Abstract
The major disorders of β-globin, sickle cell disease and β-thalassemia, may be ameliorated by expression of the fetal gene paralog γ-globin. Uncertainty regarding the mechanisms repressing fetal hemoglobin in the adult stage has served as a puzzle of developmental gene regulation as well as a barrier to rational therapeutic design. Recent genome-wide association studies implicated the zinc-finger transcriptional repressor BCL11A in fetal hemoglobin regulation. Extensive genetic analyses have validated BCL11A as a potent repressor of fetal hemoglobin level. Studies of BCL11A exemplify how contextual gene regulation may often be the substrate for trait-associated common genetic variation. These discoveries have suggested novel rational approaches for the β-hemoglobin disorders including therapeutic genome editing.
The hemoglobin disorders, the most common monogenic diseases in the world, have long served as exemplars of molecular medicine. Upon recognition of a chemically anomalous hemoglobin, Pauling dubbed sickle cell anemia the first molecular disease more than 65 years ago [1]. Less than a decade later, the defining single amino acid substitution in the β-chain was discovered as its etiology [2]. In the 1970s and 1980s, the genetic underpinnings of the thalassemia syndromes, disorders of hemoglobin production rather than structure, were unraveled by molecular cloning, revealing diverse mutations affecting the globin genes [3]. These discoveries emphasized the importance of not only coding sequences but also splice sites, 5′ and 3′ untranslated regions, proximal promoters, and distal regulatory elements in determining appropriate gene expression.
The Mendelian nature of inheritance of the hemoglobin disorders belies their phenotypic heterogeneity. The chief predictor of clinical severity of the β-hemoglobin disorders is the level of fetal hemoglobin (HbF, α2γ2). In β-thalassemia, increased levels of γ-globin substitute for absent β-globin and mitigate relative α-chain excess. In sickle cell disease (SCD), γ-globin serves as a potent anti-sickling agent. Clinical observations—including those of rare patients co-inheriting highly elevated HbF (hereditary persistence of fetal hemoglobin), large cohorts with varying HbF levels, and the natural history of infants with waning HbF— unambiguously demonstrate that HbF ameliorates the β-hemoglobin disorders.
The β-globin gene cluster includes 5 paralogous globin genes whose expression is developmentally regulated. These include ε-globin, expressed during the early first trimester from the yolk sac, two γ-globin genes (Gγ and Aγ), expressed during the remainder of fetal life, and the minor δ-globin and major β-globin adult gene products, expressed throughout postnatal life. Hemoglobin switching refers to the process of developmental changes in hemoglobin production. In a transition largely driven at the transcriptional level, HbF, the dominant hemoglobin in utero, becomes replaced by HbA (α2β2) as the γ-globin genes are progressively silenced and the β-globin gene is reciprocally activated. In the adult stage, HbA constitutes ∼97% of hemoglobin, the minor HbA2 (α2δ2) ∼2%, and HbF <1%. Residual HbF is unevenly distributed within erythrocytes, limited to a subset of cells termed F-cells.
The mechanisms controlling hemoglobin switching, particularly the fetal-to-adult transition, long remained obscure despite extensive inquiry [4]. The underlying problem is related to cellular identity, and how stage specificity for globin transcription is imposed in an erythroid cell context. More precisely, the crux is how transcript production shifts from the γ-genes to the β-gene in the β-like globin complex. As reactivation of HbF could be curative for the β-hemoglobin disorders, an understanding of the mechanisms of developmental control has immediate and direct clinical implications. An optimal therapeutic maneuver would result in measurable amounts of HbF in all erythrocytes (i.e. pancellular F-cell distribution) rather than limited to a subset (i.e. heterocellular F-cell distribution). An understanding of the molecular circuitry controlling HbF is a necessary precondition for rationally designed therapies.
Recent work has identified BCL11A as a critical modifier of the hemoglobin disorders. In addition to direct therapeutic relevance for the hemoglobinopathies, these findings emphasize the importance of contextual gene regulation in determining heritable disease severity.
Genetic evidence connecting BCL11A and HbF regulation
The initial breakthrough came from genome-wide association studies (GWAS). These studies identified common variants at three loci associated with HbF level, or the closely associated trait F-cell number [5,6]. The loci included the β-globin gene cluster, intergenic sequences between HBS1L and MYB, and BCL11A. These studies are notable for a number of reasons. First, the genetic architecture is very simple with relatively few genes of large effect. Just three loci account for about half the heritable variation in HbF level [7]. Second, the results have been robust and reproducible across populations of European, African, and Asian ancestry, as well as in healthy individuals and those with β-hemoglobin disorders [8-11]. Third, the same variants associated with HbF level are associated with the clinical severity of both SCD and β-thalassemia, emphasizing the potential therapeutic importance of the discoveries [8,9,12]. Finally, the results were concordant with prior knowledge, indicating the validity of the approach. Not only were variants recovered at the β-globin gene cluster itself, but also variants in the HBS1L–MYB intergenic interval had previously been associated with HbF level by familial linkage studies [13]. Of the three loci, only BCL11A was novel.
At the time of its implication in HbF regulation by GWAS, BCL11A was known to be a zinc finger transcriptional repressor protein and an oncogene in B-cell malignancies [14,15]. Subsequently it was found to coordinate gene expression during B-lymphopoiesis and neurogenesis [16,17]. Prior to the GWAS, it had not been suspected to play a role in erythropoiesis. Consistent with a function in repressing γ-globin expression, BCL11A is expressed in erythroid precursors [5].
Subsequent genetic experiments have demonstrated the critical role BCL11A plays as a repressor of HbF. Knockdown of BCL11A by RNA interference (RNAi) in primary human erythroid precursors leads to HbF expression without apparent detrimental effect on erythropoiesis [18]. Genetic studies in mice offer additional support but are complicated by the absence of a true fetal stage of globin expression in rodents. Transgenic mouse experiments long ago revealed that human γ-globin genes are expressed roughly coordinately with the endogenous mouse embryonic globins (βh1 and εy) and extinguished at the fetal liver stage rather than after birth [19]. Knockout of Bcl11a during mouse development prevents appropriate silencing of endogenous embryonic globins and transgenic human γ-globins [20]. A partial loss of silencing is observed in heterozygotes, suggesting BCL11A represses embryonic and fetal globins in a dose-dependent manner. Conditional erythroid knockout of Bcl11a greatly relieves repression of mouse embryonic and human fetal globin genes without negative impact on erythropoiesis [21,22]. Inducible deletion of Bcl11a in adulthood results in derepression of embryonic and fetal globin genes, indicating that BCL11A actively enforces adult-stage globin expression throughout adult life and that repression is reversible.
Additional support for the relevance of BCL11A to HbF control is provided by a “preclinical” experiment in which the phenotype of genetically engineered SCD mice is reversed in the context of erythroid-specific Bcl11a knockout, a particularly striking result indicating the therapeutic potential of targeting BCL11A [21]. In these mice, standard red blood cell parameters normalize, HbF levels are induced in an essentially pancellular fashion, circulating sickle forms are absent from the peripheral blood, and end-organ damage, as indicated by loss of urinary concentrating function, is prevented. These data offered the first experimental evidence that loss of a single gene product could thwart SCD. Overall, the preponderance of experimental genetic data validate BCL11A as a therapeutic target for the β-hemoglobin disorders.
BCL11A participates in multiprotein transcriptional complexes in erythroid cells (Figure 1) [18,23,24]. Its protein partners, as ascertained by affinity purification and protein microsequencing, include DNA-binding erythroid transcription factors (TFs) such as GATA1, FOG1, RUNX1, IKZF1, and SOX6. In addition, it binds to numerous chromatin regulators and transcriptional corepressors. These include members of the NuRD, LSD1/CoREST, NCoR/SMRT, SIN3, and SWI/SNF complexes. Notable interactors include the Mi2β (CHD4) helicase, HDAC1 and HDAC2 histone deacetylases, LSD1 lysine demethylase, DNMT1 DNA methyltransferase, and NCoR1 and BCoR corepressors. BCL11A occupies erythroid chromatin at the β-globin gene cluster [18,23]. It binds at numerous sites, including within the locus control region (LCR) distal enhancer elements as well as to the ε-globin gene but it does not bind to β-globin or the γ-globin genes themselves. Therefore it appears to exert its repressive function of γ-globin at a distance. BCL11A is required for configuring the β-globin locus in erythroid cells, promoting long-range interactions between the LCR and β-globin gene at the expense of LCR–γ-globin interactions, in keeping with a competitive mode of globin gene regulation [23]. Inhibition of many of the protein partners of BCL11A results in HbF induction, particularly when assayed by RNAi knockdown in CD34+ hematopoietic stem and progenitor cells subjected to ex vivo erythroid maturation [24,25]. However none of the partners tested to date has the specificity of BCL11A on HbF regulation. These protein partners tend to have more widespread effects on erythroid gene expression as compared to BCL11A itself whose effects are quite restricted to the globin genes. Inhibition of BCL11A's protein partners (unlike BCL11A itself) typically results in impaired erythroid maturation. These results suggest that BCL11A participates in only a subset of the functions and/or complexes of its partners. Moreover, pharmacologic HbF inducers, such as inhibitors of DNA methyltransferase, histone deacetylase, and LSD1 demethylase, are much more effective in the absence of BCL11A [21,24]. These results suggest that residual BCL11A serves as a barrier to alternative strategies for HbF reinduction.
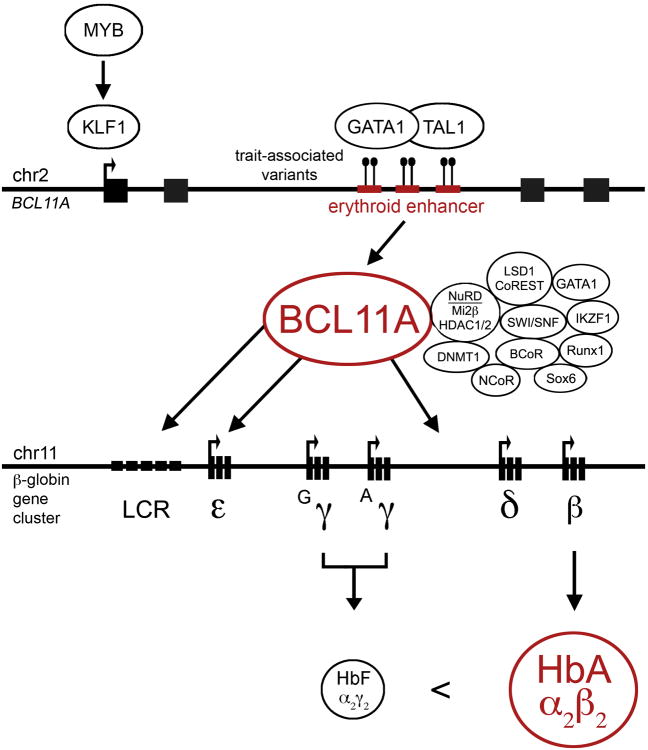
Figure 1. The BCL11A–HbF repression axis.
BCL11A itself relies on an erythroid enhancer, the subject of common trait-associated genetic variation, for its adult-stage erythroid expression pattern. GATA1 and TAL1 bind to this enhancer, and KLF1 (which may in turn be regulated by MYB) transactivates BCL11A, binding to its promoter. BCL11A is found in a multiprotein transcriptional complex in erythroid precursors, including chromatin regulators such as the NuRD complex (with Mi2β helicase and HDAC1/2 deacetylases), DNMT1 DNA methyltransferase, LSD1 lysine demethylase, SWI/SNF remodeling complex, additional corepressors such as NCoR and BCoR, and erythroid DNA-binding transcription factors such as GATA1, IKZF1, Runx1, and Sox6. BCL11A binds various regions of the β-globin gene cluster, including the locus control region (LCR) distal enhancer elements, to the ε-globin gene itself, and to intergenic sequences between γ- and δ-globin that have been implicated in γ-globin repression. BCL11A does not bind directly to the γ-globin genes. The outcome of BCL11A expression in adult-stage erythroid cells is the predominant expression of β-globin (and thus HbA) at the expense of γ-globin (and HbF).
Effects of common genetic variation on BCL11A
Although GWAS have been very successful in identifying thousands of loci associated with human traits and disease susceptibility, there remains a dearth of knowledge for most of these associations regarding the identity of the causal variants and affected genes and biologic pathways. Underscoring this challenge, due to linkage disequilibrium, it can be very difficult to distinguish causal variants from mere associated markers. Like many SNPs highlighted by GWAS, the common genetic variants associated with HbF level fall in non-coding sequences. The trait-associated variants at BCL11A reside within its large second intron. These variants, co-inherited as haplotypes, cluster at a region marked by an erythroid enhancer chromatin signature (Figure 1) [26]. This region possesses typical enhancer-associated histone modifications, such as H3K4me1 and H3K27ac, erythroid TF occupancy, DNase I sensitivity, and long-range promoter interactions. Fine-mapping of trait-associated variants demonstrates that the most highly trait-associated variants fall directly within DNase I hypersensitive sites (DHSs). The DHS residing variants fully account for the trait association of the previously identified lead variants, suggesting these were merely markers in linkage disequilibrium with enhancer variants. The most highly trait-associated variant rs1427407 disrupts a composite half E-box/GATA motif, a TF recognition site highly bound by GATA1/TAL1 complexes in erythroid cells. This variant is associated with modest reduction in TF binding and modest reduction of BCL11A expression in primary human erythroid precursors. The DHSs harboring these variants confer adult developmental stage-specific and erythroid lineage-restricted gene expression in classical ectopic enhancer assays. Genome editing experiments demonstrate that the enhancer is strictly required for BCL11A expression in the erythroid lineage but dispensable in non-erythroid contexts [26].
These results have furthered our understanding of globin gene regulation and demonstrate that the hemoglobin disorders continue to be paradigmatic of advances in molecular medicine. The role of BCL11A in repressing HbF emphasizes the importance of heritable modifiers, even for apparently monogenic conditions. The fact that common genetic variation impacts BCL11A through an erythroid-specific enhancer appears to be just one example of how context-specific gene regulation is a major determinant of human traits and disease susceptibility. Numerous chromatin mapping efforts have identified substantial enrichment of common variants related to human traits and disease susceptibility by GWAS within distal regulatory elements, particularly enhancers active in cell types relevant to the trait [27-29]. For example, one recent report estimated that 60% of the causal variants associated with autoimmunity reside within immune cell enhancers [30]. This mechanism of regulatory variation is conceptually appealing in that many genes are pleiotropic and under substantial evolutionary constraint. Therefore although trans-acting gene-encoded regulatory factors are highly conserved, the cis-regulatory landscape upon which these factors act may be subject to much greater evolutionary turnover [31,32]. This could result in both inter- and intra-species phenotypic variation that could escape excess negative selection. For example, mice with Bcl11a deficiency demonstrate perinatal lethality, presumably due to the critical role of BCL11A in development of the central nervous system. Humans haploinsufficient for BCL11A due to intrachromosomal deletions demonstrate severe neurodevelopmental phenotypes, such as autism, mental retardation, and speech and language disorder [33-35]. Analysis of exome-sequencing data from healthy individuals show that BCL11A is among the 5% of genes under the highest evolutionary constraint, with relative absence of missense as compared to synonymous mutations. Variants at BCL11A have been associated with type 2 diabetes mellitus and BCL11A coordinates pancreatic gene expression [36]. BCL11A, subject to recurrent gene amplification in triple-negative breast cancer, is required for mammary stem and progenitor cell function [37]. These results indicate that BCL11A plays varied roles in different cellular lineages and that coding variation at BCL11A is highly deleterious [38]. In contrast, HbF-associated erythroid enhancer variants at BCL11A are very common; e.g. the most highly associated variant rs1427407 has a 20% allele frequency [39].
In addition, the example of BCL11A highlights the importance of studying protective genetic variation. Many mutations and many pharmacotherapies act in a loss-of-function manner. Therefore, phenocopying protective inactivating genetic variants with rational therapeutics may be a favorable approach as compared to attempts to reverse disease-associated inactivating mutations. Other examples of protective inactivating mutations that have prompted investigation of novel therapeutic strategies include PCSK9 in heart disease and CCR5 in AIDS [40,41].
Outstanding questions
There are several questions that remain regarding mechanisms of HbF repression and how recognition of the critical role of BCL11A may be harnessed for therapeutic advantage.
First, how does BCL11A actually repress γ-globin transcription. BCL11A has several potentially functional domains, including six C2H2 zinc fingers, one C2HC zinc finger, a NuRD-interacting domain, an acidic domain, and a proline-rich domain [23]. There are several splice variants of BCL11A, with the XL-transcript producing the dominant isoform in erythroid cells. As described above, BCL11A has numerous protein partners. It remains to be defined which domains and partners are required for its function. Careful structure-function analysis of BCL11A may highlight critical protein-protein interactions. Although BCL11A has been reported to bind DNA in vitro, it is uncertain if it directly binds DNA in vivo, or is recruited by other TFs. Motif analyses have yielded conflicting results as to sequence binding preference emphasizing that an obvious DNA binding function of BCL11A has yet to be demonstrated [42-44]. The DNA binding preference for the paralog BCL11B has been similarly challenging to define [45,46]. Perhaps function of BCL11A at the LCR and putative regulatory sequences intergenic to γ- and δ-globin genes are sufficient to account for its effects [47]. It is also possible that BCL11A directly interacts with factors that in turn may bind to the γ-globin genes.
A second major unanswered question is how BCL11A is developmentally regulated. Although the identification of the role of BCL11A in hemoglobin switching was a major advance, on one level this merely redefines the problem as identifying the factors that activate BCL11A during the adult stage. A substantial component of this regulation appears to be transcriptional [23,26]. The erythroid enhancer of BCL11A restricts expression to the definitive developmental stage. Fine-mapping the BCL11A enhancer would be one approach to elucidate cis- and trans-acting determinants upstream of BCL11A. BCL11A is also expressed in hematopoietic stem and progenitor cells as well as in lymphocyte precursors and various immune cells [48,49]. With the recent appreciation for inflammatory inputs into definitive hematopoietic stem cell (HSC) formation and function [50-53], one possibility to be investigated is the interplay between inflammation, BCL11A, and HSC biology. Several regulators of BCL11A have already been identified, and ongoing work will be needed to determine the role of these during organismal development as well as erythroid maturation. KLF1 is an erythroid TF with an important role in HbF control that binds to the BCL11A promoter and positively regulates BCL11A expression [54,55]. MYB, another key HbF regulator, may itself be upstream of KLF1 [56]. Mi2β the helicase component of the NuRD complex, may be required for complete BCL11A expression, although other components of NuRD such as MBD2 appear to repress the expression of BCL11A [57]. There also appear to be post-transcriptional controls on BCL11A expression and processing, with short variants detectable prenatally, apparently due to protein processing [18,20]. The microRNA miR486-3p has been reported to destabilize the BCL11A transcript in erythroid cells [58]. An important connection to be determined is how generic developmental programs interface with BCL11A. One such candidate pathway is the heterochronic LIN28/let-7 axis, whereby LIN28 is abundant in many tissues during fetal life and reciprocally let-7 microRNAs are abundant after birth. In fact, LIN28 overexpression has been reported to increase HbF levels in CD34+ cells from healthy donors and SCD patients, although the impact of LIN28 on BCL11A expression has been inconsistent [59,60].
The advances in our knowledge of globin gene regulation and hemoglobin switching have mainly stemmed from observations of human genetics. The existing GWAS account for about half the heritable variation of HbF level, suggesting another half remains to be determined. Whether more highly powered conventional studies, novel study designs, such as restricting analyses to genes and elements most likely to be involved in adult-stage erythropoiesis, and/or investigation of rare informative patients will be required, it seems likely that human genetics has more lessons to teach.
Translating findings to the clinic
One area of clinical need is obtaining a plentiful source of human blood cells suitable for transfusion. Currently altruistic donors are used as sources for blood products. However infectious transmission risk, genetic and immune incompatibilities, and cost are major concerns. Pluripotent and hematopoietic stem cells may be cultured from various donors and induced to differentiate. Current protocols for in vitro erythroid maturation, while still not of a scale to support routine clinical transfusion, are advancing [61-63]. One challenge is that pluripotent stem cell derived progeny typically retain an embryonic character, in the case of red blood cells expressing embryonic and fetal forms of hemoglobin. A recent study showed that overexpression of BCL11A is able to convert induced pluripotent stem cell derived lines from the expression of only embryonic and fetal globin genes to predominant expression of adult globins [64]. This finding could be of relevance for efforts to use pluripotent stem cells as a source of adult blood cells.
Current treatments for the hemoglobin disorders are supportive and do not address the fundamental pathophysiologic defects. Hydroxyurea is the only FDA-approved medication for the treatment of SCD. Although this agent can modestly elevate HbF levels and mitigate painful crises, it has inconsistent efficacy, causes dose-limiting myelosuppression, and requires careful laboratory monitoring, which limit its utility. Allogeneic HSC transplantation can be curative, but immune incompatibility restricts its application. Additional therapeutic options are needed. One approach would be to target BCL11A directly at the gene level. This could be accomplished by RNA interference [65] although persistent and specific delivery to erythroid cells would be crucial.
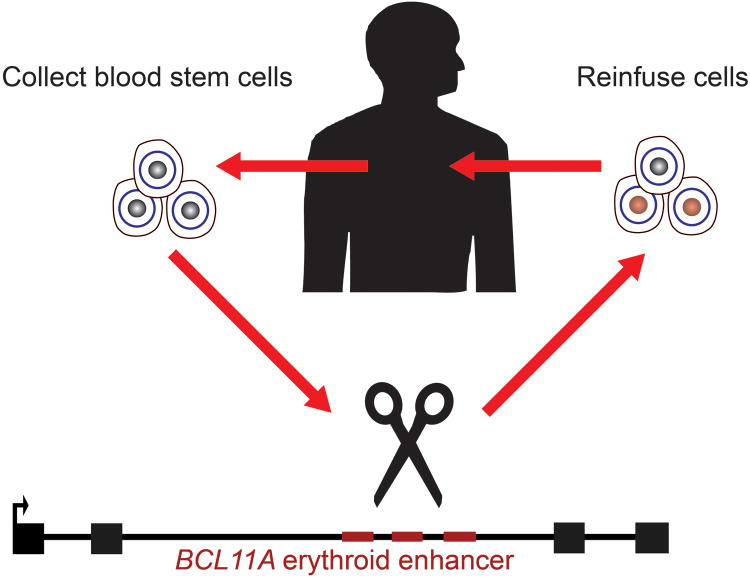
Another possibility would be therapeutic genome editing with targetable endonucleases [66]. Rapid progress in genome editing tools, particularly with the RNA-guided CRISPR/Cas systems, has made precise genome modification increasingly plausible in clinical settings [67,68]. Therapeutic genome editing would offer the potential for autologous transplantation of genetically modified HSCs (Figure 2). Although direct repair of β-globin itself would be the most direct curative strategy for the β-hemoglobinopathies, there could be several advantages to targeting BCL11A, a disease modifying gene, rather than β-globin itself. First, in contrast to the single point mutation underlying SCD, β-thalassemia may be caused by hundreds of unique mutations, so developing a universal repair strategy would be difficult. Second, HSCs appear to be particularly refractory to homology-directed repair (HDR), perhaps because HDR requires an active cell cycle whereas HSCs are quiescent cells [69]. HSCs mainly repair double strand breaks by nonhomologous end-joining, so genetic disruption rather than precise gene correction may be a much more feasible strategy for the hematopoietic system given current technology.
Figure 2. Therapeutic genome editing of BCL11A for the β-hemoglobin disorders.
Hematopoietic stem cells may be collected from a β-hemoglobin disorder patient, modified ex vivo, and then autologously re-engrafted to the patient. Genome editing technology has been rapidly advancing, and a variety of targetable endonucleases, such as CRISPR/Cas9, could be employed. The target sequences could be the erythroid enhancer of BCL11A. This strategies would allow disruption of BCL11A's repression of HbF in erythroid cells while sparing extra-erythroid functions of BCL11A. Furthermore, this genetic disruption would rely on the robust nonhomologous end-joining repair pathway, rather than on lower-frequency homology-directed repair.
Detrimental effects of loss of BCL11A in the hematopoietic compartment, either on B-lymphocytes or even on stem and progenitor cells would need to be considered [48]. Targeting the erythroid enhancer of BCL11A, or alternatively the target sequences bound by BCL11A within the β-globin gene cluster (should they be defined adequately), offers the potential for erythroid specificity [26].
Ultimately it would be highly desirable to develop small molecule inhibitors of BCL11A, as these might be suitable for use in larger numbers of patients, whereas any strategy employing stem cell transplantation, whether allogeneic or autologous (with RNA interference or genome editing), will be limited to specialized centers. Although more needs to be learned about BCL11A structure and function to allow the rational design of such an inhibitor, recent successes in targeting transcriptional regulators offer hope of feasibility [70-73]. One challenge to small molecule inhibitors of BCL11A would be to restrict effects to the erythroid lineage. It is possible that there could be a therapeutic window whereby an intermediate level of BCL11A inhibition could allow derepression of HbF but not ablate essential extra-erythroid functions of BCL11A. Alternatively, it is possible that erythroid-specific aspects of BCL11A, such as discrete protein-protein interactions, could be targeted.
Epidemiologic transitions in the developing world, with better control of infectious disease, and accompanying survival of patients with hemoglobin disorders beyond early childhood, are anticipated to result in large increases in the number of patients over the coming decades, with more than 400,000 born each year [74]. We anticipate that these conditions will continue to provide new insights about human genetics and disease and hope they could serve as successful examples of rational therapeutic design.
Acknowledgments
Thanks to Diane Chen and Jill Desimini for assistance with figure preparation and to Victoria Hargreaves for critical review. Related work from our laboratories is funded by the NIH (DK093705 to D.E.B. and HL32259, HL032262, DK49216, and HL117720 to S.H.O.) and Doris Duke Charitable Foundation. S.H.O. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel E. Bauer, Email: daniel.bauer@childrens.harvard.edu.
Stuart H. Orkin, Email: stuartorkin@dfci.harvard.edu.
References
- 1.Pauling L, Itano HA. Sickle cell anemia a molecular disease. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 2.Ingram VM. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957;180:326–328. doi: 10.1038/180326a0. [DOI] [PubMed] [Google Scholar]
- 3.Orkin SH, Kazazian HH., Jr The mutation and polymorphism of the human beta-globin gene and its surrounding DNA. Annu Rev Genet. 1984;18:131–171. doi: 10.1146/annurev.ge.18.120184.001023. [DOI] [PubMed] [Google Scholar]
- 4.Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Exp Hematol. 2005;33:259–271. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Menzel S, Garner C, Gut I, Matsuda F, Yamaguchi M, Heath S, Foglio M, Zelenika D, Boland A, Rooks H, Best S, Spector TD, Farrall M, Lathrop M, Thein SL. A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat Genet. 2007;39:1197–1199. doi: 10.1038/ng2108. [DOI] [PubMed] [Google Scholar]
- 6*.Uda M, Galanello R, Sanna S, Lettre G, Sankaran VG, Chen W, Usala G, Busonero F, Maschio A, Albai G, Piras MG, Sestu N, Lai S, Dei M, Mulas A, Crisponi L, Naitza S, Asunis I, Deiana M, Nagaraja R, Perseu L, Satta S, Cipollina MD, Sollaino C, Moi P, Hirschhorn JN, Orkin SH, Abecasis GR, Schlessinger D, Cao A. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci U S A. 2008;105:1620–1625. doi: 10.1073/pnas.0711566105. These GWAS of HbF level (or the related phenotype F-cell number) first implicated BCL11A in HbF regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galarneau G, Palmer CD, Sankaran VG, Orkin SH, Hirschhorn JN, Lettre G. Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet. 2010;42:1049–1051. doi: 10.1038/ng.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lettre G, Sankaran VG, Bezerra MA, Araujo AS, Uda M, Sanna S, Cao A, Schlessinger D, Costa FF, Hirschhorn JN, Orkin SH. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A. 2008;105:11869–11874. doi: 10.1073/pnas.0804799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nuinoon M, Makarasara W, Mushiroda T, Setianingsih I, Wahidiyat PA, Sripichai O, Kumasaka N, Takahashi A, Svasti S, Munkongdee T, Mahasirimongkol S, Peerapittayamongkol C, Viprakasit V, Kamatani N, Winichagoon P, Kubo M, Nakamura Y, Fucharoen S. A genome-wide association identified the common genetic variants influence disease severity in beta0-thalassemia/hemoglobin E. Hum Genet. 2010;127:303–314. doi: 10.1007/s00439-009-0770-2. [DOI] [PubMed] [Google Scholar]
- 10.Solovieff N, Milton JN, Hartley SW, Sherva R, Sebastiani P, Dworkis DA, Klings ES, Farrer LA, Garrett ME, Ashley-Koch A, Telen MJ, Fucharoen S, Ha SY, Li CK, Chui DH, Baldwin CT, Steinberg MH. Fetal hemoglobin in sickle cell anemia: genome-wide association studies suggest a regulatory region in the 5′ olfactory receptor gene cluster. Blood. 2010;115:1815–1822. doi: 10.1182/blood-2009-08-239517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatnagar P, Purvis S, Barron-Casella E, DeBaun MR, Casella JF, Arking DE, Keefer JR. Genome-wide association study identifies genetic variants influencing F-cell levels in sickle-cell patients. J Hum Genet. 2011;56:316–323. doi: 10.1038/jhg.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galanello R, Sanna S, Perseu L, Sollaino MC, Satta S, Lai ME, Barella S, Uda M, Usala G, Abecasis GR, Cao A. Amelioration of Sardinian beta0 thalassemia by genetic modifiers. Blood. 2009;114:3935–3937. doi: 10.1182/blood-2009-04-217901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thein SL, Menzel S, Peng X, Best S, Jiang J, Close J, Silver N, Gerovasilli A, Ping C, Yamaguchi M, Wahlberg K, Ulug P, Spector TD, Garner C, Matsuda F, Farrall M, Lathrop M. Intergenic variants of HBS1L-MYB are responsible for a major quantitative trait locus on chromosome 6q23 influencing fetal hemoglobin levels in adults. Proc Natl Acad Sci U S A. 2007;104:11346–11351. doi: 10.1073/pnas.0611393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satterwhite E, Sonoki T, Willis TG, Harder L, Nowak R, Arriola EL, Liu H, Price HP, Gesk S, Steinemann D, Schlegelberger B, Oscier DG, Siebert R, Tucker PW, Dyer MJ. The BCL11 gene family: involvement of BCL11A in lymphoid malignancies. Blood. 2001;98:3413–3420. doi: 10.1182/blood.v98.12.3413. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Yamazaki Y, Saiki Y, Moriyama M, Largaespada DA, Jenkins NA, Copeland NG. Evi9 encodes a novel zinc finger protein that physically interacts with BCL6, a known human B-cell proto-oncogene product. Mol Cell Biol. 2000;20:3178–3186. doi: 10.1128/mcb.20.9.3178-3186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu P, Keller JR, Ortiz M, Tessarollo L, Rachel RA, Nakamura T, Jenkins NA, Copeland NG. Bcl11a is essential for normal lymphoid development. Nat Immunol. 2003;4:525–532. doi: 10.1038/ni925. [DOI] [PubMed] [Google Scholar]
- 17.Avram D, Fields A, Pretty On Top K, Nevrivy DJ, Ishmael JE, Leid M. Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J Biol Chem. 2000;275:10315–10322. doi: 10.1074/jbc.275.14.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Sankaran VG, Menne TF, Xu J, Akie TE, Lettre G, Van Handel B, Mikkola HK, Hirschhorn JN, Cantor AB, Orkin SH. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322:1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 19.Chada K, Magram J, Costantini F. An embryonic pattern of expression of a human fetal globin gene in transgenic mice. Nature. 1986;319:685–689. doi: 10.1038/319685a0. [DOI] [PubMed] [Google Scholar]
- 20*.Sankaran VG, Xu J, Ragoczy T, Ippolito GC, Walkley CR, Maika SD, Fujiwara Y, Ito M, Groudine M, Bender MA, Tucker PW, Orkin SH. Developmental and species-divergent globin switching are driven by BCL11A. Nature. 2009;460:1093–1097. doi: 10.1038/nature08243. These reports demonstrated that BCL11A loss of function in human erythroid precursors and in transgenic mice is sufficient to prevent γ-globin repression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Xu J, Peng C, Sankaran VG, Shao Z, Esrick EB, Chong BG, Ippolito GC, Fujiwara Y, Ebert BL, Tucker PW, Orkin SH. Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science. 2011;334:993–996. doi: 10.1126/science.1211053. This report showed that erythroid-specific deletion of BCL11A prevents sickle cell disease in mice. This is the only example of a single gene inactivation event capable of preventing sickle cell disease. In addition, this study showed that the repression of HbF in the adult stage by BCL11A is reversible. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esteghamat F, Gillemans N, Bilic I, van den Akker E, Cantu I, van Gent T, Klingmuller U, van Lom K, von Lindern M, Grosveld F, Bryn van Dijk T, Busslinger M, Philipsen S. Erythropoiesis and globin switching in compound Klf1∷Bcl11a mutant mice. Blood. 2013;121:2553–2562. doi: 10.1182/blood-2012-06-434530. [DOI] [PubMed] [Google Scholar]
- 23*.Xu J, Sankaran VG, Ni M, Menne TF, Puram RV, Kim W, Orkin SH. Transcriptional silencing of {gamma}-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 2010;24:783–798. doi: 10.1101/gad.1897310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Xu J, Bauer DE, Kerenyi MA, Vo TD, Hou S, Hsu YJ, Yao H, Trowbridge JJ, Mandel G, Orkin SH. Corepressor-dependent silencing of fetal hemoglobin expression by BCL11A. Proc Natl Acad Sci U S A. 2013;110:6518–6523. doi: 10.1073/pnas.1303976110. These reports illuminate several puzzling features of the mechanisms of repression of HbF by BCL11A. BCL11A does not directly bind to the γ-globin genes but rather to distant elements at the β-globin cluster. Also BCL11A participates in multiprotein complexes with various partners, each of which appears to play a more widespread role in erythroid gene regulation than does BCL11A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L, Cui S, Engel JD, Tanabe O. Lysine-specific demethylase 1 is a therapeutic target for fetal hemoglobin induction. Nat Med. 2013;19:291–294. doi: 10.1038/nm.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Bauer DE, Kamran SC, Lessard S, Xu J, Fujiwara Y, Lin C, Shao Z, Canver MC, Smith EC, Pinello L, Sabo PJ, Vierstra J, Voit RA, Yuan GC, Porteus MH, Stamatoyannopoulos JA, Lettre G, Orkin SH. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342:253–257. doi: 10.1126/science.1242088. This report combines genetic fine-mapping, chromatin profiling, allele-specific analyses, transgenic enhancer assays, and genome editing to demonstrate the mechanism of the association of common genetic variation at BCL11A to HbF level. An example of what appears to be a prevalent phenomenon, the trait-associated variants modulate a context-specific (adult-stage erythroid) enhancer element. In addition to demonstrating how regulatory variation at a pleiotropic gene may escape negative selection, these studies emphasize that the effect size of a GWAS trait association is but a minimal estimate of the impact of the underlying element on the affected biologic pathway. These results suggest a novel therapeutic strategy for the hemoglobin disorders—therapeutic genome editing of the erythroid enhancer of BCL11A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, Ku M, Durham T, Kellis M, Bernstein BE. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473:43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, Reynolds AP, Sandstrom R, Qu H, Brody J, Shafer A, Neri F, Lee K, Kutyavin T, Stehling-Sun S, Johnson AK, Canfield TK, Giste E, Diegel M, Bates D, Hansen RS, Neph S, Sabo PJ, Heimfeld S, Raubitschek A, Ziegler S, Cotsapas C, Sotoodehnia N, Glass I, Sunyaev SR, Kaul R, Stamatoyannopoulos JA. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–1195. doi: 10.1126/science.1222794. This study highlights the extensive enrichment of trait-associated variants within trait-relevant context-specific regulatory DNA. Variants associated with HbF level by GWAS are just one example of this phenomenon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farh KK, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, Shoresh N, Whitton H, Ryan RJ, Shishkin AA, Hatan M, Carrasco-Alfonso MJ, Mayer D, Luckey CJ, Patsopoulos NA, De Jager PL, Kuchroo VK, Epstein CB, Daly MJ, Hafler DA, Bernstein BE. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2014 doi: 10.1038/nature13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Vierstra J, Rynes E, Sandstrom R, Zhang M, Canfield T, Hansen RS, Stehling-Sun S, Sabo PJ, Byron R, Humbert R, Thurman RE, Johnson AK, Vong S, Lee K, Bates D, Neri F, Diegel M, Giste E, Haugen E, Dunn D, Wilken MS, Josefowicz S, Samstein R, Chang KH, Eichler EE, De Bruijn M, Reh TA, Skoultchi A, Rudensky A, Orkin SH, Papayannopoulou T, Treuting PM, Selleri L, Kaul R, Groudine M, Bender MA, Stamatoyannopoulos JA. Mouse regulatory DNA landscapes reveal global principles of cis-regulatory evolution. Science. 2014;346:1007–1012. doi: 10.1126/science.1246426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Stergachis AB, Neph S, Sandstrom R, Haugen E, Reynolds AP, Zhang M, Byron R, Canfield T, Stelhing-Sun S, Lee K, Thurman RE, Vong S, Bates D, Neri F, Diegel M, Giste E, Dunn D, Vierstra J, Hansen RS, Johnson AK, Sabo PJ, Wilken MS, Reh TA, Treuting PM, Kaul R, Groudine M, Bender MA, Borenstein E, Stamatoyannopoulos JA. Conservation of trans-acting circuitry during mammalian regulatory evolution. Nature. 2014;515:365–370. doi: 10.1038/nature13972. These reports demonstrate the rapid turnover of regulatory as compared to protein coding DNA during mammalian evolution. Inter- and intra-species regulatory variation at pleiotropic genes such as BCL11A may be considered in the context of a highly constrained trans-acting landscape. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajcan-Separovic E, Harvard C, Liu X, McGillivray B, Hall JG, Qiao Y, Hurlburt J, Hildebrand J, Mickelson EC, Holden JJ, Lewis ME. Clinical and molecular cytogenetic characterisation of a newly recognised microdeletion syndrome involving 2p15-16.1. J Med Genet. 2007;44:269–276. doi: 10.1136/jmg.2006.045013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hancarova M, Simandlova M, Drabova J, Mannik K, Kurg A, Sedlacek Z. A patient with de novo 0.45 Mb deletion of 2p16.1: the role of BCL11A, PAPOLG, REL, and FLJ16341 in the 2p15-p16.1 microdeletion syndrome. Am J Med Genet A. 2013;161A:865–870. doi: 10.1002/ajmg.a.35783. [DOI] [PubMed] [Google Scholar]
- 35.Peter B, Matsushita M, Oda K, Raskind W. De novo microdeletion of BCL11A is associated with severe speech sound disorder. Am J Med Genet A. 2014;164A:2091–2096. doi: 10.1002/ajmg.a.36599. [DOI] [PubMed] [Google Scholar]
- 36*.Benitez CM, Qu K, Sugiyama T, Pauerstein PT, Liu Y, Tsai J, Gu X, Ghodasara A, Arda HE, Zhang J, Dekker JD, Tucker HO, Chang HY, Kim SK. An integrated cell purification and genomics strategy reveals multiple regulators of pancreas development. PLoS Genet. 2014;10:e1004645. doi: 10.1371/journal.pgen.1004645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Khaled WT, Choon Lee S, Stingl J, Chen X, Raza Ali H, Rueda OM, Hadi F, Wang J, Yu Y, Chin SF, Stratton M, Futreal A, Jenkins NA, Aparicio S, Copeland NG, Watson CJ, Caldas C, Liu P. BCL11A is a triple-negative breast cancer gene with critical functions in stem and progenitor cells. Nat Commun. 2015;6:5987. doi: 10.1038/ncomms6987. These functional studies following up on human genetics results have illustrated the pleiotropic nature of BCL11A. The discoveries of BCL11A's roles in pancreatic development and mammary stem cell function were spurred by GWAS of type 2 diabetes and cancer genomics of triple negative breast cancer, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samocha KE, Robinson EB, Sanders SJ, Stevens C, Sabo A, McGrath LM, Kosmicki JA, Rehnstrom K, Mallick S, Kirby A, Wall DP, MacArthur DG, Gabriel SB, DePristo M, Purcell SM, Palotie A, Boerwinkle E, Buxbaum JD, Cook EH, Jr, Gibbs RA, Schellenberg GD, Sutcliffe JS, Devlin B, Roeder K, Neale BM, Daly MJ. A framework for the interpretation of de novo mutation in human disease. Nat Genet. 2014;46:944–950. doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.1000 Genomes Project Consortium. Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, Hurles ME, McVean GA. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 41.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth RJ, Collman RG, Doms RW, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 42.Avram D, Fields A, Senawong T, Topark-Ngarm A, Leid M. COUP-TF (chicken ovalbumin upstream promoter transcription factor)-interacting protein 1 (CTIP1) is a sequence-specific DNA binding protein. Biochem J. 2002;368:555–563. doi: 10.1042/BJ20020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H, Ippolito GC, Wall JK, Niu T, Probst L, Lee BS, Pulford K, Banham AH, Stockwin L, Shaffer AL, Staudt LM, Das C, Dyer MJ, Tucker PW. Functional studies of BCL11A: characterization of the conserved BCL11A-XL splice variant and its interaction with BCL6 in nuclear paraspeckles of germinal center B cells. Mol Cancer. 2006;5:18. doi: 10.1186/1476-4598-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z, Luo HY, Steinberg MH, Chui DH. BCL11A represses HBG transcription in K562 cells. Blood Cells Mol Dis. 2009;42:144–149. doi: 10.1016/j.bcmd.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Tang B, Di Lena P, Schaffer L, Head SR, Baldi P, Thomas EA. Genome-wide identification of Bcl11b gene targets reveals role in brain-derived neurotrophic factor signaling. PLoS One. 2011;6:e23691. doi: 10.1371/journal.pone.0023691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiles ET, Lui-Sargent B, Bell R, Lessnick SL. BCL11B is up-regulated by EWS/FLI and contributes to the transformed phenotype in Ewing sarcoma. PLoS One. 2013;8:e59369. doi: 10.1371/journal.pone.0059369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Sankaran VG, Xu J, Byron R, Greisman HA, Fisher C, Weatherall DJ, Sabath DE, Groudine M, Orkin SH, Premawardhena A, Bender MA. A functional element necessary for fetal hemoglobin silencing. N Engl J Med. 2011;365:807–814. doi: 10.1056/NEJMoa1103070. This study describes HbF-associated rare genetic variation at non-coding sequencesof the β-globin gene cluster. The report suggests that these sequences may includeelements required for repression of γ-globin by BCL11A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Yu Y, Wang J, Khaled W, Burke S, Li P, Chen X, Yang W, Jenkins NA, Copeland NG, Zhang S, Liu P. Bcl11a is essential for lymphoid development and negatively regulates p53. J Exp Med. 2012;209:2467–2483. doi: 10.1084/jem.20121846. This report demonstrates that BCL11A is highly expressed in various hematopoietic lineages, including hematopoietic stem cells. The implication of the report, that BCL11A may play a role in HSC function, emphasizes the versatile roles of BCL11A and raises caution for therapeutic approaches targeting BCL11A throughout the hematopoietic compartment. Further study will be required to clarify the roles of BCL11A in various non-erythroid hematopoietic contexts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ippolito GC, Dekker JD, Wang YH, Lee BK, Shaffer AL, 3rd, Lin J, Wall JK, Lee BS, Staudt LM, Liu YJ, Iyer VR, Tucker HO. Dendritic cell fate is determined by BCL11A. Proc Natl Acad Sci U S A. 2014;111:E998–1006. doi: 10.1073/pnas.1319228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He Q, Zhang C, Wang L, Zhang P, Ma D, Lv J, Liu F. Inflammatory signaling regulates hematopoietic stem and progenitor cell emergence in vertebrates. Blood. 2014 doi: 10.1182/blood-2014-09-601542. [DOI] [PubMed] [Google Scholar]
- 51.Sawamiphak S, Kontarakis Z, Stainier DY. Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Dev Cell. 2014;31:640–653. doi: 10.1016/j.devcel.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Esain V, Teng L, Xu J, Kwan W, Frost IM, Yzaguirre AD, Cai X, Cortes M, Maijenburg MW, Tober J, Dzierzak E, Orkin SH, Tan K, North TE, Speck NA. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev. 2014;28:2597–2612. doi: 10.1101/gad.253302.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Espin-Palazon R, Stachura DL, Campbell CA, Garcia-Moreno D, Del Cid N, Kim AD, Candel S, Meseguer J, Mulero V, Traver D. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell. 2014;159:1070–1085. doi: 10.1016/j.cell.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Borg J, Papadopoulos P, Georgitsi M, Gutierrez L, Grech G, Fanis P, Phylactides M, Verkerk AJ, van der Spek PJ, Scerri CA, Cassar W, Galdies R, van Ijcken W, Ozgur Z, Gillemans N, Hou J, Bugeja M, Grosveld FG, von Lindern M, Felice AE, Patrinos GP, Philipsen S. Haploinsufficiency for the erythroid transcription factor KLF1 causes hereditary persistence of fetal hemoglobin. Nat Genet. 2010;42:801–805. doi: 10.1038/ng.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Zhou D, Liu K, Sun CW, Pawlik KM, Townes TM. KLF1 regulates BCL11A expression and gamma- to beta-globin gene switching. Nat Genet. 2010 doi: 10.1038/ng.637. These reports link the erythroid transcription factor KLF1 to BCL11A. KLF1 appears to play a dual role in HbF regulation, both activating β-globin expression directly and trans-activating BCL11A, which in turn represses γ-globin expression. [DOI] [PubMed] [Google Scholar]
- 56.Bianchi E, Zini R, Salati S, Tenedini E, Norfo R, Tagliafico E, Manfredini R, Ferrari S. c-Myb supports erythropoiesis through the transactivation of KLF1 and LMO2 expression. Blood. 2010 doi: 10.1182/blood-2009-08-238311. [DOI] [PubMed] [Google Scholar]
- 57*.Amaya M, Desai M, Gnanapragasam MN, Wang SZ, Zu Zhu S, Williams DC, Jr, Ginder GD. Mi2beta-mediated silencing of the fetal gamma-globin gene in adult erythroid cells. Blood. 2013;121:3493–3501. doi: 10.1182/blood-2012-11-466227. This study shows the critical role of the Mi2β helicase component of the NuRD complex in HbF regulation. Mi2β/NuRD physically interact with BCL11A, and may also support expression of BCL11A. Whether pharmacologic targeting of NuRD could achieve the specificity of inhibition of BCL11A itself on HbF regulation remains to be determined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lulli V, Romania P, Morsilli O, Cianciulli P, Gabbianelli M, Testa U, Giuliani A, Marziali G. MicroRNA-486-3p regulates gamma-globin expression in human erythroid cells by directly modulating BCL11A. PLoS One. 2013;8:e60436. doi: 10.1371/journal.pone.0060436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59*.Lee YT, de Vasconcellos JF, Yuan J, Byrnes C, Noh SJ, Meier ER, Kim KS, Rabel A, Kaushal M, Muljo SA, Miller JL. LIN28B-mediated expression of fetal hemoglobin and production of fetal-like erythrocytes from adult human erythroblasts ex vivo. Blood. 2013;122:1034–1041. doi: 10.1182/blood-2012-12-472308. This report demonstrates the role of the fetal factor LIN28B in promoting expression of HbF. An important task will be to determine links between generic developmental axes such as Lin28/let-7 and the proximal mechanisms coordinating globin gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Vasconcellos JF, Fasano RM, Lee YT, Kaushal M, Byrnes C, Meier ER, Anderson M, Rabel A, Braylan R, Stroncek DF, Miller JL. LIN28A expression reduces sickling of cultured human erythrocytes. PLoS One. 2014;9:e106924. doi: 10.1371/journal.pone.0106924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giarratana MC, Rouard H, Dumont A, Kiger L, Safeukui I, Le Pennec PY, Francois S, Trugnan G, Peyrard T, Marie T, Jolly S, Hebert N, Mazurier C, Mario N, Harmand L, Lapillonne H, Devaux JY, Douay L. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurita R, Suda N, Sudo K, Miharada K, Hiroyama T, Miyoshi H, Tani K, Nakamura Y. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS One. 2013;8:e59890. doi: 10.1371/journal.pone.0059890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu J, Liu J, Xue F, Halverson G, Reid M, Guo A, Chen L, Raza A, Galili N, Jaffray J, Lane J, Chasis JA, Taylor N, Mohandas N, An X. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121:3246–3253. doi: 10.1182/blood-2013-01-476390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Trakarnsanga K, Wilson MC, Lau W, Singleton BK, Parsons SF, Sakuntanaga P, Kurita R, Nakamura Y, Anstee DJ, Frayne J. Induction of adult levels of beta-globin in human erythroid cells that intrinsically express embryonic or fetal globin by transduction with KLF1 and BCL11A-XL. Haematologica. 2014;99:1677–1685. doi: 10.3324/haematol.2014.110155. This study shows that ectopic expression of BCL11A (variably in combination with KLF1) can convert cell lines such as the chronic myeloid leukemia line K562 or pluripotent stem cells from an embryonic/fetal to adult-like globin expression pattern. These findings could be useful for the engineering of systems to produce blood cells for transfusion medicine from pluripotent stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilber A, Hargrove PW, Kim YS, Riberdy JM, Sankaran VG, Papanikolaou E, Georgomanoli M, Anagnou NP, Orkin SH, Nienhuis AW, Persons DA. Therapeutic levels of fetal hemoglobin in erythroid progeny of beta-thalassemic CD34+ cells after lentiviral vector-mediated gene transfer. Blood. 2011;117:2817–2826. doi: 10.1182/blood-2010-08-300723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hardison RC, Blobel GA. Genetics. GWAS to therapy by genome edits? Science. 2013;342:206–207. doi: 10.1126/science.1245813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 69.Genovese P, Schiroli G, Escobar G, Di Tomaso T, Firrito C, Calabria A, Moi D, Mazzieri R, Bonini C, Holmes MC, Gregory PD, van der Burg M, Gentner B, Montini E, Lombardo A, Naldini L. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–240. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462:182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grembecka J, He S, Shi A, Purohit T, Muntean AG, Sorenson RJ, Showalter HD, Murai MJ, Belcher AM, Hartley T, Hess JL, Cierpicki T. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat Chem Biol. 2012;8:277–284. doi: 10.1038/nchembio.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72*.Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, Ciarlo C, Hartman E, Munshi N, Schenone M, Schreiber SL, Carr SA, Ebert BL. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73*.Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong KK, Bradner JE, Kaelin WG., Jr The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–309. doi: 10.1126/science.1244917. These elegant studies show how lenalidomide results in the proteasome-mediated degradation of the transcription factors IKZF1 and IKZF3 in multiple myeloma. Given the complexity of cellular systems for protein turnover, these results offer hope that small molecule triggered targeted destruction of non-enzymatic regulatory proteins, such as transcription factors, may be clinically practical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10:e1001484. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]