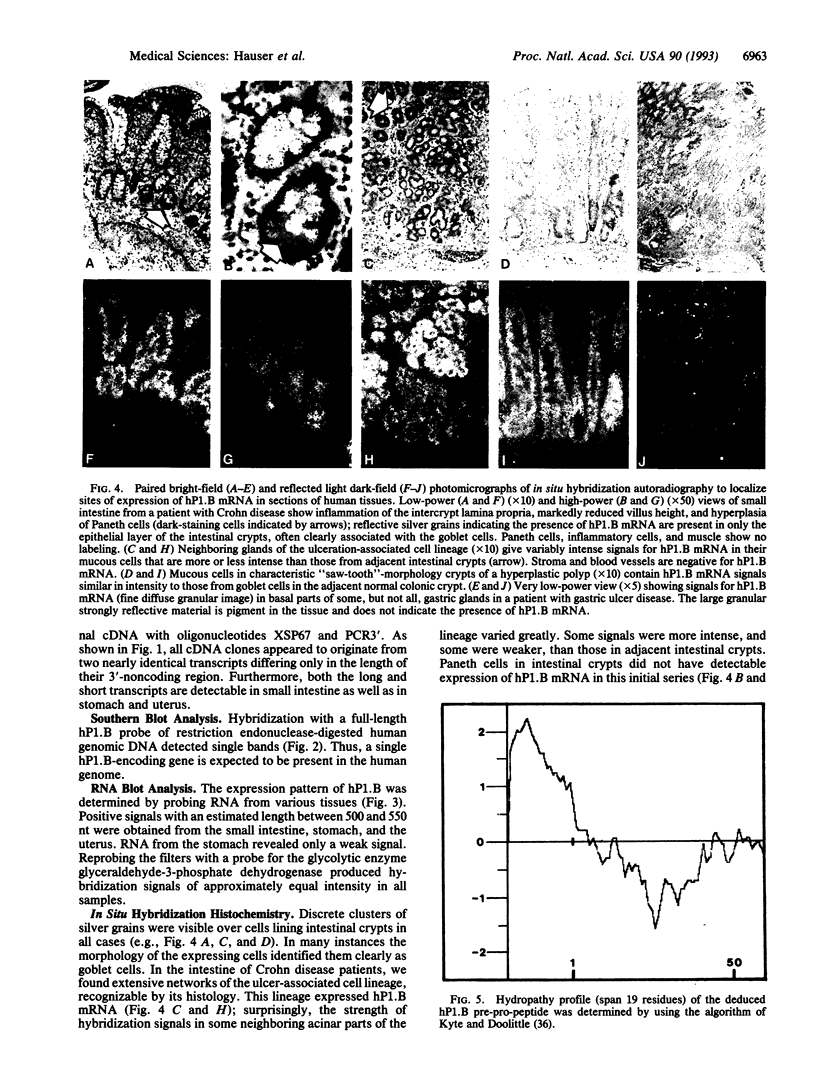
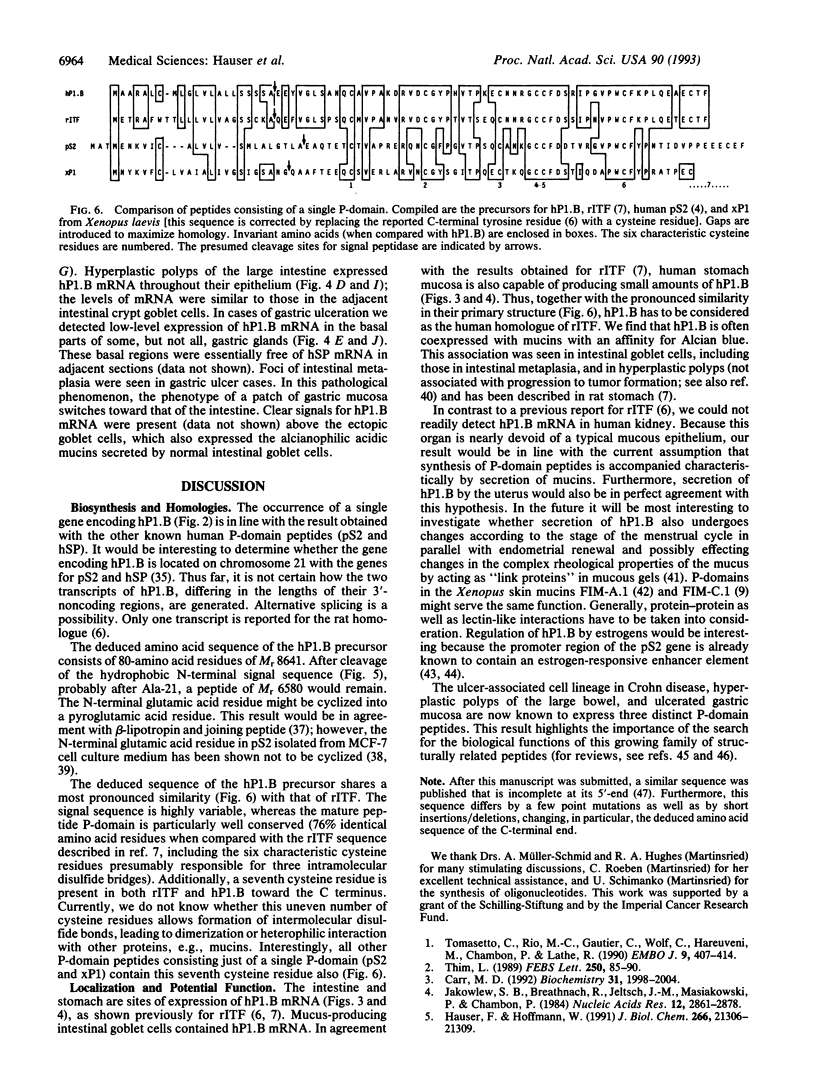
Abstract
The six-cysteine P-domain motif forms the basic repeat unit of a growing family of mucin-associated peptides. A precursor for a human secretory polypeptide has been discovered by molecular cloning and deduced to have a single P-domain, termed hP1.B. The pre-pro-peptide has 67% amino acid identity with rat intestinal trefoil factor. We find, using the techniques of RNA analysis and in situ hybridization, that this P-domain peptide is expressed in the human gastrointestinal tract, where a number of pathological conditions affect its expression, and surprisingly find it is expressed in the uterus also. In the intestine, hP1.B is expressed by goblet cells, but in Crohn disease this peptide is synthesized and secreted additionally by the ulcer-associated cell lineage that is known to secrete two other trefoil peptides, pS2 and spasmolytic polypeptide (hSP). In the stomach, hP1.B mRNA is relatively scarce but is more abundant in foci of intestinal metaplasia and near to ulceration. Mucin-rich epithelial cells in hyperplastic polyps of the colon also express this peptide. The discovery of this P-domain peptide and its expression in association with mucins support the hypothesis that P-domains with mucins may subserve related functions in the maintenance and repair of mucosal function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman A., Solomon S., Bennett H. P. Post-translational modification of bovine pro-opiomelanocortin. Tyrosine sulfation and pyroglutamate formation, a mass spectrometric study. J Biol Chem. 1990 Dec 25;265(36):22130–22136. [PubMed] [Google Scholar]
- Berry M., Nunez A. M., Chambon P. Estrogen-responsive element of the human pS2 gene is an imperfectly palindromic sequence. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1218–1222. doi: 10.1073/pnas.86.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M. D. 1H NMR-based determination of the secondary structure of porcine pancreatic spasmolytic polypeptide: one of a new family of "trefoil" motif containing cell growth factors. Biochemistry. 1992 Feb 25;31(7):1998–2004. doi: 10.1021/bi00122a015. [DOI] [PubMed] [Google Scholar]
- Chinery R., Poulsom R., Rogers L. A., Jeffery R. E., Longcroft J. M., Hanby A. M., Wright N. A. Localization of intestinal trefoil-factor mRNA in rat stomach and intestine by hybridization in situ. Biochem J. 1992 Jul 1;285(Pt 1):5–8. doi: 10.1042/bj2850005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J. A., Stone E. M., Dennison O. E., Rothblum K. N., Schwartz R. J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanby A. M., Poulsom R., Elia G., Singh S., Longcroft J. M., Wright N. A. The expression of the trefoil peptides pS2 and human spasmolytic polypeptide (hSP) in 'gastric metaplasia' of the proximal duodenum: implications for the nature of 'gastric metaplasia'. J Pathol. 1993 Mar;169(3):355–360. doi: 10.1002/path.1711690313. [DOI] [PubMed] [Google Scholar]
- Hanby A. M., Poulsom R., Singh S., Jankowski J., Hopwood D., Elia G., Rogers L., Patel K., Wright N. A. Hyperplastic polyps: a cell lineage which both synthesizes and secretes trefoil-peptides and has phenotypic similarity with the ulcer-associated cell lineage. Am J Pathol. 1993 Mar;142(3):663–668. [PMC free article] [PubMed] [Google Scholar]
- Hauser F., Gertzen E. M., Hoffmann W. Expression of spasmolysin (FIM-A.1): an integumentary mucin from Xenopus laevis. Exp Cell Res. 1990 Aug;189(2):157–162. doi: 10.1016/0014-4827(90)90230-8. [DOI] [PubMed] [Google Scholar]
- Hauser F., Hoffmann W. P-domains as shuffled cysteine-rich modules in integumentary mucin C.1 (FIM-C.1) from Xenopus laevis. Polydispersity and genetic polymorphism. J Biol Chem. 1992 Dec 5;267(34):24620–24624. [PubMed] [Google Scholar]
- Hauser F., Hoffmann W. xP1 and xP4. P-domain peptides expressed in Xenopus laevis stomach mucosa. J Biol Chem. 1991 Nov 5;266(31):21306–21309. [PubMed] [Google Scholar]
- Hauser F., Roeben C., Hoffmann W. xP2, a new member of the P-domain peptide family of potential growth factors, is synthesized in Xenopus laevis skin. J Biol Chem. 1992 Jul 15;267(20):14451–14455. [PubMed] [Google Scholar]
- Hoffmann W. A new repetitive protein from Xenopus laevis skin highly homologous to pancreatic spasmolytic polypeptide. J Biol Chem. 1988 Jun 5;263(16):7686–7690. [PubMed] [Google Scholar]
- Hoffmann W., Franz J. K. Amino acid sequence of the carboxy-terminal part of an acidic type I cytokeratin of molecular weight 51 000 from Xenopus laevis epidermis as predicted from the cDNA sequence. EMBO J. 1984 Jun;3(6):1301–1306. doi: 10.1002/j.1460-2075.1984.tb01966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W., Hauser F. The P-domain or trefoil motif: a role in renewal and pathology of mucous epithelia? Trends Biochem Sci. 1993 Jul;18(7):239–243. doi: 10.1016/0968-0004(93)90170-r. [DOI] [PubMed] [Google Scholar]
- Hoosein N. M., Thim L., Jørgensen K. H., Brattain M. G. Growth stimulatory effect of pancreatic spasmolytic polypeptide on cultured colon and breast tumor cells. FEBS Lett. 1989 Apr 24;247(2):303–306. doi: 10.1016/0014-5793(89)81357-2. [DOI] [PubMed] [Google Scholar]
- Jakowlew S. B., Breathnach R., Jeltsch J. M., Masiakowski P., Chambon P. Sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7. Nucleic Acids Res. 1984 Mar 26;12(6):2861–2878. doi: 10.1093/nar/12.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch J. M., Roberts M., Schatz C., Garnier J. M., Brown A. M., Chambon P. Structure of the human oestrogen-responsive gene pS2. Nucleic Acids Res. 1987 Feb 25;15(4):1401–1414. doi: 10.1093/nar/15.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lemoine N. R., Hughes C. M., Barton C. M., Poulsom R., Jeffery R. E., Klöppel G., Hall P. A., Gullick W. J. The epidermal growth factor receptor in human pancreatic cancer. J Pathol. 1992 Jan;166(1):7–12. doi: 10.1002/path.1711660103. [DOI] [PubMed] [Google Scholar]
- Mori K., Fujii R., Kida N., Ohta M., Hayashi K. Identification of a polypeptide secreted by human breast cancer cells (MCF-7) as the human estrogen-responsive gene (pS2) product. Biochem Biophys Res Commun. 1988 Aug 30;155(1):366–372. doi: 10.1016/s0006-291x(88)81094-5. [DOI] [PubMed] [Google Scholar]
- Müller-Schmid A., Rinder H., Lottspeich F., Gertzen E. M., Hoffmann W. Ependymins from the cerebrospinal fluid of salmonid fish: gene structure and molecular characterization. Gene. 1992 Sep 10;118(2):189–196. doi: 10.1016/0378-1119(92)90188-u. [DOI] [PubMed] [Google Scholar]
- Nunez A. M., Berry M., Imler J. L., Chambon P. The 5' flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J. 1989 Mar;8(3):823–829. doi: 10.1002/j.1460-2075.1989.tb03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L. Intron-dependent evolution: preferred types of exons and introns. FEBS Lett. 1987 Apr 6;214(1):1–7. doi: 10.1016/0014-5793(87)80002-9. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Lynch-Devaney K., Stow J. L., Oates P., Murgue B., DeBeaumont M., Sands B. E., Mahida Y. R. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993 Mar 25;268(9):6694–6702. [PubMed] [Google Scholar]
- Poulsom R., Chinery R., Sarraf C., Lalani E. N., Stamp G., Elia G., Wright N. Trefoil peptide expression in intestinal adaptation and renewal. Scand J Gastroenterol Suppl. 1992;192:17–28. doi: 10.3109/00365529209095975. [DOI] [PubMed] [Google Scholar]
- Rasmussen T. N., Raaberg L., Poulsen S. S., Thim L., Holst J. J. Immunohistochemical localization of pancreatic spasmolytic polypeptide (PSP) in the pig. Histochemistry. 1992 Sep;98(2):113–119. doi: 10.1007/BF00717002. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Daniel J. Y., Tomasetto C., Lathe R., Chenard M. P., Batzenschlager A., Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988 Aug 5;241(4866):705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Chambon P. The pS2 gene, mRNA, and protein: a potential marker for human breast cancer. Cancer Cells. 1990 Aug-Sep;2(8-9):269–274. [PubMed] [Google Scholar]
- Rio M. C., Lepage P., Diemunsch P., Roitsch C., Chambon P. Structure primaire de la protéine humaine pS2. C R Acad Sci III. 1988;307(19):825–831. [PubMed] [Google Scholar]
- Rose K., Savoy L. A., Thim L., Christensen M., Jørgensen K. H. Revised amino acid sequence of pancreatic spasmolytic polypeptide exhibits greater similarity with an inducible pS2 peptide found in a human breast cancer cell line. Biochim Biophys Acta. 1989 Oct 19;998(3):297–300. doi: 10.1016/0167-4838(89)90288-4. [DOI] [PubMed] [Google Scholar]
- Seitz G., Thelsinger B., Tomasetto G., Rio M. C., Chambon P., Blin N., Welter G. Breast cancer-associated protein pS2 expression in tumors of the biliary tract. Am J Gastroenterol. 1991 Oct;86(10):1491–1494. [PubMed] [Google Scholar]
- Senior P. V., Critchley D. R., Beck F., Walker R. A., Varley J. M. The localization of laminin mRNA and protein in the postimplantation embryo and placenta of the mouse: an in situ hybridization and immunocytochemical study. Development. 1988 Nov;104(3):431–446. doi: 10.1242/dev.104.3.431. [DOI] [PubMed] [Google Scholar]
- Suemori S., Lynch-Devaney K., Podolsky D. K. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11017–11021. doi: 10.1073/pnas.88.24.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisinger B., Welter C., Seitz G., Rio M. C., Lathe R., Chambon P., Blin N. Expression of the breast cancer associated gene pS2 and the pancreatic spasmolytic polypeptide gene (hSP) in diffuse type of stomach carcinoma. Eur J Cancer. 1991;27(6):770–773. doi: 10.1016/0277-5379(91)90186-h. [DOI] [PubMed] [Google Scholar]
- Thim L. A new family of growth factor-like peptides. 'Trefoil' disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins). FEBS Lett. 1989 Jun 19;250(1):85–90. doi: 10.1016/0014-5793(89)80690-8. [DOI] [PubMed] [Google Scholar]
- Thim L., Jørgensen K. H., Jørgensen K. D. Pancreatic spasmolytic polypeptide (PSP): II. Radioimmunological determination of PSP in porcine tissues, plasma and pancreatic juice. Regul Pept. 1982 Mar;3(3-4):221–230. doi: 10.1016/0167-0115(82)90127-6. [DOI] [PubMed] [Google Scholar]
- Tomasetto C., Rio M. C., Gautier C., Wolf C., Hareuveni M., Chambon P., Lathe R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990 Feb;9(2):407–414. doi: 10.1002/j.1460-2075.1990.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetto C., Rockel N., Mattei M. G., Fujita R., Rio M. C. The gene encoding the human spasmolytic protein (SML1/hSP) is in 21q 22.3, physically linked to the homologous breast cancer marker gene BCEI/pS2. Genomics. 1992 Aug;13(4):1328–1330. doi: 10.1016/0888-7543(92)90059-2. [DOI] [PubMed] [Google Scholar]
- Welter C., Theisinger B., Seitz G., Tomasetto C., Rio M. C., Chambon P., Blin N. Association of the human spasmolytic polypeptide and an estrogen-induced breast cancer protein (pS2) with human pancreatic carcinoma. Lab Invest. 1992 Feb;66(2):187–192. [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G., Van Noorden S., Sarraf C., Elia G., Ahnen D., Jeffery R., Longcroft J., Pike C. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993 Jan;104(1):12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]






