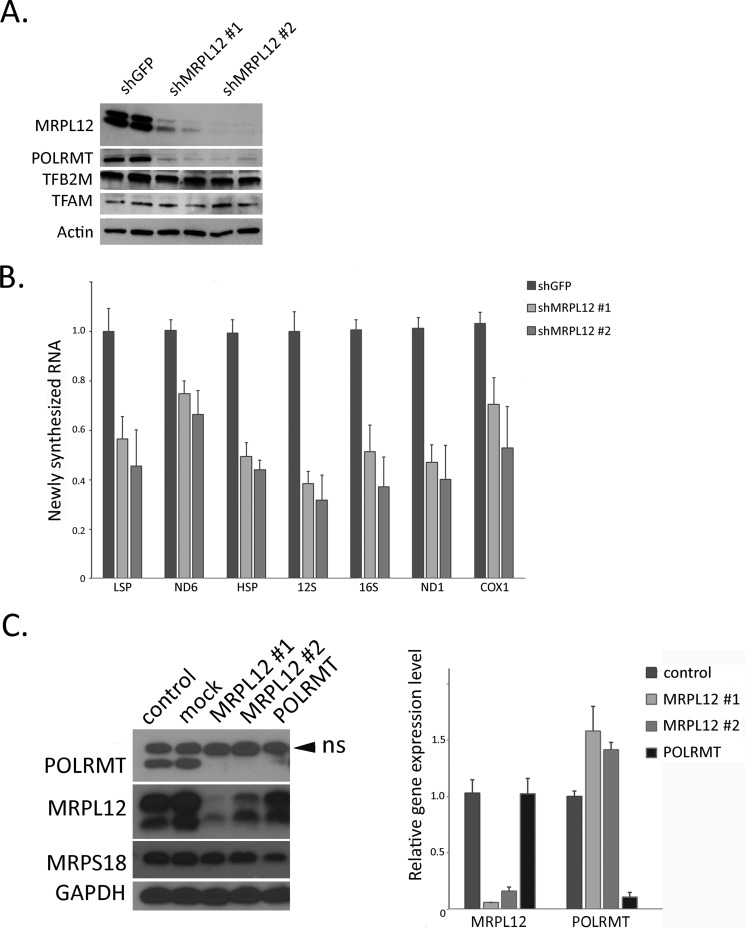
FIGURE 3.
MRPL12 knock-down leads to instability of POLRMT and reduced mitochondrial transcription. A, Western blot analysis of MRPL12, POLRMT, TFB2M, TFAM, and Actin (loading control) in HeLa cells transfected with a control shRNA (lanes 1 and 2, shGFP) or two different shRNAs against MRPL12 (lanes 3 and 4, shMRPL12 #1; lanes 5 and 6, shMRPL12 #2). B, relative amount of newly synthesized mitochondrial RNA determined by 30-min labeling with 4-TU (see “Experimental Procedures”) in HeLa cells transfected with a control shRNA or two different shRNAs against MRPL12 shMRPL12 #1 and shMRPL12 #2. The mtDNA-encoded gene measured is indicated. C, left panel: Western blot analysis of MRPL12, MRPS18, POLRMT, and GAPDH (loading control) of cell lysates from HeLa cells untreated (control), mock transfected (mock), or transfected with one of two siRNAs directed against MRPL12 (MRPL12 #1 and #2) and one siRNA directed against POLRMT (POLRMT, lane 5). The arrowhead indicates a nonspecific (ns) band that cross-reacts with the POLRMT (acknowledged by the manufacturer) and, accordingly, does not disappear upon POLRMT knockdown (lane 5). Right panel, results of quantitative RT-PCR of MRPL12 and POLRMT mRNAs (relative to GAPDH). Analysis of cells in which POLRMT is knocked down is included to demonstrate that a reduction in POLRMT protein and mRNA can be detected, but is not observed in the MRPL12 knockdown cells. Error bars represent the mean ± S.D. (n = 3).
Quantitative RT-PCR
Total RNA was isolated from cells with the RNeasy Mini plus kit (Qiagen). Trace amounts of genomic DNA contamination were removed by treatment with DNase I (Roche). Reverse transcriptase (RT) reactions were performed on 1000 ng of total RNA and with the high-performance cDNA transcription kit (Applied Biosystems). The resulting cDNA was then used for quantitative PCR with Fast SYBR Green Master Mix (Applied Biosystems) and gene-specific primers using a ViiA7 Real-time PCR system (Life Technologies). Target genes were normalized relative to 18S or GAPDH cDNA levels (as indicated in figure legends) and calculated using 2-ΔΔCt method. Experimental samples were normalized to nonspecific RNAi (e.g. shGFP) or non-transfected cells, which were given a value of 1.0.
Pulse Labeling of Mitochondrial RNA with 4-Thiouridine
Seven days after viral-mediated MRPL12 knock-down, HeLa cells were pulse-labeled with 0.2 mm 4-thiouridine (4-TU, Sigma) for 30 min. As a uridine analog 4-TU is incorporated into newly synthesized RNAs, but also has the unique property that it can be conjugated to HPDP-biotin via a disulfide linkage for purification. After 4-TU labeling, cells were collected and total cellular RNA was isolated using the RNeasy Plus Mini Kit from Qiagen. For each RNA sample, 5.5 μg of total RNA was labeled with HPDP-biotin (Sigma, 2 μl/μg of RNA) at 25 °C for 3 h. The biotinylated RNA samples were then precipitated to remove excess HPDP-biotin and resuspended in water. Biotinylated RNA was isolated from these samples using streptavidin-conjugated agarose beads (Millipore). Samples wereincubated with the agarose beads in isolation buffer (100 mm Tris-HCl, pH 7.4, 1 m NaCl, and 10 mm EDTA) for 2 h, and then washed several times to remove unbound RNA. The newly synthesized biotinylated RNA was eluted from agarose beads using 5% 2-mercaptoethanol to break the disulfide linkage, and then precipitated and resuspended in water (to concentrate the sample). Parallel vehicle control (dimethyl sulfoxide) samples that were collected and analyzed by quantitative PCR did not show any signal. This method was adapted from that developed by others (22, 23).
The entire isolated biotinylated RNA sample was used to generate cDNA with the High Capacity cDNA RT kit (Applied Biosystems). The cDNA was then used for quantitative PCR with Fast SYBR Green Master Mix (Applied Biosystems) and primers (Table 1) using the ViiA7 Real-time PCR system (Life Technologies). Mitochondrial transcripts were normalized to 18S cDNA levels, and calculated as a relative gene expression value using the 2-ΔΔCt method. Experimental samples were normalized to nonspecific RNAi (e.g. shGFP) or non-transfected cells, which were given a value of 1.0.
TABLE 1.
Primers used for quantitative RT-PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| MRPL12 | GATGGGTGGTGTGATGTCTG | AATGTGTCCGTTCTTTCGCT |
| POLRMT | AGAAGCGGAAGCTGCTCA | GGCATCCTTCACCATGAATAA |
| MRPL10 | ATTCTTCCGGTGGAGATGG | TGGAGCCATAGCGGACAG |
| ND1 | CCCTAAAACCCGCCACATCT | GAGCGATGGTGAGAGCTAAGGT |
| ND6 | CCCCGAGCAATCTCAATTACA | TGATTATGGGCGTTGATTAGTAGTAGTT |
| Cox I | CCCACCGGCGTCAAAGTAT | TGCAGCAGATCA TTTCATATTGC |
| Cyt b | AACCGCCTTTTCATCAATCG | AGCGGATGATTCAGCCATAATT |
| 12S | CGAAGGTGGATTTAGCAGTAAACTAAG | GTGTGTACGCGCTTCAGGGC |
| 16S | GTTACCCTAGGGATAACAGGCGC | GATCCAACATCGAGGTCGTAAACC |
| GAPDH | CCTGGTATGACAACGAATTTGGC | GTCTTACTCCTTGGAGGCCATG |
| LSP | GATAAAATTTGAAATCTGG | CAGCACTTAAACACATCTC |
| HSP | GACACCCCCCACAGTTTA | GGGTGATGTGAGCCCGTC |
| MIP | TCAACCACAGACATTCTCAAG | CTAGCACCATACCCCACGAG |
| 18S | CGCAGCTAGGAATAATGGAATAGG | CATGGCCTCAGTTCCGAAA |
Other Methods
Immunoprecipitation and sucrose gradient fractionation were performed as described previously (16).
Results
Two Forms of MRPL12 Protein Exist in Human and Mouse Cell Lines and Tissues That Are Not Derived from Alternative Splicing
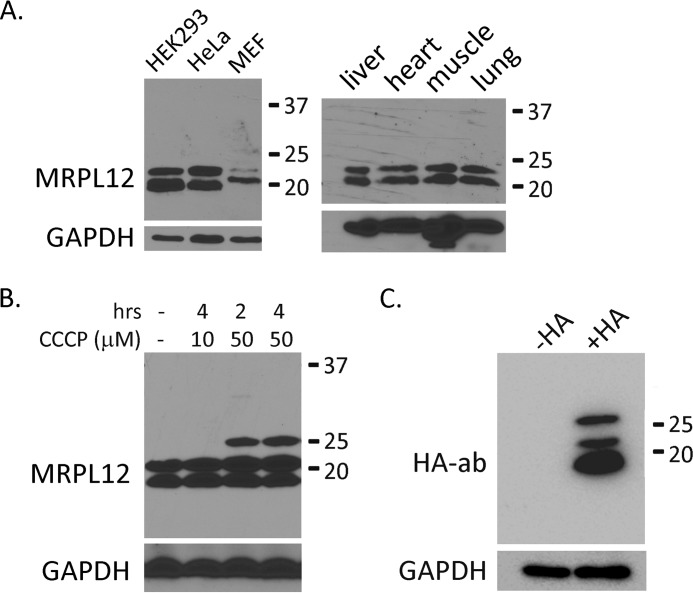
Two forms of MRPL12 have been observed by Western blot in previous studies (16, 21). However, because there is some variability within and between studies, it is not clear if these are conserved and reproducible. Thus, we first endeavored to determine whether two forms of the protein are detected in different mammalian cell lines and in mouse tissues. As shown in Fig. 1A, we can detect two forms of MRPL12 by Western blot in human HEK293 and HeLa cells, mouse embryonic fibroblasts, and in multiple mouse tissues.
FIGURE 1.
Two forms of MRPL12 in mammalian cells and tissues are not derived from alternative splicing or lack of cleavage of the N-terminal mitochondrial localization sequence. A, left panel: Western blot analysis of MRPL12 in whole cell lysates from human HEK293 and HeLa cells, and mouse embryonic fibroblasts (MEF). Right panel, Western blot analysis of MRPL12 in the indicated mouse tissues. B, Western blot analysis of HeLa cell lysates treated with the indicated amounts of CCCP (to inhibit protein import and reveal the precursor form of MRPL12) for the indicated number of hours (hrs). The minus sign indicates the vehicle-treated negative control. GAPDH was probed as the loading control in A and B. C, Western blot analysis of HEK293 cells before (−HA) or after (+HA) transfection of a vector expressing a full-length, HA-tagged MRPL12 cDNA. Numbers to the right of all of the gels in A–C indicate the location of molecular weight standards run in parallel.
One possibility is that the longer form of MRPL12 simply represents the lack of cleavage of the mitochondrial localization sequence (MLS). To test this, we treated cells with CCCP (to uncouple respiration and inhibit mitochondrial protein import and cleavage). Higher levels of CCCP resulted in the appearance of a third higher molecular weight band (Fig. 1B) that we interpret to be the precursor of MRPL12 with the MLS. Hence, under normal circumstances the longer form of MRPL12 is not due to lack of cleavage of the MLS.
Existing sequence databases do not predict any obvious alternative splice-site variants that would explain the two forms of MRPL12. Consistent with this, we observed the same two mature forms of the protein when we transfected HEK293 cells with an expression vector that encodes a full-length, HA-tagged cDNA (Fig. 1C). Here, we also observe the precursor with the MLS not cleaved (the slowest migrating band also observed in CCCP treated cells), which is a common artifact of overexpression of mitochondrial proteins.
The Two Forms of MRPL12 Are Generated by Differential Two-step Proteolytic Cleavage
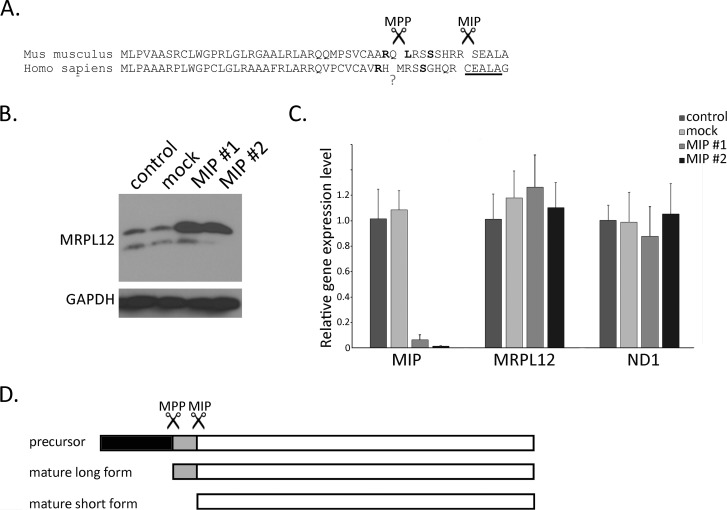
Many nucleus-encoded, mitochondria-targeted proteins are processed by proteolytic cleavage of the mitochondrial-targeting sequence during import by the mitochondrial processing protease (MPP) (24). A subset of these is subsequently cleaved by the mitochondrial intermediate protease (MIP). This two-step cleavage can be predicted to some degree by amino acid motifs, the most employed for 2-step processing is the R-10 motif: R-X↓(F/L/I)-X-X-(T/S/G)-X-X-X-X↓ (25, 26). This sequence motif can clearly be identified in the mouse MRPL12 precursor, but not in human (Fig. 2A). Nonetheless, we predict that human MRPL12 contains a degenerate or structural analog of this two-step cleavage motif. Furthermore, we hypothesized that the two forms of MRPL12 are generated by differential processing by MIP and MPP. To test this, we knocked down MIP by siRNA in HeLa cells. Using two different targets we observed efficient knock-down of MIP at the RNA level (Fig. 2C) and accumulation of the mature long form of MRPL12 protein (Fig. 2B), which is consistent with MRPL12 being subject to 2-step cleavage, with the short form of MRPL12 being generated from the long form by MIP cleavage (Fig. 2D). MIP knockdown did not result in decreased expression of MRPL12 RNA or the mtDNA-encoded ND1 RNA (Fig. 2C). Apparently, MIP knock-down did not affect MPP cleavage because the precursor with the MLS was not observed and the mature long form accumulated.
FIGURE 2.
Two forms of MRPL12 are generated in two steps, involving an efficient MPP cleavage and a second, incomplete cleavage by MIP. A, the amino-terminal sequence of the mouse (Mus musculus) and human (Homo sapiens) MRPL12 protein are shown. Scissors indicate the predicted cleavage sites by MPP and MIP proteases in the mouse protein with bold letters indicating the key predictive motif residues (see “Results” for details). The MPP cleavage site in the human protein based on mouse alignment and predictive algorithms, but is indicated by a question mark because this evidence is indirect. The MIP cleavage we propose is shown (between the R and C) is based on N-terminal sequencing of the short form of MRPL12. The underlined residues match the N-terminal sequence (XEALA) we obtained. B, Western blot analysis of MRPL12 and GAPDH (loading control) of cell lysates from HeLa cells untreated (control), mock transfected (mock), or transfected with one of two siRNAs directed against MIP (MIP #1 and #2). C, results of quantitative RT-PCR of MIP, MRPL12, and ND1 mRNAs (relative to GAPDH) in HeLa cells from B. D, schematic representation of the proposed forms of MRPL12. The precursor contains the mitochondrial localization signal (black) that is efficiently cleaved by MPP to generate the mature long form. A second cleavage by MIP generates the mature short form (gray region is removed). We propose this second MIP cleavage is inefficient or regulated, which allows the mature long and short forms to accumulate.
Next we sought direct evidence for the two-step cleavage model by attempting to sequence the N terminus of the mature long and short forms of MRPL12. We were able to immunopurify HA-tagged mature long and short forms of the protein and send them for Edman N-terminal sequencing. This analysis defined the N terminus of the mature short form as XEALA, which matches CEALA at the MIP cleavage site we predicted by sequence analysis (Fig. 2A). Unfortunately, we were not able to obtain the N-terminal sequence of the mature long form by this or mass spectrometry analysis, presumably because it was blocked/modified in some way. However, knowing the precise MIP cleavage site makes it likely that the predicted MPP cleavage site is close to where we propose (Fig. 2A), as it now precisely fulfills the R-10 rule and this site is predicted using two bioinformatics programs, MitoFates and Target P. The three MRPL12 protein forms (precursor, mature long, and mature short) are diagrammed in Fig. 2D and this nomenclature is used throughout the rest of this report.
The Stability of POLRMT Is Regulated by MRPL12
MRPL12binds POLRMT directly and activates mitochondrial transcription in vitro, and its knock-down results in decreased mtDNA transcripts in cultured cells (16, 17). However, the precise mechanisms involved in vivo are not clear. New insight was gained when we probed the steady-state protein levels of the primary mitochondrial transcription machinery after MRPL12 was knocked down by shRNA or siRNA. That is, we found that POLRMT was significantly reduced (Fig. 3, A and C), whereas the levels of TFAM and TFB2M were largely unaffected (Fig. 3A). Consistent with decreased POLRMT, there was a significant reduction in the rate of transcription of mtDNA-encoded RNAs (Fig. 3B) assayed using a 4-TU pulse labeling strategy (see “Experimental Procedures”). Knock-down of MRPL12 did not result in a reduction of POLMT mRNA (Fig. 3C), indicating down-regulation is occurring via a post-transcriptional mechanism. Knock-down of POLRMT does not drastically effect MRPL12 expression at the protein or mRNA level (Fig. 3C).
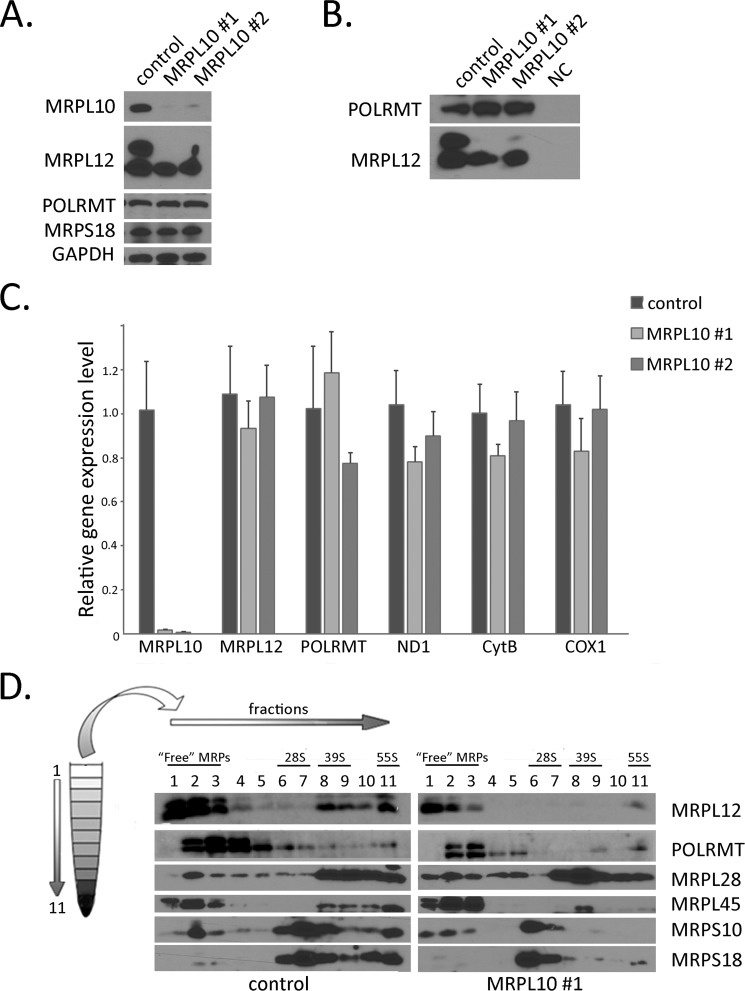
Perturbing Assembly of MRPL12 into Ribosomes Results in Loss of the Mature Long Form
In an attempt to determine whether the two forms of MRPL12 have distinct roles, we designed a strategy to disturb the assembly of MRPL12 into mitochondrial ribosomes by knocking down MRPL10, its binding partner in the large subunit. Remarkably, MRPL10 knockdown in HEK293 cells by siRNA resulted in specific loss of the mature large form of MRPL12 (Fig. 4A). This was not accompanied by a reduction of POLRMT or another mitochondrial ribosomal protein, MRPS18 (Fig. 4A). Immunoprecipitation of MRPL12 under these conditions resulted in co-immunoprecipitation of POLRMT, demonstrating that the mature short form of MRPL12 binds to POLRMT under these circumstances (Fig. 4B). The mRNA level of MRPL12 and mtDNA-encoded transcripts are not affected by MRPL10 knock-down (Fig. 4C). This indicates that loss of the mature large form of MRPL12 under these conditions is occurring by a post-transcriptional mechanism and is consistent with POLRMT being unaffected. Finally, as predicted, knock-down of MRPL10 did affect mitochondrial ribosome assembly as assessed by sucrose gradient analysis followed by probing for components of the small subunit (MRPS18 and MRPS10), which shifted significantly from fraction 7 to fraction 6, and large subunit (MRPL28, MRPL45 and MRPL48), which shifted from fractions 9, 10, and 11 to fractions 7 and 8 (Fig. 4D). However, this did not affect the migration of the mature short form of MRPL12 in the lower density fractions where POLRMT also resides (Fig. 4D).
FIGURE 4.
MRPL10 knockdown results in selective loss the mature long form of MRPL12. A, Western blot analysis of MRPL10, MRPL12, MRPS18, POLRMT, and GAPDH (loading control) of cell lysates from HEK293 cells untreated (control) or transfected with one of two siRNAs directed against MRPL10 (MRPL10 #1 and #2). B, co-immunoprecipitation of MRPL12 with POLRMT from HEK293 cells untreated (control) or in which MRPL10 is knocked down by two siRNAs (MRPL10 #1 and #2). NC indicates a negative control immunoprecipitation with no antibody. C, results of quantitative RT-PCR of MRPL10, MRPL12, POLRMT, ND1, cytochrome b (CytB), and COX1 mRNAs (relative to GAPDH) in HEK293 cells from A. Error bars represent the mean ± S.D. (n = 3). D, sucrose density gradient fractionation of untreated (control) and MRPL10 knock-down (MRPL10 #1) HEK293 cells followed by Western blot analysis of MRPL12, POLRMT, MRPL28, MRPL45, MRPS10, and MRPS18 in the indicated fractions. Samples taken from fractions 1 to 11 are of increasing density (i.e. top to bottom of the tube after separation by ultracentrifugation; as indicated by the diagram of the centrifuge tube to the left). These are loaded from left to right on the gels as indicated by the arrow on the top and lane numbering. Fractions where 28S, 39S and 55S ribosomes and non-ribosome-associated (“free”) MRPs migrate in these gradients are also indicated.
Discussion
This study provides the first concrete evidence that two forms of MRPL12 exist in mammalian mitochondria. Based on our results, we propose that mature “long” and “short” forms of MRPL12 are generated by differential proteolysis by the MPP and MIP proteases during import (Fig. 2D). That is, the initial precursor containing an N-terminal mitochondrial localization signal is cleaved first by MPP to generate the mature long form, followed by inefficient (or regulated) subsequent cleavage by MIP, which allows both forms to accumulate in the matrix. The strongest evidence for this is the accumulation of more of the mature long form when MIP is inhibited by siRNA (Fig. 2B) and our mapping of the MIP cleavage site (i.e. the N terminus of the mature short form) by Edman N-terminal sequencing (Fig. 2A). How the second MIP cleavage is prevented in a subset of molecules to allow accumulation of the long form under normal circumstances is unclear, but altered folding conformations of the protein near the cleavage site or post-translational modifications that inhibit cleavage are two possibilities. That MRPL12 might be acetylated by SIRT3 (27) is an interesting possibility to consider in this regard. Regardless of the mechanism, generation of alternative mature forms via differential, two-step processing may be a general mechanism to allow production of multiple forms of mitochondrial matrix proteins destined to perform different functions. This is especially worth considering given the large number of mitochondrial proteins subjected to cleavage upon import based on yeast proteomic studies (24). Incidentally, these studies show that both the putative yeast ortholog of MRPL12 (YGL068W) and MRPL10 are cleaved upon import and that the latter is a substrate of a yeast MIP (24).
Our results also indicate that the long and short forms of MRPL12 have distinct properties and/or functions. One striking result is the severe down-regulation of POLRMT when MRPL12 is knocked down (Fig. 3, A and C). This does not involve a down-regulation of POLRMT mRNA (Fig. 3C), thus it is occurring by a post-transcriptional mechanism. Using a 4-TU pulse labeling strategy, we found that mitochondrial transcription rates were significantly reduced in MRPL12 knock-down cells (Fig. 3B), consistent with reduced POLRMT observed and similar to the ∼50–60% reduction of steady-state transcripts we reported previously (16). Although it may seem surprising that such a severe reduction of POLRMT leads only to a 50–60% reduction in mtDNA transcription, this result is actually consistent with our previous studies showing that the mitochondrial transcription machinery is in excess of that needed to maintain normal transcriptional output (28). Furthermore, similar results were obtained by Bralha et al. (29) and Wolf and Mootha (30) who inhibited POLRMT directly, with the latter study providing some evidence for an RNA stabilization mechanism that is engaged when POLRMT levels are severely decreased. We are currently optimizing the 4-TU labeling method with even shorter incubation times (<30 min) to allow pulses of newly synthesized RNA to be measured. This should be generally useful for the field, in which inferences about mitochondrial transcriptional regulation in vivo have relied largely on measurements of steady-state RNA levels or in organello labeling strategies.
Although the instability of POLRMT was observed when both forms were knocked down with MRPL12-directed siRNA, knock-down of MRPL10 (the binding partner of MRPL12 in the mitochondrial ribosome) resulted in specific depletion of the long form of MRPL12 (Fig. 4A). Under these circumstances, POLRMT was unaffected, which suggests the mature short form of MRPL12 somehow stabilizes POLRMT in the absence of the large form. This is consistent with the results of the co-immunoprecipitation experiments that show the short form still binds to POLRMT under these circumstances (Fig. 4B) and with sucrose gradient fractionations that show the short form still migrates in lower density fractions containing POLRMT (Fig. 4D), which we showed previously contain transcription-related complexes (16). However, we observe both forms of MRPL12 in transcription complexes and ribosomes (16), so the precise relevance of their relative distribution in these higher-order assemblies remains to be determined, which could even involve mixed multimers of MRPL12.
It will be interesting to determine what destabilizes POLRMT in the absence of MRPL12. It is tempting to speculate that MRPL12 somehow protects POLRMT from protease-mediated degradation, perhaps by Lon, which conditionally degrades TFAM (31), another member of the primary transcription machinery. Similarly, disruption of ribosome biogenesis leads to the specific degradation of the mature long form of MRPL12, which could also involve Lon or some other regulated protease. Interestingly, cleavage of proteins by MIP often removes destabilizing amino acids that remain after MPP cleavage (32). Hence, lack of removal of N-terminal residues by MIP may make the mature long form of MRPL12 a better substrate for regulated proteolysis by other enzymes. That this intricate system of regulated proteolysis may be part of a larger homeostatic mechanism to coordinate import, transcription, and ribosome biogenesis to ensure proper mitochondrial OXPHOS function is intriguing to consider going forward. For example, perhaps the absence of fully processed MRPL12 (i.e. none of the short form) is a warning signal that MIP function is compromised and that mitochondrial transcription should be halted via POLRMT degradation to prevent further OXPHOS complex formation under these adverse conditions.
MRPL12 was the first human mitochondrial ribosomal subunit cloned based on its mRNA expression being positively regulated by growth factors (33). Similarly, Drosophila MRPL12 is required for cyclin-dependent growth signaling (34). Last, an in silico study identified MRPL12 as one of a small set of interacting genes that might be responsible for the longevity-promoting effects of caloric restriction (35). We speculate that the dynamic nature of the two forms of MRPL12 and the instability of POLRMT when MRPL12 levels are perturbed might contribute to these unique properties. Several other mitochondrial ribosomal proteins have been implicated in other cellular functions like cell cycle regulation and apoptosis (36). Based on the MRPL12 paradigm, it will be important to determine whether multiple forms of these are involved in mediating their diverse activities.
Author Contributions
J. N. designed and performed experiments, interpreted results, and wrote the manuscript; A. V. G. designed and performed experiments and wrote “Experimental Procedures”; M. B. designed and performed experiments and wrote “Experimental Procedures”; B. J. M. designed and performed experiments; Y. V. M. designed and performed experiments; G. S. S. designed experiments, interpreted results, and wrote the manuscript.
Acknowledgment
We thank Dr. Craig Cameron for providing human TFB2M antibody.
This work was supported, in whole or in part, by National Institutes of Health Grant AG047632 (to G. S. S.). The authors have no conflicts of interest to declare. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- OXPHOS
- oxidative phosphorylation
- MRP
- mitochondrial ribosomal protein
- 4-TU
- 4-thiouridine
- MLS
- mitochondrial localization sequence
- CCCP
- carbonyl cyanide p-chlorophenylhydrazone
- MPP
- mitochondrial processing protease
- MIP
- mitochondrial intermediate protease
- POLRMT
- mitochondrial RNA polymerase.
References
- 1.Smeitink J., van den Heuvel L., and DiMauro S. (2001) The genetics and pathology of oxidative phosphorylation. Nat. Rev. Genet. 2, 342–352 [DOI] [PubMed] [Google Scholar]
- 2.Sena L. A., and Chandel N. S. (2012) Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green D. R., Galluzzi L., and Kroemer G. (2014) Cell biology: metabolic control of cell death. Science 345, 1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkel T., Menazza S., Holmström K. M., Parks R. J., Liu J., Sun J., Liu J., Pan X., and Murphy E. (2015) The ins and outs of mitochondrial calcium. Circ. Res. 116, 1810–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West A. P., Brodsky I. E., Rahner C., Woo D. K., Erdjument-Bromage H., Tempst P., Walsh M. C., Choi Y., Shadel G. S., and Ghosh S. (2011) TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West A. P., Khoury-Hanold W., Staron M., Tal M. C., Pineda C. M., Lang S. M., Bestwick M., Duguay B. A., Raimundo N., MacDuff D. A., Kaech S. M., Smiley J. R., Means R. E., Iwasaki A., and Shadel G. S. (2015) Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace D. C. (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39, 359–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunnari J., and Suomalainen A. (2012) Mitochondria: in sickness and in health. Cell 148, 1145–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balaban R. S., Nemoto S., and Finkel T. (2005) Mitochondria, oxidants, and aging. Cell 120, 483–495 [DOI] [PubMed] [Google Scholar]
- 10.Bestwick M. L., and Shadel G. S. (2013) Accessorizing the human mitochondrial transcription machinery. Trends Biochem. Sci. 38, 283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shadel G. S., and Clayton D. A. (1997) Mitochondrial DNA maintenance in vertebrates. Annu. Rev. Biochem. 66, 409–435 [DOI] [PubMed] [Google Scholar]
- 12.Calvo S. E., and Mootha V. K. (2010) The mitochondrial proteome and human disease. Annu. Rev. Genomics Hum. Genet. 11, 25–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonawitz N. D., Clayton D. A., and Shadel G. S. (2006) Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol. Cell 24, 813–825 [DOI] [PubMed] [Google Scholar]
- 14.Rebelo A. P., Dillon L. M., and Moraes C. T. (2011) Mitochondrial DNA transcription regulation and nucleoid organization. J. Inherit. Metab. Dis. 34, 941–951 [DOI] [PubMed] [Google Scholar]
- 15.O'Brien T. W. (2003) Properties of human mitochondrial ribosomes. IUBMB Life 55, 505–513 [DOI] [PubMed] [Google Scholar]
- 16.Surovtseva Y. V., Shutt T. E., Cotney J., Cimen H., Chen S. Y., Koc E. C., and Shadel G. S. (2011) Mitochondrial ribosomal protein L12 selectively associates with human mitochondrial RNA polymerase to activate transcription. Proc. Natl. Acad. Sci. U.S.A. 108, 17921–17926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z., Cotney J., and Shadel G. S. (2007) Human mitochondrial ribosomal protein MRPL12 interacts directly with mitochondrial RNA polymerase to modulate mitochondrial gene expression. J. Biol. Chem. 282, 12610–12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surovtseva Y. V., and Shadel G. S. (2013) Transcription-independent role for human mitochondrial RNA polymerase in mitochondrial ribosome biogenesis. Nucleic Acids Res. 41, 2479–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bocharov E. V., Gudkov A. T., Budovskaya E. V., and Arseniev A. S. (1998) Conformational independence of N- and C-domains in ribosomal protein L7/L12 and in the complex with protein L10. FEBS Lett. 423, 347–350 [DOI] [PubMed] [Google Scholar]
- 20.Han M. J., Cimen H., Miller-Lee J. L., Koc H., and Koc E. C. (2011) Purification of human mitochondrial ribosomal L7/L12 stalk proteins and reconstitution of functional hybrid ribosomes in Escherichia coli. Protein Expr. Purif. 78, 48–54 [DOI] [PubMed] [Google Scholar]
- 21.Serre V., Rozanska A., Beinat M., Chretien D., Boddaert N., Munnich A., Rötig A., and Chrzanowska-Lightowlers Z. M. (2013) Mutations in mitochondrial ribosomal protein MRPL12 leads to growth retardation, neurological deterioration and mitochondrial translation deficiency. Biochim. Biophys. Acta 1832, 1304–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleary M. D., Meiering C. D., Jan E., Guymon R., and Boothroyd J. C. (2005) Biosynthetic labeling of RNA with uracil phosphoribosyltransferase allows cell-specific microarray analysis of mRNA synthesis and decay. Nat. Biotechnol. 23, 232–237 [DOI] [PubMed] [Google Scholar]
- 23.Cleary M. D. (2008) Cell type-specific analysis of mRNA synthesis and decay in vivo with uracil phosphoribosyltransferase and 4-thiouracil. Methods Enzymol. 448, 379–406 [DOI] [PubMed] [Google Scholar]
- 24.Vögtle F. N., Wortelkamp S., Zahedi R. P., Becker D., Leidhold C., Gevaert K., Kellermann J., Voos W., Sickmann A., Pfanner N., and Meisinger C. (2009) Global analysis of the mitochondrial N-proteome identifies a processing peptidase critical for protein stability. Cell 139, 428–439 [DOI] [PubMed] [Google Scholar]
- 25.Hendrick J. P., Hodges P. E., and Rosenberg L. E. (1989) Survey of amino-terminal proteolytic cleavage sites in mitochondrial precursor proteins: leader peptides cleaved by two matrix proteases share a three-amino acid motif. Proc. Natl. Acad. Sci. U.S.A. 86, 4056–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavel Y., and von Heijne G. (1990) Cleavage-site motifs in mitochondrial targeting peptides. Protein Eng. 4, 33–37 [DOI] [PubMed] [Google Scholar]
- 27.Hebert A. S., Dittenhafer-Reed K. E., Yu W., Bailey D. J., Selen E. S., Boersma M. D., Carson J. J., Tonelli M., Balloon A. J., Higbee A. J., Westphall M. S., Pagliarini D. J., Prolla T. A., Assadi-Porter F., Roy S., Denu J. M., and Coon J. J. (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 49, 186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seidel-Rogol B. L., and Shadel G. S. (2002) Modulation of mitochondrial transcription in response to mtDNA depletion and repletion in HeLa cells. Nucleic Acids Res. 30, 1929–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bralha F. N., Liyanage S. U., Hurren R., Wang X., Son M. H., Fung T. A., Chingcuanco F. B., Tung A. Y., Andreazza A. C., Psarianos P., Schimmer A. D., Salmena L., and Laposa R. R. (2015) Targeting mitochondrial RNA polymerase in acute myeloid leukemia. Oncotarget 6, 37216–37228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf A. R., and Mootha V. K. (2014) Functional genomic analysis of human mitochondrial RNA processing. Cell Rep. 7, 918–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu B., Lee J., Nie X., Li M., Morozov Y. I., Venkatesh S., Bogenhagen D. F., Temiakov D., and Suzuki C. K. (2013) Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Mol. Cell 49, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vögtle F. N., Prinz C., Kellermann J., Lottspeich F., Pfanner N., and Meisinger C. (2011) Mitochondrial protein turnover: role of the precursor intermediate peptidase Oct1 in protein stabilization. Mol. Biol. Cell 22, 2135–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marty L., and Fort P. (1996) A delayed-early response nuclear gene encoding MRPL12, the mitochondrial homologue to the bacterial translational regulator L7/L12 protein. J. Biol. Chem. 271, 11468–11476 [DOI] [PubMed] [Google Scholar]
- 34.Frei C., Galloni M., Hafen E., and Edgar B. A. (2005) The Drosophila mitochondrial ribosomal protein mRpL12 is required for cyclin D/Cdk4-driven growth. EMBO J. 24, 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goertzel B., Pennachin C., de Alvarenga Mudado M., and de Souza Coelho L. (2008) Identifying the genes and genetic interrelationships underlying the impact of calorie restriction on maximum lifespan: an artificial intelligence-based approach. Rejuvenation Res. 11, 735–748 [DOI] [PubMed] [Google Scholar]
- 36.Shutt T. E., and Shadel G. S. (2007) Expanding the mitochondrial interactome. Genome Biol. 8, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]