Abstract
Inhibition of root elongation is one of the most distinct symptoms of aluminium (Al) toxicity. Although putrescine (Put) has been identified as an important signaling molecule involved in Al tolerance, it is yet unknown how Put mitigates Al-induced root inhibition. Here, the possible mechanism was investigated by using two wheat genotypes differing in Al resistance: Al-tolerant Xi Aimai-1 and Al-sensitive Yangmai-5. Aluminium caused more root inhibition in Yangmai-5 and increased ethylene production at the root apices compared to Xi Aimai-1, whereas the effects were significantly reversed by ethylene biosynthesis inhibitors. The simultaneous exposure of wheat seedlings to Al and ethylene donor, ethephon, or ethylene biosynthesis precursor, 1-aminocyclopropane-1-carboxylic acid (ACC), increased ethylene production and aggravated root inhibition, which was more pronounced in Xi Aimai-1. In contrast, Put treatment decreased ethylene production and alleviated Al-induced root inhibition in both genotypes, and the effects were more conspicuous in Yangmai-5. Furthermore, our results indicated that Al-induced ethylene production was mediated by ACC synthase (ACS) and ACC oxidase, and that Put decreased ethylene production by inhibiting ACS. Altogether, these findings indicate that ethylene is involved in Al-induced root inhibition and this process could be alleviated by Put through inhibiting ACS activity.
Aluminium (Al) toxicity is a major constraint limiting crop growth and yield on acid soils, which occupy approximately 50% of the world’s potentially arable land1,2. Most Al exists in soils in non-toxic complexed forms; however, when soil pH drops below 5.0, phytotoxic forms of Al as hexaaquaaluminium [Al(H2O2)6]3+, or Al3+ ions may appear3. Low concentrations of Al rapidly inhibit root growth and function, subsequently leads to poor nutrient acquisition and reduced crop production4,5. Because Al is such a reactive element, a number of possible mechanisms for Al toxicity have been proposed. For example, Al may interact with multiple root cell sites, including the cell wall, plasma membrane, and symplasm, or it may interact with intracellular components, such as enzymes and proteins, which lead to the disruption of their functions4,6,7. Aluminium may also interfere with signal cascades in plants, such as cytosolic Ca2+ and 1,4,5-trisphosphate8,9. Plants have numerous strategies to withstand Al stress, among which the most well-characterised mechanism is Al exclusion from the root tips based on root exudation of organic acid2. Recently, genes involved in the Al-activated organic acid exudation have been identified in several plant species2,3. For example, TaALMT1 (Triticum aestivum Al-activated malate transporter), which underpins the Al-induced wheat root malate exudation, has been identified as the major gene conferring Al resistance in wheat10. Although extensive progresses have been made during the past few years, the mechanisms of Al toxicity and tolerance remain elusive.
Ethylene, a gaseous plant hormone, is gradually becoming established as a vital co-regulator of plant growth and development under optimal and stressful conditions11,12. Rapidly increased ethylene production has frequently been observed in plant roots under Al stress13,14,15. Previous studies using ethylene synthesis inhibitors or ethylene-insensitive mutants demonstrated that the rapidly produced ethylene contributes to Al-induced root inhibition and, thus, relate to Al sensitivity, as demonstrated in Lotus japonicas, Arabidopsis, Glycine max13,16,17. In other plant species, however, it has been reported that enhanced ethylene production did not play a role in Al toxicity, for example in the roots of maize18. It is possible that the discrepancy between the studies is related to different plant species used and the kinetics of ethylene production. In plants, ethylene is synthesized from S-adenosylmethionine (SAM) and 1-aminocylopropane-1carboxylic acid (ACC), catalysed by ACC synthase (ACS) and ACC oxidase (ACO), respectively19. Although the cellular ethylene biosynthesis in higher plants is under strict metabolic regulation, the enzymes may change to some extent in response to abiotic stress20. Down-regulation of ethylene production through manipulating its biosynthesis enzymes has been considered as an essential strategy to enhance Al tolerance of crops, for example, in Medicago21. However, the mechanism and/or signal molecules involved in the modulation of Al-induced ethylene biosynthesis are largely unknown.
Putrescine (Put) is an essential signaling molecule involved in modulating plant resistance to Al stress22. For instance, exogenous Put promoted root growth under Al stress in saffron plants23, whereas Put biosynthesis inhibitors exacerbated the effects of Al in red kidney bean plants24. Currently available data also suggest that Put may interfere with ethylene biosynthesis or signaling transductions under stress conditions25,26. Hyodo and Tanaka27 found that Put suppressed ethylene production in a non-competitive manner. Further studies show that under osmotic stress, Put decreased stress-induced ethylene production through reducing the level of reactive oxygen species26. Since both of Put and ethylene have been implicated in the regulation of Al-induced root elongation inhibition, it is reasonable to assume that Put may alleviate Al-induced root inhibition, and subsequently Al toxicity through a mechanism of modulating ethylene production.
In this study, the above hypothesis was addressed by using pharmacological agents and two wheat genotypes differing in Al tolerance (Al-sensitive, Yangmai-5; Al-tolerant, Xi Aimai-1). We found that the differential Al sensitivity between the wheat genotypes was associated with their different ethylene production capacities, and that Put promoted root growth under Al stress by inhibiting ACS-mediated ethylene production.
Results
Putrescine alleviates Al-induced inhibition of root elongation
Root elongation inhibition increased as the Al concentration rose (0, 10, 20, 30, 40, and 50 μM) in both genotypes. Al resistance, as determined by the RRE values, was more pronounced in Xi Aimai-1 than in Yangmai-5. Exposure to 30 μM AlCl3 resulted in the largest RRE difference between Yangmai-5 (30%) and Xi Aimai-1 (51%) (Fig. 1a). Thus, 30 μM Al was used in this study. Experiments on the effects of 0.5, 1, 2, 5, and 10 mM Put and 30 μM Al on root elongation were conducted in order to investigate whether Al-induced inhibition of root elongation can be alleviated by Put. A dose-dependent alleviation of Put on Al-induced root inhibition was observed at all concentrations tested. The 2 mM Put treatment had the most significant effect, and the amelioration was more efficient in Yangmai-5 than in Xi Aimai-1 (Fig. 1b). However, in the Al-free control, the 2 mM Put treatment slightly inhibited root elongation in both genotypes (Fig. 2a) (two-way ANOVA interaction, P < 0.001 for genotype, treatment and interactions). An ameliorating response by Put against Al-induced root inhibition was also morphologically observed (Fig. 2b).
Figure 1. Root growth was measured.
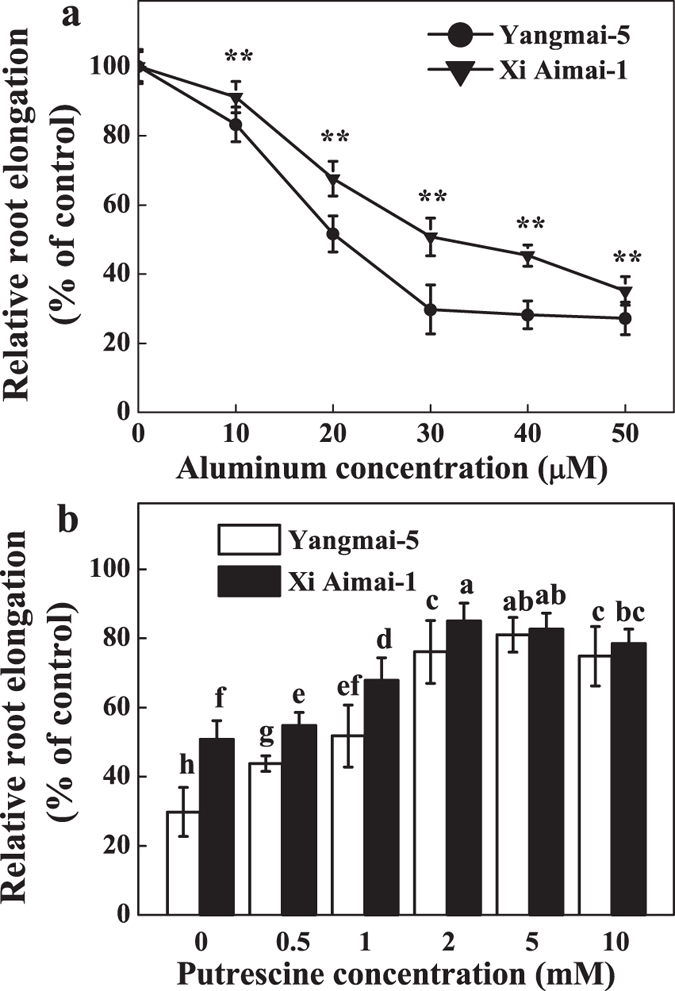
(a) Effects of increasing AlCl3 concentrations (0 ~ 50 μM) on root elongation of wheat seedlings. The primary root lengths were measured after the seedlings were exposed to Al for 24 h. (b) Effect of Put concentrations (0 ~ 10 mM) on Al-induced root inhibition of wheat seedlings. Seedlings were treated with Put at the indicated concentrations in the presence of 30 μM Al for 24 h. The control solution contained 0.5 mM CaCl2, pH 4.3 ± 0.1. Data are presented as the relative root elongation (RRE) compared to the control (CK) values. All values are means ± SD (n = 20). ** indicates a significant difference at P < 0.01. Columns with different letters are significantly different at P < 0.05. “n” refers to the number of independent experiments, same as follows.
Figure 2. Effect of Put on Al-induced root inhibition of wheat seedlings.

The 3-d-old seedlings were treated with 2 mM Put in the absence or presence of 30 μM Al for 24 h. (a) Root elongation. RRE was expressed relative to root elongation in the control solutions, which contained 0.5 mM CaCl2 (pH 4.3 ± 0.1). The values shown are means ± SD (n = 20). Columns with different letters are significantly different at P < 0.05. (b) Corresponding photographs. Bar, 2 cm.
Effects of ethylene and Put on Al-induced inhibition of root elongation
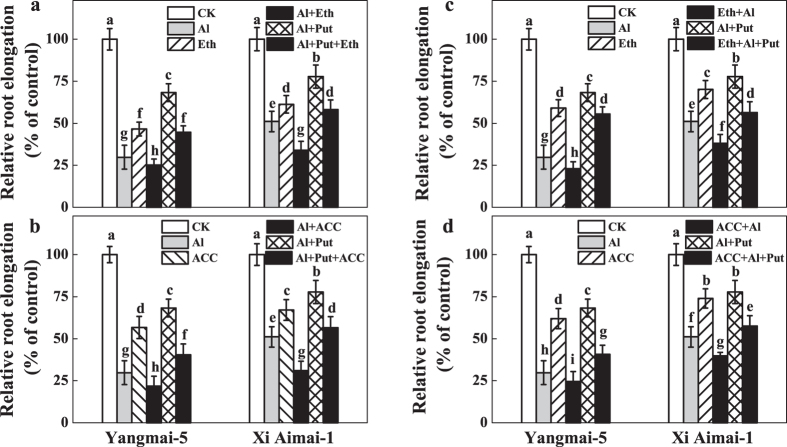
To confirm the role of ethylene in Al-induced root growth inhibition, further experiments were performed that manipulated ethylene levels or ethylene signaling by using ethylene biosynthesis inhibitors (AVG and CoCl2) or an ethylene perception blocker (AgNO3), ethephon, and ACC and Put separately or simultaneously. As shown in Fig. 3, treatment with 30 μM ethephon or 10 μM ACC inhibited root elongation and aggravated Al-induced root inhibition in both wheat genotypes, but the aggravation was more serious in Xi Aimai-1 (two-way ANOVA interaction, P < 0.001 for genotype, treatment and interactions). In contrast, pretreatment with 1 μM AVG, 10 μM CoCl2, or 10 μM AgNO3, which had little effect on root elongation under the control conditions, significantly alleviated root inhibition under Al stress by 26%, 21%, and 27% for Yangmai-5, and 14%, 11%, and 18% for Xi Aimai-1, respectively (Fig. 4) (two-way ANOVA interaction, P < 0.001 for genotype, treatment and interactions). Interestingly, Put treatment alone also alleviated Al-induced root inhibition by 38% and 27% in Yangmai-5 and Xi Aimai-1, respectively. In addition, application of ethephon/ACC partially reversed the alleviation of root inhibition by Put under Al stress in both wheat genotypes (Fig. 3). To avoid a direct interaction between ethephon/ACC and Put, an ethephon/ACC pretreatment experiment was also conducted and similar effects to roots treated with ethephon/ACC, and Al or Al + Put simultaneously, were observed (Fig. 3c,d).
Figure 3. Effects of Al, ethylene-releasing substance ethephon (Eth), and ethylene biosynthesis precursor 1-aminocylopropane-1carboxylic acid (ACC) on wheat plant root growth.
Seedlings were treated with 30 μM Eth (a), 10 μM ACC (b) in the absence or presence of Al or Al + Put for 24 h, or pretreated with 30 μM Eth (c) or 10 μM ACC (d) for 3 h and then exposed to Al for 24 h. RRE was expressed relative to root elongation in control solutions containing 0.5 mM CaCl2 (pH 4.3 ± 0.1). The values shown are means ± SD (n = 20). Columns with different letters are significantly different at P < 0.05.
Figure 4. Effects of ethylene synthesis inhibitors (AVG, AgNO3, and CoCl2) on Al-induced root inhibition of wheat seedlings.
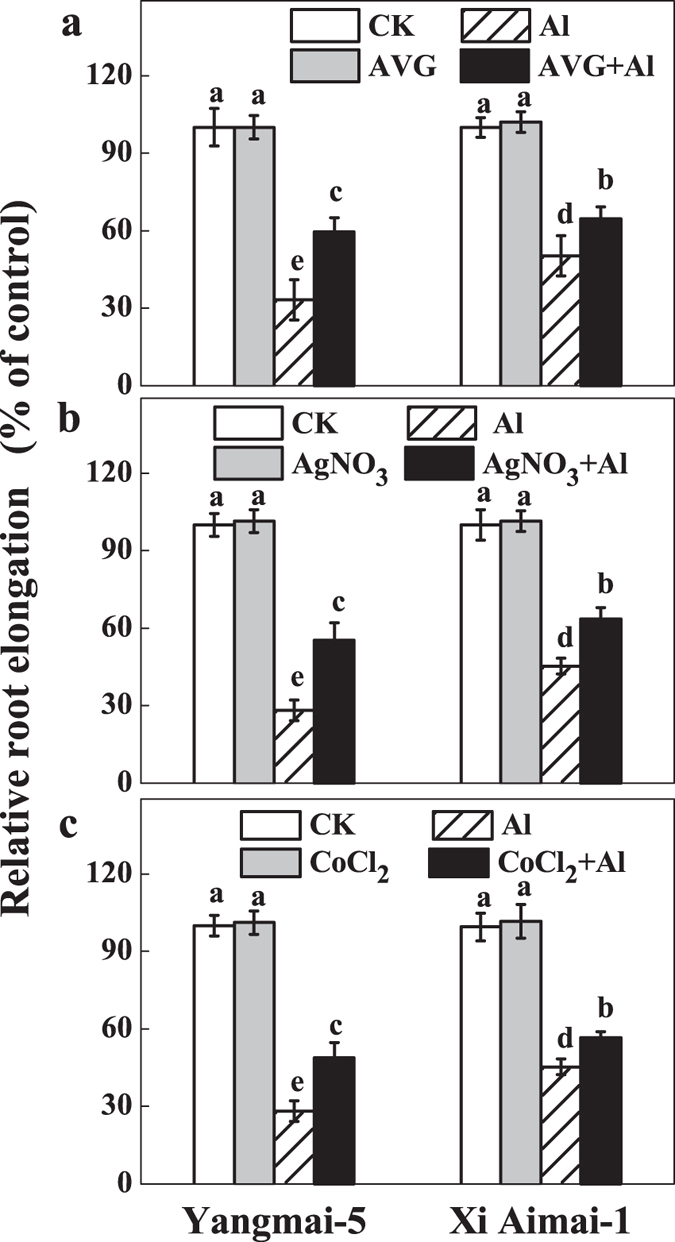
The 3-d-old seedlings were pretreated with 1 μM AVG (a), 10 μM AgNO3 (b), or 10 μM CoCl2 (c) for 3 h and then exposed to Al for 24 h. RRE was expressed relative to root elongation in control solutions containing 0.5 mM CaCl2 (pH 4.3 ± 0.1). The values shown are means ± SD (n = 20). Columns with different letters are significantly different at P < 0.05.
Effects of Al and Put on ethylene production in the root tips of wheat seedlings
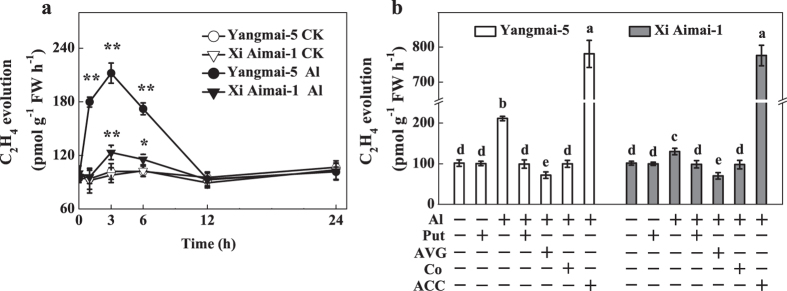
The effect of Al3+ on ethylene evolution from the root tips of wheat plants was analyzed to further clarify whether Al-induced root inhibition is related to the induction of ethylene production. The roots of the control samples represented the basal ethylene levels. After Al treatment, significant differences in ethylene production were observed in the root tips of the two wheat genotypes (Fig. 5). A significant burst of ethylene was observed in Al-sensitive Yangmai-5 after 1.5 h Al exposure and the maximum ethylene content was reached at 3 h, but then production declined to a relatively steady level after 12 h. However, in the Al-tolerant Xi Aimai-1, ethylene had only slightly increased after 3 h Al treatment and the level was 1.7-fold lower than that in Yangmai-5 (Fig. 5a). Treatment with exogenous Put significantly reduced Al-induced ethylene production by approximately 106% and 36% in Yangmai-5 and Xi Aimai-1, respectively. A similar effect was also observed in the CoCl2 and AVG treatments (Fig. 5b), which suggested that the ameliorating effects of CoCl2 and AVG on Al-induced root inhibition were associated with Al-elicited ethylene biosynthesis. This effect might also be responsible for the improved root growth induced by Put.
Figure 5. Ethylene production in root tips of wheat plants.
(a) Time-course for ethylene production from root apexes of wheat seedlings exposed to 30 μM AlCl3. (b) Effects of Al or Put alone, Al + Put, ethylene synthesis or perception inhibitors (AVG, AgNO3, and CoCl2), or ethylene precursor (ACC) on ethylene production. Roots of 3-d-old seedlings were pretreated with 1 μM AVG or 10 μM CoCl2 for 3 h and then exposed to Al for another 3 h, or directly treated with Al + Put for 3 h. Root apexes were excised to determine ethylene production. Data are means ± SD (n = 3). * and ** indicate significant differences between CK and Al treatments at P < 0.05 and P < 0.01, respectively. Columns with different letters are significantly different at P < 0.05.
Effects of Put on the activities of ethylene synthesis enzymes
ACS and ACO are two key enzymes responsible for ethylene production in plants28. We measured the ACS and ACO activities to determine the possible mechanism underlying Put decreased ethylene production in the root tips of wheat. As shown in Fig. 6, the activities of both ACS and ACO increased in the root tips of the two wheat genotypes, with the former increasing much more than the latter. Furthermore, both enzyme activities in Yangmai-5 were much higher than in Xi Aimai-1 (Fig. 6). Application of Put significantly reduced ACS activity, but it had little effect on ACO activity, which suggested that Put may reverse Al-induced ethylene production by inhibiting the activity of ACS.
Figure 6. Effects of Put and ethylene synthesis inhibitors (AVG and CoCl2, respectively) on ACC synthase (ACS) (a) and ACC oxidase activity (b) in root apexes of wheat seedlings.
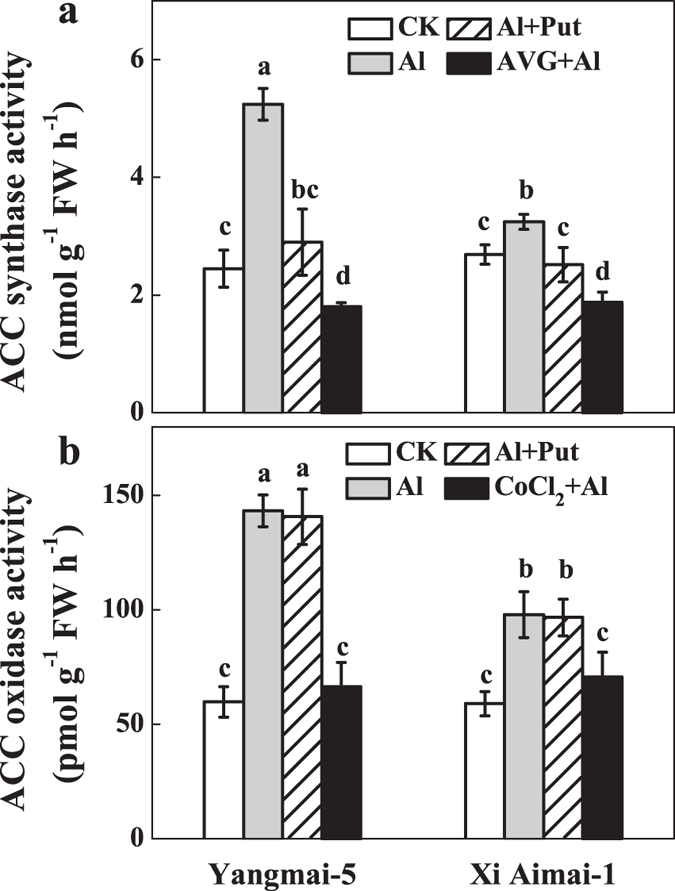
The 3-d-old seedlings were pretreated with 1 μM AVG (an ACS inhibitor) or 10 μM CoCl2 (an ACO inhibitor) for 3 h, and then exposed to Al or Al + Put for another 3 h. Then, the root apexes were collected for assaying ACS and ACO activity. Data are means ± SD (n = 3). Columns with different letters are significantly different at P < 0.05.
Effects of Put on the content of ethylene precursor ACC
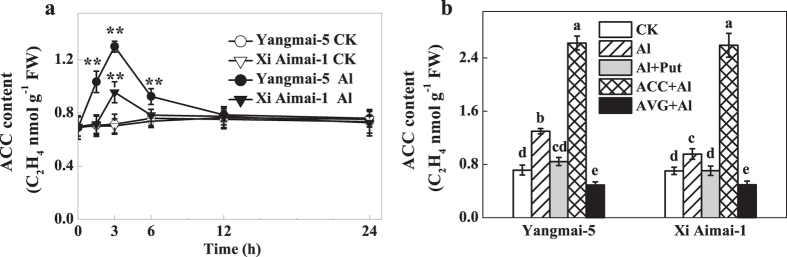
Changes in ACC levels were analyzed to further confirm whether the Put-reduced ethylene production in wheat roots was related to ACC biosynthesis. Figure 7a shows that the increases in ACC content caused by Al were similar to the ethylene production changes seen in wheat plants, whereas the application of AVG or Put suppressed the increase (Fig. 7b). In addition, ACC application further increased endogenous ACC content in both genotypes. It has been reported that ACC per se has a short-term (a few hours) influence on root cell elongation29. In this study, ACC application further enhanced Al-induced ethylene production (Fig. 5b) and the inhibitory effect of ACC on RRE was mostly alleviated in the presence of AgNO3 (Fig. S1), which suggested that ACC exerts its effect mainly after conversion to ethylene.
Figure 7. ACC content in root tips of wheat plants.
(a) Time-course for ACC content in wheat root tips upon exposure to 30 μM AlCl3. (b) Effect of Put, ACC, and AVG on ACC content in wheat root tips under Al stress. The 3-d-old seedlings were pretreated with 10 μM ACC or 1 μM AVG for 3 h, and then exposed to Al for 3 h, or they were treated with Al + Put for 3 h. Root apexes were collected to determine the ACC content. Data are means ± SD (n = 3). ** indicate significant differences between CK and Al treatments at P < 0.01. Columns with different letters are significantly different at P < 0.05.
Discussion
Plant responses to Al toxicity are expressed by a myriad of changes in the physiological processes, and also involve various signal components7,23. As a crucial signaling molecule, Put plays essential roles for plant performance under stress conditions30,31. In the present study, Put was found to significantly alleviate the growth inhibition of wheat plants subjected Al stress. Al tolerance in wheat plants was shown to be highly associated with the TaALMT1-mediated malate exudation from the root tips2,10. However, application of Put had little effect on the expression of TaALMT1 in the root tips of both wheat genotypes under Al stress (Fig. S2), suggesting that the Put-related improved Al tolerance might not be associated with TaALMT1-mediated malate efflux. Furthermore, in our previous study, we showed that Put did not affect Al-induced malate secretion in wheat plants34, providing wfurther evidence for the above assumption. In this study, we found that Put alleviates root growth inhibition through inhibiting ethylene biosynthesis, which is rapidly produced and behaved as a negative regulator of root elongation.
Inhibition of root elongation is the primary manifestation of Al toxicity, and RRE has been frequently used to assess Al resistance in plants32,33. In this study, We found that inhibition of root elongation increased with increasing external Al concentrations in both Al-sensitive (Yangmai-5) and Al-tolerant (Xi Aimai-1) wheat genotypes, with the effect being much less pronounced in the latter (Fig. 1a). Recently, there has been some experimental evidence supporting the involvement of Put in plant tolerance to Al stress. Our previous study revealed a marked Put accumulation in the root tips of wheat plants34. Here, exogenous Put significantly alleviated Al-induced root inhibition in both wheat genotypes, and the effect was more pronounced in Yangmai-5 than in Xi Aimai-1 (Fig. 2). Similar results were observed in red kidney bean22,24, saffron23, and Salvinia35, in which exogenous Put alleviated while Put biosynthesis inhibitors exaggerated Al toxicity.
Ethylene is a plant hormone that plays prominent roles in modulating root growth36. Recent studies suggested that ethylene may also regulate root response to Al in plants37,38. In our study, a similar effect on root growth in wheat plants to Al stress was caused by an ethylene-releasing substance (ethephon) and an ethylene precursor (ACC) (Fig. 3). We also observed that Al caused a rapid production of ethylene in the root tips of Yangmai-5 after 1.5 h. The Al-elicited ethylene production reached a maximum after 3 h exposure to Al, and the level was significantly higher in Yangmai-5 than in Xi Aimai-1 (Fig. 5). The difference in Al content may not be responsible for the variation in ethylene production since there was no significant difference in Al content in the root tips of the two wheat genotypes before 6 h (Fig. S3). This suggests that ethylene may be involved in Al-induced root inhibition of wheat seedlings and that the genotypic variations in ethylene production may contribute to their difference in Al sensitivity. The observation that application of ACC, which increased ethylene production in the root tips of both wheat genotypes (Fig. 5b), and aggravated Al-induced root inhibition more in Xi Aimai-1 than in Yangmai-5 (Fig. 3b) is in line with this proposition. This was further supported by the application of ethylene biosynthesis (AVG and CoCl2) or ethylene perception (AgNO3) antagonists, which markedly reduced Al-induced ethylene production (Fig. 5b) or blocked its action and efficiently promoted root growth of both wheat genotypes under Al stress (Figs 3 and 4). Therefore, our results indicated that the ethylene production induced by Al occurred very rapidly and might be responsible for Al-induced root inhibition.
Many studies have suggested that Put and ethylene have opposite effects in many different plant processes and abiotic stress responses39,40,41. Put has also been reported to interact with ethylene biosynthesis under various stress conditions27,42. However, the interaction between Put and ethylene under Al stress remains undetermined. Our results showed that exogenous Put inhibited Al-induced ethylene production in the roots of wheat seedling subjected to Al stress, which was similar to the effects of AVG or CoCl2 (Fig. 5b). Importantly, these changes were related to the recovery from root inhibition caused by Al exposure (Figs 2 and 3). Furthermore, pretreatment with ethephon or ACC was able to counteract the Put-induced alleviation of root inhibition by Al stress (Fig. 3c,d). These results clearly suggest that, at least partially, Put improved root growth by inhibiting ethylene production under Al stress. The fact that the ameliorative effect of Put was more prominent in Yangmai-5, which produced higher levels of ethylene than Xi Aimai-1, further proved this conjecture. In terrestrial plants, the ethylene and polyamine pathways are generally considered to be competitive43,44. Although exogenous Put slightly increased spermidine (Spd) content (data not shown) and decreased ethylene production, the two pathways were not strictly antagonistic in our study because the addition of AVG, which inhibits the conversion of SAM (the common precursor of Spd and ethylene) to ethylene, did not enhance polyamine biosynthesis (Fig. S4). A better explanation could be that the availability of SAM in vivo is not rate limiting during the biosynthesis of either ethylene or Spd, and that both pathways could run simultaneously45,46. Evaluation of the potential sources of ethylene revealed that Al-induced root inhibition might be due to the increase in both ACS and ACO activities (Fig. 6). However, ACS and ACO have been identified as two sites where Put can affect ethylene biosynthesis25,47. The effects of Put on the activities of ACS and ACO, and ACC content were examined to further unravel how Put decreases ethylene production under Al stress. Our results suggested that Put inhibited ethylene production by directly suppressing ACS activity at the step where SAM was converted to ACC (Figs 6a and 7).
In summary, our study reveals the protective role of Put on Al-induced root inhibition of wheat plants. We also demonstrated that ethylene may be involved in Al-induced root inhibition and the different ethylene production profiles may be due to the differential Al sensitivity between the two wheat genotypes. Most importantly, Put application reduced ACS activity, and thus ethylene production, which may explain how Put alleviated root inhibition under Al tress. Our results not only suggested a potential mechanism for Al-induced root inhibition, but also provided a possible explanation for the function of Put in plants. We therefore proposed a simple model to explain the integration between Put and ethylene under Al stress (Fig. 8).
Figure 8. Proposed model of how Put alleviates Al-induced root inhibition.
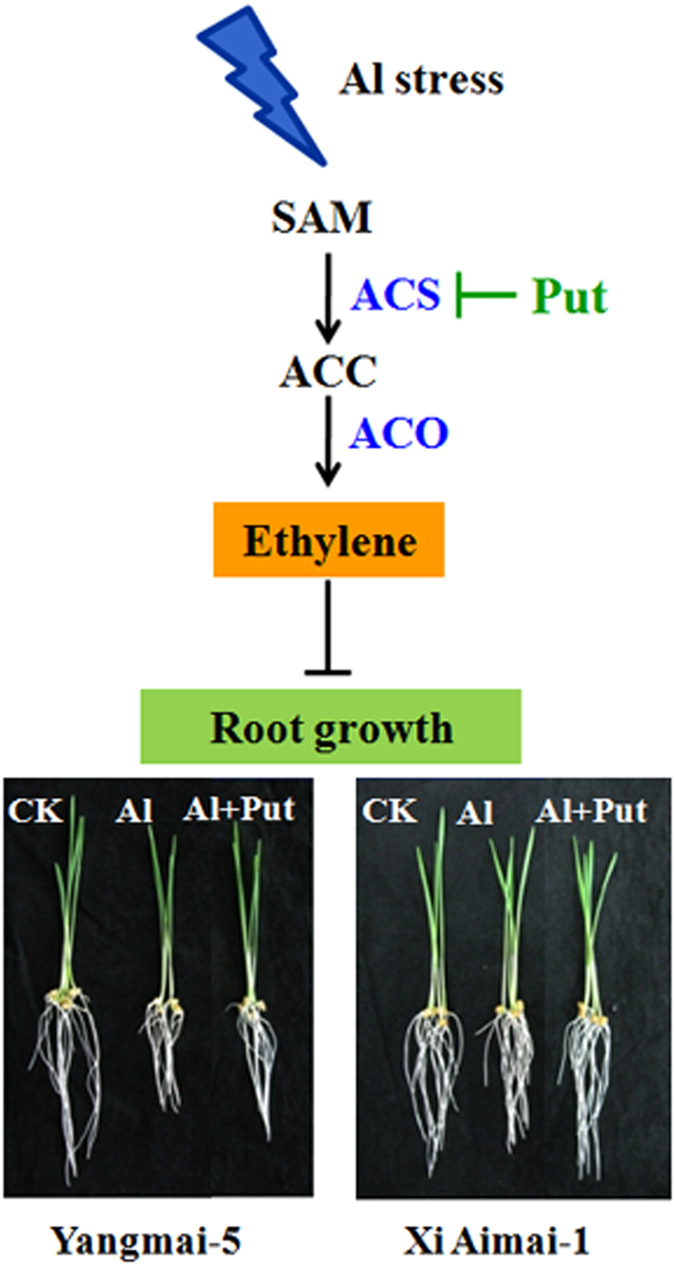
Al activates ACS and ACO, which promote ethylene biosynthesis and inhibit root elongation, whereas Put suppresses ACS, which blocks the beginning of ethylene biosynthesis and thus reduces Al-elicited ethylene production, leading to increased root growth.
Materials and Methods
Plant materials
Seeds of two wheat genotypes Yangmai-5 and Xi Aimai-1, which are classified as Al-sensitive and Al-tolerant genotypes previously48,49, were used in this study. The seeds were surface sterilized with 1% NaClO for 20 min and rinsed in distilled water overnight. After germination in the dark for 12 h at 25 °C, the seeds were transplanted to plastic screens floating on 0.5 mM CaCl2 solution (pH 4.3 ± 0.1) in a growth chamber under a 12 h/25 °C day and 12 h/22 °C night regime, a light intensity of 300 μmol m−2 s−1, and a relative humidity of 70%. The solutions were renewed daily. The seedlings were exposed to the various treatments when the average root length was about 45 mm. After that, the roots at selected time points after treatment were washed with distilled water several times and the root apexes (~10 mm) were excised for further analysis.
Measurement of root elongation
To study the effect of ethylene, aminoethoxyvinylglycine (AVG), CoCl2, and AgNO3 on root elongation in the absence and presence of Al, 3-d-old seedlings were treated with 30 μM ethephon (an ethylene-releasing substance) or 10 μM ACC (an ethylene precursor), 10 μM AVG (an inhibitor of ACS), 10 μM CoCl2 (an inhibitor of ACO) and 10 μM AgNO3 (an antagonist of ethylene perception) together with 30 μM AlCl3 for 24 h or pretreated with above reagents for 3 h and then exposed to 30 μM AlCl3 for another 24 h. Elongation of the primary root was measured with a ruler before and after treatment. Relative root elongation (RRE, %) was calculated as the percentage of the root elongated by various treatments normalised over the Al-free control. Values of RRE were given as means ± SD of at least twenty independent measurements. All experiments were repeated at least three times.
Measurement of ethylene production
Ethylene production was determined according to Sun et al.13, with slight modifications. Briefly, root tips (~10 mm in length) were excised. To minimize the wounding effect, the excised root apexes were put into 6 mL gas-tight vials for 0.5 h before they were sealed. After incubation at room temperature for 2 h in the dark, a 1 mL gas sample of the headspace was taken from the vials with a syringe. The ethylene concentration in the samples was measured by injecting the sample into a gas chromatograph (Agilent 7890 A, USA) equipped with a Porapak Q column and a flame ionization detector (FID).
Extraction and analysis of ACC
The ACC contents in wheat roots were extracted and analyzed according to Wang et al.50, with slight modifications. Root apexes (~10 mm) were pulverized in liquid nitrogen and homogenized in 1 mL of 80% ethanol at 55 °C for 10 min. The slurry was then centrifuged at 10,000 g for 10 min. The supernatant was saved and the extraction was repeated twice. The final samples were combined and evaporated to dryness in vacuum at 55 °C, and then resuspended with 2.5 mL distilled water. The extracted ACC in the aqueous part was quantified indirectly by converting ACC to ethylene. The ethylene evolved from ACC was assayed using gas chromatography as described above.
Assays for ACS and ACO activity
The ACS and ACO activity was determined as described by Woeste et al.51 and Tian et al.36. For ACS determination, root apexes (~10 mm) were ground with liquid nitrogen, and resuspended in 1.5 mL extract buffer containing 200 mM phosphate buffer (pH 8.0), 10 μM pyridoxal phosphate, 1 mM EDTA, 2 mM phenylmethylsulfonyl fluoride (PMSF), and 5 mM dithiothreitol (DTT). The extract was centrifuged at 15,000 g for 15 min at 4 °C. A 600 μL aliquot of the supernatant was transferred to a 6 mL gas-tight vial containing 200 μL 5 mM S-(5′-Adenosyl)-L-methionine (Sigma-Aldrich). The reaction was carried out for 1 h at 22 °C. The ACC formed was converted to ethylene by adding 200 μL of a 1:1 (v:v) mixture containing saturated NaOH:bleach. The reaction vials were quickly sealed and kept in ice for 20 min. Ethylene produced in the head space was determined as described above.
To determine ACO activity, root apexes (~10 mm) were ground with liquid nitrogen and resuspended in 1.5 mL extract buffer containing 0.1 M Tris-HCl (pH 7.2), 10% (w:v) glycerol, 30 mM sodium ascorbate, and 5% (w:v) polyvinyl polypyrolidine (PVPP). After centrifuging at 15,000 g for 20 min at 4 °C, the ACO activity was assayed immediately by mixing 1 mL of the supernatant with 1.7 mL of extraction buffer (without PVPP), 50 μM FeSO4, and 2 mM ACC. Then the mixture was incubated at 30 °C for 1 h. The ethylene produced in the head space was determined as described earlier.
Statistical analysis
The data were subjected to analysis of variance (ANOVA), and the least significant difference (LSD) test was employed to determine differences among the treatments at P = 0.01 and P = 0.05 levels.
Additional Information
How to cite this article: Yu, Y. et al. Inhibition of ethylene production by putrescine alleviates aluminium-induced root inhibition in wheat plants. Sci. Rep. 6, 18888; doi: 10.1038/srep18888 (2016).
Supplementary Material
Acknowledgments
This work was financially supported by the National Basic Research Program (973 Program) of China (No. 2013CB127403), the National Natural Science Foundation of China (Nos 31272237 and 30771292), and the Foundation for University Ph.D. Granting Discipline of the Ministry of Education (No. 20120101110130) and IPNI.
Footnotes
Author Contributions Y.Y., X.Y.L. and C.W.J. designed research; Y.Y., C.L.S., J.H.W., Y.Q.Y. and W.W.Z. performed research; Y.Y., C.W.J., X.Y.L. and C.L.S. analyzed data; Y.Y., X.Y.L. and C.W.J. wrote the paper. All authors discussed the data and made comments on the manuscript.
References
- Kochian L. V., Hoekenga O. A. & Piñeros M. A. How do crop plants tolerate acid soils ? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 55, 459–493 (2004). [DOI] [PubMed] [Google Scholar]
- Kochian L., Piñeros M., Liu J. & Magalhaes J. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 66, 571–598 (2015). [DOI] [PubMed] [Google Scholar]
- Ma J., Chen Z. & Shen R. Molecular mechanisms of Al tolerance in gramineous plants. Plant Soil 381, 1–12 (2014). [Google Scholar]
- Ma J. F. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int. Rev. Cytol 264, 225–252 (2007). [DOI] [PubMed] [Google Scholar]
- Sun C. et al. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol. 201, 1240–1250 (2014). [DOI] [PubMed] [Google Scholar]
- Zheng S. & Yang J. Target sites of aluminum phytotoxicity. Biol. Plant. 49, 321–331 (2005). [Google Scholar]
- Liu J., Piñeros M. A. & Kochian L. V. The role of aluminum sensing and signaling in plant aluminum resistance. J. Integr. Plant Biol. 56, 221–230 (2014). [DOI] [PubMed] [Google Scholar]
- Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. Int. Rev. Cytol. 200, 1–46 (2000). [DOI] [PubMed] [Google Scholar]
- Rengel Z. & Zhang W. Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phyt. 159, 295–314 (2003). [DOI] [PubMed] [Google Scholar]
- Sasaki T. et al. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 37, 645–653 (2004). [DOI] [PubMed] [Google Scholar]
- Lin Z., Zhong S. & Grierson D. Recent advances in ethylene research. J. Exp. Bot. 60, 3311–3336 (2009). [DOI] [PubMed] [Google Scholar]
- Shan X., Yan J. & Xie D. Comparison of phytohormone signaling mechanisms. Curr. Opin. Plant Biol. 15, 84–91 (2012). [DOI] [PubMed] [Google Scholar]
- Sun P. et al. Aluminum-induced ethylene production is associated with inhibition of root elongation in Lotus japonicus L. Plant Cell Physiol. 48, 1229–1235 (2007). [DOI] [PubMed] [Google Scholar]
- Tian Q. et al. Ethylene negatively regulates aluminium-induced malate efflux from wheat roots and tobacco cells transformed with TaALMT1. J. Exp. Bot. 65, 2415–2426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massot N., Nicander B., Barceló J., Poschenrieder C. & Tillberg E. A rapid increase in cytokinin levels and enhanced ethylene evolution precede Al3+ -induced inhibition of root growth in bean seedlings (Phaseolus vulgaris L.). Plant Growth Regul. 37, 105–112 (2002). [Google Scholar]
- Sun P., Tian Q., Chen J. & Zhang W. Aluminium-induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. J. Exp. Bot. 61, 347–356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopittke P. M. et al. Identification of the primary lesion of toxic aluminum in plant roots. Plant Physiol. 167, 1402–1411 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsé B., Poschenrieder C. & Barceló J. The role of ethylene metabolism in the short-term responses to aluminium by roots of two maize cultivars different in Al-resistance. Environ. Exp. Bot. 43, 73–81 (2000). [Google Scholar]
- Wang K. L., Li H. & Ecker J. R. Ethylene biosynthesis and signaling networks. Plant Cell 14 suppl 1, S131–S151 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellingen K. et al. Cadmium-induced ethylene production and responses in Arabidopsis thaliana rely on ACS2 and ACS6 gene expression. BMC Plant Biol. 14, 214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran D. et al. Transcriptome profiling identified novel genes associated with aluminum toxicity, resistance and tolerance in Medicago truncatula. Planta 228, 151–166 (2008). [DOI] [PubMed] [Google Scholar]
- Wang H., Huang J., Liang W., Liang X. & Bi Y. Involvement of putrescine and nitric oxide in aluminum tolerance by modulating citrate secretion from roots of red kidney bean. Plant Soil 366, 479–490 (2013). [Google Scholar]
- Chen W., Xu C., Zhao B., Wang X. & Wang Y. Improved Al tolerance of saffron (Crocus sativus L.) by exogenous polyamines. Acta Physiol. Plant. 30, 121–127 (2008). [Google Scholar]
- Wang H., Liang W. & Huang J. Putrescine mediates aluminum tolerance in red kidney bean by modulating aluminum-induced oxidative stress. Crop Sci. 53, 2120–2128 (2013). [Google Scholar]
- Lutts S., Kinet J.-M. & Bouharmont J. Ethylene production by leaves of rice (Oryza sativa L.) in relation to salinity tolerance and exogenous putrescine application. Plant Sci. 116, 15–25 (1996). [Google Scholar]
- Li C., Jiao J. & Wang G. The important roles of reactive oxygen species in the relationship between ethylene and polyamines in leaves of spring wheat seedlings under root osmotic stress. Plant Sci. 166, 303–315 (2004). [Google Scholar]
- Hyodo H. & Tanaka K. Inhibition of 1-aminocyclopropane-l-carboxylic acid synthase activity by polyamines, their related compounds and metabolites of S-adenosylmethionine. Plant Cell Physiol. 27, 391–398 (1986). [Google Scholar]
- Bleecker A. B. & Kende H. Ethylene: A gaseous signal molecule in plants. Ann. Rev. Cell Dev. Biol. 16, 1–18 (2000). [DOI] [PubMed] [Google Scholar]
- Tsang D. L., Edmond C., Harrington J. L. & Nühse T. S. Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiol. 156, 596–604 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H. & Chan Z. Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J. Integr. Plant Biol. 56, 114–121 (2014). [DOI] [PubMed] [Google Scholar]
- Pál M., Szalai G. & Janda T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 237, 16–23 (2015). [DOI] [PubMed] [Google Scholar]
- Kochian L. V., Pineros M. A. & Hoekenga O. A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274, 175–195 (2005). [Google Scholar]
- Sun C. et al. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J. Integr. Plant Biol. 57, 550–561 (2014). [DOI] [PubMed] [Google Scholar]
- Yu Y. et al. Elevation of arginine decarboxylase-dependent putrescine production enhances aluminum tolerance by decreasing aluminum retention in root cell walls of wheat. J. Hazard. Mater. 299, 280–288 (2015). [DOI] [PubMed] [Google Scholar]
- Mandal C. et al. Antioxidative responses of Salvinia (Salvinia natans Linn.) to aluminium stress and it’s modulation by polyamine. Physiol. Mol. Biol. Plants 19, 91–103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q., Sun P. & Zhang W. Ethylene is involved in nitrate-dependent root growth and branching in Arabidopsis thaliana. New Phytol. 184, 918–931 (2009). [DOI] [PubMed] [Google Scholar]
- Eticha D. et al. Transcriptomic analysis reveals differential gene expression in response to aluminium in common bean (Phaseolus vulgaris) genotypes. Ann. Bot. 105, 1119–1128 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. et al. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 26, 2889–2904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L. et al. Silicon-mediated changes in polyamine and 1-aminocyclopropane-1-carboxylic acid are involved in silicon-induced drought resistance in Sorghum bicolor L. Plant Physiol. Biochem. 80, 268–277 (2014). [DOI] [PubMed] [Google Scholar]
- Tang W. & Newton R. J. Polyamines promote root elongation and growth by increasing root cell division in regenerated Virginia pine (Pinus virginiana Mill.) plantlets. Plant Cell Rep. 24, 581–589 (2005). [DOI] [PubMed] [Google Scholar]
- Swarup R. et al. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19, 2186–2196 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinet M. et al. Putrescine differently influences the effect of salt stress on polyamine metabolism and ethylene synthesis in rice cultivars differing in salt resistance. J. Exp. Bot. 61, 2719–2733 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margret S., Barbara M., Maye C. S., Rudiger H. & Markus W. Methionine salvage and S-adenosylmethionine: essential links between sulfur, ethylene and polyamine biosynthesis. Biochem. J. 451, 145–154 (2013). [DOI] [PubMed] [Google Scholar]
- Chen T. et al. Polyamines and ethylene interact in rice grains in response to soil drying during grain filling. J. Exp. Bot. 64, 2523–2538 (2013). [DOI] [PubMed] [Google Scholar]
- Mehta R. A. et al. Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat. Biotechnol. 20, 613–618 (2002). [DOI] [PubMed] [Google Scholar]
- Dias L. L. et al. Ethylene and polyamine production patterns during in vitro shoot organogenesis of two passion fruit species as affected by polyamines and their inhibitor. Plant Cell Tiss. Org. 99, 199–208 (2009). [Google Scholar]
- Parra-Lobato M. C. & Gomez-Jimenez M. C. Polyamine-induced modulation of genes involved in ethylene biosynthesis and signalling pathways and nitric oxide production during olive mature fruit abscission. J. Exp. Bot. 62 4447–4465 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Zhang Y. & Tao Q. Adaption of wheat genotypes with differential aluminum tolerance to acis, aluminum toxic soil in relation to their nutrient composition in shoots. J. Zhejiang Univ. (Agric. & Life Sci.) 26, 635–639 (1999). [Google Scholar]
- Lin X., Zhang Y. & Luo A. Difference of aluminum tolerance on wheat genotypes and its screening techniques. Plant Nutr. Fertilizer Sci. 7, 64–70 (2001). [Google Scholar]
- Wang H. & Woodson W. R. Reversible inhibition of ethylene action and interruption of petal senescence in carnation flowers by norbornadiene. Plant Physiol. 89, 434–438 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeste K. E., Ye C. & Kieber J. J. Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 119, 521–530 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.