Abstract
Hepatic fibrosis is a wound healing response to insults and as such affects the entire world population. In industrialized countries, the main causes of liver fibrosis include alcohol abuse, chronic hepatitis virus infection and non-alcoholic steatohepatitis. A central event in liver fibrosis is the activation of hepatic stellate cells, which is triggered by a plethora of signaling pathways. Liver fibrosis can progress into more severe stages, known as cirrhosis, when liver acini are substituted by nodules, and further to hepatocellular carcinoma. Considerable efforts are currently devoted to liver fibrosis research, not only with the goal of further elucidating the molecular mechanisms that drive this disease, but equally in view of establishing effective diagnostic and therapeutic strategies. The present paper provides a state-of-the-art overview of in vivo and in vitro models used in the field of experimental liver fibrosis research.
Keywords: liver fibrosis, animal models, in vitro models, hepatic stellate cells
1. Introduction
Liver fibrosis basically is a wound healing response to various types of injury, which can progress into liver cirrhosis and even to hepatocellular carcinoma (HCC). The most common causes of liver fibrosis in industrialized countries are alcohol abuse, viral hepatitis B (HBV) and C (HCV) infections, and metabolic syndromes due to obesity, insulin resistance and diabetes (Blachier et al 2013). In non-industrialized countries, parasitic infections, such as Schistosoma species, are also included in liver injury cases (Stensgaard et al 2013). In the European Union, 0.1% of the population is affected by cirrhosis, the most advanced stage of liver fibrosis with full architectural disturbances, leading to 170.000 deaths each year (Blachier et al 2013). According to the World Health Organization, HCC currently is the fifth most common cause of cancer, resulting in 47.000 deaths each year in Europe (Blachier et al. 2013). Besides the epidemiological relevance, liver fibrosis and hence cirrhosis also impose a considerable economic burden on society. Indeed, when conventional treatment fails, the only curative therapy for decompensated cirrhosis is liver transplantation (Pedersen et al 2015). More than 5.500 orthotopic liver transplantations are currently performed in Europe on a yearly basis, costing up to €100.000 the first year and €10.000 yearly thereafter (van Agthoven et al. 2001). Thus, it is clear that there is an urgent need for new therapies for the treatment of liver disease, in casu fibrosis (Kisseleva and Brenner 2011) as well as for novel strategies allowing early diagnosis of this disease (Karsdal et al. 2014; Sharma et al. 2014). This has been, and still is, a major driver for many fundamental and translational researchers in the hepatology field to devote their work to liver fibrosis. As a result, a variety of in vitro and in vivo models are nowadays used in this area. The purpose of the present paper is to provide an overview of these experimental settings.
2. Pathogenesis of liver fibrosis
2.1. General overview
The process following liver injury involves an acute and a chronic response (Bataller and Brenner 2005). When acute liver injury is not severe, neighboring adult hepatocytes are able to regenerate and to replace apoptotic and necrotic cells (Bataller and Brenner 2005). If the insult persists, the regenerative process fails and hepatocytes become substituted by extracellular matrix (ECM) proteins, accompanied by inflammation (Fig. 1). Furthermore, during chronic disease, the composition of the ECM changes from collagens type IV and VI, glycoproteins and proteoglycans into collagens type I and III and fibronectin (Brown et al. 2006; Hahn et al. 1980; Rojkind et al. 1979). In healthy liver, quiescent hepatic stellate cells (HSCs), residing in the space of Disse, serve as storehouses of vitamin A in the form of retinol esters and express glial fibrillary acidic protein (GFAP) (Geerts 2001; Niki et al. 1996). A key event in liver fibrosis includes the activation of HSCs, whereby these cells adopt a myofibroblast-like phenotype. Activated HSCs are proliferating and contractile, and are characterized by the loss of vitamin A storage and GFAP expression (Neubauer et al. 1996; Niki et al. 1996), high production of alpha smooth muscle actin (αSMA) (Ramadori et al. 1990; Schmitt-Gräff et al. 1991), secretion of collagens type I and III (Maher and McGuire 1990), and expression of matrix metalloproteinases (MMPs) and their specific tissue inhibitors (TIMPs) (Benyon and Arthur 2001). The activation of HSCs involves a complex process that consists of 2 major phases, namely initiation and perpetuation, followed by resolution of fibrosis if the injury subsides (Fig. 2) (Friedman 2008). The initiation stimuli involve the generation of apoptotic bodies, reactive oxygen species (ROS) and paracrine activation in conjunction with the release of lipopolysaccharide from the gut after liver injury (Lee and Friedman 2011). These stimuli sensitize cells and if persistent, HSCs maintain the activated phenotype, promoting ECM accumulation and chronic inflammation. In this scenario, other ECM-producing cells contribute to scar formation in the liver, including portal fibroblasts (Lemoinne et al. 2013), myofibroblasts derived from bone marrow (Kisseleva et al. 2006) and epithelial cells that undergo epithelial-to-mesenchymal transition (Zeisberg et al. 2007). Regarding the latter, some in vitro evidence has highlighted the possibility that in the presence of transforming growth factor (TGF)β, oval cells can enter epithelial-to-mesenchymal transition to enhance the expression of HSC markers (Wang et al. 2009). Nevertheless, this mechanism is surrounded by quite some controversy, as it has been shown that hepatocytes and cholangiocytes do not follow this process during liver fibrosis (Chu et al. 2011; Taura et al. 2010). In contrast, the resolution of fibrosis refers to pathways involved in HSC apoptosis or reversion into a more quiescent phenotype (Gaça et al. 2003; Iredale et al. 1998; Issa et al. 2001; Kisseleva et al. 2012). In parallel, the recruitment of inflammatory cells plays a crucial role in the initiation and persistence stages as well as in the resolution phase. The presence of macrophages leads to the development of the fibrotic response in the liver (Ide et al. 2005), while the enhanced production of cytokines, such as interleukin (IL)-13, has been proven to induce fibrosis in a Schistosoma mansoni model (Chiaramonte et al. 2001). Alternatively, macrophages may regulate the reversibility of the disease by ECM degradation, production of tumor necrosis factor (TNF)α-related apoptosis-inducing ligand, phagocytosis of the apoptotic myofibroblasts and recruitment of other inflammatory cells (Pellicoro et al. 2014).
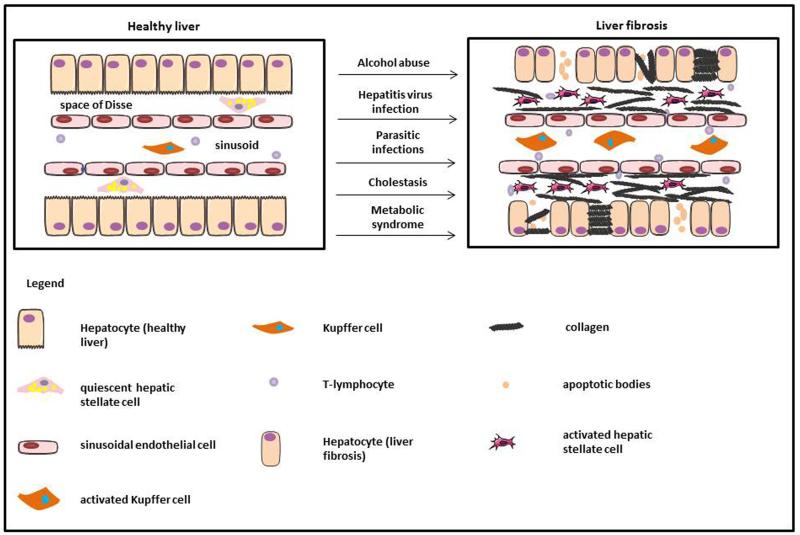
Fig. 1. Pathogenesis of liver fibrosis.
In healthy liver, hepatocytes are studded with microvilli, HSCs store retinol and sinusoidal endothelial cells display fenestrae. During liver injury by a variety of causes, hepatocytes lose microvilli and may undergo apoptosis. The sinusoidal endothelial cells become devoid of fenestrae allowing inflammatory lymphocytes to infiltrate in the hepatic parenchyma. Furthermore Kupffer cells are activated, which in turn trigger HSC activation. As a result, large amounts of ECM proteins, including fibrillar collagens, are deposited in the Space of Disse.
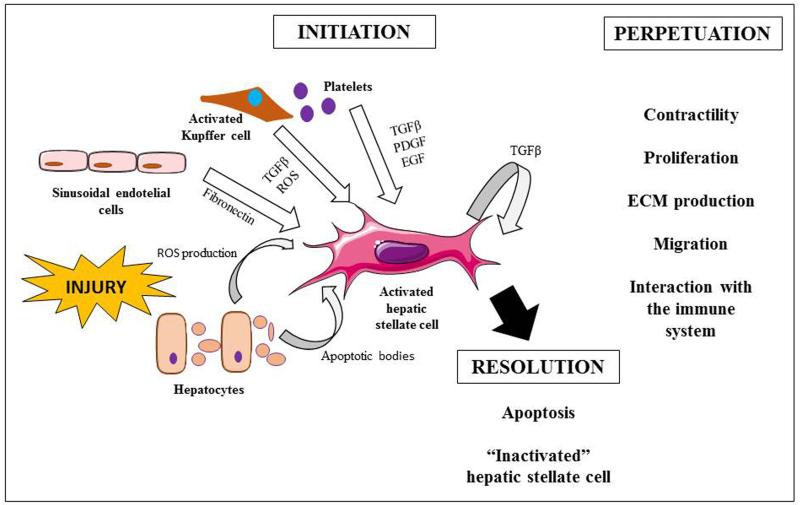
Fig. 2. Process of hepatic stellate cell activation.
Upon insult, the stimuli that involve hepatic stellate cell (HSC) activation come from the injured hepatocytes, sinusoidal cells, Kupffer cells and platelets. Due to this interaction, HSCs are able to produce transforming growth factor β. The perpetuation of the injury leads to more active cells with the ability to contract, proliferate, produce extracellular matrix proteins, migrate and interact with the immune system. This triggers inflammatory and fibrogenic responses and decreases blood supply. Withdrawal of the injury may lead to the resolution of the disease by apoptosis of the activated HSCs and the reversion of the active into an inactivated HSC phenotype. (ECM, extracellular matrix; EGF, epithelial growth factor; PDGF, platelet derived growth factor; ROS, reactive oxygen species; TGFβ, transforming growth factor β)
2.2. Initiation of hepatic stellate cell activation
Stimuli triggering HSC activation originating from injured hepatocytes, sinusoidal endothelial cells, Kupffer cells and platelets lead to a morphological changes in HSC shape, loss of vitamin A and the expression of cell surface receptors for growth factors and cytokines. Hepatocytes are the main source of lipid peroxides and apoptotic bodies in injured liver, thus stimulating the expression of collagen I (Bedossa et al. 1994) and increase in ROS production (MacDonald et al. 2001), in turn inducing collagen synthesis and chemotaxis in a dose-dependent manner (Novo et al. 2006). ROS generation by cytochrome P450 2E1 (CYP2E1) in hepatocytes can also induce collagen synthesis and proliferation of HSCs (Nieto et al. 2002a, 2002b), which is typically seen in alcoholic liver disease (ALD) (Niemelä et al. 2000). Hepatocellular apoptosis after injury may also contribute to liver inflammation and fibrosis (Canbay et al. 2002; Ogasawara et al. 1993). The engulfment of apoptotic bodies by HSCs induces intracellular signaling cascades that promote the expression of collagen type I secretion, monocyte chemo-attractant protein-1 and TGFβ (Lee et al. 2011). The latter is considered as the main fibrogenic molecule involved in the induction of collagen I by HSCs (Bissell et al. 2001; Breitkopf et al. 2006). Nevertheless, TGFβ can act synergistically with platelet derived growth factor (PDGF) to promote collagen I expression (Yoshida and Matsuzaki 2012) and the migration of HSCs to the site of injury (Yoshida et al. 2005). Early injury promotes the secretion of fibronectin by sinusoidal endothelial cells, which has an activating effect on HSCs (Jarnagin et al. 1994). In addition, the activation of Kupffer cells facilitates HSC activation by secretion of TGFβ and ROS in the extracellular environment (Kolios et al. 2006). This paracrine activation induced by platelets is mediated by PDGF, TGFβ and epidermal growth factor (Bachem et al. 1989). These autocrine and paracrine signals contribute to transient HSC activation that corresponds with an initial inflammatory reaction and collagen deposition in the liver.
2.3. Perpetuation of hepatic stellate cell activation
In this second step, HSCs acquire a more myofibroblastic phenotype and become more proliferative and contractile, leading to enhanced production of ECM proteins, angiogenesis regulation and the amplification of the immune response. The proliferative stage that accompanies activation of HSCs is governed by PDGF, which signaling underlies the activation of the Ras/mitogen activated protein kinase and the phosphatidylinositol 3 kinase/Akt pathways involved in HSC growth and chemotaxis (Chen et al. 2008; Marra et al. 1997). This has been observed in patients with non-alcoholic fatty liver disease (NAFLD) in conjunction with collagen I production (Svegliati-Baroni et al. 1999). There is some evidence that PDGF may act in concert with TGFβ to activate HSCs during liver fibrosis (Yoshida et al. 2005). Other mitogens that can modulate HSC proliferation via paracrine signaling are TGFα, epidermal growth factor (Lee et al. 1995; Svegliati-Baroni et al. 2005) and the HBV proteins c and x (Bai et al. 2012). In parallel to this proliferative stage, the acquisition of contractility is a determinant in intrahepatic vascular resistance during liver fibrosis (Rockey 1997). This contraction capacity leads to modulation of the blood flow via sinusoidal constriction. Activated HSCs express receptors from a variety of vasoconstrictor substances, especially endothelin-1 (Rockey and Weisiger 1996; Shibamoto et al. 2008), which may induce cell contraction through calcium-dependent and calcium-sensitizing mechanisms (Iizuka et al. 2011). Additionally, the contractibility can also be regulated by nitric oxide synthase, which is involved in the relaxation of HSCs and that can be inhibited by TGFβ (Rockey and Chung 1995). TGFβ is a key molecule during the progression of chronic liver disease, as it is the most potent stimulus for the production of collagen I (Breitkopf et al. 2006) and other ECM components, including fibronectin (Date et al. 2000) and proteoglycans (Krull et al. 1993). Moreover, in cases of chronic HCV infection, TGFβ expression levels can be modulated by the presence of the HCV core protein, which triggers HSC activation (Wu et al. 2013). The maintenance of these ECM proteins in the fibrotic liver is due to the interplay between MMPs and TIMPs secreted by activated HSCs, resulting in the deterioration of the healthy ECM and concomitant fibrous scar formation (Benyon and Arthur 2001). In chronic disease, activated HSCs play a role in inflammatory and immune-mediated responses, which can enhance hepatocellular necrosis and apoptosis, and perpetuate the stimuli of fibrogenesis (Czaja 2014; Friedman 2008). In this context, activated HSCs are characterized by the production of chemokines, the expression of adhesion molecules and the presentation of antigens to T-lymphocytes and natural killer cells. Chemokines promote the migration of activated HSCs to the site of injury, thereby boosting the inflammatory response (Seki et al. 2009). Other chemokines, such as vascular endothelial growth factor, PDGF, monocyte chemo-attractant protein-1 and chemokine C-X-C receptor 3, are also involved in cell chemotaxis. On the other hand, degradation of the basement membrane-like matrix through MMPs and the interaction mediated by α1β1 integrin may assist in cell migration within the space of Disse during liver injury (Yang et al. 2003). In contrast, activated HSCs secrete pro-inflammatory cytokines that behave as chemo-attractants in the recruitment of inflammatory cells (Kharbanda et al. 2001; Marra et al. 1998). This production of pro-inflammatory cytokines is promoted by ethanol consumption (Kharbanda et al. 2001) and by the presence of lipopolysaccharide secreted by gut bacteria upon binding to Toll-like receptor 4 (Paik et al. 2003). The gathering of immune cells at the site of injury together with the interaction of activated HSCs with T-lymphocytes via antigen-presenting receptors and co-stimulatory proteins may result in the modulation of lymphocyte proliferation (Viñas et al. 2003), which triggers the perpetuation of the immune response. The chronicity of the injury allows full transdifferentiation of HSCs into myofibroblastic cells, which interact with a number of factors and cells to enhance scar formation, the reduction of liver blood flow and the amplification of the immune response.
2.4. Resolution of liver fibrosis
The resolution of liver fibrosis and cirrhosis observed in animals and humans has been well studied (Iredale et al. 1998; Marcellin et al. 2013). This process may be explained by the HSC reversion into a quiescent stage and/or apoptosis. The reversibility of activated HSCs after eradication of hepatic injury has been assessed in vitro (Gaça, et al. 2003) and in vivo (Kisseleva et al. 2012; Troeger et al. 2012). Nevertheless, full recovery is not achieved and the cells remain in a stage that predisposes them to rapidly reactivate into myofibroblasts in the presence of a deteriorative stimulus with facilitated development of a more severe stage of fibrosis (Kisseleva et al. 2012; Troeger et al. 2012). A body of evidence supports the role of HSC apoptosis in the regression of fibrosis (Iredale et al. 1998; Issa et al. 2001). Signals mediating HSC apoptosis include Fas ligand (Saile et al. 1997) and TNFα-related apoptosis-inducing ligand (Taimr et al. 2003). The latter can be released from Kupffer cells (Tang et al. 2009) and natural killer cells (Radaeva et al. 2006), yet the signaling pathway inducing HSC apoptosis remains largely unknown. Recent studies suggest the importance of endoplasmic reticulum stress in this process because of the relationship between calpain/caspase activation and c-Jun N-terminal kinases/p38 mitogen-activated protein kinase phosphorylation (Huang et al. 2014) and by the downregulation of heat shock protein 47 (Kawasaki et al. 2014). On the other hand, Kupffer cells and activated natural killer cells can also cause HSC apoptosis. The former may involve caspase 9-dependent and receptor-interacting protein-dependent mechanisms (Fischer et al. 2002), while the latter is related to the natural killer group 2D receptor pathway (Radaeva et al. 2006).
3. In vivo models of liver fibrosis
3.1. Chemical-based models
A number of chemicals are known to induce liver fibrosis and hence are commonly used to set up experimental animal models to study this particular pattern of lesions. In most cases, intraperitoneal injection of these chemicals triggers liver fibrosis on a relatively short-term basis (Smith 2013). When administered orally or via inhalation, fibrosis is limited and takes more time to develop (Smith 2013). These chemical-based animal models are popular because of their high reproducibility, ease of use and appropriate reflection of the mechanisms involved in human liver fibrosis (Smith 2013) (Table 1).
Table 1. In vivo models of liver fibrosis.
(ALD, alcoholic liver disease; aHSCs, activated hepatic stellate cells; CYP2E1, cytochrome 2E1; HCC, hepatocellular carcinoma; I.p. intraperitoneal; NASH, non-alcoholic steatohepatitis; ROS, reactive oxygen species; S.c., subcutaneous; TNF-α, tumor necrosis factor alpha).
| Model | Mechanistic basis | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Ethanol | CYP450-mediated biotransformation to reactive metabolites Enhanced immune response Increased collagen synthesis |
- | Lack of ability to develop ALD due to alcohol consumption | (Beier and McClain 2010; Best and Hartroft 1949; DeCarli and Lieber 1967; French 2001; Keegan et al 1995; Leo and Lieber 1983; Lieber 1997; Tsukamoto et al 1984) |
| Carbon tetrachloride | CYP2E1-mediated biotransformation to reactive metabolites | High reproducibility Close to human liver fibrosis |
Intraperitoneal administration can induce chronic peritonitis Subcutaneous administration can induce necrosis at the site of injection |
(Basu 2003; Weber et al 2003) |
| Thiocetamide | CYP450-mediated biotransformation to reactive metabolites Immunological response |
Can be used to confirm results obtained from other models | Long time to develop Slow reversibility |
(Low et al 2004) |
| Dimethylnitrosamine and diethylnitrosamine | CYP2E1-mediated biotransformation to reactive metabolites | Good model to study HCC | Not ideal for the study of liver fibrosis | (Aparicio-Bautista et al 2013; Jin et al 2010; Sánchez-Pérez et al 2005; Verna et al 1996; Yoshida, et al 2004) |
| Methionine choline-deficient diet | Lipotoxicity Kupffer cells activation and monocytes recruitment HSC activation Hepatocyte apoptosis and release of danger signals |
Close to human NASH | Lack of obesity and peripheral insulin resistance | (Jha et al 2014; Rinella and Green 2004; Tosello-Trampont et al 2012) |
| High fat diet | Unknown | Obesity and peripheral insulin resistance | Long time to develop mild fibrosis in mouse Rats do not develop fibrosis Not close to human NASH |
(Ito et al 2007) |
| High cholesterol diet | Unknown | Induces NASH and in some cases cirrhosis. | Lack of obesity and peripheral insulin resistance | (Ichimura et al 2014) |
| Choline deficient L-amino acid defined diet | Unknown | Mimics the human main characteristics, namely obesity and peripheral insulin resistance | Development of HCC can hinder the study of liver fibrosis | (De Minicis et al 2014; Denda et al 2002; Nakae et al 1992) |
| Common bile duct ligation | Increased biliary pressure Infiltration of inflammatory cells ROS generation Portal fibroblast activation |
Reversibility after relief of the obstruction Close to human cholestatic injury |
High mortality rate Variability between animals |
(Abdel-Aziz et al 1990; Aronson et al 1993; Chang et al 2005; Georgiev et al 2008) |
| Multidrug resistance-associated protein 2-deficient mice | Lack of phospholipid secretion into the bile Hepatocyte necrosis HSCs activation Canalicular and small bile ductular destruction Inflammatory cells infiltration |
Similar to human chronic biliary disease | Long time to develop | (Fickert et al 2002; Morita and Terada, 2014; Popov, et al 2005) |
| Alms1 Fat ausi mutant mice | Lipotoxicity Inflammatory cells infiltration Ballooned hepatocytes HSC activation |
Close to human NASH | No reversibility Long time to develop |
(Arsov et al 2006; Larter et al 2013) |
| Hepatitis virus models | Immune response | Similar to human viral infections | Variability in the response to the infection between animals | (Cheever et al 2002; Sitia et al 2012) |
| Schistosoma spp. | Cytokines production | Similar to human parasitic infections | Variability in fibrosis development | (Cheever et al 2002; Zhang et al 2015) |
3.1.1. Ethanol
Alcohol consumption is a worldwide cause of chronic liver disease. ALD usually starts with hepatic steatosis that may progress into fibrosis and subsequent cirrhosis. In the liver, ethanol is mainly metabolized by alcohol dehydrogenases and CYP450 enzymes. This process is associated with several deleterious events, such as the production of ROS, glutathione depletion, lipid peroxidation and increased collagen synthesis (Beier and McClain 2010; Lieber 1997). Collectively, these mechanisms induce hepatocyte apoptosis, inflammation and the activation of HSCs. Although rodents have a natural aversion for alcohol consumption, with the exception of HAP-2 (Lopez et al. 2011) and C57BL/6 (Metten and Crabbe 2005) mice, they remain the most routinely used model in the study of ALD. Mice are more prone to alcohol-induced ALD than rats (Shinohara et al. 2010), with female mice being most susceptible (Melón et al. 2013). There is, however, not a single rodent model that fully mirrors human ALD by alcohol consumption. The Lieber-DeCarli full liquid diet (DeCarli and Lieber 1967; Leo and Lieber 1983), alcohol administration in drinking-water (Best and Hartroft 1949; Keegan et al. 1995) and Tsukamoto-French intragastric feeding model (French 2001; Tsukamoto et al. 1984) failed to develop liver fibrotic stages. In order to overcome these limitations, new techniques have been introduced, such as the combination of ethanol administration with a second stimulus, including specific diets, pharmacological agents, CYP450 inducers, hormones, Toll-like receptor ligands, genetic manipulation or viral infection (Brandon-Warner et al. 2012; Enomoto et al. 1998). However, these combinational models are driven by a plethora of mechanisms that can complicate the interpretation of results.
3.1.2. Carbon tetrachloride
Carbon tetrachloride (CCl4) is the most widely used hepatotoxin in the study of liver fibrosis and cirrhosis in rodents. In many aspects, it mimics human chronic disease associated with toxic damage. Hepatic biotransformation of CCl4 relies on CYP2E1 and yields the trichloromethyl radical, which is involved in several free radical reactions and lipid peroxidation processes (Basu 2003; Weber et al. 2003) that contribute to an acute phase reaction characterized by necrosis of centrilobular hepatocytes, the activation of Kupffer cells and the induction of an inflammatory response (Heindryckx et al. 2009). This sequence is associated with the production of several cytokines, which promote activation of HSCs and hence liver fibrosis (Iwaisako et al. 2014). The CCl4 model can be applied to both rats and mice. However, mice are preferred, because of a higher metabolic rate of CCl4 compared to rats (Thrall et al. 2000). The susceptibility of mice to CCl4-induced liver fibrosis is strain-dependent. Thus, BALB/c mice manifest more liver fibrosis upon CCl4 administration compared to C57BL/6 and DBA/2 counterparts (Shi et al. 1997; Walkin et al. 2013). In the most routinely followed strategy, CCl4 is injected intraperitoneally 2 to 3 times per week during 4 to 6 weeks at a dose range of 300 to 1000 μl/kg (Constandinou et al. 2005). Recently, a C57BL/6 mouse model was standardized relying on intraperitoneal administration of CCl4 in a concentration range between 0.5 to 0.7 μl/g body weight 2 times per week for 6 weeks or 3 times per week for 4 weeks. Alternatively, CCl4 can be administered orally, subcutaneously or through inhalation 2 times per week 10 weeks, between 4 and 8 weeks or between 2 and 6 weeks, respectively. There is a lot of discussion about oral administration of CCl4, as some authors claim to show the highest reproducibility of liver fibrosis with acceptable animal survival rates (Jang et al. 2008), while others do not recommend the oral administration unless it is strongly required due to high rates of early mortality (Scholten et al. 2015). Subcutaneous injection represents a decrease in mouse mortality. However, animals grow granulomas at the site of injection (Domenicali et al. 2009; Geerts et al. 2008). Although administration through inhalation carries a number of disadvantages, including the necessity of appropriate equipment and operator training (Tsujimura et al. 2008), it was described as the best model to study complications of cirrhosis, such as portal hypertension and ascites formation (Domenicali et al. 2009; Liedtke et al. 2013).
3.1.3. Thioacetamide
Like CCl4, thioacetamide requires metabolic activation to become toxic. This bioactivation process, which is catalyzed by CYP450 isoenzymes, results in the formation of thioacetamide sulphur dioxide, responsible for the overall toxicity. The mechanisms underlying the induction of liver fibrosis through thioacetamide sulphur dioxide are not fully understood, but may imply downregulation of enzymes involved in fatty acid β-oxidation, branched chain amino acids and methionine breakdown, and upregulation of proteins related to lipid peroxidation and oxidative stress (Low et al. 2004). Anyhow, the final outcome includes severe oxidative damage associated with HSC activation. Rats are the first-rank species for establishing thioacetamide-mediated liver fibrosis models, yet it is also frequently applied to mice. Typically, thioacetamide is administered intraperitoneally in doses between 100 and 200 mg/kg body weight 3 times per week for a period of 6 to 8 weeks. These animals show an enlarged liver with centrilobular necrosis and mild inflammatory cell infiltration along with elevated alanine aminotransferase and aspartate aminotransferase serum levels (Chen et al. 2012). More recently, this model has been standardized at a dose of 150 mg/kg 3 times per week for a period between 8 and 12 weeks (Wallace et al. 2015). When administered orally, higher doses of 200 to 300 mg/kg body weight are used for 16 weeks (Salguero Palacios et al. 2008). Moreover, C57BL/6 mice require 2 to 4 months to develop significant fibrosis when orally administered 300 mg/l in drinking water (Wallace et al. 2015).
3.1.4. Dimethylnitrosamine and diethylnitrosamine
Dimethylnitrosamine (DMN) and diethylnitrosamine (DEN) are carcinogenic compounds that are frequently used to experimentally induce liver fibrosis in animals. As a consequence of their biotransformation, ROS are abundantly produced, all which react with nucleic acids (Verna et al. 1996), proteins (Aparicio-Bautista et al. 2013) and lipids (Sánchez-Pérez et al. 2005), causing cell malfunction and triggering the development of centrilobular necrosis (Oh et al. 2009). The susceptibility of mice to develop HCC due to DEN administration is determined, at least in part, by the strain. In this respect, C3H and B6C3F1 mice are most likely to develop tumors compared to C57BL mice (Buchmann et al. 1991). In rats, the R16 strain is most susceptible to carcinogenic chemicals (Melhem et al. 1989). DEN is routinely administered orally to mice at a dose of 100 μl/kg body weight for 12 weeks (Starkel and Leclercq 2011). DEN is administered to rats with weekly oral gavage of 5 ml of 1.5 %/kg DEN during 3 to 11 weeks (Jin et al. 2010) or intraperitoneally once per week for 2 weeks, applying doses between 40 and 100 mg/kg (Starkel and Leclercq 2011). DMN is administered intraperitoneally to mice 10 μg/g 3 times per week during 3 weeks (Yoshida et al. 2004).
3.2. Diet-based models
A number of specific diets can be used to induce progression of NAFLD to non-alcoholic steatohepatitis (NASH) in experimental animals (Anstee and Goldin 2006). It seems that the rodent strain is the major determinant of liver fibrosis caused by dietary ingredients. Overall, C57BL/6 mice are more susceptible to develop diet-induced fibrosis compared to the BALB/c strain (Farrell et al. 2014; Walkin et al. 2013). Nevertheless, these diet-based models fail to mimic the typical characteristics of the human pathology, thus restricting interspecies extrapolation of results (Anstee and Goldin 2006) (Table 1).
3.2.1. Methionine-deficient and choline-deficient diet
Mice fed a methionine-deficient and choline-deficient (MCD) diet constitute a frequently addressed model to study NASH. However, this dietary model lacks some of the major human pathological features, including obesity and pronounced peripheral insulin resistance (Rinella and Green 2004). MCD diets mimic the hepatic stress caused by the fatty acid flux from adipose tissue to the liver as well as increased production of triglycerides, resulting in liver steatosis and lipotoxicity (Jha et al. 2014). Kupffer cells may play a role in the initiation and progression of MCD diet-induced liver steatosis, as they are the firsts to respond to hepatocyte injury. Activated Kupffer cells increase the production of TNFα, the recruitment of monocytes (Tosello-Trampont et al. 2012) and may control collagen deposition by secreting high levels of MMP-13 (Itagaki et al. 2013). In addition, the infiltration of these macrophages can also promote the upregulation of pro-inflammatory pathways and mediators, including nuclear factor kappa-light-chain-enhancer of activated B-cells, intracellular adhesion molecule 1, cyclo-oxygenase 2, monocyte chemo-attractant protein-1 and IL6 (Ramadori et al. 2015). In a following next step, HSCs become activated, which directs the pathology into a more fibrotic stage. Mice fed a MCD diet present steatohepatitis after 8 weeks, whereas the more fibrotic stage, in particular affecting the portal and bridging areas, is only observed after 16 weeks (Itagaki et al. 2013).
3.2.2. High-fat diet
High-fat (HF) diets overcome the shortcomings of the MCD diet, since animals gain body weight and develop peripheral insulin resistance. Although this model has phenotypic hallmarks similar to human NASH, it requires 50 weeks to develop steatohepatitis with merely mild fibrosis in mice (Ito et al. 2007). Male inbred C57BL/6 mice are the most suitable rodents to develop NASH using a HF diet (Ganz et al. 2014). This is in contrast to rats, which are not responsive to HF diets. Because of this flaw, an alternative high-cholesterol diet has been proposed for rats. This high-cholesterol diet induces fibrotic NASH in 9 weeks, whereby the rats occasionally develop cirrhosis, reminiscent of human NASH (Ichimura et al. 2014). Nonetheless, the main disadvantage of this high-cholesterol diet model is the lack of both obesity and insulin resistance.
3.2.3. Choline-deficient L-amino acid defined diet
The choline-deficient L-amino acid defined diet causes a similar phenotype as the MCD diet, though animals also gain weight and develop peripheral insulin resistance (De Minicis et al. 2014; Denda et al. 2002). Choline-deficient L-amino acid-fed rats and C57BL/6J mice frequently produce liver tumors associated with fibrosis (Denda et al. 2002; Nakae et al. 1992), rendering these models eligible to study the progression from NAFLD to NASH and further to HCC (Denda et al. 2002). Mice fed this diet develop evident liver fibrosis after 22 weeks and HCC after 84 weeks (Denda et al. 2002).
3.3. Surgery-based models
Common bile duct ligation (BDL) is well known to cause cholestatic injury and periportal biliary fibrosis. This model was first established in rats and was later applied to mice (Miyoshi et al. 1999; Rodríguez-Garay et al. 1996). As such, BDL consists of a doubly ligated bile duct transected between 2 ligatures (Rodríguez-Garay et al. 1996). The obstruction of the bile duct evokes increases in biliary pressure, mild inflammation and cytokine secretion by biliary epithelial cells, thus generating cholestasis. This results in proliferation of biliary epithelial cells, an increase of expression of fibrogenic markers, including TIMP-1, α-SMA, collagen 1 and TGFβ1, and accumulation of B-cells and T-cells in the portal tracts (Georgiev et al. 2008), generating ROS and liver damage. A recent report claims that, besides the relevant role of HSCs in fibrogenesis, portal fibrosis might be produced by another cell type, active portal fibroblasts (Iwaisako et al. 2014). The latter are a source of myofibroblasts in BDL and may activate HSCs through IL13 (Iwaisako et al. 2014). These events are reversible up to 2 weeks after relief of the obstruction (Abdel-Aziz et al. 1990; Aronson et al. 1993). The applicability of BDL in mice is restricted by frequent perforation of the bilioperitoneum and the variability in the dilatation of the gall bladder, which induces different parenchyma responses (Starkel and Leclercq 2011). In general, early mortality in rodents may ensue after BDL due to bile leakage, rupture of biliary cysts or gall bladder. The mortality rate 5 to 6 weeks after BDL in rats is about 20% and peaks in mice after 10 days. BDL can be particularly used for short-term studies of liver fibrosis associated with cholestatic injury (Chang et al. 2005; Iwaisako et al. 2014; Park et al. 2014).
3.4. Genetically modified models
Genetically modified animals have become powerful research models in the past decade. In particular, they allow to gain insight into the involvement of specific proteins and signaling pathways in the generation of liver fibrosis and thus facilitate the identification of potentially new drug targets (Hayashi and Sakai 2011). Nevertheless, genetic models rarely develop liver fibrosis due to the genetic manipulation as such and need a second stimulus for disease induction (Larter and Yeh 2008) (Table 1). This indicates interaction between the environment and the genotype to manifest the disease, which is the case for NASH.
3.4.1. Multidrug resistance-associated protein 2-deficient mice
Mouse multidrug resistance-associated protein 2 (Mdr2) is the homolog of the human adenosine triphosphate-binding cassette subfamily B member 4 gene, which codes for P-glycoprotein that is involved in biliary phospholipid excretion (Morita et al. 2013). The lack of P-glycoprotein impedes phospholipid secretion into the bile. Consequently, Mdr2-deficient mice develop a phenotype resembling human primary sclerosing cholangitis, including hepatocyte necrosis, strong portal inflammation and proliferation, destruction of the canalicular and small bile ductular tracts, and onion-skin-type periductal fibrosis (Fickert et al. 2004; Morita and Terada 2014). Mdr2-deficient mice develop biliary fibrosis at 4 to 8 weeks of age. Already at 4 weeks, increased expression of TGFβ and HSC activation markers, including α-SMA, MMP-2 and PDGFRβ, is observed (Popov et al. 2005). This is accompanied by periductal fibroblast proliferation and fibrosis, granulocytic infiltration and partial necrosis of the bile duct (Fickert et al. 2002). Abundant presence of collagen is seen at week 8, leading to fibrous scar formation with obliteration of the bile duct lumen. Mdr2-deficient mice aged 4 to 6 months can develop HCC (Mauad et al. 1994).
3.4.2. Alms1Fat ausi mutant mice
Fat ausi (foz/foz) mouse present a spontaneous deletion of 11-base pair (foz) in the Alms1 gene that is responsible for Alstrom’s disease in humans When fed a HF diet, these animals show hyperphagic obesity, insulin resistance, hepatomegaly, diabetes, hypoadiponectinemia, high serum levels of alanine transaminase, inflammatory cells, numerous ballooned hepatocytes and pericellular and pericentral fibrosis (Arsov et al. 2006). After 24 weeks of HF diet, Alms1Fat ausi mutant mice develop adipose restriction, which promotes the flux of lipids to the liver and a decrease in serum adiponectin levels, in turn causing adipose inflammation, hepatocellular injury, hepatomegaly and liver inflammation (Larter et al. 2009). In addition, it has been documented that the presence of cholesterol in the diet could underlie the transition of the disease from NAFLD to NASH (Van Rooyen et al. 2011). This model relies on the interaction between diet and genotype in order to promote liver injury. Accordingly, this is an attractive model for the study of NAFLD progression into NASH due to the presence of different factors. In intervention studies, where the normal diet is recovered, remaining obesity and adipose inflammation has been noticed in this model (Larter et al. 2013).
3.5. Infection-based models
Infection-based models have aided researchers in the elucidation of the mechanisms mediated by the immune system, which occur during liver fibrosis and that can not be reproduced in other models (Starkel and Leclercq 2011). Hepatitis virus infection induces liver fibrosis in humans, but not in rodents. Therefore, genetically engineered animals able to express the HBV envelope coding region under the constitutive transcriptional control of the mouse albumin promoter are typically used (Chisari et al. 1986). These mice do not spontaneously develop liver hepatitis unless their immune system is compromised and replaced by non-transgenic bone marrow cells and spleen cells previously immunized with the HBV antigen (Chisari et al. 1986; Nakamoto et al. 2004). This model has shown the importance of immune reactions in the progression of the disease to HCC (Sitia et al. 2012) (Table 1). An alternative to this model is the use of immunodeficient mice transfected with a HBV plasmid (McCaffrey et al. 2003). Schistosoma mansoni infection is readily established in mice due to high resemblance to human infection and high reproducibility (Cheever et al. 2002). Nevertheless, different mouse strains can show great variations in hepatic fibrosis levels, with the C3H/HeN strain being the most prone to develop higher levels of fibrosis (Cheever et al. 1987; Chiaramonte et al. 2001). Alternatively, animals can be infected by percutaneous administration of 35 cercarias through the tail (Chiaramonte et al. 2001) or by intravenous administration of 10.000 viable eggs (Cheever et al. 2002). The cercarias evolve into adults and can produce more than 100 eggs per day, which can be trapped in the liver. This forms the main cause for the development of granulomas associated with liver fibrosis (Cheever et al. 2002; Chiaramonte et al. 2001). Development of the latter is mediated by the action of T-helper 2 cytokines (Wynn et al. 1995), especially IL13 in a Schistosoma mansoni model (Chiaramonte et al. 2001) and IL17A in a Schistosoma japonicum infection (Zhang et al. 2015), which highlights the role of cytokines in the development of this chronic liver disease. Moreover, the presence of activated HSCs in the periphery of the egg granulomas from Schistosoma japonicum has been observed in rodents and humans (Bartley et al. 2006). Collectively, the role of the cytokines in these infection models contributes to the activation of the HSCs and thus to the progression of liver fibrosis.
4. In vitro models of liver fibrosis
4.1. Primary hepatic stellate cells
Primary HSCs, directly derived from healthy liver tissue, provide a good reflection of the hepatic in vivo situation. However, primary HSCs cope with a number of issues, which originate from isolation and cultivation procedures (Table 2). The classical methodology for the isolation of HSCs is based on a density gradient centrifugation method using Percoll, Nycodenz, Stractan or metrizamide. HSC density is low because of the abundant lipid content. This facilitates separation from other liver cell types, yielding cell suspensions containing up to 75% HSCs with a high viability (Weiskirchen and Gressner 2005). The density gradient centrifugation method can not be used to isolate HSCs from young animals or animals suffering from liver disease due to low lipid content and poor purity. This can be overcome, at least in part, by using fluorescence-activated cell sorting with an ultraviolet laser able to excite vitamin A and thereby to isolate HSCs with high selectivity (Geerts et al. 1998; Tacke and Weiskirchen 2012). However, this procedure is time-consuming and only produces limited amounts of HSCs. A possible solution to the latter includes intravenous injection of liposome-encapsulated dichloromethylene diphosphatein, which eliminates Kupffer cells, in mice prior to HSC isolation (Chang et al. 2014). This results in higher quantities of pure HSC populations upon isolation. When seeded on a plastic culture dish, freshly isolated HSCs spontaneously activate and turn into myofibroblast-like cells as also occurring during liver fibrosis in vivo. This spontaneous in vitro activation triggers a differential gene expression profile in comparison with the in vivo counterpart process, which may not reflect the pathophysiological mechanisms manifested during liver fibrogenesis (De Minicis et al. 2007). Consequently, different strategies have developed to counteract spontaneous HSC activation, including culturing primary HSCs on Matrigel®, which mimics the ECM scaffold in liver (Gaça et al. 2003), or the maintenance of the cells in suspension cultures (Friedman et al. 1994). Like other primary cells, the lifespan of cultured HSCs is limited, which impedes their use. Furthermore, despite improvement of isolation techniques and increased purity, HSC cultures may be contaminated with other liver cell types. Finally, the establishment of human HSC cultures is restricted by the general lack of human biological material for research purposes (Herrmann et al. 2007).
Table 2. In vitro models of liver fibrosis.
(GFP, green fluorescent protein; HSCs, hepatic stellate cells; IL6, interleukin 6; TNF-β1, tumor necrosis factor beta 1; TSV40, large T-antigen of simian virus 40; UV, ultraviolet light; VSV-G, vesicular stomatitis virus protein G.)
| Model | Origin | Characteristics | Advantages | Disadvantages | References | |
|---|---|---|---|---|---|---|
| Primary stellate cells | Rodent Human |
Cells derived from healthy liver: quiescent HSCs Cells derived from injured liver: myofibroblasts |
Close link with the in vivo situation | Activation occurs when seeded on plastic culture dishes Limited life span Cell culture heterogeneity Restricted human material |
(Herrmann et al 2007; Weiskirchen and Gressner 2005) | |
|
| ||||||
| Cell lines | GRX | C3H/HeN mice infected with Shistosoma mansoni 2 phenotypes: myofibroblasts and lipocyte-like cells |
Myofibroblasts resemble activated HSCs | Of use for the study of lipid-related changes and anti-fibrotic molecules. | No difference between both phenotypes at the expression level as occur in vivo | (Borojevic et al 1985; de Mesquita et al 2013; Fortuna et al 2001; Guimarães et al 2007; Guma et al 2001; Pinheiro-Margis et al 1992) |
|
| ||||||
| A640-IS | HSCs from ICR mice transfected with TSV40 | 33 °C: myofibroblastic phenotype 39 °C: HSC-like phenotype |
Myofibroblasts resemble activated HSCs | No difference between both phenotypes at the expression level as occur in vivo | (Kitamura et al 1997) | |
|
| ||||||
| SV68c-IS | HSCs from ICR mice transfected with TSV40 | Myofibroblastic phenotype | - | Lack of correlation with activated HSCs in vivo | (Horie et al 2000) | |
|
| ||||||
| M1-4HSC | HSCs from male p19ARF null mice | Absence of TNF-β1: HSC-like phenotype Presence of TNF-β1: Myofibroblastic phenotype |
Myofibroblast resembles activated HSCs | Lack of correlation with the in vivo situation | (Proell et al 2005) | |
|
| ||||||
| JS1 | HSCs from C57BL/6 transfected with TSV40 | Myofibroblast | Easily transfected Of use in studies of apoptotic mechanisms |
Required characterization | (Guo et al 2009; Lim et al 2011) | |
|
| ||||||
| Col-GFP | HSCs from transgenic mice expressing GFP under the control of collagen I gene promoter and transfected with TSV40 and the hygromycin resistance gene | Myofibroblast phenotype | Of use for drug screening | Lack of correlation with activated HSCs in vivo | (Meurer et al 2013) | |
|
| ||||||
| NFSC | HSCs from Wistar rats spontaneously immortalized | Fusiform phenotype | Of use for study of ECM compound secretion. Secretion of IL-6 | Lack of correlation with activated HSCs in vivo. | (Greenwel et al 1991) | |
|
| ||||||
| CFSC | HSCs from cirrhotic Wistar rats spontaneously immortalized | Fusiform phenotype | Of use for study of ECM Clone heterogeneity | Lack of correlation with activated HSCs in vivo | (Greenwel et al 1993; Greenwel et al 1991) | |
|
| ||||||
| HSC-T6 | HSCs from Wistar rats transfected with TSV40. | Absence of retinol: Myofibroblast phenotype Presence of retinol: myofibroblast phenotype and lipid droplets in the cytoplasm |
Can behave as activated and quiescent HSCs Of use for the study of signaling pathways involved in collagen expression, chemotaxis and contraction |
Lack of correlation with activated HSCs in vivo | (Fang et al 2014; Kim et al 1998; Li et al 2013; Liu and Huang 2014; Vogel et al 2000; Yang et al 2008) | |
|
| ||||||
| BSC | HSCs from rats with biliary fibrosis and spontaneously immortalized | Myofibroblast phenotype | Of use for the study the molecular pathways involved in HSC activation | Lack of correlation with activated HSCs in vivo | (Sung et al 2004) | |
|
| ||||||
| PAV-1 | HSCs from Wistar rats spontaneously immortalized | Absence of retinol: Myofibroblast phenotype Presence of retinol: myofibroblast phenotype with lipid droplets in the cytoplasm. |
Of use for the study of free fatty acids role during liver fibrosis | Lack of correlation with activated HSCs in vivo | (Sauvant et al 2002) | |
|
| ||||||
| HSC-T6/Cl6 | HSCs from Wistar rats transfected with TSV40 and neomycin resistance gene | Myofibroblast phenotype | Of use for the study of apoptosis mechanisms in activated HSCs. | Lack of correlation with activated HSCs in vivo | (Kim et al 2003) | |
| MFBY2 | HSCs from cirrhotic rat liver. | Myofibroblast phenotype When transfected with the terminal latency associated peptide: HSC-like phenotype |
Of use for the study of signaling pathways in HSCs activation | Lack of correlation with activated HSCs in vivo. | (Isono et al 2003) | |
|
| ||||||
| HSC-PQ | HSCs from rat were expose to UV illumination. | Myofibroblast phenotype | Similar to activated HSCs | Doubtful immortalization of cells | (Pan et al 2005) | |
|
| ||||||
| RNPC | HSCs from Wistar rats transfected with TSV40 and neomycin resistance gene. | Epithelial-like morphology | - | Lack of correlation with activated HSCs in vivo | (Takenouchi et al 2010) | |
|
| ||||||
| RGF-N2 | Portal myofibroblast from Wistar rats sorted and transfected with TSV40 | Myofibroblast | Close link with active portal myofibroblast | Further studies are required | (Fausther et al 2015) | |
|
| ||||||
| RGF | Portal myofibroblast from Wistar rats transfected with TSV40 | Myofibroblast | Close link with active portal myofibroblast | Further studies are required | (Fausther et al 2015) | |
|
| ||||||
| LI90 | Human HSCs obtained following cholecystectomy | Absence of vitamin A: Myofibroblast phenotype Presence of vitamin A: myofibroblast phenotype with lipid droplets in the cytoplasm |
Similar to activated HSCs Transfectable Of use for the study of signaling pathways in HSCs activation |
Senescence | (Murakami et al 1995) | |
|
| ||||||
| TWNT-4 | LI90 transfected with hTERT | Myofibroblast phenotype. | Transfectable Of use for the study of signaling pathways in HSCs activation |
Lack of correlation with activated HSCs in vivo | (Shibata et al 2003) | |
|
| ||||||
| GREF-X | Human HSCs isolated from explants of a normal human liver | Absence of retinol: Myofibroblast phenotype Presence of retinol: myofibroblast phenotype with lipid droplets in the cytoplasm |
Of use for the study of signaling pathways in HSCs activation | No expression of activation markers of activated HSCs in vivo | (Weill et al 1997) | |
|
| ||||||
| hTERT-HSC | Human HSCs isolated normal human liver with a VSV-G vector | Absence of membrane-like matrix: myofibroblast phenotype Presence of membrane-like matrix: quiescent HSCs-like phenotype |
Of use for the study of signaling pathways of pro-inflammatory cytokine production | Lack of correlation with activated HSCs in vivo | (Schnabl et al 2002) | |
|
| ||||||
| LX-1 | Human HSCs isolated normal human liver transfected with TSV40 | Myofibroblast phenotype Quiescent behavior when grow in Matrigel® |
Close link to primary HSCs at gene expression level | Not transfectable Unviable in serum-free media |
(Xu et al 2005) | |
|
| ||||||
| LX-2 | Human HSCs isolated normal human liver transfected with TSV40 and subsequent propagation in low serum conditions | Myofibroblast phenotype Quiescent behavior when grown in Matrigel®. |
Close link to primary HSCs at gene expression level Of use for the study of ECM component secretion. Easily transfectable Viable in serum-free media. |
- | (Cao et al 2006; Xu et al 2005) | |
|
| ||||||
| Co-cultures | Rodent and human primary cells and cell lines | Combination of liver cell types | Establishment of cell to cell interactions | Restricted to HSCs and hepatocytes | (Bhatia et al 1999; Giraudi et al 2014; Thomas et al 2005) | |
|
| ||||||
| Precision-cut liver slices | Liver explants from rodents and humans | Liver explants with different cell types | Establishment of cell to cell interactions. | Limited human supply Limited viability |
(Fisher and Vickers, 2013; Olinga et al 1997; Westra et al 2014a) | |
4.2. Cell lines
Cell lines appeared as an alternative to primary cells and offer advantages, such as ease of use, unlimited supply and high interlaboratory reproducibility of results (Herrmann et al. 2007). However, cell lines may lose differentiated functionality and morphology, thus questioning their in vivo relevance (Herrmann et al. 2007). Nevertheless, a variety of HSC cell lines from murine, rat and human origin has been developed and are abundantly used by fundamental liver fibrosis researchers (Table 2).
4.2.1. Mouse cell lines
One of the first described HSC cell line is the murine cell line (GRX) obtained from hepatic fibrotic granulomas of C3H/HeN mice infected with Shistosoma mansoni (Borojevic et al. 1985). In culture, GRX cells show a myofibroblastic phenotype and overgrow into typical hills and valleys because of low contact inhibition. However, when transferred to cell culture media containing insulin and iodomethacin or retinol, GRX cells adopt a fat-storing phenotype and are organized in a regular monolayer. Both GRX phenotypes are able to express collagens type I, III and IV, fibronectin, laminin, vimentin, desmin, GFAP and α-SMA (Pinheiro-Margis et al. 1992), yet production of the different collagen types, desmin and GFAP in the lipocyte-like phenotype is low (Guma et al. 2001; Pinheiro-Margis et al. 1992). This lipocyte-like phenotype has the ability to take up and metabolize retinol similar to HSCs (Guma et al. 2001; Pinheiro-Margis et al. 1992). Therefore, the GRX cell line is a useful tool in the study of lipid-related changes as also occurring during liver fibrosis (Fortuna et al. 2001; Guimarães et al. 2007) and the action of molecules in the reversion of the activated phenotype (de Mesquita et al. 2013; Stefano et al. 2011).
A640-IS cells are HSCs isolated from male imprinting control region (ICR) mice that have been subsequently transfected with the large T-antigen of simian virus 40 (TSV40). This cell line is temperature-sensitive, implying that cells acquire a myofibroblastic and proliferative phenotype at 33°C and a more HSC-like morphology at 39°C. Both A640-IS phenotypes produce collagens type I, III and IV, fibronectin, laminin, vimentin, desmin and α-SMA. Desmin is, however, highly expressed at 39°C, while α-SMA is present in low density cultures at both temperatures (Kitamura et al. 1997). An alternative cell line with similar origin is SV68c-IS. SV68c-IS cells display a myofibroblastic shape and express collagen III, desmin, α-SMA and GFAP (Horie et al. 2000). Both A640-IS and SV68c-IS cells show characteristics reminiscent of activated HSCs in rodents (Horie et al. 2000; Kitamura et al. 1997). However, none of them fully correlates with liver fibrosis in vivo, resulting in their restricted use by researchers.
The M1-4HSC line originates from male p19ARF null mice. These cells appear in 2 different phenotypes depending on the presence of TNFβ1. In the absence of TNFβ1, M1-4HSC cells resemble quiescent HSCs with an epithelial-like phenotype and expression of procollagen I, vimentin, desmin, α-SMA and GFAP. In the presence of TNFβ1, M1-4HSC cells adopt a more myofibroblastic morphology and produce pro-collagen I, vimentin, α-SMA and GFAP (Proell et al. 2005). However, these cells do not manifest other markers of HSC activation (Proell et al. 2005).
The immortalized cell lines JS1, JS2 and JS3 were obtained from isolated HSCs from wild-type, Toll-like receptor 4-deficient and myeloid differentiation primary response gene 88-deficient C57/B16 mice, respectively. These cells were subsequently transfected with the cytomegalovirus promoter TSV40. They were created in order to explore the different pathways involved in HSC activation due to the presence of lipopolysaccharide (Guo et al. 2009). Their most important characteristic lies in their high capacity to be transfected. Although 3 lines were developed, only JS1 cells are extensively used. Because of the high transfection potential, the JS1 cell line is considered as a useful tool to test the efficiency in the expression of different vectors (Ghiassi-Nejad et al. 2013), but also in the selectivity-induced expression or inhibition of specific genes (Guo et al. 2009; Lim et al. 2011). Consequently, this has helped researchers in the elucidation of apoptotic mechanisms of activated HSCs (Lim et al. 2011).
More recently, a new mouse cell line, called Col-green fluorescence protein (GFP), has been described. Col-GFP cells are HSCs isolated from transgenic mice expressing GFP under the control of the collagen I gene promoter and treated with CCl4 for 8 weeks (Meurer et al. 2013). To immortalize these cells, a lentivirus vector containing the TSV40 and the hygromycin resistance gene have been used (Meurer et al. 2013). The resulting cells are characterized by expression of collagen types I and IV, fibronectin, desmin, α-SMA, GFAP, the fibrosis-associated protein connective tissue growth factor (CTGF) and the inhibitor of differentiation-2 (Id2) (Meurer et al. 2013). These Col-GFP cells are considered promising for the screening of potential anti-fibrogenic drugs (Meurer et al. 2013).
4.2.2. Rat cell lines
Normal fat-storing cells (NFSC) and cirrhotic fat-storing cells (CFSC) arose from spontaneous immortalization of a normal and cirrhotic liver, respectively, from male Wistar rats. Both cell lines show a fusiform phenotype and express collagens types I and III, fibronectin, laminin, vimentin, desmin and TGFβ1 (Greenwel et al. 1991). Unlike CFSC cells, NFSC cells produce IL6. Because of collagen expression, both lines can be addressed to investigate collagen secretion by HSCs. The selection of 4 clones from the CFSC line, named CFSC-8B, CFSC-2G, CFSC-3H and CFSC-5H, resulted in the heterogeneous expression of α 1 (I), α 1 (III) and α 1 (IV) procollagen, IL6, TGFβ and connexin43 (Greenwel et al. 1993), suggesting that genetic differences define the ECM composition. This phenomenon can also occur in vivo and thus different clones might be useful in the study of the role of defined ECM scaffolds (Greenwel et al. 1993).
The HSC-T6 cell line was developed by transfection of HSCs from male retired-breeder Sprague-Dawley rats with TSV40 (Vogel et al. 2000). These cells present a myofibroblastic phenotype and are able to form lipid droplets and accumulate retinyl esters in the cytoplasm in the presence of retinol. The expression of collagens type I, III and IV, fibronectin, laminin, vimentin, desmin, α-SMA, GFAP, TIMP-1, TIMP-2 and TGFβ1, suggests a link with activated HSCs (Kim et al. 1998; Li et al. 2013; Vogel et al. 2000). Furthermore, 6 nuclear retinoid receptors, including retinoid acid receptor α, β and γ, and retinoid X receptor α, β and γ can be detected in HSC-T6 cells (Vogel et al. 2000), which is a typical hallmark of quiescent HSCs. Hence, HSC-T6 cells can behave both as activated and quiescent HSCs. HSC-T6 cells have been successfully used for examining signaling pathways involved in collagen expression and for identifying novel targets for liver fibrosis therapy (Fang et al. 2014; Li et al. 2013; Yang et al. 2008). This cell line was also evaluated to express chemotactic, proliferative, adhesion molecules and inflammatory genes in the presence of lipopolysaccharide (Liu and Huang 2014).
The biliary stellate cell (BSC) line came from isolated HSCs from rats with biliary liver fibrosis (Sung et al. 2004). One of the BSC clones generated by spontaneous immortalization includes BSC-C10, which expresses markers of HSC activation, such as α 1 procollagen, desmin, α-SMA, GFAP, neural cell adhesion molecule, vascular cell adhesion molecule and synaptophysin (Sung et al. 2004). The BSC line has been used to investigate the molecular pathways involved in HSC activation (Ramani and Tomasi 2012; Sung et al. 2004).
PAV-1 cells are immortalized cells with a myofibroblastic appearance. PAV-1 cells express the same HSC activation markers as HSC-T6 cells, but lack production of collagen III, GFAP, TIMP-1 and TIMP-2 (Sauvant et al. 2002). Moreover, PAV-1 cells also express RARα and RXRα, and are able to take up and metabolize retinol present in cell culture media, which can be improved by adding free fatty acids (Abergel et al. 2006; Sauvant et al. 2002). This cell line has been used in ALD research. In the presence of ethanol, retinol metabolism in PAV-1 cells is disrupted, thereby decreasing levels of lipid droplets in the cytoplasm, in turn leading to a more active phenotype (Sauvant et al. 2002). Therefore, this cell line is of use for studying the role of free fatty acids in ALD.
The immortalized T-HSC/Cl6 cell line was created in view of unveiling the apoptotic mechanisms involved in HSC activation. These cells express collagen type I, desmin, α-SMA, GFAP and TGFβ (Kim et al. 2003). Over the years, T-HSC/Cl6 cells have been particularly used for investigating molecular actions of anti-fibrotic drugs (Bai et al. 2013; Kim et al. 2003; Yin et al. 2007).
Spontaneously immortalized MFBY2 cells have been isolated from a cirrhotic rat liver and show typical HSC activation markers, including neural cell adhesion molecule, α-SMA, collagens type I and III, fibronectin and TIMP-1 (Isono et al. 2003). When transfected with an adenovirus containing the terminal latency-associated peptide of TGFβ1, MFBY2 cells present a HSC-like cell shape with arrested proliferation. In this transduced cell line, production of collagens, fibronectin and TIMP-1 levels drastically decreases, while GFAP production, uptake and esterification of retinol become manifested (Isono et al. 2003).
The immortalized HSC-PQ cell line arose from ultaviolet illumination of confluent rat HSC cultures. The myofibroblastic phenotype together with expression of collagens type I and III, fibronectin, laminin, desmin and α-SMA (Pan et al. 2005) indicate similarity with activated HSCs.
The RNPC cell line was immortalized according to a protocol identical to that used for T-HSC/Cl6 cells, however, this cell line only expresses α-SMA and desmin in low levels (Takenouchi et al. 2010), thus limiting their use for liver fibrosis research.
More recently, 2 rat portal myofibroblast cell lines were established from male Sprague-Dawley rats, namely RGF-N2 and RGF. Both cell lines express myofibroblasts markers, such as collagen type I and XV, elastin, vimentin, α-SMA, TIMP-1, fibulin-2, lysyl oxidase-like 1-4and cytoglobin. In contrast, they lack of the expression of HSC markers, including desmin and lecithin-retinol acyltransferase. Moreover, they also express membrane receptors characteristic of myofibroblasts, including the TGFβ receptor 1, PDGF receptor β, epidermal growth factor receptor, insulin growth factor 1 receptor, TNF receptor 1a and 1b and other receptors, such as IL4 receptor α, IL13 receptor α1, Cd200 and Cd9. The difference between both cell lines lies with the expression of vascular endothelium growth factor receptor 2, which is only present in RGF cells (Fausther et al. 2015).
4.2.3. Human cell lines
The LI90 cell line was the first human HSC immortalized cell line originating from an epithelioid hemangioendothelioma from the right liver lobe of 55 year old Japanese female following cholecystectomy. LI90 cells display a polygonal shape and a high proliferation rate, and have the ability to overgrow because of the lack of contact inhibition. LI90 cells produce collagens type I, III, IV, V and VI, fibronectin, laminin, vimentin and α-SMA. Moreover, upon addition of vitamin A to the cell culture medium, LI90 cells form lipid droplets in the cytoplasm (Murakami et al. 1995). This cell line constitutes a promising model for the characterization of drug targets in HSC activation. However, after a number of passages, these cells undergo senescence. This can be counteracted by introduction of the human telomerase reverse transcriptase (hTERT) gene using a retroviral vector. By doing so, a new cell line, called TWNT-4, was generated. TWNT-4 cells express several HSC activation markers, including collagen I, α-SMA and PDGFβR (Shibata et al. 2003). TWNT-4 cells have been utilized in anti-fibrotic drug testing (Zhen et al. 2006).
Spontaneously immortalized GREF-X cells are HSCs isolated from the explants of a normal human liver. These myofibroblast-like cells express collagens type I, IV, V and VI, fibronectin, laminin, vimentin and α-SMA, and secrete MMP-2 (Weill et al. 1997). In addition, they retain the capacity to take up and esterify retinol present in the cell culture medium (Weill et al. 1997).
The hTERT-HSC line was developed to tackle the senescence of HSCs in culture. This cell line comes from HSCs isolated from surgical specimens of normal human liver, which have been infected with a VSV-G pseudotyped vector encoding hTERT with a cytomegalovirus promoter (Schnabl et al. 2002). hTERT-HSC cells produce IL6, IL8, IL10, PDGFRα and β, GFAP, vimentin, fibulin 2 and vascular cell adhesion molecule-1. These cells maintain retinol uptake and metabolism capacity (Schnabl et al. 2002). Undoubtedly, the most commonly used human HSC cell line is the Lieming Xu (LX)-2, which was created together with the LX-1 line. LX-1 and LX-2 cell lines were generated by TSV40 transfection, and, in the case of LX-2, by subsequent propagation in low serum conditions (Xu et al. 2005). Both cell lines show a phenotype similar to activated HSCs in vivo and express collagen types I and IV, fibronectin, endoglobin, vimentin, desmin, α-SMA, GFAP, CTGF, survivin, p21, βPDGFR, TGFβ receptor types I and II, DDR2 and Ob-RL (Weiskirchen et al. 2013; Xu et al. 2005). LX-2, but not LX-1, secretes MMP-2 as well as TIMP-1 upon stimulation with leptin (Xu et al. 2005). LX-2 cells have been recently used to study secretion of ECM compounds. Despite the active phenotype, LX-2 and LX-1 cells display a quiescent behavior when grown in Matrigel® (Xu et al. 2005). Because of the capacity to resemble in vivo HSC activation, LX-2 cells are considered as a model of first choice for investigating the signaling pathways in HSC activation (Cao et al. 2006).
4.3. Co-cultures
Although useful, cultures consisting of only 1 cell type are merely of limited use for studying HSC activation and liver fibrosis. These monocultures indeed do not consider interactions between different cell types, which are critical for disease progression. Therefore, co-cultures, joining 2 cell types, have been developed (Table 2). These mixed cultures typically maintain functionality over extended periods of time. The use of co-cultures consisting of primary hepatocytes and primary HSCs is rare (Krause et al. 2009; Thomas et al. 2005). Rather, HSC cell lines are used to set up such co-culture systems with hepatocytes (Abu-Absi et al. 2004). The co-culture configuration keeps the HSCs in a quiescent state (Abu-Absi et al. 2004; Krause et al. 2009; Thomas et al. 2005). These hepatocyte-HSC co-culture systems have been improved by applying a number of strategies, including seeding between 2 layers of ECM compounds or by culturing in spheroids, both which favor the tridimensional architecture of cells (Bhatia et al. 1999). In spheroid co-cultures of rat hepatocytes and HSCs, abundant expression of ECM proteins has been observed, which supports phenotypic hepatocyte stability (Thomas et al. 2005). The latter has also been observed in the spheroid co-culture on a chip model (Lee et al. 2013). Recently, the use of co-culture systems based on hepatocytes and HSC cell lines demonstrated that the cell-to-cell proximity is of high importance to initiate the fibrotic process induced by fatty accumulation (Giraudi et al. 2014). By contrast, co-cultures based on primary HSCs and Kupffer cells reflect the role of immune cells in the regulation of fibrotic responses (Nieto 2006), while co-cultures consisting of HSCs and endothelial cells have shown the importance of HSCs in angiogenesis (Wirz et al. 2008).
4.4. Precision-cut liver slices
Precision-cut liver slices (PCLS) are appropriate systems for the in vitro study of liver fibrosis, as they maintain the complex and many cellular interactions that occur in vivo, which also lack in co-cultures. PCLS are liver explants with a normal thickness of 100 to 250 μm and a diameter of 5 mm, which allows oxygen and nutrients to diffuse. PCLS can be incubated in cell culture dishes, which in turn may be incorporated in dynamic organ culture systems (Fisher and Vickers 2013; Olinga et al. 1997). In such dynamic cultures, PCLS are intermittently exposed to a gas phase or cell culture medium by placing them in a glass vial. PCLS prepared from healthy and fibrotic livers can be used for investigating the early and late phases, respectively, of liver fibrosis (Guo et al. 2007; van de Bovenkamp et al. 2006; Westra et al. 2014b). PCLS are particularly interesting for scrutinizing the different mechanisms involved in chemical induction and reversion of fibrosis (Olinga and Schuppan 2013). A general shortcoming of PCLS is the limited viability, thus restricting their use to short-term purposes (Westra et al. 2014a) (Table 2).
5. Conclusions and perspectives
Liver fibrosis results from a sustained wound healing response to chronic injury. The progression of the disease is commonly related to hepatitis virus infection, alcohol abuse and NAFLD (Blachier et al. 2013). The only treatment currently available is liver transplantation, which is, however, hampered by high treatment costs and the limited number of liver donors (van Agthoven et al. 2001). Thus, there is an urgent need for clinical strategies to manage liver fibrosis. Such research necessitates the establishment of experimental systems to study liver fibrosis. Today, different in vivo and in vitro models are available that try to mimic the complex hepatic cell-cell interactions and signaling pathways, which are involved in all aspects of the disease. Ideally, each liver fibrosis model should reflect major pathological and molecular features of the human disease, such as parenchymatous centered fibrosis in chronic hepatitis. Moreover, in vivo models of liver fibrosis should be easy to set up and should be highly reproducible. Unfortunately, such model is presently lacking. The available chemical-induced fibrosis models are the closest to these ideal characteristics (Smith 2013). They are commonly obtained by administration of CCl4 to mice and rats, and are popular among researchers because of their reproducibility and ease of handling. Furthermore, these models show great similarities with human liver fibrosis and can progress from a fibrotic into a cirrhotic stage, and reverse the fibrotic process upon withdrawal of the insult (Jiang et al. 2004). Diet-based animal models are not able to reproduce human NAFLD progression into NASH. The absence of reproducibility of the main human disease features, namely obesity and insulin resistance, renders these models unsuitable to study the development of liver fibrosis caused by dietary ingredients (Anstee and Goldin 2006). Genetically modified animals have been routinely used to confirm results obtained with other models and have great potential for drug target discovery (Zhang et al. 2014). By contrast, the generation of liver fibrosis due to genetic manipulation is not possible, with the exception of Mdr2-deficient mice that develop biliary fibrosis (Fickert et al. 2002). Nevertheless, upon a second insult, such as provided by a HF diet, genetically modified animals develop characteristics of human NAFLD (Sahai et al. 2004; Wouters et al. 2008), suggesting a close link between the environment and the genetic background of the animals, which has also been noticed in humans (Naik et al. 2013). Due to the high prevalence of hepatitis virus infections worldwide, infection-based models have become increasingly important. These models are valuable tools to study the involvement of the immune system in liver fibrosis and have even been successfully used in drug target discovery (McCaffrey et al. 2003). In recent years, the use of humanized animal models has allowed researchers to gain more mechanistic and clinically relevant insight into the development of liver fibrosis. In this context, a protocol to generate humanized mice with human immune and liver cells has been described, enabling the establishment of viral infections, including HCV (Bility et al. 2012) and long-term HBV infection that induces human immune and fibrotic responses (Bility et al. 2014; Bility et al. 2012). Although these models closely resemble human liver disease during hepatitis infection, they present several limitations, including low hepatocyte repopulation of the liver and limited anti-viral immune response in comparison with the human situation (Bility et al. 2012).
In vitro models are indispensable for in-depth investigation of the mechanisms that drive liver fibrosis. Monoculture HSC systems possess a number of limitations, including the restricted primary cell supply and the absence of heterotypic crucial cell-cell interactions. Co-cultures may be better in vitro systems in this regard, as they allow interaction between HSCs and other hepatic cells, necessary to initiate the fibrotic process. Such co-cultures should preferably consist of quiescent primary HSCs rather than activated HSC cell lines. The latter can be used to study the reversibility of the disease in vitro. It can be anticipated that new in vitro models of liver fibrosis will be introduced in the upcoming years. In this light, a very promising group includes stem cell-based systems, involving differentiation of stem cells of different origin into mature and inactivated HSCs (Asahina et al. 2009; Baba et al. 2004; Miyata et al. 2008). Furthermore, great promise lies in the use of tridimensional human bio-artificial devices that reproduce all aspects of liver physiology and hence of liver pathology (Nedredal et al. 2007; Wen et al. 2008). Such sophisticated models are of utmost fundamental and translational research interest. Indeed, these systems will undoubtedly assist in the development of efficient strategies for the clinical therapy of liver fibrosis, which in turn will benefit human health worldwide.
Acknowledgements
This work was financially supported by the grants of the University Hospital of the Vrije Universiteit Brussel-Belgium (Willy Gepts Fonds UZ-VUB), the Fund for Scientific Research-Flanders (FWO grants G009514N and G010214N), the European Research Council (ERC Starting Grant 335476), the University of São Paulo-Brazil (USP) and the Foundation for Research Support of the State of São Paulo (FAPESP SPEC grant 2013/50420-6).
Abbreviations
- ALD
alcohol liver disease
- αSMA
alpha smooth muscle actin
- BDL
bile duct ligation
- CCl4
carbon tetrachloride
- CFSC
cirrhotic fat storing cells
- CYP2E1
cytochrome P450 2E1
- DEN
diethylnitrosamine
- DMN
dimethylnitrosamine
- ECM
extracellular matrix
- GFP
green fluorescent protein
- GFAP
glial fibrillary acidic protein
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HF
high-fat
- HSCs
hepatic stellate cells
- hTERT
human telomerase reverse transcriptase
- IL
interleukin
- LX
Lieming Xu
- MCD
methionine-deficient and choline-deficient
- Mdr2
multidrug resistance-associated protein 2
- MMPs
matrix metalloproteinases
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NFSC
normal fat storing cells
- PCLS
precision-cut liver slices
- PDGF
platelet-derived growth factor
- ROS
reactive oxygen species
- TIMPs
tissue inhibitors metalloproteinases
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- TSV40
large T-antigen of simian virus 40
References
- Abdel-Aziz G, Lebeau G, Rescan PY, et al. Reversibility of hepatic fibrosis in experimentally induced cholestasis in rat. Am J Pathol. 1990;137(6):1333–1342. [PMC free article] [PubMed] [Google Scholar]
- Abergel A, Sapin V, Dif N, et al. Growth arrest and decrease of alpha-SMA and type I collagen expression by palmitic acid in the rat hepatic stellate cell line PAV-1. Dig Dis Sci. 2006;51(5):986–995. doi: 10.1007/s10620-005-9031-y. [DOI] [PubMed] [Google Scholar]
- Abu-Absi SF, Hansen LK, Hu WS. Three-dimensional co-culture of hepatocytes and stellate cells. Cytotechnology. 2004;45(3):125–140. doi: 10.1007/s10616-004-7996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87(1):1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio-Bautista DI, Pérez-Carreón JI, Gutiérrez-Nájera N, et al. Comparative proteomic analysis of thiol proteins in the liver after oxidative stress induced by diethylnitrosamine. Biochim Biophys Acta. 2013;1834(12):2528–2538. doi: 10.1016/j.bbapap.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Aronson DC, Chamuleau RA, Frederiks WM, et al. Reversibility of cholestatic changes following experimental common bile duct obstruction: fact or fantasy? J Hepatol. 1993;18(1):85–95. doi: 10.1016/s0168-8278(05)80014-5. [DOI] [PubMed] [Google Scholar]
- Arsov T, Larter CZ, Nolan CJ, et al. Adaptive failure to high-fat diet characterizes steatohepatitis in Alms1 mutant mice. Biochem Biophys Res Commun. 2006;342(4):1152–1159. doi: 10.1016/j.bbrc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Asahina K, Tsai SY, Li P, et al. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatol. 2009;49(3):998–1011. doi: 10.1002/hep.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba S, Fujii H, Hirose T, et al. Commitment of bone marrow cells to hepatic stellate cells in mouse. J Hepatol. 2004;40(2):255–260. doi: 10.1016/j.jhep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Bachem MG, Melchior R, Gressner AM. The role of thrombocytes in liver fibrogenesis: effects of platelet lysate and thrombocyte-derived growth factors on the mitogenic activity and glycosaminoglycan synthesis of cultured rat liver fat storing cells. J Clin Chem Clin Biochem. 1989;27(9):555–565. doi: 10.1515/cclm.1989.27.9.555. [DOI] [PubMed] [Google Scholar]
- Bai Q, An J, Wu X, et al. HBV promotes the proliferation of hepatic stellate cells via the PDGF-B/PDGFR-β signaling pathway in vitro. Int J Mol Med. 2012;30(6):1443–1450. doi: 10.3892/ijmm.2012.1148. [DOI] [PubMed] [Google Scholar]
- Bai T, Lian LH, Wu YL, et al. Thymoquinone attenuates liver fibrosis via PI3K and TLR4 signaling pathways in activated hepatic stellate cells. Int Immunopharmacol. 2013;15(2):275–281. doi: 10.1016/j.intimp.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Bartley PB, Ramm GA, Jones NK, et al. A contributory role for activated hepatic stellate cellsin the dynamics of Schistosoma japonicum egg-induced fibrosis. Int J Parasitol. 2006;36:993–1001. doi: 10.1016/j.ijpara.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189(1-2):113–127. doi: 10.1016/s0300-483x(03)00157-4. [DOI] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedossa P, Houglum K, Trautwein C, et al. Stimulation of collagen alpha 1(I) gene expression is associated with lipid peroxidation in hepatocellular injury: a link to tissue fibrosis? Hepatol. 1994;19(5):1262–1271. [PubMed] [Google Scholar]
- Beier JI, McClain CJ. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391(11):1249–1264. doi: 10.1515/BC.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21(3):373–384. doi: 10.1055/s-2001-17552. [DOI] [PubMed] [Google Scholar]
- Best CH, Hartroft WS. Liver damage produced by feeding alcohol or sugar and its prevention by choline. Br Med J. 1949;2(4635):1002–1006. doi: 10.1136/bmj.2.4635.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia SN, Balis UJ, Yarmush ML, et al. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13(14):1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- Bility MT, Cheng L, Zhang Z, et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10(3):e1004032. doi: 10.1371/journal.ppat.1004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bility MT, Zhang L, Washburn ML, et al. Generation of a humanized mouse model with both human immune system and liver cells to model hepatitis C virus infection and liver immunopathogenesis. Nat Protoc. 2012;7(9):1608–1617. doi: 10.1038/nprot.2012.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell DM, Roulot D, George J. Transforming growth factor beta and the liver. Hepatol. 2001;34(5):859–867. doi: 10.1053/jhep.2001.28457. [DOI] [PubMed] [Google Scholar]
- Blachier M, Leleu H, Peck-Radosavljevic M, et al. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58(3):593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Borojevic R, Monteiro AN, Vinhas SA, et al. Establishment of a continuous cell line from fibrotic schistosomal granulomas in mice livers. In Vitro Cell Dev Biol. 1985;21(7):382–390. doi: 10.1007/BF02623469. [DOI] [PubMed] [Google Scholar]
- Brandon-Warner E, Schrum LW, Schmidt CM, et al. Rodent models of alcoholic liver disease: of mice and men. Alcohol. 2012;46(8):715–725. doi: 10.1016/j.alcohol.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkopf K, Godoy P, Ciuclan L, et al. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol. 2006;44(1):57–66. doi: 10.1055/s-2005-858989. [DOI] [PubMed] [Google Scholar]
- Brown B, Lindberg K, Reing J, et al. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12(3):519–526. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- Buchmann A, Bauer-Hofmann R, Mahr J, et al. Mutational activation of the c-Ha-ras gene in liver tumors of different rodent strains: correlation with susceptibility to hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1991;88(3):911–915. doi: 10.1073/pnas.88.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canbay A, Higuchi H, Bronk SF, et al. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterol. 2002;123(4):1323–1330. doi: 10.1053/gast.2002.35953. [DOI] [PubMed] [Google Scholar]
- Cao Q, Mak KM, Lieber CS. Leptin enhances alpha1(I) collagen gene expression in LX-2 human hepatic stellate cells through JAK-mediated H2O2-dependent MAPK pathways. J Cell Biochem. 2006;97(1):188–197. doi: 10.1002/jcb.20622. [DOI] [PubMed] [Google Scholar]
- Chang ML, Yeh CT, Chang PY, et al. Comparison of murine cirrhosis models induced by hepatotoxin administration and common bile duct ligation. World J Gastroenterol. 2005;11(27):4167–4172. doi: 10.3748/wjg.v11.i27.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Yang M, Song L, et al. Isolation and culture of hepatic stellate cells from mouse liver. Acta Biochim Biophys Sin (Shanghai) 2014;46(4):291–298. doi: 10.1093/abbs/gmt143. [DOI] [PubMed] [Google Scholar]
- Cheever AW, Duvall RH, Hallack TA, et al. Variation of hepatic fibrosis and granuloma size among mouse strains infected with Shistosoma mansoni. Am J Trop Med Hyg. 1987;37:85–97. doi: 10.4269/ajtmh.1987.37.85. [DOI] [PubMed] [Google Scholar]
- Cheever AW, Lenzi JA, Lenzi HL, et al. Experimental models of Schistosoma mansoni infection. Mem Inst Oswaldo Cruz. 2002;97(7):917–940. doi: 10.1590/s0074-02762002000700002. [DOI] [PubMed] [Google Scholar]
- Chen IS, Chen YC, Chou CH, et al. Hepatoprotection of silymarin against thioacetamide-induced chronic liver fibrosis. J Sci Food Agric. 2012;92(7):1441–1447. doi: 10.1002/jsfa.4723. [DOI] [PubMed] [Google Scholar]
- Chen SW, Zhang XR, Wang CZ, et al. RNA interference targeting the platelet-derived growth factor receptor beta subunit ameliorates experimental hepatic fibrosis in rats. Liver Int. 2008;28(10):1446–1457. doi: 10.1111/j.1478-3231.2008.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaramonte MG, Cheever AW, Malley JD, et al. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatol. 2001;34:273–282. doi: 10.1053/jhep.2001.26376. [DOI] [PubMed] [Google Scholar]
- Chisari FV, Filippi P, McLachlan A, et al. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J Virol. 1986;60(3):880–887. doi: 10.1128/jvi.60.3.880-887.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu AS, Diaz R, Hui JJ, et al. Lineage tracing demonstrates no evidence of cholagiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatol. 2011;53(5):1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constandinou C, Henderson N, Iredale JP. Modeling liver fibrosis in rodents. Methods Mol Med. 2005;117:237–250. doi: 10.1385/1-59259-940-0:237. [DOI] [PubMed] [Google Scholar]
- Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014;20(10):2515–2532. doi: 10.3748/wjg.v20.i10.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date M, Matsuzaki K, Matsushita M, et al. Modulation of transforming growth factor beta function in hepatocytes and hepatic stellate cells in rat liver injury. Gut. 2000;46(5):719–724. doi: 10.1136/gut.46.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mesquita FC, Bitencourt S, Caberlon E, et al. Fructose-1,6-bisphosphate induces phenotypic reversion of activated hepatic stellate cell. Eur J Pharmacol. 2013;720(1-3):320–325. doi: 10.1016/j.ejphar.2013.09.067. [DOI] [PubMed] [Google Scholar]
- De Minicis S, Agostinelli L, Rychlicki C, et al. HCC development is associated to peripheral insulin resistance in a mouse model of NASH. PLoS One. 2014;9(5):e97136. doi: 10.1371/journal.pone.0097136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Minicis S, Seki E, Uchinami H, et al. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterol. 2007;132(5):1937–1946. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- DeCarli LM, Lieber CS. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr. 1967;91(3):331–336. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- Denda A, Kitayama W, Kishida H, et al. Development of hepatocellular adenomas and carcinomas associated with fibrosis in C57BL/6J male mice given a choline-deficient, L-amino acid-defined diet. Jpn J Cancer Res. 2002;93(2):125–132. doi: 10.1111/j.1349-7006.2002.tb01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenicali M, Caraceni P, Giannone F, et al. A novel model of CCl4-induced cirrhosis with ascites in the mouse. J Hepatol. 2009;51(6):991–999. doi: 10.1016/j.jhep.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Enomoto N, Ikejima K, Bradford B, et al. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterol. 1998;115(2):443–451. doi: 10.1016/s0016-5085(98)70211-2. [DOI] [PubMed] [Google Scholar]
- Fang L, Huang C, Meng X, et al. TGF-β1-elevated TRPM7 channel regulates collagen expression in hepatic stellate cells via TGF-β1/Smad pathway. Toxicol Appl Pharmacol. 2014;280(2):335–344. doi: 10.1016/j.taap.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Farrell GC, Mridha AR, Yeh MM, et al. Strain dependence of diet-induced NASH and liver fibrosis in obese mice is linked to diabetes and inflammatory phenotype. Liver Int. 2014;34(7):1084–1093. doi: 10.1111/liv.12335. [DOI] [PubMed] [Google Scholar]
- Fausther M, Goree JR, Lavoie EG, et al. Establishment and characterization of rat portal myofibroblasts cell lines. PLoS One. 2015;10(3):e0121161. doi: 10.1371/journal.pone.0121161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Fuchsbichler A, Wagner M, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterol. 2004;127(1):261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Fickert P, Zollner G, Fuchsbichler A, et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterol. 2002;123(4):1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- Fischer R, Cariers A, Reinehr R, et al. Caspase 9-dependent killing of hepatic stellate cells by activated Kupffer cells. Gastroenterol. 2002;123(3):845–861. doi: 10.1053/gast.2002.35384. [DOI] [PubMed] [Google Scholar]
- Fisher RL, Vickers AE. Preparation and culture of precision-cut organ slices from human and animal. Xenobiotica. 2013;43(1):8–14. doi: 10.3109/00498254.2012.728013. [DOI] [PubMed] [Google Scholar]
- Fortuna VA, Trugo LC, Borojevic R. Acyl-CoA: retinol acyltransferase (ARAT) and lecithin:retinol acyltransferase (LRAT) activation during the lipocyte phenotype induction in hepatic stellate cells. J Nutr Biochem. 2001;12(11):610–621. doi: 10.1016/s0955-2863(01)00179-6. [DOI] [PubMed] [Google Scholar]
- French SW. Intragastric ethanol infusion model for cellular and molecular studies of alcoholic liver disease. J Biomed Sci. 2001;8(1):20–27. doi: 10.1007/BF02255967. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Yamasaki G, Wong L. Modulation of transforming growth factor beta receptors of rat lipocytes during the hepatic wound healing response. Enhanced binding and reduced gene expression accompany cellular activation in culture and in vivo. J Biol Chem. 1994;269(14):10551–10558. [PubMed] [Google Scholar]
- Ganz M, Csak T, Szabo G. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol. 2014;20(26):8525–8534. doi: 10.3748/wjg.v20.i26.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaça MD, Zhou X, Issa R, et al. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol. 2003;22(3):229–239. doi: 10.1016/s0945-053x(03)00017-9. [DOI] [PubMed] [Google Scholar]
- Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21(3):311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- Geerts A, Niki T, Hellemans K, et al. Purification of rat hepatic stellate cells by side scatter-activated cell sorting. Hepatol. 1998;27(2):590–598. doi: 10.1002/hep.510270238. [DOI] [PubMed] [Google Scholar]
- Geerts AM, Vanheule E, Praet M, et al. Comparison of three research models of portal hypertension in mice: macroscopic, histological and portal pressure evaluation. Int J Exp Pathol. 2008;89(4):251–263. doi: 10.1111/j.1365-2613.2008.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev P, Jochum W, Heinrich S, et al. Characterization of time-related changes after experimental bile duct ligation. Br J Surg. 2008;95(5):646–656. doi: 10.1002/bjs.6050. [DOI] [PubMed] [Google Scholar]
- Ghiassi-Nejad Z, Hernandez-Gea V, Woodrell C, et al. Reduced hepatic stellate cell expression of KLF6 tumor suppressor isoforms amplifies fibrosis during acute and chronic rodent liver injury. Hepatol. 2013;57(2):786–796. doi: 10.1002/hep.26056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudi PJ, Barbero Becerra VJ, Marin V, et al. The importance of the interaction between hepatocyte and hepatic stellate cells in fibrogenesis induced by fatty accumulation. Exp Mol Pathol. 2014;98(1):85–92. doi: 10.1016/j.yexmp.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Greenwel P, Rubin J, Schwartz M, et al. Liver fat-storing cell clones obtained from a CCl4-cirrhotic rat are heterogeneous with regard to proliferation, expression of extracellular matrix components, interleukin-6, and connexin 43. Lab Invest. 1993;69(2):210–216. [PubMed] [Google Scholar]
- Greenwel P, Schwartz M, Rosas M, et al. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest. 1991;65(6):644–653. [PubMed] [Google Scholar]
- Guimarães EL, Franceschi MF, Andrade CM, et al. Hepatic stellate cell line modulates lipogenic transcription factors. Liver Int. 2007;27(9):1255–1264. doi: 10.1111/j.1478-3231.2007.01578.x. [DOI] [PubMed] [Google Scholar]
- Guma FCR, Mello TG, Mermelstein CS, et al. Intermediate filaments modulation in an in vitro model of the hepatic stellate cell activation or conversion into the lipocyte phenotype. Biochem Cell Biol. 2001;79(4):409–417. [PubMed] [Google Scholar]
- Guo J, Loke J, Zheng F, et al. Functional linkage of cirrhosis-predictice single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatol. 2009;49(3):960–968. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wang H, Zhang C. Establishment of rat precision-cut fibrotic liver slice technique and its application in verapamil metabolism. Clin Exp Pharmacol Physiol. 2007;34(5-6):406–413. doi: 10.1111/j.1440-1681.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- Hahn E, Wick G, Pencev D, et al. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980;21(1):63–71. doi: 10.1136/gut.21.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Sakai T. Animal models for the study of liver fibrosis: new insights from knockout mouse models. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):G729–738. doi: 10.1152/ajpgi.00013.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90(4):367–386. doi: 10.1111/j.1365-2613.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J, Gressner AM, Weiskirchen R. Immortal hepatic stellate cell lines: useful tools to study hepatic stellate cell biology and function? J Cell Mol Med. 2007;11(4):704–722. doi: 10.1111/j.1582-4934.2007.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie S, Kitamura Y, Kawasaki H, et al. Inhibitory effects of antisense oligonucleotides on the expression of procollagen type III gene in mouse hepatic stellate cells transformed by simian virus 40. Pathol Int. 2000;50(12):937–944. doi: 10.1046/j.1440-1827.2000.01146.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li X, Wang Y, et al. Endoplasmic reticulum stress-induced hepatic stellate cell apoptosis through calcium-mediated JNK/P38 MAPK and Calpain/Caspase-12 pathways. Mol Cell Biochem. 2014;394(1-2):1–12. doi: 10.1007/s11010-014-2073-8. [DOI] [PubMed] [Google Scholar]
- Ichimura M, Kawase M, Masuzumi M, et al. High-fat and high-cholesterol diet rapidly induces non-alcoholic steatohepatitis with advanced fibrosis in Sprague-Dawley rats. Hepatol Res. 2014 doi: 10.1111/hepr.12358. [DOI] [PubMed] [Google Scholar]
- Iizuka M, Murata T, Hori M, et al. Increased contractility of hepatic stellate cells in cirrhosis is mediated by enhanced Ca2+-dependent and Ca2+-sensitization pathways. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G1010–1021. doi: 10.1152/ajpgi.00350.2010. [DOI] [PubMed] [Google Scholar]
- Iredale JP, Benyon RC, Pickering J, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102(3):538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono M, Soda M, Inoue A, et al. Reverse transformation of hepatic myofibroblast-like cells by TGFbeta1/LAP. Biochem Biophys Res Commun. 2003;311(4):959–965. doi: 10.1016/j.bbrc.2003.10.093. [DOI] [PubMed] [Google Scholar]
- Issa R, Williams E, Trim N, et al. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48(4):548–557. doi: 10.1136/gut.48.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki H, Shimizu K, Morikawa S, et al. Morphological and functional characterization of non-alcoholic fatty liver disease induced by a methionine-choline-deficient diet in C57BL/6 mice. Int J Clin Exp Pathol. 2013;6(12):2683–2696. [PMC free article] [PubMed] [Google Scholar]
- Ito M, Suzuki J, Tsujioka S, et al. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res. 2007;37(1):50–57. doi: 10.1111/j.1872-034X.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- Iwaisako K, Jiang C, Zhang M, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A. 2014;111(32):E3297–3305. doi: 10.1073/pnas.1400062111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH, Kang KJ, Kim YH, et al. Reevaluation of experimental model of hepatic fibrosis induced by hepatotoxic drugs: an easy, applicable, and reproducible model. Transplant Proc. 2008;40(8):2700–2703. doi: 10.1016/j.transproceed.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Jarnagin WR, Rockey DC, Koteliansky VE, et al. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127(6):2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha P, Knopf A, Koefeler H, et al. Role of adipose tissue in methionine-choline-deficient model of non-alcoholic steatohepatitis (NASH) Biochim Biophys Acta. 2014;1842(7):959–970. doi: 10.1016/j.bbadis.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liu J, Waalkes M, et al. Changes in the gene expression associated with carbon tetrachloride-induced liver fibrosis persist after cessation of dosing in mice. Toxicol Sci. 2004;79(2):404–410. doi: 10.1093/toxsci/kfh120. [DOI] [PubMed] [Google Scholar]
- Jin N, Deng J, Chadashvili T, et al. Carbogen gas-challenge BOLD MR imaging in a rat model of diethylnitrosamine-induced liver fibrosis. Radiology. 2010;254(1):129–137. doi: 10.1148/radiol.09090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal MA, Krarup H, Sand JM, et al. Review article: the efficacy of biomarkers in chronic fibroproliferative diseases - early diagnosis and prognosis, with liver fibrosis as an exemplar. Aliment Pharmacol Ther. 2014;40(3):233–249. doi: 10.1111/apt.12820. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Ushioda R, Ito S, et al. Deletion of the Collagen-specific Molecular Chaperone Hsp47 Causes Endoplasmic Reticulum Stress-mediated Apoptosis of Hepatic Stellate Cells. J Biol Chem. 2014 doi: 10.1074/jbc.M114.592139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan A, Martini R, Batey R. Ethanol-related liver injury in the rat: a model of steatosis, inflammation and pericentral fibrosis. J Hepatol. 1995;23(5):591–600. doi: 10.1016/0168-8278(95)80067-0. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, Todero SL, Shubert KA, et al. Malondialdehyde-acetaldehyde-protein adducts increase secretion of chemokines by rat hepatic stellate cells. Alcohol. 2001;25(2):123–128. doi: 10.1016/s0741-8329(01)00174-4. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim KM, Nan JX, et al. Induction of apoptosis by tanshinone I via cytochrome c release in activated hepatic stellate cells. Pharmacol Toxicol. 2003;92(4):195–200. doi: 10.1034/j.1600-0773.2003.920410.x. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ratziu V, Choi SG, et al. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem. 1998;273(50):33750–33758. doi: 10.1074/jbc.273.50.33750. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Brenner DA. Anti-fibrogenic strategies and the regression of fibrosis. Best Pract Res Clin Gastroenterol. 2011;25(2):305–317. doi: 10.1016/j.bpg.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Cong M, Paik Y, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109(24):9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in the pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Tanigawa T, Katsumoto T, et al. Cell growth and differentiation of a novel mouse Ito (fat-storing) cell line transformed by a temperature-sensitive mutant of simian virus 40. Hepatol. 1997;26(2):323–329. doi: 10.1002/hep.510260211. [DOI] [PubMed] [Google Scholar]
- Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol. 2006;12(46):7413–7420. doi: 10.3748/wjg.v12.i46.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P, Saghatolislam F, Koenig S, et al. Maintaining hepatocyte differentiation in vitro through co-culture with hepatic stellate cells. In Vitro Cell Dev Biol Anim. 2009;45(5-6):205–212. doi: 10.1007/s11626-008-9166-1. [DOI] [PubMed] [Google Scholar]
- Krull NB, Zimmermann T, Gressner AM. Spatial and temporal patterns of gene expression for the proteoglycans biglycan and decorin and for transforming growth factor-beta 1 revealed by in situ hybridization during experimentally induced liver fibrosis in the rat. Hepatol. 1993;18(3):581–589. [PubMed] [Google Scholar]
- Larter CZ, Yeh MM. Animal models of NASH: getting both pathology and metabolic context right. J Gastroenterol Hepatol. 2008;23(11):1635–1648. doi: 10.1111/j.1440-1746.2008.05543.x. [DOI] [PubMed] [Google Scholar]
- Larter CZ, Yeh MM, Haigh WG, et al. Dietary modification dampens liver inflammation and fibrosis in obesity-related fatty liver disease. Obesity (Silver Spring) 2013;21(6):1189–1199. doi: 10.1002/oby.20123. [DOI] [PubMed] [Google Scholar]
- Larter CZ, Yeh MM, Van Rooyen DM, et al. Roles of adipose restriction and metabolic factors in progression of steatosis to steatohepatitis in obese, diabetic mice. J Gastroenterol Hepatol. 2009;24(10):1658–1668. doi: 10.1111/j.1440-1746.2009.05996.x. [DOI] [PubMed] [Google Scholar]
- Lee KS, Buck M, Houglum K, et al. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96(5):2461–2468. doi: 10.1172/JCI118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, No dY, Kang E, et al. Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte-hepatic stellate cell interactions and flow effects. Lab Chip. 2013;13(18):3529–3537. doi: 10.1039/c3lc50197c. [DOI] [PubMed] [Google Scholar]
- Lee TF, Lin YL, Huang YT. Kaerophyllin inhibits hepatic stellate cell activation by apoptotic bodies from hepatocytes. Liver Int. 2011;31(5):618–629. doi: 10.1111/j.1478-3231.2011.02485.x. [DOI] [PubMed] [Google Scholar]
- Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25(2):195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo MA, Lieber CS. Hepatic fibrosis after long-term administration of ethanol and moderate vitamin A supplementation in the rat. Hepatol. 1983;3(1):1–11. doi: 10.1002/hep.1840030101. [DOI] [PubMed] [Google Scholar]
- Li Y, Luo Y, Zhang X, et al. Combined taurine, epigallocatechin gallate and genistein therapy reduces HSC-T6 cell proliferation and modulates the expression of fibrogenic factors. Int J Mol Sci. 2013;14(10):20543–20554. doi: 10.3390/ijms141020543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;257(1):59–84. doi: 10.1016/s0009-8981(96)06434-0. [DOI] [PubMed] [Google Scholar]
- Liedtke C, Luedde T, Sauerbruch T, et al. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 2013;6(1):19. doi: 10.1186/1755-1536-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MP, Devi LA, Rozenfeld R. Cannabinoil causes activated hepatic stellate cell death through a mechanism of endoplasmic reticulum stress-induced apoptosis. Cell Death Dis. 2011;2:e170. doi: 10.1038/cddis.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YW, Huang YT. Inhibitory effect of tanshinone IIA on rat hepatic stellate cells. PLoS One. 2014;9(7):e103229. doi: 10.1371/journal.pone.0103229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoinne S, Cadoret A, El Mourabit H, et al. Origins and functions of liver myofibroblasts. Biochim Biophys Acta. 2013:948–954. doi: 10.1016/j.bbadis.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Grahame NJ, Becker HC. Development of ethanol withdrawal-related sensitization and relapse drinking in mice selected for high- or low-ethanol preference. Alcohol Clin Exp Res. 2011;35(5):953–962. doi: 10.1111/j.1530-0277.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low TY, Leow CK, Salto-Tellez M, et al. A proteomic analysis of thioacetamide-induced hepatotoxicity and cirrhosis in rat livers. Proteomics. 2004;4(12):3960–3974. doi: 10.1002/pmic.200400852. [DOI] [PubMed] [Google Scholar]
- MacDonald GA, Bridle KR, Ward PJ, et al. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J Gastroenterol Hepatol. 2001;16(6):599–606. doi: 10.1046/j.1440-1746.2001.02445.x. [DOI] [PubMed] [Google Scholar]
- Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- Marra F, DeFranco R, Grappone C, et al. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol. 1998;152(2):423–430. [PMC free article] [PubMed] [Google Scholar]
- Marra F, Gentilini A, Pinzani M, et al. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor’s actions on hepatic stellate cells. Gastroenterol. 1997;112(4):1297–1306. doi: 10.1016/s0016-5085(97)70144-6. [DOI] [PubMed] [Google Scholar]
- Mauad TH, van Nieuwkerk CM, Dingemans KP, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145(5):1237–1245. [PMC free article] [PubMed] [Google Scholar]
- McCaffrey AP, Nakai H, Pandey K, et al. Inhibition of hepatitis B virus in mice by RNA interference. Nat Biotechnol. 2003;21(6):639–644. doi: 10.1038/nbt824. [DOI] [PubMed] [Google Scholar]
- Melhem MF, Rao KN, Kunz HW, et al. Genetic control of susceptibility to diethylnitrosamine carcinogenesis in inbred ACP (grc+) and R16 (grc) rats. Cancer Res. 1989;49(23):6813–6821. [PubMed] [Google Scholar]
- Melón LC, Wray KN, Moore EM, et al. Sex and age differences in heavy binge drinking and its effects on alcohol responsivity following abstinence. Pharmacol Biochem Behav. 2013;104:177–187. doi: 10.1016/j.pbb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119(4):911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Meurer SK, Alsamman M, Sahin H, et al. Overexpression of endoglin modulates TGF-β1-signalling pathways in a novel immortalized mouse hepatic stellate cell line. PLoS One. 2013;8(2):e56116. doi: 10.1371/journal.pone.0056116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata E, Masuya M, Yoshida S, et al. Hematopoietic origin of hepatic stellate cells in the adult liver. Blood. 2008;111(4):2427–2435. doi: 10.1182/blood-2007-07-101261. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Rust C, Roberts PJ, et al. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterol. 1999;117(3):669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- Morita SY, Terada T. Molecular Mechanisms for Biliary Phospholipid and Drug Efflux Mediated by ABCB4 and Bile Salts. Biomed Res Int. 2014;2014:954781. doi: 10.1155/2014/954781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita SY, Tsuda T, Horikami M, et al. Bile salt-stimulated phospholipid efflux mediated by ABCB4 localized in nonraft membranes. J Lipid Res. 2013;54(5):1221–1230. doi: 10.1194/jlr.M032425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Abe T, Miyazawa M, et al. Establishment of a new human cell line, LI90, exhibiting characteristics of hepatic Ito (fat-storing) cells. Lab Invest. 1995;72(6):731–739. [PubMed] [Google Scholar]
- Naik A, Košir R, Rozman D. Genomic aspects of NAFLD pathogenesis. Genomics. 2013;102(2):84–95. doi: 10.1016/j.ygeno.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Nakae D, Yoshiji H, Mizumoto Y, et al. High incidence of hepatocellular carcinomas induced by a choline deficient L-amino acid defined diet in rats. Cancer Res. 1992;52(18):5042–5045. [PubMed] [Google Scholar]
- Nakamoto Y, Suda T, Momoi T, et al. Different procarcinogenic potentials of lymphocyte subsets in a transgenic mouse model of chronic hepatitis B. Cancer Res. 2004;64(9):3326–3333. doi: 10.1158/0008-5472.can-03-3817. [DOI] [PubMed] [Google Scholar]
- Nedredal GI, Elvevold K, Ytrebø LM, et al. Significant contribution of liver nonparenchymal cells to metabolism of ammonia and lactate and cocultivation augments the functions of a bioartificial liver. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G75–83. doi: 10.1152/ajpgi.00245.2006. [DOI] [PubMed] [Google Scholar]
- Neubauer K, Knittel T, Aurisch S, et al. Glial fibrillary acidic protein--a cell type specific marker for Ito cells in vivo and in vitro. J Hepatol. 1996;24(6):719–730. doi: 10.1016/s0168-8278(96)80269-8. [DOI] [PubMed] [Google Scholar]
- Niemelä O, Parkkila S, Juvonen RO, et al. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33(6):893–901. doi: 10.1016/s0168-8278(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of [corrected] Kupffer cells on stellate cells. Hepatol. 2006;44(6):1487–1501. doi: 10.1002/hep.21427. [DOI] [PubMed] [Google Scholar]
- Nieto N, Friedman SL, Cederbaum AI. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J Biol Chem. 2002a;277(12):9853–9864. doi: 10.1074/jbc.M110506200. [DOI] [PubMed] [Google Scholar]
- Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatol. 2002b;35(1):62–73. doi: 10.1053/jhep.2002.30362. [DOI] [PubMed] [Google Scholar]
- Niki T, De Bleser PJ, Xu G, et al. Comparison of glial fibrillary acidic protein and desmin staining in normal and CCl4-induced fibrotic rat livers. Hepatol. 1996;23(6):1538–1545. doi: 10.1002/hep.510230634. [DOI] [PubMed] [Google Scholar]
- Novo E, Marra F, Zamara E, et al. Dose dependent and divergent effects of superoxide anion on cell death, proliferation, and migration of activated human hepatic stellate cells. Gut. 2006;55(1):90–97. doi: 10.1136/gut.2005.069633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364(6440):806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Oh SW, Kim DH, Ha JR, et al. Anti-fibrotic effects of a methylenedioxybenzene compound, CW209292 on dimethylnitrosamine-induced hepatic fibrosis in rats. Biol Pharm Bull. 2009;32(8):1364–1370. doi: 10.1248/bpb.32.1364. [DOI] [PubMed] [Google Scholar]
- Olinga P, Groen K, Hof IH, et al. Comparison of five incubation systems for rat liver slices using functional and viability parameters. J Pharmacol Toxicol Methods. 1997;38(2):59–69. doi: 10.1016/s1056-8719(97)00060-9. [DOI] [PubMed] [Google Scholar]
- Olinga P, Schuppan D. Precision-cut liver slices: a tool to model the liver ex vivo. J Hepatol. 2013;58(6):1252–1253. doi: 10.1016/j.jhep.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Paik YH, Schwabe RF, Bataller R, et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatol. 2003;37(5):1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- Pan Q, Li DG, Lu HM, et al. A new immortalized rat cell line, hepatic stellate cell-PQ, exhibiting characteristics of hepatic stellate cell. Hepatobiliary Pancreat Dis Int. 2005;4(2):281–284. [PubMed] [Google Scholar]
- Park KC, Park JH, Jeon JY, et al. A new histone deacetylase inhibitor improves liver fibrosis in BDL rats through suppression of hepatic stellate cells. Br J Pharmacol. 2014;171(21):4820–4830. doi: 10.1111/bph.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen JS, Bendtsen F, Moller S. Management of cirrhotic ascites. Ther Adv Chronic Dis. 2015;6(3):124–137. doi: 10.1177/2040622315580069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicoro A, Ramachandran P, Iredale JP, et al. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- Pinheiro-Margis M, Margis R, Borojevic R. Collagen synthesis in an established liver connective tissue cell line (GRX) during induction of the fat-storing phenotype. Exp Mol Pathol. 1992;56(2):108–118. doi: 10.1016/0014-4800(92)90028-a. [DOI] [PubMed] [Google Scholar]
- Popov Y, Patsenker E, Fickert P, et al. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43(6):1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Proell V, Mikula M, Fuchs E, et al. The plasticity of p19 ARF null hepatic stellate cells and the dynamics of activation. Biochim Biophys Acta. 2005;1744(1):76–87. doi: 10.1016/j.bbamcr.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Radaeva S, Sun R, Jaruga B, et al. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterol. 2006;130(2):435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Veit T, Schwögler S, et al. Expression of the gene of the alpha-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(6):349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- Ramani K, Tomasi ML. Transcriptional regulation of methionine adenosyltransferase 2A by peroxisome proliferator-activated receptors in rat hepatic stellate cells. Hepatol. 2012;55(6):1942–1953. doi: 10.1002/hep.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40(1):47–51. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Rockey D. The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatol. 1997;25(1):2–5. doi: 10.1053/jhep.1997.v25.ajhep0250002. [DOI] [PubMed] [Google Scholar]
- Rockey DC, Chung JJ. Inducible nitric oxide synthase in rat hepatic lipocytes and the effect of nitric oxide on lipocyte contractility. J Clin Invest. 1995;95(3):1199–1206. doi: 10.1172/JCI117769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DC, Weisiger RA. Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatol. 1996;24(1):233–240. doi: 10.1002/hep.510240137. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Garay EA, Agüero RM, Pisani G, et al. Rat model of mild stenosis of the common bile duct. Res Exp Med (Berl) 1996;196(2):105–116. doi: 10.1007/BF02576832. [DOI] [PubMed] [Google Scholar]
- Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterol. 1979;76(4):710–719. [PubMed] [Google Scholar]
- Sahai A, Malladi P, Pan X, et al. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287(5):G1035–1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- Saile B, Knittel T, Matthes N, et al. CD95/CD95L-mediated apoptosis of the hepatic stellate cell. A mechanism terminating uncontrolled hepatic stellate cell proliferation during hepatic tissue repair. Am J Pathol. 1997;151(5):1265–1272. [PMC free article] [PubMed] [Google Scholar]
- Salguero Palacios R, Roderfeld M, Hemmann S, et al. Activation of hepatic stellate cells is associated with cytokine expression in thioacetamide-induced hepatic fibrosis in mice. Lab Invest. 2008;88(11):1192–1203. doi: 10.1038/labinvest.2008.91. [DOI] [PubMed] [Google Scholar]
- Sauvant P, Abergel A, Partier A, et al. Treatment of the rat hepatic stellate cell line, PAV-1, by retinol and palmitic acid leads to a convenient model to study retinoids metabolism. Biol Cell. 2002;94(6):401–408. doi: 10.1016/s0248-4900(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Sauvant P, Sapin V, Abergel A, Schmidt CK, et al. PAV-1, a new rat hepatic stellate cell line converts retinol into retinoic acid, a process altered by ethanol. Int J Biochem Cell Biol. 2002;34(8):1017–1029. doi: 10.1016/s1357-2725(02)00023-7. [DOI] [PubMed] [Google Scholar]
- Schmitt-Gräff A, Krüger S, Bochard F, et al. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am J Pathol. 1991;138(5):1233–1242. [PMC free article] [PubMed] [Google Scholar]
- Schnabl B, Choi YH, Olsen JC, et al. Immortal activated human hepatic stellate cells generated by ectopic telomerase expression. Lab Invest. 2002;82(3):323–333. doi: 10.1038/labinvest.3780426. [DOI] [PubMed] [Google Scholar]
- Scholten D, Trebicka J, Liedtke C, et al. The carbon tetrachloride model in mice. Lab Anim. 2015;49(S1):4–11. doi: 10.1177/0023677215571192. [DOI] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Gwak GY, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119(7):1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Khalili K, Nguyen GC. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol. 2014;20(45):16820–16830. doi: 10.3748/wjg.v20.i45.16820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci U S A. 1997;94(20):10663–10668. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto T, Kamikado C, Koyama S. Increased sinusoidal resistance is responsible for the basal state and endothelin-induced venoconstriction in perfused cirrhotic rat liver. Pflugers Arch. 2008;456(3):467–477. doi: 10.1007/s00424-007-0437-6. [DOI] [PubMed] [Google Scholar]
- Shibata N, Watanabe T, Okitsu T, et al. Establishment of an immortalized human hepatic stellate cell line to develop antifibrotic therapies. Cell Transplant. 2003;12(5):499–507. doi: 10.3727/000000003108747064. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Ji C, Kaplowitz N. Differences in betaine-homocysteine methyltransferase expression, endoplasmic reticulum stress response, and liver injury between alcohol-fed mice and rats. Hepatol. 2010;51(3):796–805. doi: 10.1002/hep.23391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia G, Aiolfi R, Di Lucia P, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A. 2012;109(32):E2165–2172. doi: 10.1073/pnas.1209182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP. Animal models for the study of human disease. Elsevier; China: 2013. [Google Scholar]
- Starkel P, Leclercq IA. Animal models for the study of hepatic fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:319–333. doi: 10.1016/j.bpg.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Stefano JT, Cogliati B, Santos F, et al. S-Nitroso-N-acetylcysteine induces de-differentiation of activated hepatic stellate cells and promotes antifibrotic effects in vitro. Nitric Oxide. 2011;25(3):360–365. doi: 10.1016/j.niox.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Stensgaard AS, Utzinger J, Vounatsou P, et al. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: does climate matter? Acta Trop. 2013;128(2):378–390. doi: 10.1016/j.actatropica.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Sung CK, She H, Xiong S, et al. Tumor necrosis factor-alpha inhibits peroxisome proliferator-activated receptor gamma activity at a posttranslational level in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;286(5):G722–729. doi: 10.1152/ajpgi.00411.2003. [DOI] [PubMed] [Google Scholar]
- Svegliati-Baroni G, Ridolfi F, Di Sario A, et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatol. 1999;29(6):1743–1751. doi: 10.1002/hep.510290632. [DOI] [PubMed] [Google Scholar]
- Svegliati-Baroni G, Ridolfi F, Hannivoort R, et al. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterol. 2005;128(4):1042–1055. doi: 10.1053/j.gastro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pérez Y, Carrasco-Legleu C, García-Cuellar C, et al. Oxidative stress in carcinogenesis. Correlation between lipid peroxidation and induction of preneoplastic lesions in rat hepatocarcinogenesis. Cancer Lett. 2005;217(1):25–32. doi: 10.1016/j.canlet.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Tacke F, Weiskirchen R. Update on hepatic stellate cells: pathogenic role in liver fibrosis and novel isolation techniques. Expert Rev Gastroenterol Hepatol. 2012;6(1):67–80. doi: 10.1586/egh.11.92. [DOI] [PubMed] [Google Scholar]
- Taimr P, Higuchi H, Kocova E, et al. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatol. 2003;37(1):87–95. doi: 10.1053/jhep.2003.50002. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Yoshioka M, Yamanaka N, et al. Reversible conversion of epithelial and mesenchymal phenotypes in SV40 large T antigen-immortalized rat liver cell lines. Cell Biol Int Rep. 2010;17(1):e00001. doi: 10.1042/CBR20100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Yang J, Li J. Accelerative effect of leflunomide on recovery from hepatic fibrosis involves TRAIL-mediated hepatic stellate cell apoptosis. Life Sci. 2009;84(15-16):552–557. doi: 10.1016/j.lfs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Taura K, Miura K, Iwaisako K, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatol. 2010;51(3):1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RJ, Bhandari R, Barrett DA, et al. The effect of three-dimensional co-culture of hepatocytes and hepatic stellate cells on key hepatocyte functions in vitro. Cells Tissues Organs. 2005;181(2):67–79. doi: 10.1159/000091096. [DOI] [PubMed] [Google Scholar]
- Thrall KD, Vucelick ME, Gies RA, et al. Comparative metabolism of carbon tetrachloride in rats, mice, and hamsters using gas uptake and PBPK modeling. J Toxicol Environ Health A. 2000;60(8):531–548. doi: 10.1080/00984100050082085. [DOI] [PubMed] [Google Scholar]
- Tosello-Trampont AC, Landes SG, Nguyen V, et al. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem. 2012;287(48):40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger JS, Mederacke I, Gwak GY, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterol. 2012;143(4):1073–1083. e1022. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura K, Ichinose F, Hara T, et al. The inhalation exposure of carbon tetrachloride promote rat liver carcinogenesis in a medium-term liver bioassay. Toxicol Lett. 2008;176(3):207–214. doi: 10.1016/j.toxlet.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Reidelberger RD, French SW, et al. Long-term cannulation model for blood sampling and intragastric infusion in the rat. Am J Physiol. 1984;247(3):R595–599. doi: 10.1152/ajpregu.1984.247.3.R595. [DOI] [PubMed] [Google Scholar]
- van Agthoven M, Metselaar HJ, Tilanus HW, et al. A comparison of the costs and effects of liver transplantation for acute and for chronic liver failure. Transpl Int. 2001;14(2):87–94. doi: 10.1007/s001470050852. [DOI] [PubMed] [Google Scholar]
- van de Bovenkamp M, Groothuis GM, Meijer DK, et al. Precision-cut fibrotic rat liver slices as a new model to test the effects of anti-fibrotic drugs in vitro. J Hepatol. 2006;45(5):696–703. doi: 10.1016/j.jhep.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Van Rooyen DM, Larter CZ, Haigh WG, et al. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterol. 2011;141(4):1393–1403. doi: 10.1053/j.gastro.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996;71(1-2):57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- Viñas O, Bataller R, Sancho-Bru P, et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatol. 2003;38(4):919–929. doi: 10.1053/jhep.2003.50392. [DOI] [PubMed] [Google Scholar]
- Vogel S, Piantedosi R, Frank J, et al. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41(6):882–893. [PubMed] [Google Scholar]
- Walkin L, Herrick SE, Summers A, et al. The role of mouse strain differences in the susceptibility to fibrosis: a systematic review. Fibrogenesis Tissue Repair. 2013;6(1):18. doi: 10.1186/1755-1536-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MC, Hamesch K, Lunova M, et al. Standard operating procedures in experimental liver research: thioacetamide model in mice and rats. Lab Anim. 2015;49(S1):21–29. doi: 10.1177/0023677215573040. [DOI] [PubMed] [Google Scholar]
- Wang P, Liu T, Cong M, et al. Expression of extracellular matrix genes in cultured hepatic oval cells: an origin of hepatic stellate cells through transforming growth factor beta? Liver Int. 2009;29(4):575–584. doi: 10.1111/j.1478-3231.2009.01992.x. [DOI] [PubMed] [Google Scholar]
- Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- Weill FX, Blazejewski S, Blanc JF, et al. Characterization of a new human liver myofibroblast cell line: transcriptional regulation of plasminogen activator inhibitor type I by transforming growth factor beta 1. Lab Invest. 1997;77(1):63–70. [PubMed] [Google Scholar]
- Weiskirchen R, Gressner AM. Isolation and culture of hepatic stellate cells. Methods Mol Med. 2005;117:99–113. doi: 10.1385/1-59259-940-0:099. [DOI] [PubMed] [Google Scholar]
- Weiskirchen R, Weimer J, Meurer SK, et al. Genetic characteristics of the human hepatic stellate cell line LX-2. PLoS One. 2013;8(10):e75692. doi: 10.1371/journal.pone.0075692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen F, Chang S, Toh YC, et al. Development of dual-compartment perfusion bioreactor for serial coculture of hepatocytes and stellate cells in poly(lactic-co-glycolic acid)-collagen scaffolds. J Biomed Mater Res B Appl Biomater. 2008;87(1):154–162. doi: 10.1002/jbm.b.31086. [DOI] [PubMed] [Google Scholar]
- Westra IM, Oosterhuis D, Groothuis GM, et al. Precision-cut liver slices as a model for the early onset of liver fibrosis to test antifibrotic drugs. Toxicol Appl Pharmacol. 2014a;274(2):328–338. doi: 10.1016/j.taap.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Westra IM, Oosterhuis D, Groothuis GM, et al. The effect of antifibrotic drugs in rat precision-cut fibrotic liver slices. PLoS One. 2014b;9(4):e95462. doi: 10.1371/journal.pone.0095462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz W, Antoine M, Tag CG, et al. Hepatic stellate cells display a functional vascular smooth muscle cell phenotype in a three-dimensional co-culture model with endothelial cells. Differentiation. 2008;76(7):784–794. doi: 10.1111/j.1432-0436.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- Wouters K, van Gorp PJ, Bieghs V, et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatol. 2008;48(2):474–486. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- Wu CF, Lin YL, Huang YT. Hepatitis C virus core protein stimulates fibrogenesis in hepatic stellate cells involving the obese receptor. J Cell Biochem. 2013;114(3):541–550. doi: 10.1002/jcb.24392. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Cheever AW. Cytokine regulation of granuoma formation in shistosomiasis. Curr Opin Immunol. 1995;7:505–511. doi: 10.1016/0952-7915(95)80095-6. [DOI] [PubMed] [Google Scholar]
- Xu L, Hui AY, Albanis E, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54(1):142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zeisberg M, Mosterman B, et al. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterol. 2003;124(1):147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- Yang KL, Chang WT, Chuang CC, et al. Antagonizing TGF-beta induced liver fibrosis by a retinoic acid derivative through regulation of ROS and calcium influx. Biochem Biophys Res Commun. 2008;365(3):484–489. doi: 10.1016/j.bbrc.2007.10.203. [DOI] [PubMed] [Google Scholar]
- Yin MF, Lian LH, Piao DM, et al. Tetrandrine stimulates the apoptosis of hepatic stellate cells and ameliorates development of fibrosis in a thioacetamide rat model. World J Gastroenterol. 2007;13(8):1214–1220. doi: 10.3748/wjg.v13.i8.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Matsuzaki K. Differential Regulation of TGF-β/Smad Signaling in Hepatic Stellate Cells between Acute and Chronic Liver Injuries. Front Physiol. 2012;3:53. doi: 10.3389/fphys.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Matsuzaki K, Mori S, et al. Transforming growth factor-beta and platelet-derived growth factor signal via c-Jun N-terminal kinase-dependent Smad2/3 phosphorylation in rat hepatic stellate cells after acute liver injury. Am J Pathol. 2005;166(4):1029–1039. doi: 10.1016/s0002-9440(10)62324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ogata H, Kamio M, et al. SOCS1 is a suppressor of liver fibrosis and hepatitis-induced carcinogenesis. J Exp Med. 2004;199(12):1701–1707. doi: 10.1084/jem.20031675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Yang C, Martino M, et al. Fibroblasts derived from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282(32):23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang D, Gao W, et al. Lack of IL-17 signaling decreases liver fibrosis in murine shistosomiasis japonica. Int Immunol. 2015 doi: 10.1093/intimm/dxv017. doi:10.1093/intimm/dxv017. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shen J, Man K, et al. CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis. J Hepatol. 2014;61(6):1365–1375. doi: 10.1016/j.jhep.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Zhen MC, Huang XH, Wang Q, et al. Green tea polyphenol epigallocatechin-3-gallate suppresses rat hepatic stellate cell invasion by inhibition of MMP-2 expression and its activation. Acta Pharmacol Sin. 2006;27(12):1600–1607. doi: 10.1111/j.1745-7254.2006.00439.x. [DOI] [PubMed] [Google Scholar]