Abstract
Immaturities in cognitive shifting are associated with adolescent risk behaviors. The orbital frontal cortex (OFC) regulates reward processing and response inhibition. This study tested the relationship between cognitive shifting, OFC activity, and reward-modulated response inhibition in young adolescents. An fMRI antisaccade (AS) paradigm examined the effects of reward conditions on inhibitory response and OFC processing. A validated self-report inventory assessed cognitive shifting. Compared to neutral, reward trials showed better AS performance and increased OFC activation. Cognitive shifting positively associated with AS performance in reward and neutral trials. Poorer cognitive shifting predicted greater OFC activation. Results indicate lower OFC efficiency, as greater activation to achieve correct performance, underlies cognitive shifting problems. These neurocognitive impairments are relevant for understanding adolescent risk behaviors.
During adolescence, immaturities in executive functioning and development of brain regions underlying reinforcement processing contribute to psychological dysregulation. Psychological dysregulation involves impairment in the ability to modulate reactivity to environmental challenges for optimal reward gain, defined as maximization of the reward opportunities that can be achieved by behavioral responses (Clark, Thatcher, & Tapert, 2008). Specifically, psychological dysregulation includes deficiencies in, inhibition and cognitive shifting. These executive functions develop throughout adolescence and are thought to be subserved by the frontal cortex (Spear, 2000). As such, variations in frontal cortical development in adolescents may underlie differences in psychological dysregulation (Clark, Chung, Thatcher, Pajtek, & Long, 2012; Habeych, Sclabassi, Charles, Kirisci, & Tarter, 2005; Rubia et al., 2000). Psychological dysregulation in early adolescence, a period of heightened vulnerability towards disturbances in neurocognitive development, predicts high risk behaviors (Clark & Winters, 2002; Giancola, Martin, Tarter, Pelham, & Moss, 1996; Giancola, Mezzich, & Tarter, 1998).
An integral component of psychological regulation, encompassing inhibition and cognitive shifting, is the ability to flexibly alter behavior and problem solving strategies depending on changes in reward contingencies (Miyake et al., 2000; Muller, et al., 2007). The antisaccade (AS) task employed in this study may be used to measure this ability (Luna, Velanova, & Geier, 2008). The AS task requires participants to inhibit a prepotent eye movement to a salient visual stimulus in favor of a voluntary eye movement to the mirror direction (Hallett, 1978). While AS has traditionally been linked to inhibitory abilities, conceptually, AS performance may rely, in part, on cognitive shifting to alter response strategies, which supports the ability to selectively attend and respond to reward relevant cues while ignoring irrelevant stimuli and inhibiting prepotent behavior. In adolescents, problems in cognitive shifting characterized by inflexible behavioral patterns regardless of consequences, as measured in this study, has been found to be associated with AS task performance rates (Agam, Joseph, Barton, & Manoach, 2010; Mosconi et al., 2009).
The ability to accurately perform AS and activation of brain regions supporting AS performance matures from late childhood through late adolescence and early adulthood (Luna et al., 2001). Response patterns in older individuals that are typical of younger developmental periods are identified as being immature. Immaturities in the related ability to suppress prepotent responses, a specific case of behavioral inhibition integral to AS performance, along with other aspects of psychological regulation reflects differences in functional development in frontal, parietal, striatal, and thalamic brain regions during adolescence (Luna et al., 2001). Brain regions that subserve AS performance include the supplementary eye field, dorsolateral prefrontal cortex, posterior parietal cortex, basal ganglia, thalamus, superior colliculus, and cerebellum (Munoz & Everling, 2004; Luna, Garver, Urban, Lazar, & Sweeney, 2004). Various neuroimaging approaches have underscored the importance of these regions in psychological regulation and risk for psychiatric outcomes (Clark et al., 2013).
Of the multiple brain regions that underlie AS performance and other cognitive abilities, the orbital frontal cortex (OFC) is most relevant in psychopathology of a litany of cognitive and psychological regulatory disorders involving reward, behavior inhibition, and cognitive shifting that manifest by late adolescence and early adulthood. The clinical importance of the OFC has been subject of several recent reviews (Goldstein & Volkow, 2002; Schoenbaum & Shaham, 2008; Volkow & Fowler, 2000). To illustrate, hyper OFC activation has been highlighted in individuals with substance use disorder (SUD), a disorder involving reward driven behavior and preservative responding (Volkow & Folwer 2000). However, little is known about OFC functionality underlying psychological regulation, especially cognitive shifting, emerging in early adolescence; when there is a heightened vulnerability to disturbances in neurocognitive development that predicts risk of dysregulatory psychiatric disorders in early adulthood. Of relevance, orbital frontal cortex impairments have been found to contribute to maladaptive perseverance in learned behavioral patterns (Ragozzino, 2007). In addition, OFC lesions are associated with response perseveration and impaired cognitive shifting (Rolls, 2006).
In addition, immature reward processing, which interacts with cognitive shifting and behavioral inhibition, may contribute to psychological dysregulation. Frontal cortical regions associated with higher-order cognitive functioning and reward processing, including the OFC, mature throughout adolescence and into early adulthood (Gogtay et al., 2004; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999). Different models similarly indicate that adult level processing of reward incentives differ from adolescents. One model suggests that relative to adults, adolescents possess hyper-active reward driven systems and less efficient regulatory executive control systems mediated by the prefrontal cortex (Ernst, Pine, & Hardin, 2006). Reward anticipation has been demonstrated to modify cerebral activity in regions supporting behavioral inhibition (Geier & Luna, 2009). In this AS task variant, trials were preceded by a reward cue indicating a possible reward for correct performance (i.e., reward trial) or a neutral cue indicating no potential reward (i.e., neutral trial). Reward incentives were demonstrated to enhance activity in regions supporting AS (Luna et al., 2008; Amador, Schlag-Rey, & Schlag, 2000; Johnston & Everling, 2006).
The OFC has been shown to be a key region in the neurocircuitry that mediates emotional processes critical to responses to changes in reward contingencies (Schultz, 2000; O’Doherty, Kringelbach, Rolls, Hornack, & Andrews, 2001; Schultz & Tremblay, 2006). The OFC is connected to a number of limbic structures and is regarded as the “gateway between the limbic system and representational memory” (Roesch & Schoenbaum, 2006; Price, 2006). While connectivity studies have separately illustrated circuits supporting inhibitory and cognitive shifting abilities, as well as reward processing, together, they highlight the importance of the OFC as a functional locus that integrates cognitive abilities and reward incentives. However, little is known about the differences among adolescents with varying levels of cognitive shifting abilities in regards to reward incentive on AS processing and performance.
In non-human primates, neurons associated with reward expectations and valuations are found in the OFC (Tremblay & Schultz, 1999; 2000). Furthermore, OFC vision-related neurons have been shown to respond differently to images depending on the associated rewards (Rolls, Critchley, Mason, & Wakeman, 1996). OFC processes reward expectations, receipt, and valence, and can maintain reward representations (O’Doherty & Dolan, 2006). In response to changing reward contingencies, OFC influences the choice between repeating a prior response or adopting to a new strategy, which critically involves cognitive shifting abilities (O’Doherty, Critchley, Deichmann, & Dolan, 2003). OFC facilitates selective attention to salient reward cues (Hooker & Knight, 2006), directing attention towards reward relevant stimuli. Furthermore, OFC activation during the preparation phase for response inhibition is associated with lower response inhibition reaction time (Hu & Li, 2012). Thus, problems in cognitive shifting may reflect delays in OFC development of reward processing and underlie poor AS performance. Considering the clinical importance of OFC functioning in previous reports, the limited attention paid to the OFC by previous studies using the AS task must be addressed. Additionally, the OFC functioning associated with reward incentive and response inhibition during early adolescence, as a critical age of development and risk for maladaptive outcomes, is not well understood. It is of critical importance to expand the understanding of the OFC with AS paradigms, such as the task in the current study to allow elucidation of reward incentive and response inhibition in early adolescents with varying executive cognitive functioning.
The aim of this study is to determine, in early adolescents prior to onset of major psychiatric disorders of psychological dysregulation, the effect of reward anticipation on OFC activation and AS performance, and test separately the relationship between reported cognitive shifting problems and OFC activation, as well as the relationship between cognitive shifting problems and AS performance. Reward cue has been shown to facilitate AS performance (Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010). We hypothesize that improved AS performance, as well as increased OFC activation, in response to reward cue, relative to neutral cue. Since we suspect that this laboratory prompted OFC response may reflect a functionally meaningful endophenotype, we hypothesize that this observed OFC activation will be associated with naturally occurring behavioral indicators reflecting problems in cognitive shifting. Additionally, poorer laboratory AS performance is expected to be associated with more reported problems in cognitive shifting.
Methods
Participants
Young adolescent participants (n = 59; 51% male; ages 12–15 years; mean = 13.81 years, sd = 1.15) were recruited from the Pittsburgh area as part of a broader study on alcohol and adolescent brain development. The study group was a community sample stratified by age and ethnicity, and composed of 73% whites and 27% African Americans. The mean Full Scale IQ was 108.2 (sd = 14.7), determined by Wechsler Abbreviated Scale of Intelligence (Psychological Corporation, 1999). To be included in this sample, participants were required to have no lifetime history of substance use. 13.6% of participants had a diagnosis of disruptive behavior disorder (ADHD, oppositional defiant disorder, conduct disorder).
These adolescents were identified through a neighborhood-based targeted random dialing telephone procedure. Successfully contacted families were screened for eligibility by staff at the University Center for Social and Urban Research (UCSUR) at the University of Pittsburgh. Eligible participants and their parents completed informed consent, a psychosocial assessment, and MRI procedures. Written informed consent was obtained in person from a parent and assent from the minor adolescents prior to conducting any of the study procedures. The study protocol was approved by the university’s Institutional Review Board. Prior to MRI scanning, the adolescents were screened for any contraindications to participation such as metal objects that could not be removed or pregnancy.
Measures
Cognitive Shifting
The 5-item Cognitive Shift clinical subscale of the larger Behavior Rating Inventory of Executive Function-Self-Report (BRIEF-SR: Guy, Isquith, & Gioia, 2004), which was designed for application in older children and adolescents (ages 11–18 years) and useful in identifying youths with clinical disorders of executive function, measured participants’ self-reported problems in daily life cognitive shifting on a Likert scale. An example item is as follows: “I try the same approach to a problem over and over even when it does not work.” The BRIEF Cognitive Shift subscale thus assesses the ability to flexibly adapt to different situations, activities, or problem solving strategies as circumstances change. To reduce sample specific effects and allow findings to be more applicable to general populations, the scores were standardized to a normative adolescent population, which produced T-scores for further statistical analyses with fMRI and AS data. To ensure the validity of and remove potential bias scoring on the BRIEF Cognitive Shift measure, participants included in this study were required to meet criteria scores on the BRIEF Negativity (≤5 out of 10) and Inconsistency (≤8 out of 10) validity scales that measure the extent to which items were answered in an unusually negative or inconsistent manner to ensure subjects were not providing invalid and unreliable self-reported cognitive shifting replies to the BRIEF scale. Based on Negativity and Inconsistency scores, 2 subjects were excluded from analyses.
Anti-Saccade Paradigm
The procedure and data acquisition parameters are described in detail in prior publications (Chung et al., 2011; Geier et al., 2010). One of the advantages of using this AS paradigm is that the distinct perceptual, cognitive and behavioral task components (i.e., stimuli presentation, response preparation, AS execution) can be characterized. Additionally, this AS paradigm has the advantage of allowing distinct characterization of activity under reward and neutral cue conditions between individuals with varying cognitive shifting difficulties without reward feedback that may obscure activation results in the OFC. Briefly, participants were trained on the AS task prior to entering the scanner. All trials began with presentation of a reward cue (cue condition) image for 1.5 s consisting of a white fixation cross surrounded by either green dollar signs ($) for reward trials or isoluminant blue number signs (#) for neutral trials (termed the “Cue epoch”). Next, adolescents fixated on a central red cross in a black background for 1.5 s, indicating an AS was to be performed (“Response Preparation epoch”). Finally, a peripheral target appeared at an unpredictable location at a visual angle (± 4° or 8°) on the horizontal meridian for 1.5 s (“Saccade Response epoch”). No explicit visual or auditory feedback was provided regarding whether a trial was successfully performed. A fast event-related design (Dale & Buckner, 1997), which varies the time for baseline recovery, was used to minimize the time needed to present an optimal number of trials in the session. To capture activity uniquely related to each component of the trials (i.e., activity associated with response preparation can be estimated uniquely from reward image processing and motor processing), 30% partial or “catch” trials were included as well as a jittered fixation period between 1.5 and 4.5 s (Ollinger, Shullman, & Corbetta, 2001). There were two catch trial variants where the trial terminated after the Response Preparation epoch or after presentation of the incentive cue. For each incentive, there were 14 trials and 6 partial trials (3 for each variant). Each run lasted 5 min 9 s and was presented 4 times, for a total of 56 reward trials and 56 neutral trials. The order of reward and neutral trials were randomly determined. Participants were told that they could win up to $20, with no monetary loss for incorrect response, if they correctly performed the task over a threshold value. As in previous studies employing this AS paradigm (Geier et al., 2010), the value was intentionally ambiguous to prevent participants from keeping a running total of earning during the task. All participants were compensated.
Anti-Saccade Eye Tracking and Behavioral Analysis
Subject’s eye movements were monitored using the ASL System Model 504LRO (Applied Science Laboratories - Bedford, MA), a video-based eye tracking system that provided video images captured through a small relay mirror in the head coil (Gitelman, Parrish, LaBar, & Mesulam, 2000). Video monitoring provided data on task compliance. Stimuli were presented using E-prime (Psychological Software Tools Inc., Pittsburgh, PA), projected onto a flat screen positioned behind the magnet and viewed on a mirror mounted on the head coil. Eye movements were scored off-line using a combination of ILAB software (Gitelman, 2002) and in-house scoring programs written in MATLAB (MathWorks, Inc., Natick, Massachusetts). A correct AS response was defined as a trial in which the first eye movement during the Saccade epoch with a velocity greater that 30° per second was made towards the mirror direction of the peripheral stimulus and extended beyond a 2.5° visual angle from the central fixation point. An incorrect pro-saccade response was defined as a trial in which the first eye movement during the Saccade epoch was directed towards the stimulus and extended beyond a 2.5° visual angle from the central fixation point. Incorrect pro-saccades were consistently followed by redirection towards the correct location (mirror location of the peripheral stimulus), suggesting that the subject understood and were complying with the task, but was ineffective at inhibiting the pre-potent response (Velanova, Wheeler, & Luna, 2008). The AS performance rate was calculated as a percentage of the number of correct AS responses divided by the number of scorable trials. Trials with no eye movements were excluded from all analyses.
Anti-saccade BOLD fMRI Acquisition and Processing
Data were collected with a Siemens Trio 3T scanner optimized for functional imaging. An automated shim procedure was applied to minimize magnetic field inhomogeneities. In-plane T2 structural images were acquired for visualization and normalization of functional data. Blood oxygenation-level dependent (BOLD) functional images were acquired with a gradient echo EPI sequence, covering 34 axial-oblique slices (3 mm thick, 0 mm gap) oriented to the AC-PC line. This encompassed the entire cerebrum and the majority of the cerebellum (TR = 2000, TE = 25 ms, FOV = 24 cm, matrix = 64 × 64). All scanning parameters were selected to optimize the BOLD signal quality.
Image processing was done using the Functional MRI of the Brain software library (FSL) (Smith et al., 2004) following previously described methods (Geier et al., 2010). Briefly, structural images (MPRAGE) were brain extracted (BET) and registered and then transformed to standard Talairach space using a combination of linear (FLIRT) and nonlinear registration (FNIRT). Functional images were slice-time corrected to adjust for interleaved slice acquisition, rotational and translational motion estimates were calculated and images were motion corrected by aligning each volume in the time series to the middle slice. Functional images were transformed to Talairach space and spatially smoothed with a 5 mm full-width at half maximum kernel and subjected to high-pass temporal filtering (sigma = 37.5 s) to remove low-frequency scanner drift. Signal intensity for each run was scaled to a mean of 100 and multiple runs were concatenated. As an additional step, functional images were also visually inspected for motion artifacts (banding), and slices where they occurred (mean percentage of TRs = 6.3%) were excluded from deconvolution analyses.
Individual subject deconvolution (regression) and group-based analyses were performed with Analysis of Functional Neuroimages (AFNI; Cox, 1996). The regression model consisted of six task regressors (reward cue, neutral cue, reward preparation, neutral preparation, reward saccade response, and neutral saccade response), regressors for reward and neutral error trials (for each epoch), regressors modeling baseline, linear, and non-linear trends, as well as motion parameters that were included as ‘nuisance’ regressors. Analyses of fMRI data included only correct AS trials. Gamma basis functions were used to estimate a unique estimated impulse response function (i.e., hemodynamic response function [HRF]) for each regressor of interest (reward and neutral cue, preparation, and saccade response). For fMRI group-level analyses, we estimated the HRF duration for each epoch of the trial (18 s from onset; 13 TR), using an assumed common shape (Gamma, p = 8.6, q = 0.547). The baseline was calculated as the mean activation for each voxel across all fixation time points. Goodness of fit statistics were calculated from the deconvolution, including partial F-statistics for each task regressor (e.g., reward cue, response preparation, response execution) and t-scores comparing each of the 5 estimated beta weights with zero.
fMRI Group-Level Analyses
Voxel-wise linear mixed-effects models (3dLME program in AFNI) used the subjects’ mean estimated impulse response (beta weights from deconvolution scaled to reflect percent signal change) maps with cue condition (neutral and reward) as a fixed factor and subjects as a random factor for each trial epoch. Analyses of fMRI data were conducted only with correct AS trials. We assessed cue condition effects on cerebral activation in the AS task Response Preparation epoch, which is critical to effective response inhibition (Everling, Dorris, & Munoz, 1998). The main effects of cue condition maps were used to define regions of interest (ROIs). First, peak voxels exceeding a threshold of p < 0.001 (uncorrected) were identified and a 9 mm radius sphere mask was centered on each peak. The main effect of cue condition image was corrected for multiple comparisons using criteria from a Monte Carlo simulation (AFNI AlphaSim). This indicated that a minimum cluster size of 8 contiguous voxels was required and an individual voxel threshold of p = 0.001 in order to achieve a corrected cluster-level significance of p < 0.05. ROIs were defined as significant voxel clusters that were included in non-overlapping spheres with a 9 mm radius centered on the maximum voxel in the corrected main effect of cue condition maps ensuring that the same anatomical regions were examined across subjects and conditions (Velanova et al., 2008; Geier et al., 2010). The functionally defined clusters were then used as masks to extract mean activation values and time courses from the constituent voxels over the 13 TR epochs for each subject in each of the two reward cue conditions.
Statistical Analyses
To facilitate interpretations, additional analyses based on mean activation were conducted in SPSS only with correct AS trials. Given that our hypothesis is region specific, our analyses were focused on the ROI encompassed within the OFC. Due to the diversity of functions mediated by different regions of the OFC, an a priori anatomically defined ROI encompassing the OFC was not employed to exclude activation to stimuli not specifically related to the conditions of the AS task. For the purposes of describing and confirming the cue condition effects on OFC function (Kriegeskorte, Simmons, Bellgowan, & Baker, 2009), we performed analyses with time course data from correctly performed AS trials extracted from deconvolution replicated with cubic spline basis functions, where no assumptions were made about shape. Repeated measures analysis of variance was used to test for interactions between cue condition and time (0–12 TR). Given that the temporally later peak (i.e., peak > 6 s after onset of the epoch) was observed for the ROI, we used a more conservative approach and focused on estimated responses at TRs 3–6. We restricted the Cue Condition X Time to these early time points (i.e., 3–7.5 s after epoch onset) because this period includes the initial peak in a stereotyped hemodynamic response, which generally occurs between 4 and 6 s after stimulus presentation (Geier et al., 2010). Greenhouse-Geisser sphericity corrected levels of significance are reported. The Cue Condition X Time interaction provided information on how brain regions differed in terms of percent signal change between the two cue conditions over time. Regression analyses were performed between individual subjects' BRIEF Cognitive Shift score as the independent variable and dependent variables of mean activations extracted from ROI encompassed within the OFC for both reward and neutral cue trials that were correctly performed to determine the relationship between OFC activation and problems in cognitive shifting. Additional regression analyses were performed between the BRIEF Cognitive Shift score as the independent variable and the dependent variables of AS performance rate for each cue condition (reward vs. neutral) to determine the relationship between problems in cognitive shifting and AS performance. Tests with p < 0.05 were interpreted as significant for these analyses.
Results
Table 1 shows the sample means, standard deviations, and intercorrelations of the main measures: AS performance rate and latency, and lateral (l)OFC activation during correctly performed neutral and reward cue conditions as well as scores on BRIEF Cognitive Shift scale. Cognitive shifting significantly correlated with AS performance rate and lOFC activation during the neutral cue condition.
Table 1.
Means, standard deviations (SD), and intercorrelations of antisaccade (AS) performance and latency, left-orbital frontal cortex (lOFC) activation, and BRIEF Cognitive Shift score
| Main Measures | Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|
| 1. AS Performance Rate - Neutral | 59.4 | 18.4 | ||||||
| 2. AS Performance Rate - Reward | 69.8 | 15.9 | 0.81*** | |||||
| 3. AS Latency - Neutral (ms) | 439.5 | 64.1 | −0.20 | −0.03 | ||||
| 4. AS Latency - Reward (ms) | 420.9 | 57.7 | −0.27* | −0.17 | 0.83*** | |||
| 5. lOFC Activation - Neutral | −0.04 | 0.11 | −0.13 | −0.05 | −0.04 | −0.13 | ||
| 6. lOFC Activation - Reward | 0.02 | 0.11 | −0.13 | −0.04 | 0.13 | 0.08 | 0.26* | |
| 7. BRIEF Cognitive Shifting | 44.5 | 9.7 | −0.32** | 0.33** | 0.19 | 0.17 | 0.29* | 0.14 |
p ≤ .05
p ≤ .01
p ≤ .001.
Behavioral Results
Correct AS task performance rate, calculated as the percentage of correctly performed AS trials out of all scorable trials, was examined in relation to demographic characteristics and the AS trial cue condition (i.e., reward or neutral). Regression analysis showed that correct AS performance rate was significantly associated with age (b = 0.35, t(58) = 2.79, p = 0.007). Age was not associated with other main variables. There was no significantly difference in correct AS performance rate between gender (female: mean = 68.27, sd = 1.55; male: mean = 60.88, sd = 1.66; t = 1.77, df = 57, p = 0.08) or race (Caucasian: mean = 63.33, sd = 1.75; African American: mean = 67.97, sd = 1.32; t = −0.936, df = 56, p = 0.35). Compared to neutral trials, the correct AS performance rate was significantly higher during reward trials (t = −7.35, df = 58, p < 0.001). Correct AS performance rate between neutral and reward trials remained significantly different after controlling for age (f(1, 58) = 11.96, p = .001). Compared to neutral trials, the latency to correct AS performance was significantly shorter during the reward trials (t = 4.004, df = 58, p < 0.001).
fMRI Activation Results
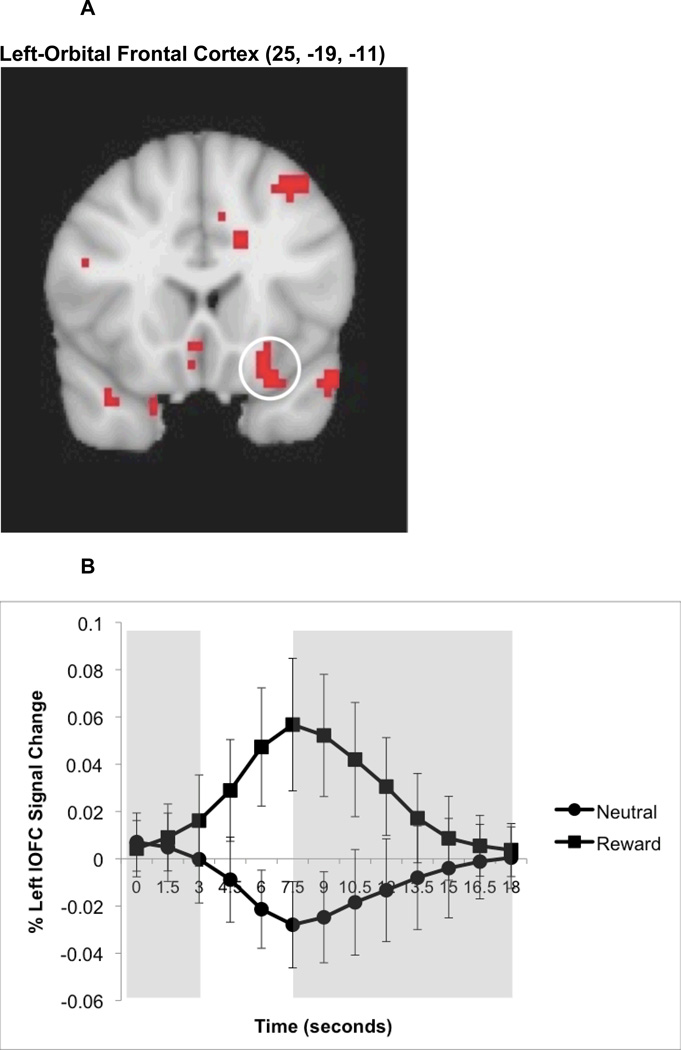
A cluster ROI (Talairach RAI coordinates: 25, −19, −11; Volume: 675ml) within the left lateral Orbital Frontal Cortex (lOFC; Brodmann’s Area 45, 11) was identified in AFNI to have a significant cue condition effect (shown in Fig. 1a,b for descriptive purposes only). This cluster ROI (white circle in Fig. 1a) was used in all subsequent left lOFC analyses in this study. RM ANOVA with lOFC activation during early time points (TR 3–6) across cue condition types determined a significant Cue Condition X Time interaction (f (1, 58) = 4.06, p = 0.046), and a significant main effect of Cue Condition (f(1, 58) = 5.04, p = 0.029). Particularly during early time points, the percent signal change was greater for rewarded trials than during neutral trials (Fig. 1B).
Figure 1.
For illustrative purposes; the effect of Cue Condition (reward vs. neutral) during preparation epoch: Left Lateral Orbital Frontal Cortex (lOFC) mean activation and time courses. A. Activation map during Response Preparation epoch illustrating main effect of Reward Cue Condition in left lOFC (white circle), Talairach RAI coordinates of max voxel (25, −19, −11). B. Left lOFC percent signal change Time X Reward Cue Condition interaction graph, p = 0.046.
Cognitive Shifting Results
Regression analysis was used to examine the association between correct AS performance rate for reward and neutral cue conditions and the BRIEF Cognitive Shift scale, where higher score reflect greater reported problems in the cognitive shifting dimension of executive functioning. As hypothesized, BRIEF Cognitive Shift scores were significantly negatively associated with correct AS performance rates during both rewarded (b = −0.33, t(58) = −2.60, p = 0.012) and neutral (b = −0.32, t(58) = −2.57, p = 0.013) trials. After controlling for age, BRIEF Cognitive Shift score retained significant negative associations with AS performance rates during both reward (b = −0.30, t(58) = −2.51, p = 0.015) and neutral (b = −0.30, t(58) = −2.48, p = 0.016) trials.
BRIEF Cognitive Shift score was significantly positively associated with lOFC activation (beta value from gamma HRF) during the neutral cue condition (b = 0.29, t(58) = 2.325, p = 0.02) indicating that adolescents with higher BRIEF Cognitive Shift scores (greater reported difficulties) demonstrated greater lOFC activation. A significant association was not observed between lOFC activation and BRIEF Cognitive Shift score in the rewarded cue condition (b = 0.14, t(137) = 1.05, p = 0.30). However, Wolfe's Test (1976) of difference between two dependent correlation coefficients showed that the correlations of lOFC-BRIEF Cognitive Shift during neutral and reward cue conditions (Table 1) were not significantly different (t(58) = 1.09, p = 0.14). Regression analyses did not reveal significant associations between BRIEF Cognitive Shift scores and activation during correctly performed trials in other frontal cortical regions that were demonstrated in AFNI to have significant cue condition effects.
Discussion
This study examined the effects of reward anticipation on response inhibition and OFC activation in young adolescents. Adolescents had more successful AS performance and lower response latency during reward trials compared to neutral trials. The reward and neutral cues elicited different lOFC responses during cognitive preparation to inhibit a prepotent saccade response. During neutral trials, adolescents showed lower lOFC activation compared to that in reward trials. Successful AS performance rate was related to fewer reported cognitive shifting problems. In neutral trials, lOFC response was directly related to cognitive shifting, where adolescents reporting greater overall cognitive shifting difficulty exhibited greater lOFC activation when preparing for response inhibition. However, the correlation between lOFC responses and cognitive shifting in reward trials were not significantly different from the correlation in neutral trials.
Consistent with other AS studies (Geier et al., 2010; Hardin, Schroth, Pine, & Ernst, 2007), the reward cue facilitated AS performance. Maturation of inhibitory abilities, along with other processes supporting executive functioning, occurs in late childhood and adolescence (Luna et al., 2004). These data confirm that the neurocognitive systems supporting inhibitory control in response to reward stimuli were engaged by this AS task (Geier & Luna, 2009). The AS latency data reported here are also consistent with previous studies (Geier et al., 2010; Chung et al., 2011). These young adolescents were responsive to the reward cue, which may have heightened motivation to correctly respond to this somewhat difficult task.
LOFC activation levels during cognitive preparation in the correctly performed neutral AS trials were positively associated with reported cognitive shifting difficulties. Exaggerated cerebral activations in critical inhibitory task regulatory regions such as the OFC may indicate more effortful neural responses were required to achieve correct cognitive task performance in inhibition impaired individuals (Fisher, Munoz, & Hariri, 2008; Schulz et al., 2004). Impaired OFC functionality reduces the ability to recruit subsidiary brain regions involved in inhibitory control. This may contribute to inflexibility in adapting to environmental challenges and rigid response strategies despite negative outcomes. Our data suggests that young adolescents with greater difficulties in cognitive shifting posses lower OFC efficiency in inhibitory processing, indicated as greater activation to achieve correct AS task performance. However, as the subjects in the current study were still in the early adolescent phase of development, differences in lOFC activation in neutral trials between those with high and low cognitive shifting scores may not have diverged enough to detect significant differences in the lOFC-cognitive shifting correlation between neutral and reward trails.
No association was found between lOFC activation in correct AS performance and BRIEF Cognitive Shift score during reward trials. Sensitivity to reward can modulate cognitive control processes (Hardin et al., 2007). Compared to adults, adolescents may exhibit heightened incentive responding, with recruitment of OFC and striatum during anticipation of reward (Geier & Luna, 2009). Our data indicated that lOFC activation during reward trials was similar in adolescents with varying degrees of cognitive shifting abilities. Taken in consideration of the greater OFC responses during neutral trials in early adolescents with lower cognitive shifting abilities, these results suggest that the OFC functioning in adolescents with greater cognitive shifting abilities can better discriminate between neutral and reward contexts. When inhibiting prepotent responses, as opposed to hyperactivation during neutral and reward trails, the increased OFC activation during reward laden contexts, relative to neutral, represents more efficient neurocognitive processing such that OFC functioning upregulates according to the demands of reward contingencies. This may underlie the greater abilities for cognitive shifting.
The relationship between BRIEF Cognitive Shift score and AS performance rate was observed in both reward and neutral trials. Consistent with prior research (Miyake et al., 2000), our data showed greater reported cognitive shifting difficulties resulted in poorer AS performance rate. As measured by the BRIEF Cognitive Shift scale, cognitive shifting involves a variety of executive functioning dimensions, including working memory and response inhibition. Our data suggest that successful AS performance may depend on multiple psychological dimensions in cognitive shifting. Initiation of voluntary eye movements, as in the AS task, relies on response inhibition to suppress prepotent responses, and working memory to manipulate information for planned responding (Luna et al., 2008).
While not directly measured in the current study, these results may be pertinent, at least conceptually, to understanding the relationship between psychological dysregulation in young adolescence and later clinical outcomes such as SUD (Clark et al., 2008), as well as inform prevention methodology, including targeting and outcome measurement for preventive interventions (Clark, et al., 2013). As an example, the OFC has a central role in SUD etiology (Goldstein & Volkow, 2002; Schoenbaum & Shaham, 2008; Volkow & Fowler, 2000). Adults with SUD have been shown to exhibit greater OFC activation associated with poorer response inhibition and cognitive shifting on the Stroop task (Goldstein, Volkow, Wang, Fowler, & Rajaram, 2001). Furthermore, lOFC facilitates selective attention and inhibiting interference by irrelevant stimuli critical to response inhibition and cognitive shifting (Hooker & Knight, 2006). Our data extends these findings by demonstrating a similar relationship between lOFC functioning and reported cognitive shifting in young adolescents prior to significant substance use. To elaborate, a primary characteristic of individuals at high risk for SUD is poor cognitive shifting underlied by prefrontal cortical dysfunction, in response to changing reward contexts, especially perseveration towards prepotent responses (Giancola, Peterson, & Pihl, 1993; Wiers, Sergeant, & Gunning, 1994). LOFC functioning was lower in adolescents with greater cognitive shifting abilities relative to adolescents with poor cognitive shifting abilities during neutral trials, but similar during reward trials. This suggests that greater OFC efficiency, operationalized as activation according the demands of the reward contingency and discrimination of reward cues relative to neutral, underlies cognitive shifting. Hence the association between more effortful OFC responding during neutral trails, in which the reward cue condition is not presented, and poor cognitive shifting, as well as the similar OFC activation across different levels of cognitive shifting during reward trails corresponds to the neurocognitive underpinnings of psychological dysregulatory factors that may contribute dysregulatory disorders including SUD. While speculative, these data, together, suggest that elevated OFC activation during challenges to response inhibition and problematic cognitive shifting may be part of the pathophysiology contributing to SUD risk as well as other dysregulatory problems. Cognitive shifting impairment associated with immaturities in OFC is an important component of psychological dysregulation, suggesting a possible neurocognitive endophenotype predicting SUD. Future studies may confirm this relationship by testing whether OFC activation patterns during correct AS trails in young adolescents in the current study predicts odds of substance use and SUD at later follow-up assessments during peak risk ages.
The findings of this study must be considered in the context of several limitations. Cognitive shifting difficulties in these young adolescents were self-reported and not verified by other observers. However, biases in cognitive shifting reports were controlled for by removing subjects demonstrating disproportionately high Negativity and Inconsistency scores in the BRIEF validity scales as an internal check of the data accuracy. Thus, there is some verification supporting scale validity and accuracy of results. Brain activation patterns during unsuccessful AS trials and the potential influence of performance feedback were not studied but may be directions for future research. Laboratory based assessment of executive functioning and cognitive control may have limited generalizability to real-world contexts that contain multiple social and incentive factors (Steinberg, 2010). However, the use of BRIEF scores derived from daily life executive functioning difficulties represents a step towards understanding the AS paradigm in "every-day" behavior. The adolescents included in this study were a community sample before the typical age of onset of significant substance use. Interpretation about AS task responses and cerebral activation patterns potentially predicting SUD require further empirical investigation. The correlation between cognitive shifting and lOFC activation in neutral trials was not significantly different from the correlation between cognitive shifting and activation during reward trials. This may partly be due to the earliness of the assessment ages, prior to manifestation of phenotypic differences in the rate of cognitive development and reward processing. Nevertheless, greater problems in cognitive shifting is associated with elevated lOFC activation with neutral cue condition.
In summary, this study examined relationships among OFC activation, cognitive shifting, and response inhibition in young adolescents. Adolescents with greater difficulties in cognitive shifting produced more effortful OFC activation responses during successful AS response inhibition, and poorer AS performance while OFC activation in adolescent with greater cognitive shifting abilities can better discriminate reward from non-reward contexts in changing task requirements. Reward cues enhanced successful AS performance by increasing accuracy and reducing latency. These results suggest that difficulties in cognitive shifting may be underlied by inefficient OFC discrimination and response to reward stimuli from non-reward stimuli. Furthermore, this expands the applications of the AS paradigm to include assessment of cognitive shifting abilities. These results advance understanding of the neurobiological basis for observations that adolescents with psychological dysregulation may benefit from prevention approaches with clear reward contingencies.
Acknowledgments
This research was supported by grants from NIH (R21AA017312, P50DA005605) and the Commonwealth of Pennsylvania (PA-HEAL SPH00010).
References
- Agam Y, Joseph RM, Barton JJ, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage. 2010;52(1):336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. Journal of Neurophysiology. 2000;84(4):2166–2170. doi: 10.1152/jn.2000.84.4.2166. Retrieved from: http://jn.physiology.org/ [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. Retrieved from: http://www.journals.elsevier.com/journal-of-biomedical-informatics/ [DOI] [PubMed] [Google Scholar]
- Chung T, Geier CF, Luna B, Pajtek S, Terwilliger R, Thatcher DL, Clark DB. Enhancing response inhibition by incentive: Comparison of adolescents with and without substance use disorder. Drug and Alcohol Dependence. 2011;115(1–2):43–50. doi: 10.1016/j.drugalcdep.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Chung T, Pajtek S, Zhai Z, Long E, Hasler B. Neuroimaging methods for adolescent substance use disorder prevention science. Prevention Science. 2013;14(3):300–309. doi: 10.1007/s11121-012-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Chung T, Thatcher DL, Pajtek S, Long EC. Psychological dysregulation, white matter disorganization and substance use disorders in adolescence. Addiction. 2012;107(1):206–214. doi: 10.1111/j.1360-0443.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation and adolescent brain development. Alcoholism: Clinical and Experimental Research. 2008;32(3):375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Clark DB, Winters KC. Measuring risks and outcomes in substance use disorders prevention research. Journal of Consulting and Clinical Psychology. 2002;70(6):1207–1223. doi: 10.1037//0022-006x.70.6.1207. [DOI] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5(5):329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. http://dx.doi.org/10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Munoz DP. Reflexive suppression in the anti-saccade task is dependent on prestimulus neural processes. Journal of Neurophysiology. 1998;80(3):1584–1589. doi: 10.1152/jn.1998.80.3.1584. Retrieved from: http://jn.physiology.org/ [DOI] [PubMed] [Google Scholar]
- Fisher PM, Munoz KE, Hariri AR. Identification of neurogenetic pathways of risk for psychopathology. American Journal of Medical Genetics Part C Seminars in Medical Genetics. 2008;148C(2):147–153. doi: 10.1002/ajmg.c.30173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Luna B. The maturation of incentive processing and cognitive control. Pharmacology, Biochemistry, and behavior. 2009;93(3):212–221. doi: 10.1016/j.pbb.2009.01.021. Retrieved from: http://www.journals.elsevier.com/pharmacology-biochemistry-and-behavior/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Martin CS, Tarter RE, Pelham WE, Moss HB. Executive cognitive functioning and aggressive behavior in preadolescent boys at high risk for substance abuse/dependence. Journal of Studies on Alcohol. 1996;57(4):352–359. doi: 10.15288/jsa.1996.57.352. Retrieved from: http://www.jsad.com/ [DOI] [PubMed] [Google Scholar]
- Giancola PR, Mezzich AC, Tarter RE. Executive cognitive functioning, temperament, and antisocial behavior in conduct-disordered adolescent females. Journal of Abnormal Psychology. 1998;107(4):629–641. doi: 10.1037//0021-843x.107.4.629. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Peterson JB, Pihl RO. Risk for alcoholism, antisocial behavior, and response perseveration. Journal of Clinical Psychology. 1993;49(3):423–428. doi: 10.1002/1097-4679(199305)49:3<423::aid-jclp2270490317>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Gitelman DR. ILAB: a program for post-experimental eye movement analysis. Behavior Research Methods, Instruments, and Computers. 2002;34(4):605–612. doi: 10.3758/bf03195488. Retrieved from: http://link.springer.com/journal/13428. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Parrish TB, LaBar KS, Mesulam MM. Real-time monitoring of eye movements using infrared video-oculography during functional magnetic resonance imaging of the frontal eye fields. Neuroimage. 2000;11(1):58–65. doi: 10.1006/nimg.1999.0517. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Science USA. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: Involvement in response inhibition. Neuroreport. 2001;12(11):2595–2599. doi: 10.1097/00001756-200108080-00060. Retrieved from: http://journals.lww.com/neuroreport/pages/default.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy SC, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function Self-Report Version. Odessa, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- Habeych ME, Sclabassi RJ, Charles PJ, Kirisci L, Tarter RE. Association among parental substance use disorder, p300 amplitude, neurobehavioral disinhibition in preteen boys at high risk for substance use disorder. Psychology of Addictive Behaviors. 2005;19(2):123–30. doi: 10.1037/0893-164X.19.2.123. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18(10):1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: Development and psychopathology related differences. Journal of Child Psychology and Psychiatry. 2007;48(5):446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Knight RT. The role of lateral orbitofrontal cortex inhibitory control of emotion. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford, England: Oxford University Press; 2006. pp. 307–324. [Google Scholar]
- Hu S, Li CS. Neural processes of preparatory control for stop signal inhibition. Human Brain Mapping. 2012;33(12):2785–2796. doi: 10.1002/hbm.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K, Everling S. Neural activity in monkey prefrontal cortex is modulated by task context and behavioral instruction during delay-match-to-sample and conditional prosaccade-antisaccade tasks. Journal of Cognitive Neuroscience. 2006;18(5):749–765. doi: 10.1162/jocn.2006.18.5.749. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nature Neuroscience. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B, Velanova K, Geier CF. Development of eye-movement control. Brain and Cognition. 2008;68(3):293–308. doi: 10.1016/j.bandc.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex "Frontal Lobe" tasks: A latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D’Cruz AM, Seidenfeld A, Guter S, Stanford LD, Sweeney JA. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychological Medicine. 2009;39(9):1559–1566. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Dreisbach G, Goschke T, Hensch T, Lesch KP, Brocke B. Dopamine and cognitive control: The prospect of monetary gains influences the balance between flexibility and stability in a set-shifting paradigm. European Journal of Neuroscience. 2007;26(12):3661–3668. doi: 10.1111/j.1460-9568.2007.05949.x. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: The anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience. 2004;5(3):218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. Journal of Neuroscience. 2003;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. Retrieved from: http://www.jneurosci.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP, Dolan RJ. The role of human orbitofrontal cortex in reward prediction and behavioral choice: Insights from neuroimaging. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford, England: Oxford University Press; 2006. pp. 265–283. [Google Scholar]
- O’Doherty JP, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13(1):210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Price JL. Connections of orbital cortex. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford, England: Oxford University Press; 2006. pp. 39–55. [Google Scholar]
- Psychological Corporation. Weschler Abbreviated Scale of Intelligence (WASI) Manual. San Antonio, TX: Author; 1999. [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York Academy of Sciences. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Roesch M, Schoenbaum G. From associations to expectancies: Orbitofrontal cortex as gateway between limbic system and representational memory. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford, England: Oxford University Press; 2006. pp. 199–235. [Google Scholar]
- Rolls ET. The neurophysiology and functions of the orbitofrontal cortex. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford, England: Oxford University Press; 2006. pp. 95–124. [Google Scholar]
- Rolls ET, Critchley HD, Mason R, Wakeman EA. Orbitofrontal cortex neurons: role in olfactory and visual association learning. Journal of Neurophysiology. 1996;75(5):1970–1981. doi: 10.1152/jn.1996.75.5.1970. Retrieved from: http://jn.physiology.org/ [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Functional frontalisation with age: Mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Reviews. 2000;24(1):13–19. doi: 10.1016/s0149-7634(99)00055-x. http://dx.doi.org/10.1016/S0149-7634(99)00055-X. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L. Involvement of primate orbitofrontal neurons in reward, uncertainty, and learning. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford, England: Oxford University Press; 2006. pp. 173–198. [Google Scholar]
- Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, Halperin JM. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event-related fMRI study. American Journal of Psychiatry. 2004;161(9):1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: A review of preclinical studies. Biological Psychiatry. 2008;63(3):256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansesn-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. Retrieved from: http://www.journals.elsevier.com/neuroscience-and-biobehavioral-reviews/ [DOI] [PubMed] [Google Scholar]
- Steinberg L. A dual system model of adolescent risk-taking. Developmental Psychobiology. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398(6729):704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Reward-related neuronal activity during go-nogo task performance in primate orbitofrontal cortex. Journal of Neurophysiology. 2000;83(4):1864–1876. doi: 10.1152/jn.2000.83.4.1864. Retrieved from: http://jn.physiology.org/ [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18(11):2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Sergeant JA, Gunning WB. Psychological mechanisms of enhanced risk of addiction in children of alcoholics: A dual pathway? Acta Paediatrica Supplement. 1994;404:9–13. doi: 10.1111/j.1651-2227.1994.tb13377.x. [DOI] [PubMed] [Google Scholar]
- Wolfe DA. On testing equality of related correlation coefficients. Biometrika. 1976;63:214–215. [Google Scholar]