Abstract
Many studies suggest that trimethylamine-N-oxide (TMAO), a gut-flora-dependent metabolite of choline, contributes to the risk of cardiovascular diseases, but little is known for non-alcoholic fatty liver disease (NAFLD). We examined the association of circulating TMAO, choline and betaine with the presence and severity of NAFLD in Chinese adults. We performed a hospital-based case-control study (CCS) and a cross-sectional study (CSS). In the CCS, we recruited 60 biopsy-proven NAFLD cases and 35 controls (18–60 years) and determined serum concentrations of TMAO, choline and betaine by HPLC-MS/MS. For the CSS, 1,628 community-based adults (40-75 years) completed the blood tests and ultrasonographic NAFLD evaluation. In the CCS, analyses of covariance showed adverse associations of ln-transformed serum levels of TMAO, choline and betaine/choline ratio with the scores of steatosis and total NAFLD activity (NAS) (all P-trend <0.05). The CSS revealed that a greater severity of NAFLD was independently correlated with higher TMAO but lower betaine and betaine/choline ratio (all P-trend <0.05). No significant choline-NAFLD association was observed. Our findings showed adverse associations between the circulating TMAO level and the presence and severity of NAFLD in hospital- and community-based Chinese adults, and a favorable betaine-NAFLD relationship in the community-based participants.
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in Western and Asian countries1. It may progress to chronic liver disease, culminating in end-stage liver disease. The prevalence of NAFLD in China is increasing at an alarming rate and currently ranges from 11.5%–27%2,3. Although the exact causes of this disease are uncertain, many studies have shown that food components and their metabolites may play important roles in its development and progression4.
Choline is an essential nutrient that serves as a component of phosphatidylcholine (PC), a precursor of the neurotransmitter acetylcholine. Choline can be oxidized to betaine in humans. Choline and betaine function as methyl donors in pathways involving the re-methylation of homocysteine to methionine to diminish blood homocysteine5 and in DNA and histone methylation, which may play potential roles in NAFLD and other cardio-metabolic diseases6. A few studies have examined the association of choline and betaine with fatty liver disease in animals and humans. Raubenheimer et al. have found that a choline-deficient diet greatly exacerbates fatty liver induced by high-fat diet consumption in rodents7. A small number of human studies have shown that the consumption of a low-choline diet promotes fatty liver and liver damage8,9. However, some epidemiological studies have found that a high blood choline level is positively associated with NAFLD10, metabolic syndrome (or dyslipidemia)11 and major adverse cardiovascular events (MACEs)12,13. It is unclear whether choline itself or its metabolite, which is produced by humans and gut microbiota, contributes to the adverse events experienced by individuals with high choline intake.
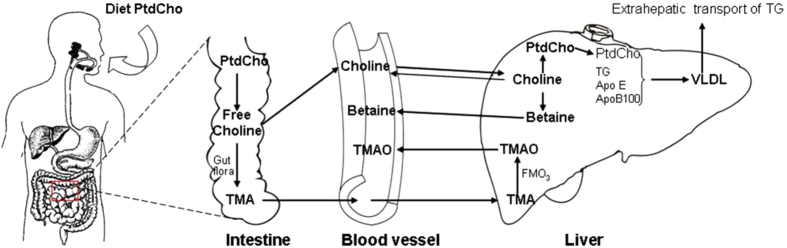
Choline can be metabolized to trimethylamine (TMA) by the gut microbiota (Fig. 1). TMA is subsequently oxidized by hepatic flavin-containing monooxygenases in the liver, forming trimethylamine-N-oxide (TMAO), which is then released into circulation14,15. Previous studies have revealed that TMAO may affect lipid absorption and cholesterol homeostasis and modulate glucose and lipid metabolism by decreasing the total bile acid pool size16. An animal study has suggested that TMAO may exacerbate impaired glucose tolerance by blocking the hepatic insulin signaling pathway and promoting the development of fatty liver in mice fed a high-fat diet17. Several human studies have shown that an elevated level of TMAO may increase the risk of cardiovascular disease (CVD) and exacerbate the effects of high choline and betaine levels on the risk of MACEs in a large-scale clinical cohort14,18. These studies have suggested that high circulating TMAO, a gut-flora-dependent choline metabolite, may increase the risk of cardiometabolic disease. However, to our knowledge, no study has examined the association between TMAO and NAFLD in humans to date, and few studies have determined the relationship between blood betaine and NAFLD in humans either.
Figure 1. Schematic chart of choline metabolism.
The chart was drawn by Y.L. and H.L.Z. Abbreviations: PtdCho: phosphatidylcholine; TG: Triglyceride; TMA: Trimethylamine; TMAO: Trimethylamine N-oxide; VLDL: Very low density lipoprotein.
The aims of the present study were to evaluate the association of circulating TMAO, choline and betaine with the presence and severity of NAFLD in clinical and community-based Chinese adults.
Participants and Methods
Study participants
We conducted a case-control study (CCS) and cross-sectional study (CSS). For the CCS, we recruited 60 adults (age: 18–60 years) with biopsy-proven NAFLD from outpatients with NAFLD evaluated firstly by ultrasonography, in addition to 35 controls with the same age rage at the Affiliated Dongnan Hospital of Xiamen University and Sun Yat-Sen Memorial Hospital from April 2012 to June 2013. The controls were patients who had undergone partial hepatectomy due to hepatic hemangioma or hepatolithiasis and had normal liver histopathology. Subjects with the following conditions were be excluded: confirmed heart diseases, stroke, cancer, excessive alcohol consumption, autoimmune liver disease or other conditions19 that may result in fatty liver. For the CSS, participants were drawn from a community-based cohort, from which 3,169 participants (40–75 years) were recruited among apparently healthy residents of Guangzhou, China between July 2008 and June 201020. Of them, 1,996 participants without the following exclusion criteria underwent testing to determine the plasma TMAO, choline and betaine levels: a history of excessive alcohol consumption, the use of steatogenic medication, autoimmune liver disease, chronic viral hepatitis, and hepatic carcinoma or decompensation. In addition, 1,628 of the 1996 subjects underwent further NAFLD testing by ultrasonography between April 2011 and January 2013. Baseline data, including general information, plasma levels of TMAO, choline and betaine, and NAFLD measurements at follow-up were used in the CSS. The abovementioned exclusion criteria were used in both studies. The study protocol complied with all provisions of the 1975 Declaration of Helsinki and its current amendment, and was approved by the Ethics Committee of the School of Public Health of Sun Yat-sen University. Written informed consent was obtained from all participants.
Data collection
For both the CCS and CSS, we collected general information using the same structured questionnaires and performed body measurements and biochemical tests of fasting serum lipid and glucose levels and plasma levels of TMAO, choline and betaine. Histopathological evaluation of liver biopsy samples and blood AST and ALT tests were conducted for the CCS, and ultrasonographic evaluation of NAFLD was performed in the CSS.
Questionnaire interview and Anthropometric measurements
Face-to-face interviews were conducted to collect the following information: socio-demographic characteristics (e.g., age, gender, level of education, occupation and other factors), lifestyle habits (e.g., consumption of alcohol, tobacco and tea), physical activities, and history of chronic diseases and medication20. Body weight and height and waist circumferences (WC) were measured while the participants were barefoot and wearing light clothing. Body mass index (BMI, kg/m2) was also calculated. Two consecutive blood pressure (BP) measurements were performed on the right arm after the participant had been sitting for at least 10 min, and the mean value was used in the further analyses.
Laboratory analysis
Fasting serum was isolated and stored at −80 °C until analysis. Serum concentrations of TMAO, choline and betaine were quantified by high-performance liquid chromatography with online electrospray ionization tandem mass spectrometry (HPLC-MS/MS) (Agilent 6400 Series Triple Quad LCMS; CA, USA)20. A volume of 60 μl of either the serum sample or standards was combined with 100 μl of acetonitrile containing 10 μM of internal standards [d9-choline, d9-betaine (Sigma-Aldrich, St. Louis, USA) and 9-TMAO (Toronto Research Chemicals Inc, Toronto, Canada)], and the sample was centrifuged at 13,000 × g for 10 min to precipitate the proteins. Finally, after an additional centrifugation step, the supernatant was analyzed after injection into a normal-phase silica column (2.1 mm × 100 mm, 5 μm) and equilibrated with 30% solution A (15 mmol/L ammonium formate in water, pH 3.0) and 70% solution B (acetonitrile) under isocratic elution with the flow rate of 0.2 mL/min. The coefficients of variation for the between-run assays were 6.0%, 4.91% and 6.21% for TMAO, choline and betaine, respectively.
Overnight fasting serum total cholesterol (TC), triglyceride (TG), LDL cholesterol (LDLc), HDL cholesterol (HDLc) and fasting blood glucose were measured by colorimetric methods using a Hitachi 7600-010 automated analyzer.
Histopathologic evaluation
Liver biopsy samples were stained using both hematoxylin and eosin (H&E) and Masson’s trichrome methods. Histological assessment was performed according to the nonalcoholic steatohepatitis (NASH) Clinical Research Network Scoring System21 by two experienced pathologists who were unaware of the participants’ information. Fatty liver was categorized as follows, according to the proportion of steatosis22: grade 0 (<5%), 1 (5%–33%), 2 (33%–66%), or 3 (>66%). Hepatocellular ballooning was graded as none (grade 0), few balloons (grade 1), and many cells/prominent (grade 2). According to the number of inflammatory foci per field of view at a magnification of 200×, lobular inflammation was classified as grade 0 (none), 1 (<2 foci/field), 2 (2–4 foci/field) and 3 (>4 foci/field). The total NAFLD activity score (NAS) was calculated as the sum of the scores for steatosis, hepatocellular ballooning and lobular inflammation and was then categorized as no NASH (<3), borderline NASH (3−4) and NASH (≥5).
Abdominal ultrasonography and Diagnosis of NAFLD
Ultrasonography of the upper abdominal organs was performed using a color Doppler ultrasound (Sonoscape SSI-5500, Shenzhen, China) with a 3.5 MHz probe by experienced physicians who blinded to the participants’ information. Fatty liver disease was diagnosed according to Graif’s criteria23, which has been adopted by the Chinese Society of Herpetology24. The degree of steatosis was assessed semi-quantitatively based on fatty-fibrotic patterns as described by Graif et al.23, and reported in our previous article25. In brief, the ultrasonic diagnosis was based on follows25: 1) diffuse enhancement of the near-field echo in the hepatic region (stronger than in the kidney and spleen regions) and gradual attenuation of the far-field echo; 2) unclear display of intra-hepatic lacuna structures; 3) mild to moderate hepatomegaly with a round and blunt border; 4) color Doppler ultrasonography showing a reduction of the blood flow signal in the liver or a difficult-to-display signal with a normal distribution of blood flow; and 5) unclear or non-intact display of the envelope of the right liver lobe and diaphragm. Subjects were graded into: absent (none of above items); mild (1 and any one of item 2–4); moderate (1 and any two of item 2–4); and severe (1, 5 and any two of item 2–4). NAFLD was then confirmed after excluding the following conditions: significant alcohol consumption for hepatic steatosis and chronic liver disease1.
Statistical analysis
All analyses were conducted using SPSS statistical software (version 13.0, SPSS Inc., Chicago, IL), and P values of below 0.05 (two-tailed) were considered significant in all the statistical analyses. Skewed variables were natural logarithm (ln)-transformed before subsequent analyses. Analyses of variance (ANOVA) and covariance (ANCOVA) and t-tests were used to compare mean differences in the participants’ characteristics, and chi-square (or Fisher’s exact) tests were used to analyze differences in the frequencies of categorical variables among the NAFLD groups. ANOVA and ANCOVA were used to compare mean differences, and the sub-command of “Polynomial contrasts” was used to test the linear trend across the serum levels of TMAO, choline and betaine across the NAFLD groups in the CCS and CSS. Bonferroni t test was used for the multiple comparisons between the NAFLD groups. For the multivariate models, we adjusted for age, sex (in total men and women), waist circumference, SBP, blood cholesterol, triglyceride, HDLc, LDLc, glucose, uric acid, education levels (secondary or below, high school, college or above), job (light, moderate and heavy, in physcial labor work), household income (<4000, 4000-6000, >6000, yuan/month/person), smoking (current or /no), alcohol intake statuses (current or /no), physical activity (in MET h/week, excluding sleeping and sitting), and dietary intakes of total energy, fat, and fiber (continous variables except those defined). Logistic regression analyses were used to calculate the odds ratios (OR) and their 95% confidence interval (CI) of NAFLD (or related indices) for the quartiles of serum values of TMAO, betaine, and choline in the CSS, and for each one unit increase in ln-transformed serum values of TMAO, betaine and choline in the CCS.
Results
Case-control study
The NAFLD patients included 48 men and 12 women, with a mean (SD) age of 34.8 (10.2) years. There were 22 men and 13 women in the control group, with a mean age of 44.8 (10.8) years. The NAFLD patients had a younger mean age and higher levels of BMI, WC, DBP, and blood lipids (TC, TGs, LDLc, and HDLc) compared with the controls (all p < 0.05) (Table 1). The ln-transformed levels of TMAO were positively associated with the corresponding values of choline (pearson correlation coefficient (r) = 0.487, 0.203) and betaine (r = 0.294, 0.159) in the CCS and CSS (all p < 0.05) (data not shown).
Table 1. Characteristics of the study population in the case-control study1.
| Normal subjects (n = 35) | NAFLD patients (n = 60) | P | Adjusted P1 | Adjusted P2 | |
|---|---|---|---|---|---|
| General characteristics | |||||
| Age, years | 44.8 ± 10.8 | 34.8 ± 10.2 | <0.001 | – | – |
| Male/female, n | 22/13 | 48/12 | 0.067 | – | – |
| BMI, kg/m2 | 22.6 ± 3.2 | 27.9 ± 3.1 | <0.001 | <0.001 | – |
| Waist circumference, cm | 84.6 ± 9.1 | 95.6 ± 7.4 | <0.001 | <0.001 | – |
| SBP, mmHg | 120.8 ± 11.4 | 125.4 ± 13.5 | 0.117 | 0.180 | 0.892 |
| DBP, mmHg | 78.0 ± 9.3 | 82.6 ± 10.9 | 0.044 | 0.131 | 0.472 |
| Current smoker, n(%) | 10(30.3) | 17(29.8) | 0.962 | 0.470 | – |
| Passive smoker, n(%) | 17(56.67) | 30(56.60) | 0.996 | 0.393 | – |
| Physical activity, MET | 37.7 ± 21.1 | 35.3 ± 23.4 | 0.619 | 0.269 | – |
| Biochemical characteristics | |||||
| AST, U/L | 26.0(20.7,44.5) | 30.1(22.6,42.1) | 0.871 | 0.049 | 0.951 |
| ALT, U/L | 41.8(24.0,73.6) | 56.5(31.1,87.7) | 0.206 | 0.321 | 0.807 |
| Blood glucose, mg/dL | 5.30(4.97,5.85) | 5.09(4.76,5.95) | 0.502 | 0.215 | 0.344 |
| Triglycerides, mmol/L | 1.15(0.76,1.69) | 1.79(1.18,2.71) | 0.005 | 0.039 | 0.925 |
| Cholesterol, mmol/L | 3.95 ± 1.50 | 5.22 ± 1.13 | <0.001 | <0.001 | 0.062 |
| HDLc, mg/dL | 1.00 ± 0.31 | 1.25 ± 0.26 | <0.001 | 0.001 | 0.140 |
| LDLc, mg/dL | 2.49 ± 1.02 | 2.92 ± 0.83 | 0.028 | 0.025 | 0.152 |
| HBV( + ), n(%) | 12(35.3) | 16(27.1) | 0.659 | 0.122 | – |
1The data are expressed as the mean ± SD or median (interquartile range) according to the distribution of variables.
The P values in bold indicates significant differences. Adjusted P1: Adjusted for age and gender by analysis of covariance (ANCOVA) for continous variables, and multivariate logistic regression analysis for binary variables. Adjusted P2: Adjusted for age, gender, HB virus, MET, BMI, waist circumference, and smoking status by using ANCOVA or logistic regression analysis. Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure; HDLc: high-density lipoprotein cholesterol; LDLc: low-density lipoprotein cholesterol; and MET: metabolic equivalent task.
ANOVA showed significantly higher scores for steatosis, NAS, and lobular inflammation in association with greater ln-transformed levels of TMAO, choline and betaine, except for the steatosis scores associated with betaine. The ratio of betaine to choline was inversely associated with the steatosis and NAS (all p < 0.05) (STable 1). After adjusting for the potential covariates (age, sex, smoking status, alcohol intake status, physical activity and WC), the associations were attenuated. However, significant associations remained between the serum ln-transformed level of TMAO, betaine/choline ratio, choline level, and steatosis and NAS (ANCOVA). The serum levels of betaine and choline were positively correlated with the lobular inflammation score. To determine whether the associations between the choline level and the scores for steatosis, NAS and lobular inflammation were mediated by TMAO, we further adjusted for TMAO in analysis of choline. The associations of choline with the steatosis, NAS and inflammation scores were attenuated to non- or marginally significant levels. (Table 2).
Table 2. Comparison of the covariate-adjusted means of serum TMAO, betaine, choline and betaine to choline ratio according to histologic features of NAFLD in the case-control study.
| n | mean | SE | n | mean | SE | n | mean | SE | ANCOVA |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P-Diff | P-trend | ||||||||||
| Steatosis score | 0 | 1 | 2–3 | ||||||||
| Ln(TMAO, μM) | 35 | 2.20 | 0.20 | 31 | 3.09 | 0.18** | 25 | 2.95 | 0.22 | 0.011 | 0.026 |
| Ln(Betaine, μM) | 35 | 3.553 | 0.77 | 31 | 3.552 | 0.071 | 25 | 3.566 | 0.085 | 0.990 | 0.920 |
| Ln(Choline, μM) | 35 | 2.209 | 0.087 | 31 | 2.444 | 0.080 | 25 | 2.543 | 0.096 | 0.072 | 0.025 |
| Ln(Choline, μM) # | 2.308 | 0.082 | 2.371 | 0.075 | 2.496 | 0.088 | 0.346 | 0.174 | |||
| Betaine/Choline | 35 | 1.344 | 0.083 | 31 | 1.107 | 0.076 | 25 | 1.023 | 0.091 | 0.063 | 0.023 |
| Betaine/Choline# | 1.313 | 0.086 | 1.131 | 0.078 | 1.038 | 0.091 | 0.160 | 0.058 | |||
| NAFLD activity score | 0 | 1–2 | 3–5 | ||||||||
| Ln(TMAO, μM) | 23 | 1.767 | 0.245 | 28 | 2.963 | ** | 40 | 3.068 | 0.157** | <0.001 | <0.001 |
| Ln(Betaine, μM) | 23 | 3.384 | 0.098 | 28 | 3.625 | 0.071 | 40 | 3.608 | 0.063 | 0.160 | 0.089 |
| Ln(Choline, μM) | 23 | 1.929 | 0.103 | 28 | 2.488 | 0.075** | 40 | 2.566 | 0.066** | <0.001 | <0.001 |
| Ln(Choline, μM) # | 2.166 | 0.109 | 2.474 | 0.081 | 2.435 | 0.066 | 0.104 | 0.065 | |||
| Betaine/Choline | 23 | 1.455 | 0.106 | 28 | 1.137 | 0.077 | 40 | 1.041 | 0.068* | 0.016 | 0.004 |
| Betaine/Choline# | 1.419 | 0.116 | 1.147 | 0.078 | 1.055 | 0.071 | 0.071 | 0.022 | |||
| Lobular inflammation score | 0 | 1 | 2–3 | ||||||||
| Ln(TMAO, μM) | 30 | 2.196 | 0.216 | 33 | 3.169 | 0.193* | 26 | 2.667 | 0.194 | 0.015 | 0.128 |
| Ln(Betaine, μM) | 30 | 3.338 | 0.079 | 33 | 3.662 | 0.070* | 26 | 3.640 | 0.071* | 0.015 | 0.009 |
| Ln(Choline, μM) | 30 | 2.058 | 0.090 | 33 | 2.600 | 0.080** | 26 | 2.469 | 0.081** | 0.001 | 0.002 |
| Ln(Choline, μM) # | 2.180 | 0.088 | 2.507 | 0.082>* | 2.428 | 0.075 | 0.054 | 0.042 | |||
| Betaine/Choline | 30 | 1.280 | 0.092 | 33 | 1.062 | 0.082 | 26 | 1.172 | 0.083 | 0.303 | 0.407 |
| Betaine/Choline# | 1.239 | 0.094 | 1.101 | 0.085 | 1.169 | 0.082 | 0.647 | 0.595 | |||
*,**compared with histologic features of “0” group; P < 0.05 and P < 0.01, respectively.
ANCOVA (analysis of covariance): adjusted for age, sex, smoking status (yes/no), alcohol intake status (yes/no), physical activity (in MET [hour/day], excluding sleeping and sitting), and waist circumference. Bonferroni t test was used for the multiple comparisons between the NAFLD groups. P values of below 0.05 (two-tailed) were considered significant.
P-Diff: P value for the difference among the groups.
#: further adjusted for ln-TMAO.
ORs (95%CI) of high score (≥1 vs. 0) of NAFLD indices for each one unit inrease in ln-transformed values of TMAO (μM) were 3.58 (1.38–9.26) for the statosis score, 3.34 (1.44–7.78) for the NASH score, and 2.37 (1.10–5.09) for the lobular inflammation score, respectively. Higher choline values were also associatied with greater risks of having high scores of statosis, NASH, and lobular inflmmation. (STable 3).
In consistent with the results NAFLD, ln-TMAO and ln-choline were positively and significantly correlated to serum levels of TC, TG and HDLc (all p < 0.05), but not to LDLc after adjusted for age and sex. (data not shown).
Cross-sectional study
Table 3 shows the characteristics of the participants. Among 1628 participants, 43.2%, 10.3% and 2.6% had mild, moderate and severe NAFLD, respectively, and 79.4% were women. The severity of NAFLD was positively associated with age, BMI, WC, blood pressure, TGs, LDLc and glucose, but negatively correlated with HDLc in both the males and females (all p < 0.05). More NAFLD patients than normal subjects were smokers.
Table 3. Characteristics of the study participants in the cross-sectional study (CSS)1.
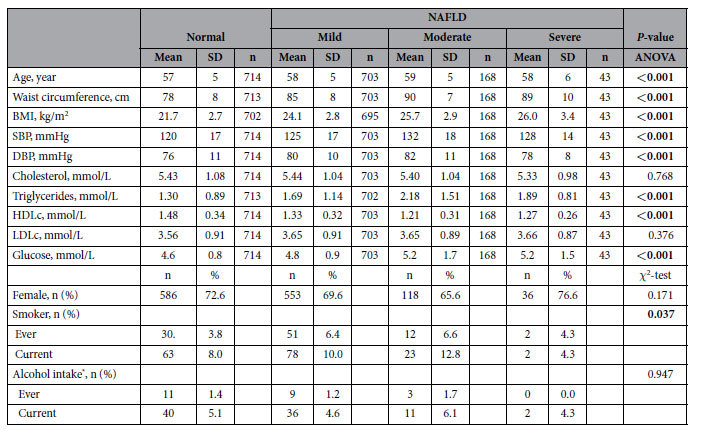
1The data are expressed as the mean ± SD or n (%) according to the measurements or frequencies of the variables.
The P values in bold indicate significant differences. Abbreviations: BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure; HDLc: high-density lipoprotein cholesterol; and LDLc: low-density lipoprotein cholesterol.
*Drinking alcohol beverages ≥1 times/week in the past year. The fisher’s exact test was used.
Table 4 shows that a higher concentration of ln-transformed TMAO and a lower concentration of ln-transformed betaine and betaine to choline ratio were associated with a greater severity of NAFLD after adjusting for age, sex, WC, metabolic risk factors, socioeconomic factors, smoking status and physical activity for all participants and for the women (all p-trend < 0.05). The associations were similar in women and in men. Univariate analysis resulted in more pronounced associations (STable 2). We did not observe any significant differences in ln-transformed the plasma choline concentration among NAFLD groups by either univariate or multivariate analysis of variance (Table 4, STable 2).
Table 4. Comparison of covariate-adjusted means of serum TMAO, betaine, choline and betaine to choline ratio with NAFLD groups in the cross-sectional study.
| NAFLD |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal |
Mild |
Moderate & Severe |
ANCOVA |
||||||||
| n | Mean | SE | n | Mean | SE | n | Mean | SE | P-Diff | P-trend | |
| Total | |||||||||||
| Ln(TMAO, μM) | 643 | 0.104 | 0.040 | 643 | 0.160 | 0.038 | 197 | 0.434 | 0.073**,## | <0.001 | <0.001 |
| Ln(Betaine, μM) | 662 | 3.840 | 0.017 | 671 | 3.809 | 0.016 | 208 | 3.667 | 0.030**,## | <0.001 | <0.001 |
| Ln(Choline, μM) | 662 | 3.179 | 0.018 | 671 | 3.169 | 0.017 | 208 | 3.092 | 0.032 | 0.065 | 0.026 |
| Betaine/Choline | 662 | 2.156 | 0.037 | 671 | 2.096 | 0.035 | 208 | 1.987 | 0.065 | 0.104 | 0.034 |
| Women | |||||||||||
| Ln(TMAO, μM) | 457 | 0.096 | 0.049 | 437 | 0.134 | 0.047 | 135 | 0.382 | 0.089* | 0.021 | 0.007 |
| Ln(Betaine, μM) | 470 | 3.792 | 0.021 | 453 | 3.755 | 0.020 | 140 | 3.601 | 0.038**,## | <0.001 | <0.001 |
| Ln(Choline, μM) | 470 | 3.165 | 0.022 | 453 | 3.158 | 0.022 | 140 | 3.071 | 0.041 | 0.120 | 0.053 |
| Betaine/Choline | 470 | 2.084 | 0.042 | 453 | 2.010 | 0.041 | 140 | 1.930 | 0.077 | 0.243 | 0.106 |
| Men | |||||||||||
| Ln(TMAO, μM) | 186 | 0.118 | 0.075 | 206 | 0.222 | 0.066 | 62 | 0.539 | 0.130* | 0.032 | 0.009 |
| Ln(Betaine, μM) | 192 | 3.948 | 0.028 | 218 | 3.925 | 0.025 | 68 | 3.822 | 0.048 | 0.096 | 0.035 |
| Ln(Choline, μM) | 192 | 3.208 | 0.030 | 218 | 3.188 | 0.027 | 68 | 3.154 | 0.051 | 0.699 | 0.399 |
| Betaine/Choline | 192 | 2.325 | 0.074 | 218 | 2.287 | 0.064 | 68 | 2.116 | 0.124 | 0.377 | 0.175 |
*,**compared with “Normal”; P < 0.05 and P < 0.01, respectively;
##compared with “Mild NAFLD”; P < 0.01, respectively.
The following covariates were adjusted for: age, sex (male/female) (in total), waist circumference, SBP, blood cholesterol, triglyceride, HDL, LDL glucose, uric acid, and education levels (secondary or below, high school, college or above), job (light, moderate and heavy, in physcial labor work), household income (<4000, 4000-6000, >6000, yuan/month/person), smoking (yes/no), and alcohol intake statuses (yes /no), and physical activity (in MET h/week, excluding sleeping and sitting), and dietary intakes of total energy, fat, and fiber (continous variables except those defined). Bonferroni t test was used for the multiple comparisons between the NAFLD groups. P values of below 0.05 (two-tailed) were considered significant.
ORs (95%CI) of NAFLD (none vs. moderate or severe) for the highest (vs. lowest) quartile of TMAO, betaine and choline were 3.25 (1.61-6.56) , 0.13 (0.06-0.26) , and 0.47 (0.25–0.89), respectively. Similar trends were observed for the different cutoffs of NAFLD. (STable 4).
Positive association of ln-TAMO were observed with serum levels of TC, TG and LDLc (p < 0.05); while ln-betaine were inversely associated with TC, TG and LDLc (p < 0.05). No significant associations between ln-choline and lipids were observed . (data not shown).
Discussion
Main findings
In the present study, we found consistently adverse associations between the plasma TMAO level and the presence and severity of NAFLD in both the CCS and CSS and a favorable betaine-NAFLD association in the CSS. To our knowledge, the present study is the first to report the adverse association of TMAO with NAFLD in humans. Our findings suggest that TMAO, an intestinal microbiota-dependent metabolite of phosphatidylcholine (PC)/choline, may be an independent risk marker (possible risk factor) for NAFLD.
TMAO and NAFLD
In recent years, a few studies have examined the association between TMAO and cardiovascular risk in both animals and humans. Wang et al.14 have identified a novel pathway (dietary PC/choline → gut-flora-formed TMA → hepatic-flavin monooxygenase-formed TMAO) linking the dietary intake of PC/choline, intestinal microflora and atherosclerosis in mice, in which TMAO promotes the upregulation of multiple macrophage scavenger receptors involved in atherosclerosis. This association has been further confirmed in humans13,18. Tang et al.18 have found that an increased TMAO level is correlated to an increased risk of incident MACE (HR for quartile 4 vs. 1: 2.54, 95%CI: 1.96-3.28) in a 3-year follow-up study of 4007 patients who underwent elective coronary angiography. In addition, an elevated plasma TMAO level has been associated with a 2.2-fold (95%CI, 1.42–3.43) increased risk of mortality after adjusting for traditional risk factors in 720 patients with stable heart failure26. Similar results have been observed in other studies27,28. Further, TMAO may decrease long-term survival in stable subjects with chronic kidney disease, and contribute to progressive renal fibrosis and dysfunction in a murine model29. However, limited data are available for NAFLD. Dumas et al.30 have found that a high urinary excretion of TMAO is associated with insulin resistance and NAFLD in mice (129S6) prone to these diseases. In agreement with these previous studies, we showed an adverse association of the plasma TMAO level with the presence and severity of NAFLD in both clinical patients and community-based participants. The findings of our study and previous studies suggested that TMAO might play diverse adverse roles in the development and progression of various chronic diseases.
The increased risk of fatty liver disease might be caused by TMAO due to its effect on decreasing the total bile acid pool size16 via the following mechanisms: 1) by decreasing the synthesis of bile acids due to the inhibition of the key enzymes CYP7A1 and CYP27A116; and 2) by limiting the enterohepatic circulation of bile acids between the liver and intestines due to the repression of organic anion transporter (oapt) and multidrug resistance protein (MRP) family protein expression16. In addition to their well-established roles in dietary lipid absorption and cholesterol homeostasis, bile acids also act as metabolically active signaling molecules to modulate glucose and lipid metabolism. Therefore, it is possible that TMAO may affect hepatic TGs levels, and reverse the directions of cholesterol transport and glucose and energy homeostasis by altering the synthesis and transport of bile acids, indicating that it is potential risk factor for fatty liver disease.
Choline and NAFLD
Choline is a constituent of cell and mitochondrial membranes. Choline deficiency may affect liver from steatosis to carcinomas via diverse processes, including abnormal phospholipid synthesis, defective very-low-density lipoprotein (VLDL) secretion, aberrations in the methylation-dependent biosynthesis of molecules and the enterohepatic circulation of bile and cholesterol, etc.8. A large number of studies have demonstrated that a choline-deficient diet may cause NAFLD in animals and humans8,31,32. It has been reported that a low dietary choline intake is associated both with a higher risk of NAFLD in normal-weight Chinese women33 and with worse liver fibrosis in postmenopausal US women with NAFLD9. On the other hand, high plasma choline levels have been associated with an unfavorable cardiovascular risk factor profile11 and higher MACE risk12,13. To our knowledge, only one study has examined circulating choline levels and NAFLD, showing that plasma free choline levels are positively related to the severity of liver steatosis, fibrosis and NASH in Japanese10. In this study, we observed an unfavorable association in the hospital-based patients, but not in the community-based adults. The adverse association between circulating choline and NAFLD detected in the CCS may have been due to the TMAO level because this significant association was almost attenuated to null after analysis was adjusted for TMAO.
Betaine and NAFLD
Betaine is an important methyl donor that may be obtained from dietary sources or synthesized endogenously from choline. It may supply its methyl group for the remethylation of homocysteine (Hcy) to methionine and the further regeneration of S-adenosylmethionine (SAM). SAM may subsequently facilitate the metabolic conversion of phosphatidylethanolamine (PE) to form Phosphatidylcholine (PC). Plenty of betaine may partially spare choline34 which is a basic component for the synthesis of PC. PC is necessary for the packaging of VLDL and then promoting lipid exportation from the liver35, subsequently attenuating fatty liver36. Several animal studies have shown that betaine supplementation protects the liver from fat accumulation in rodent models induced by high-fat diets37,38. A small number of randomized controlled trials (RCT) have also reported such beneficial effects of betaine. In a short-term RCT, betaine supplementation for 8 weeks has been found to reduce hepatic steatosis by 25% and attenuate the hepatic concentrations of AST and ALT in 191 patients with NASH39. Another RCT has shown that betaine treatment (20 g/d vs. placebo) for 12 months improves hepatic steatosis and may protect against the worsening of steatosis in 55 patients with biopsy-proven NASH40. Consistent with these results, we observed a significantly inverse association between the plasma betaine concentration and the severity of NAFLD in 1628 community-based adults. Due to the limited evidence in humans, further prospective studies are needed to confirm the potential benefits of betaine in NAFLD.
Strengths and limitations
The strengths of our study include the following: we examined the associations of the plasma levels of TMAO, choline and betaine with NAFLD in both clinical patients with/without biopsy-proven NAFLD and in community-based adults evaluated by ultrasound. In addition, this is the first report of a consistently adverse association between TMAO and NAFLD. Liver biopsy, which was performed in the CCS, is the gold standard for evaluating hepatic steatosis. In addition, the relatively large study size allowed us to have higher power to determine these associations in the CSS, and a variety of covariates including various cardio-metabolic risk factors were adjusted for to avoid potential confounding biases in both studies. However, some residual confounders might be existed due to unmeasured covariates or the potential measurement errors in our studies.
Several limitations should be acknowledged. First, we could not infer a causal relationship between TMAO and NAFLD due to the limitations associated with the study designs used. In addition, we measured the plasma levels of choline and its metabolites in samples obtained at baseline and assessed NAFLD at the follow-up in the CSS. The reported associations are dependent on the stability of the blood levels of TMAO, choline and betaine over an interval of 3.2 years. However, the potential changes in the relevant blood concentrations over the 3.2 years tended to dilute (and not increase) the reported associations, and the delayed evaluation of the dependent variable decreased the possibility of inverse causality in the CSS. Third, liver tissue evaluation is the gold standard method for the assessment of fatty live. Ultrasonography conducted in our CSS may be less sensitive than magnetic resonance spectroscopy in comparison to histology41. A meta-analysis in 2011 reported that liver ultrasound has acceptable sensitivity (84%, 95%CI: 0.79%−0.89%) and excellent specificity (94%, 95%CI: 0.87%−0.97%) compared with liver biopsy42. The misclassification caused by relatively low sensitivity of ultrasound at a low liver fat content41 was unlikely to overestimate the observed association in this study. Fourth, a limited study size was used for the biopsy-proven NAFLD cases and controls due to the difficulty in obtaining such samples. Fifth, inconsistent results for the betaine and choline levels were observed in the CCS and CSS, likely due to the different populations assessed. Lastly, we could not determine whether TMAO was a risk factor or just a marker of co-existing risk factor(s). Howevr, our findings provided a useful clue for further studies of identification of NAFLD causes in humans.
Conclusions
Our findings showed an inverse association of circulating TMAO level with the presence and severity of NAFLD in both the clinical patients and community-based adults, and a favorable relationship between the blood betaine concentration and the severity of NAFLD in the community-based participants but not in the clinical patients. Further studies, particularly inventional human studies or animal models, are needed to clarify if there is a causal relationship between TMAO and NAFLD.
Additional Information
How to cite this article: Chen, Y.-m. et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci. Rep. 6, 19076; doi: 10.1038/srep19076 (2016).
Supplementary Material
Acknowledgments
This study was jointly supported by the National Natural Science Foundation of China (No. 81472966, 81273050, and 81472965) and the 5010 Program for Clinical Studies of Sun Yat-sen University, Guangzhou, China (No. 2007032).
Footnotes
Author Contributions H.L.Z. and Y.M.C. conceived and designed the research; Y.M.C. and H.L.Z. wrote the manuscript; Y.M.C. analyzed the data; Y.L., X.L.C., C.W., X.Y.T., L.J.W., R.D.Z. and H.W.Z. collected the data; W.H.L. gave critical comments; H.L.Z. have the primary responsibility for the final content. All of the authors have read and approved the final manuscript.
References
- Farrell G. C., Wong V. W. & Chitturi S. NAFLD in Asia–as common and important as in the West. Nat Rev Gastroenterol Hepatol 10, 307–18 (2013). [DOI] [PubMed] [Google Scholar]
- Chen C. H. et al. Prevalence and risk factors of nonalcoholic fatty liver disease in an adult population of taiwan: metabolic significance of nonalcoholic fatty liver disease in nonobese adults. J Clin Gastroenterol 40, 745–52 (2006). [DOI] [PubMed] [Google Scholar]
- Fan J. G. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol 28, Suppl 1 11–7 (2013). [DOI] [PubMed] [Google Scholar]
- Fan J. G. & Cao H. X. Role of diet and nutritional management in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 28, Suppl 4 81–7 (2013). [DOI] [PubMed] [Google Scholar]
- Wallace J. M. et al. Choline supplementation and measures of choline and betaine status: a randomised, controlled trial in postmenopausal women. Br J Nutr 108, 1264–71 (2012). [DOI] [PubMed] [Google Scholar]
- Wang L. J. et al. Betaine attenuates hepatic steatosis by reducing methylation of the MTTP promoter and elevating genomic methylation in mice fed a high-fat diet. J Nutr Biochem 25, 329–36 (2014). [DOI] [PubMed] [Google Scholar]
- Raubenheimer P. J., Nyirenda M. J. & Walker B. R. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes 55, 2015–20 (2006). [DOI] [PubMed] [Google Scholar]
- Corbin K. D. & Zeisel S. H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol 28, 159–65 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrerio A. L. et al. Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am J Clin Nutr 95, 892–900 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajo K. et al. Plasma free choline is a novel non-invasive biomarker for early-stage non-alcoholic steatohepatitis: A multi-center validation study. Hepatol Res 42, 757–66 (2012). [DOI] [PubMed] [Google Scholar]
- Konstantinova S. V. et al. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr 138, 914–20 (2008). [DOI] [PubMed] [Google Scholar]
- Danne O., Lueders C., Storm C., Frei U. & Mockel M. Whole blood choline and plasma choline in acute coronary syndromes: prognostic and pathophysiological implications. Clin Chim Acta 383, 103–9 (2007). [DOI] [PubMed] [Google Scholar]
- Wang Z. et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-Noxide. Eur Heart J 35, 904–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B. J. et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 17, 49–60 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth R. A. et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19, 576–85 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X. et al. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng 118, 476–81 (2014). [DOI] [PubMed] [Google Scholar]
- Tang W. H. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368, 1575–84 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N. et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55, 2005–23 (2012). [DOI] [PubMed] [Google Scholar]
- Chen Y. M. et al. Higher serum concentrations of betaine rather than choline is associated with better profiles of DXA-derived body fat and fat distribution in Chinese adults. Int J Obes (Lond) 39, 465–71 (2015). [DOI] [PubMed] [Google Scholar]
- Kleiner D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–21 (2005). [DOI] [PubMed] [Google Scholar]
- Sanyal A. J. et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 54, 344–53 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graif M. et al. Quantitative estimation of attenuation in ultrasound video images: correlation with histology in diffuse liver disease. Invest Radiol 35, 319–24 (2000). [DOI] [PubMed] [Google Scholar]
- Zeng M. D. et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis 9, 108–12 (2008). [DOI] [PubMed] [Google Scholar]
- Huang B. X. et al. Neck Circumference, along with Other Anthropometric Indices, Has an Independent and Additional Contribution in Predicting Fatty Liver Disease. PLoS One 10, e0118071 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H. et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 64, 1908–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troseid M. et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med 277, 717–26 (2015). [DOI] [PubMed] [Google Scholar]
- Lever M. et al. Betaine and Trimethylamine-N-Oxide as Predictors of Cardiovascular Outcomes Show Different Patterns in Diabetes Mellitus: An Observational Study. PLoS One 9, e114969 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H. et al. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ Res 116, 448–55 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas M. E. et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA 103, 12511–6 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. L. et al. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology 22, 1399–403 (1995). [PubMed] [Google Scholar]
- Ueland P. M. Choline and betaine in health and disease. J Inherit Metab Dis 34, 3–15 (2011). [DOI] [PubMed] [Google Scholar]
- Yu D. et al. Higher dietary choline intake is associated with lower risk of nonalcoholic fatty liver in normal-weight Chinese women. J Nutr 144, 2034–40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger R. N., Garrow T. A. & Baker D. H. Betaine can partially spare choline in chicks but only when added to diets containing a minimal level of choline. J Nutr 137, 2224–8 (2007). [DOI] [PubMed] [Google Scholar]
- Noga A. A. & Vance D. E. A gender-specific role for phosphatidylethanolamine N-methyltransferase-derived phosphatidylcholine in the regulation of plasma high density and very low density lipoproteins in mice. J Biol Chem 278, 21851–9 (2003). [DOI] [PubMed] [Google Scholar]
- Kharbanda K. K. et al. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J Hepatol 46, 314–21 (2007). [DOI] [PubMed] [Google Scholar]
- Deminice R. et al. Betaine supplementation prevents fatty liver induced by a high-fat diet: effects on one-carbon metabolism. AminoAcids 47, 839–46 (2015). [DOI] [PubMed] [Google Scholar]
- Kawakami S. et al. Effects of dietary supplementation with betaine on a nonalcoholic steatohepatitis (NASH) mouse model. J Nutr Sci Vitaminol (Tokyo) 58, 371–5 (2012). [DOI] [PubMed] [Google Scholar]
- Miglio F., Rovati L. C., Santoro A. & Setnikar I. Efficacy and safety of oral betaine glucuronate in non-alcoholic steatohepatitis. A double-blind, randomized, parallel-group, placebo-controlled prospective clinical study. Arzneimittelforschung 50, 722–7 (2000). [DOI] [PubMed] [Google Scholar]
- Abdelmalek M. F. et al. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology 50, 1818–26 (2009). [DOI] [PubMed] [Google Scholar]
- Bril F. et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int 35, 2139–46 (2015). [DOI] [PubMed] [Google Scholar]
- Hernaez R. et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 54, 1082–90 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.