Abstract
Treatment of cancer patients by adoptive T cell therapy has yielded promising results. In solid tumors, however, T cells encounter a hostile environment, in particular with increased inflammatory activity as a hallmark of the tumor milieu that goes along with abundant reactive oxygen species (ROS) that substantially impair antitumor activity. We present a strategy to render antitumor T cells more resilient toward ROS by coexpressing catalase along with a tumor specific chimeric Ag receptor (CAR) to increase their antioxidative capacity by metabolizing H2O2. In fact, T cells engineered with a bicistronic vector that concurrently expresses catalase, along with the CAR coexpressing catalase (CAR-CAT), performed superior over CAR T cells as they showed increased levels of intracellular catalase and had a reduced oxidative state with less ROS accumulation in both the basal state and upon activation while maintaining their antitumor activity despite high H2O2 levels. Moreover, CAR-CAT T cells exerted a substantial bystander protection of nontransfected immune effector cells as measured by CD3ζ chain expression in bystander T cells even in the presence of high H2O2 concentrations. Bystander NK cells, otherwise ROS sensitive, efficiently eliminate their K562 target cells under H2O2-induced oxidative stress when admixed with CAR-CAT T cells. This approach represents a novel means for protecting tumor-infiltrating cells from tumor-associated oxidative stress–mediated repression.
Introduction
Tumor-infiltrating lymphocytes (TILs) have long been recognized as a prognostic factor for cancer patients in a variety of tumor types (1). This has spurred the development of adoptive cell therapy with TILs, which in combination with non-myeloblative lymphodepletion regimens has resulted in some remarkable clinical response rates in metastatic melanoma patients (2, 3). Isolation and expansion of TILs from cancer patients is however not feasible for all tumor types, and genetic transfer of tumor specificity with TCRs and chimeric Ag receptors (CARs) into T cells from peripheral blood is an attractive alternative. Similar to conventional T cells, the limitation of TCR-transduced T cells are in their inability to recognize tumors that have downregulated their MHC class I molecules (4, 5). CARs circumvent this by providing specificity by a single-chain fragment of a variable Ab region specific for a surface tumor Ag. CARs activate T cells through intracellular signaling domains such as CD3ζ, which is improved by costimulation including CD28 or 4-1BB (6). Recently, transfer of such second generation CAR T cells targeting CD19+ B cell lymphoid leukemia has shown encouraging clinical results in treating patients with bulky tumors (7–10). Although these results are galvanizing the field of adoptive cell therapy, clinical trials focusing on solid tumors have seen less success (11–13). The challenge for T cell–based therapies of solid tumors lies in that T cells, in addition to reaching their targets, are required to survive and function within the unfavorable tumor microenvironment.
Tumor cells have long been known to have high levels of oxidative stress and reactive oxygen species (ROS), which have been shown to play key roles in many aspects of tumorigenesis (14). Reactive oxygen intermediaries (ROIs) and ROS, such as superoxide and hydrogen peroxide, are produced by all mammalian cells mainly as part of normal mitochondrial metabolic processes. Innate phagocytic immune cells produce high levels of ROS through the NADPH oxidase complex as their primary mechanisms of clearing bacterial infections. Oxidative stress exists when the balance between ROS production and antioxidant function is shifted in favor of ROS. Increased production of ROI in tumor cells can be attributed to alterations in metabolic pathways, as exemplified by glucose deprivation in breast carcinomas leading to decrease in intracellular pyruvate preventing decomposition of ROI (15).
Also, tumor-infiltrating immune cells may be responsible for a large part of the ROS production. Thus, immature myeloid cells found in tumors effectuate their suppressive function on the immune system via ROS (16, 17). Cancer patients have been found to have increased levels of activated granulocytes (18), subsequently defined as granulocytic myeloid-derived suppressor cells (MDSCs) (19). High concentrations of ROS can lead to necrotic cell death, although there is a window of ROS-induced oxidative stress in which lymphocytes are still viable but become unresponsive (18). This has been linked to blockage of NF-κB activation due to protein oxidation, resulting in deficient IFN-γ, TNF-α, and IL-2 production (20, 21). ROS-induced alterations in T cell and NK cell functions may also be attributed to the decreased TCRζ- and CD16ζ-chain levels found in tumor-bearing patients and mice (22–24), which is associated with tumor accumulation of myeloid cells (25).
We have shown that T cells transduced with catalase survive and function in toxic concentrations of H2O2 (26). To adapt the approach to cell therapy, we sought to enhance persistence and function of tumor-redirected T cells in the environment of high oxidative stress. In this study, we demonstrate that T cells modified with a bicistronic expression vector CAR coexpressing catalase (CAR-CAT) produce increased amounts of intracellular catalase and have a reduced intracellular oxidative state. This improves protection of the CAR-CAT–transduced T cells from intrinsic oxidative stress, which is a result of T cell stimulation, as well as from extrinsic, especially tumor-associated, ROS. Such CAR-CAT T cells are able to lyse tumor cells in an Ag-specific manner under H2O2-induced oxidative stress, under which CAR T cells failed to do so. Furthermore, CAR-CAT T cells elicited a protective bystander effect allowing neighboring NK cells to kill tumor cells within a detrimental environment. CAR-CAT T cells provide a strategy to maintain antitumor activity of resident and adoptively transferred immune cells within the oxidative stress environment of tumors.
Materials and Methods
Cells and reagents
HEK293T and SkoV3 cells were maintained in complete RPMI 1640 l-glutamine plus medium supplemented with 10% (v/v) FCS, 100 U/ml penicillin and 100 U/ml streptomycin, 1% MEM nonessential amino acids, 1 mM sodium pyruvate, and 50 μM 2-ME (Life Technologies). PBMCs were obtained from healthy donors and used for transduction as well as the lymphocytic fraction from a healthy leukapheresis donor. These were either cultured in activation medium consisting of complete RPMI 1640 GlutaMAX with the addition of 500 U/ml IL-2 (Proleukin) or stimulation medium consisting of complete RPMI 1640 supplemented with 500 U/ml IL-2 and 100 ng/ml anti-CD3 (Orthoclone OKT3).
CAR engineering of T cells
T cells were transduced with recombinant retroviruses as described previously (27). T cells were purified from healthy donor PBMCs followed by a Pan T Cell Isolation kit (Miltenyi Biotec) or by leukapheresis followed by elutriation and collection of the second fraction containing >95% lymphocytes. Elutriated lymphocytes were frozen down in 10% DMSO FCS and thawed prior to use. Lymphocytes were cultured in stimulation medium for 2 d and then transferred to activation medium for 4 d. In parallel HEK293T cells were transfected with retroviral packaging plasmids pCOLT and pHIT60 along with a CAR expression vector using JetPrime (Polyplus). Activated lymphocytes were cocultured with virus producing transiently transfected HEK293T cells for 48 h. Transduction efficiency was analyzed by flow cytometry with goat F(ab′)2 anti-human IgG-PE (SouthernBiotech) and mouse anti-human CD3-allophycocyanin (BioLegend).
Catalase detection
Transduced T cells were sorted using anti-PE MACS (Miltenyi Biotec) beads in combination with F(ab′)2 anti-human IgG-PE (Southern Biotech). Sorted CAR+ lymphocytes were lysed in CelLytic M (Sigma-Aldrich) at a concentration of 1 × 106 cells/100 μl. Protein concentrations of lysates were measured using Pierce BCA assay (Thermo Scientific). Catalase activity assays were performed as per the manufacturer’s instructions after normalization based on BCA assay (Life Technologies). To evaluate catalase protein expression, Western blots were performed as follows. Lysate (20 μg) was loaded into 10% NuPAGE Bis-Tris acrylamide gels (Invitrogen) and run for 45 min at 200 V followed by transfer onto polyvinylidene difluoride membrane for 3 h at 40 V. Catalase was stained using 1:1000 dilution of rabbit anti-human catalase (GenScript, A01202), and actin was stained using mouse anti–β-actin at a dilution of 1:25,000 (Sigma-Aldrich) overnight at 4°C. HRP-linked anti-rabbit and anti-mouse Abs were used for secondary Abs (Cell Signaling Technology) and membranes were developed with ECL Prime Western blotting detection reagent (GE Healthcare). Intracellular staining and detection of catalase was done using a Cytofix/Cytoperm kit (BD Biosciences); catalase was stained using 2.5 μg/μl rabbit anti-human catalase Ab (GenScript) followed by anti-rabbit IgG-FITC (BD Pharmingen)–conjugated Ab.
Cell death assay
Lymphocytes were cultured in RPMI 1640 plus l-glutamine supplemented with 500 U/ml IL-2 at a concentration of 1 × 106 cells/ml in the presence of H2O2. After 24 h cells were washed with PBS and then stained with anti-human CD3-allophycocyanin (BioLegend), 7-aminoactinomycin D (BioLegend), and annexin V–FITC (BioLegend) and acquired on an LSR II (BD Biosciences).
Detection of thiols, ROS, and oxidative state
Cell surface thiols were evaluated using Alexa Fluor 488–C5-maleimide (Life Technologies). Staining was done by washing cells three times with 4°C PBS followed by labeling with 5 μM maleimide for 20 min at 4°C in the dark. Cells were labeled with Abs prior to acquisition on an LSR II (BD Biosciences). Free ROS were measured using L-012 luminol probe (Wako Chemicals). The final concentration of L-012 in medium with cells was 0.5 mg/ml. Luminescence was measured using a Centro LB 960 plate reader. CellROX (Invitrogen) was used to determine intracellular oxidative states. Lymphocytes (1 × 105) were loaded with 5 μM CellROX for short-term assays or 0.5 μM CellROX for long-term assays at 37°C for 10 min. For basal ROS measurements, cells were incubated at 37°C for the indicated duration followed by staining with anti-human CD3-Pacific Orange (BioLegend) and F(ab′)2 anti-human IgG-PE (SouthernBiotech) prior to acquisition with an LSR II. To stimulate intracellular ROS, cells were activated by PMA (3 ng/μl) or dihydroxynaphthoquinone (DHNQ) (20 μM) for 2 h prior to Ab labeling and acquisition by flow cytometry.
Cytotoxicity and proliferation assay
Her2+ SkoV3 cells were used as targets for Her2-specific CAR-transduced T cells in standard chromium release assays. K562 cells were used as targets for NK cells. Target cells were loaded with [51Cr] (50 μCi) (PerkinElmer) for 1 h and then washed of excess [51Cr]. Target cells were incubated for 1 h in complete medium prior to being plated out into 96-well plates together with effector cells at different E:T ratios. Following coculture with effector cells for 18 h, 25 μl supernatant was transferred onto LumaPlates (PerkinElmer) and after desiccation were analyzed on the MicroBeta scintillation (TriLux 1450, PerkinElmer) plate reader. For proliferation, 5 × 103 T cells were seeded in a 96-well U-bottom plate and stimulated to proliferate with 1 μl anti-CD3/CD28 mAb–coated beads per well. Every 24 h cells were collected by a MicroBeta scintillation counter (TriLux 1450, PerkinElmer) after pulsing with [3H]thymidine (31 μCi/well) (PerkinElmer) for 4 h.
Results
CAR-redirected T cells engineered with catalase
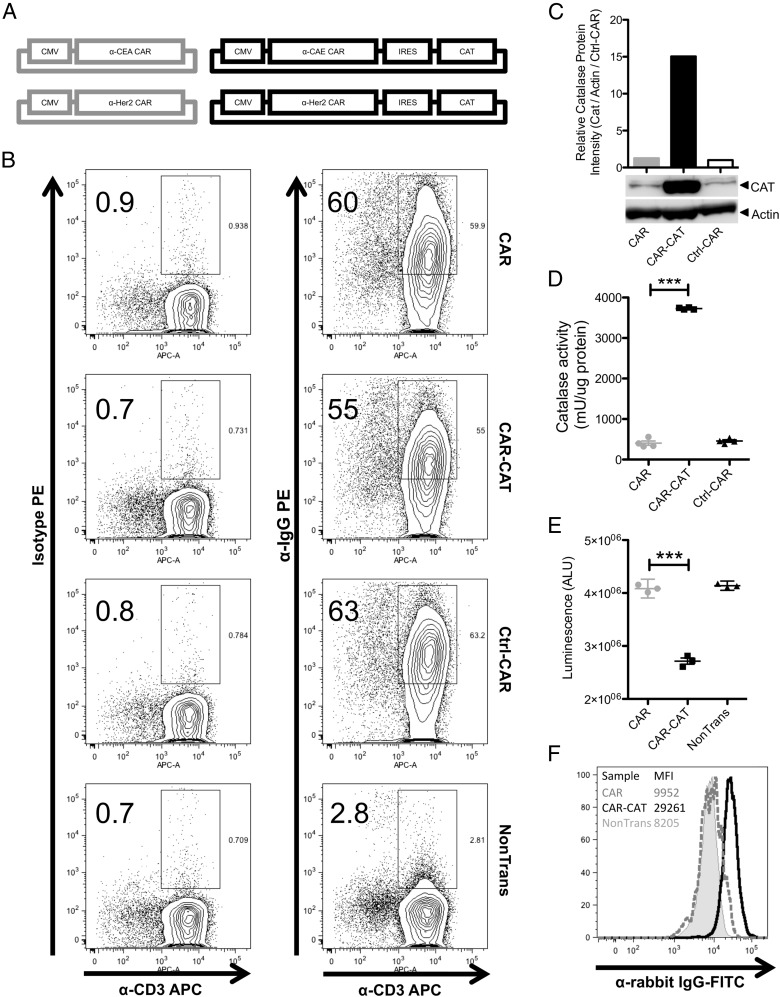
Human peripheral blood T cells from healthy donors were engineered with a CAR specific for CEA or Her2 with or without a bicistronic cassette to coexpress catalase. CAR-CAT were based on the CEA-specific CAR BW431/26scFv-IgG1-CD28-CD3ζ and the Her2-specific CAR C6-B1.D2-IgG1-CD28-CD3ζ (27, 28) by inserting the full-length human catalase cDNA downstream of the internal ribosome entry site (Fig. 1A). Nontransduced T cells and T cells modified with a truncated nerve growth factor receptor–specific control CAR (Ctrl-CAR) lacking internal T cell signaling domains were used as controls. Retroviral transduction of these constructs into T cells gave efficiencies of ∼55% CAR+ cells (Fig. 1B). To determine the level of catalase present in the transduced T cells, CAR+ and CAR− cells were sorted from freshly transduced T cells using MACS beads specific for the CAR IgG linker region and the catalase recorded by Western blot analysis (Fig. 1C). No differences were seen in the negative fractions of transduced T cells (Supplemental Fig. 1A). To confirm the function of catalase in CAR+ cells, a catalase activity assay was performed on lysates from CAR-, CAR-CAT–, and Ctrl-CAR–sorted cells. CAR-CAT–transduced T cells were found to contain >7-fold higher catalase activity than CAR T cells without transduced catalase or Ctrl-CAR T cells (Fig. 1D). Catalase activity was also measured in the lysate of CAR− T cells and found not to significantly differ between samples (Supplemental Fig. 1B). Freshly transduced T cells were evaluated for their ability to neutralize H2O2. L-012, an ROS-sensitive luminol (29), was added to T cells prior to addition of 50 μM H2O2. Significantly reduced luminescence of CAR-CAT T cells (Fig. 1E), indicating reduced ROS activity in the presence of CAT-CAT, was recorded in CAR-CAT T cells upon permeabilization, indicating intracellular localization (Fig. 1F).
FIGURE 1.
Design of bicistronic expression vector for CARs and catalase expression. (A) Schematic diagram depicting the two sets of CARs used. (B) PBMCs from healthy donors were cultured for a 4 d and transduced using either bicistronic retroviral expression vectors for CARs and catalase or retroviral expression vector for CARs alone. Expression of CARs on transduced T cells was assessed by staining with PE conjugated F(ab′)2 anti-human IgG that binds to the extracellular Fc region of the CAR and allophycocyanin-conjugated anti-CD3. PE-conjugated isotype Abs were used to confirm lack of nonspecific binding. CAR cells were gated on lymphocyte population in forward scatter and side scatter prior to gating CAR+ cells. (C) Protein lysates from MACS-sorted transduced T cells were analyzed by Western blot. Relative protein expression was determined by ImageJ analysis of the intensity of the bands from the Western blot. (D) MACS-sorted lysates were used to measure catalase activity. (E) Luminescence from 105 transduced or nontransduced cells was measured after adding L-012 and H2O2. (F) Transduced T cells were permeabilized and rabbit polyclonal anti-human catalase Ab was used to stain for intracellular catalase. FITC-conjugated anti-rabbit IgG Ab was used to analyze the samples with flow cytometry. Data are presented as means ± SD. ***p < 0.005 by Student t test using GraphPad Prism 5.
CAR-CAT T cells display an increased antioxidant capacity against intrinsic ROS upon T cell activation
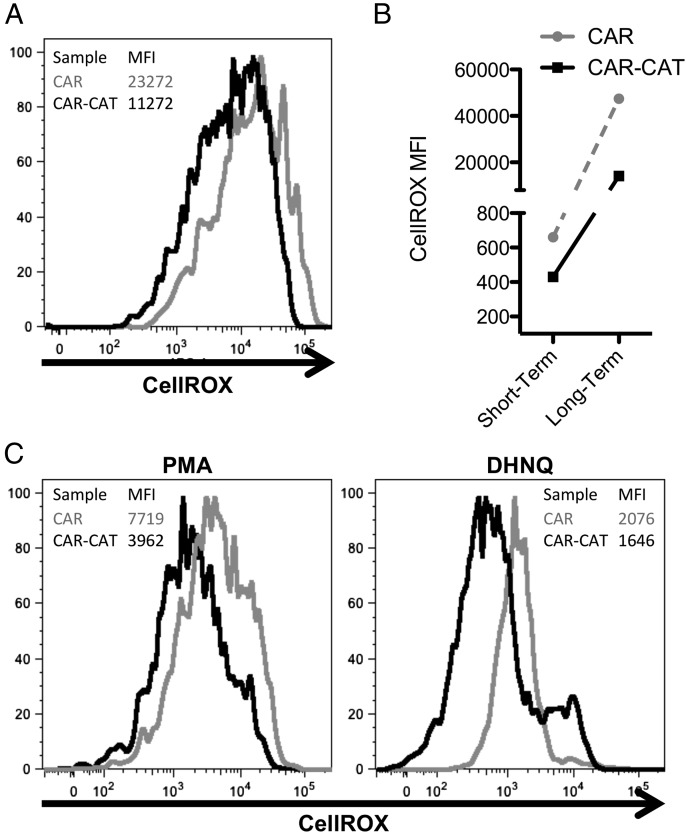
Activation of lymphocytes induces increased mitochondrial activity, resulting in oxidative stress (30). We next asked whether CAR-CAT T cells are more resistant to this type of cellular stress. Freshly transduced T cells were labeled with the oxidative stress indicator CellROX directly after transduction. The basal oxidative state was found to be lower in the CAR-CAT T cells compared with CAR T cells without transduced catalase (Fig. 2A). This was also found to be the case after long-term culture (Fig. 2B). We additionally stained cells with maleimide–Alexa Fluor 488 and found that the differences between CAR-CAT and CAR T cells were minimal at 0 mM H2O2, but at 1 mM H2O2 CAR T cells were not able to maintain cell surface thiols with a mean fluorescence intensity (MFI) decrease of 20%, whereas CAR-CAT maintained cell surface thiols with a decrease of 0.7%. A reduced oxidative state in the CAR-CAT T cells compared with CAR only–transduced cells was also found after coculture for 24 h with SkoV3 tumor cells (Supplemental Fig. 2).
FIGURE 2.
Oxidative state is reduced in CAR-CAT T cells compared with CAR T cells. (A) Directly after coculture with HEK293T cells, transduced T cells were labeled with CellROX (5 μM) in complete medium. (B) After short-term 1-h culture, or long-term 18-h culture, cells were labeled with PE anti-human IgG and Pacific Orange anti-human CD3 and samples were acquired on an LSR II. (C) To induce oxidative stress, freshly transduced T cells were loaded with CellROX and stimulated with PMA (3 μg/ml) and DHNQ (20 μM) for 2 h before acquiring samples by flow cytometry. Samples were analyzed using FlowJo.
T cells encountering Ag at the tumor site will induce oxidative stress in the engaged T cells. To simulate this effect we stimulated CellROX-labeled T cells by incubation with PMA or DHNQ (Fig. 2C). CAR-CAT T cells displayed a decreased CellROX MFI compared with CAR T cells following PMA or DHNQ stimulation, similar to the differences found between their levels of basal oxidative states, indicating less oxidative stress of catalase-engineered T cells upon activation.
CAR-CAT T cells maintain their activity under H2O2 stress
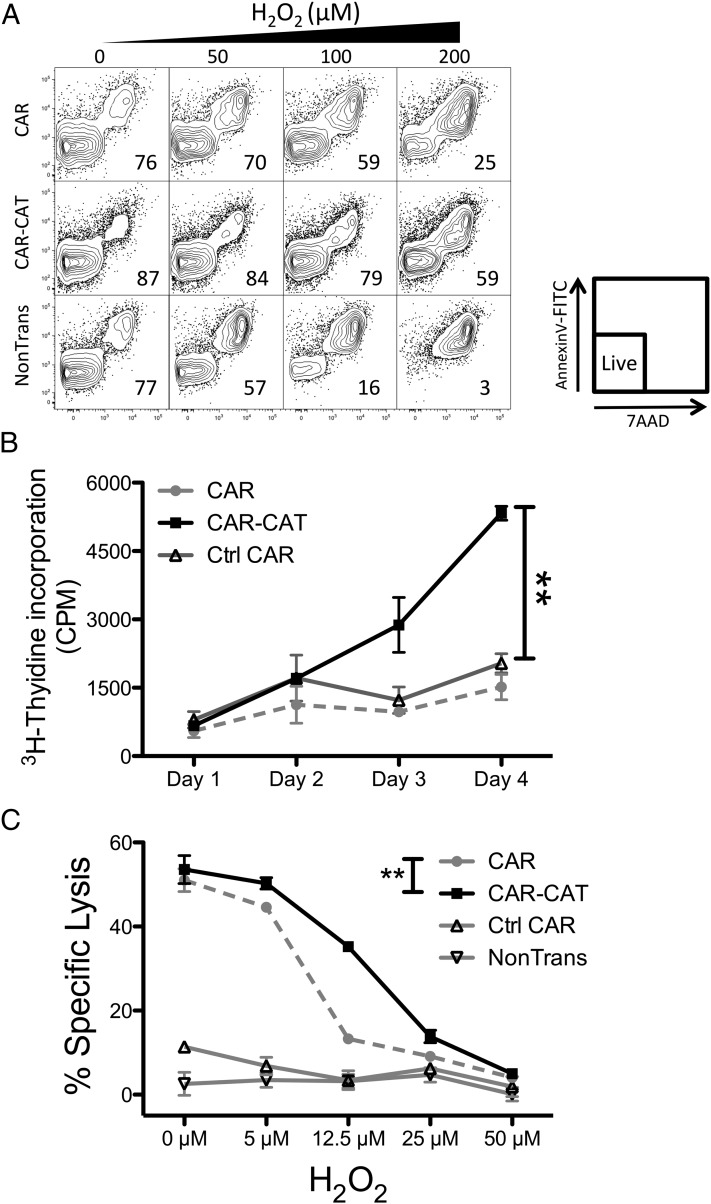
To examine whether CAR-CAT–transduced T cells were more resistant to high levels of oxidative stress, cells were cultured in increasing concentrations of H2O2 (Fig. 3A). T cells were freshly transduced and not sorted for CAR T cells. At 100 μM H2O2, CAR T cells were only 59% viable, which dropped to <30% viability at 200 H2O2. CAR-CAT T cells faired best, retaining their viability at 200 μM H2O2. Of note, activation of T cells for retroviral modification itself increased the resistance of T cells to oxidative stress compared with nonmodified T cells.
FIGURE 3.
CAR-CAT T cells maintain viability and functionality under H2O2-induced oxidative stress. (A) After transduction with CAR or CAR-CAT, T cells were resuspended to 2 × 105 in 200 μl complete medium RPMI 1640 containing 10% FCS and exposed to increasing concentrations of H2O2. After 24 h cells were stained with annexin V–FITC and 7-aminoactinomycin D and analyzed by FACS. (B) H2O2 (50 μM) was used to induce oxidative stress in 5 × 103 engineered or nonmodified T cells being stimulated with CD3-CD28 proliferation beads for 4 d and cell proliferation was measured by [3H]thymidine incorporation for 4 h. (C) Transduced T cells were used as effectors for targeting Her2+ tumors at an E:T ratio of 1:2. Freshly transduced T cells were cultured overnight with increasing concentrations of H2O2 to induce oxidative stress. After 18 h, target cells were labeled with [51Cr] and transferred into effector cell–containing wells. Supernatant was collected after 24 h coculture and transferred to LumaPlates and read out on MicroBeta. The percentage specific lysis was calculated using the following formula: (CPMsample − CPMspontaneous)/(CPMmaximum − CPMspontaneous). Data are presented as means ± SD. **p < 0.005 by two-way ANOVA using GraphPad Prism 5 for (B) and (C) between CAR and CAR-CAT.
Even if adoptively transferred T cells are able to survive in the presence of increased ROS levels, maintaining their function with respect to redirected cytolysis and amplifications remains crucial for their antitumor efficacy. When T cells recognize their target Ag they become strongly proliferative, as shown for CD19-specific CAR adoptively transferred into chronic lymphocytic leukemia patients (31). To address this issue we assayed the ability of T cells to proliferate at a level of ROS insult at which T cell viability is not affected. Transduced T cells were pulsed with 50 μM H2O2 and incubated with anti-CD3 and anti-CD28 beads for CAR-independent stimulation. At this concentration of H2O2, CAR-CAT T cells still maintained their proliferative potential after 4 d compared with control T cells (p < 0 0.005, Fig. 3B).
We asked whether these cells retain their CAR-endowed tumor-specific effector functions. To address this, we studied the ability of Her2-specific CAR T cells to lyse the Her2+ SkoV3 ovarian carcinoma cells under oxidative stress. CAR-mediated specific lysis of SkoV3 cells was almost abolished in the CAR T cells at a concentration of 12.5 μM H2O2, whereas the CAR-CAT–transduced T cells efficiently lysed the SkoV3 cells (Fig. 3C). Data demonstrate the superior capacity of CAR-CAT–transduced T cells to maintain their tumor-specific cytotoxic function under conditions of oxidative stress where this function is lost in nonmodified T cells.
CAR-CAT T cells mediate a protective bystander effect
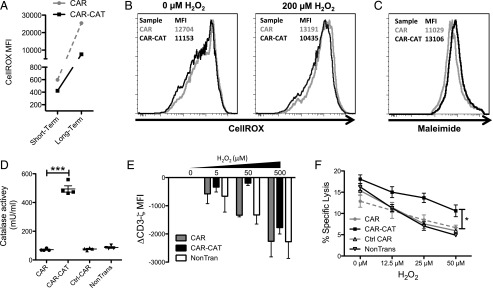
The reduction of ROS by the CAR-CAT T cells may protect also nonmodified immune cells in their vicinity. To investigate whether CAR-CAT T cells provide protection to bystanders, autologous nonmodified T cells were stained by CellROX. CAR− cells had decreased CellROX MFI for 1 h as well as well as for overnight staining (Fig. 4A). To address whether this would be the case when oxidative stress was introduced, autologous T cells were labeled with CFSE and admixed with CAR- or CAR-CAT–engineered lymphocytes. CAR-CAT bystander cells maintained basal oxidative stress levels, whereas those admixed with CAR had increased oxidative stress (Fig. 4B). Additionally, the level of surface thiols in CAR-CAT− autologous T cells was reduced when compared with CAR− autologous T cells (Fig. 3C). The results showed that these nontransduced T cells cocultured together with the CAR-CAT–transduced cells had a reduced oxidative state when compared with their counterparts cocultured together with CAR T cells. The supernatant had a 5-fold increase in catalase activity in CAR-CAT T cell culture compared with that from the supernatant of CAR T cells (Fig. 4D). We conclude that CAR-CAT T cells released catalase in substantial amounts, which reduced the oxidative state of cocultured cells.
FIGURE 4.
A bystander effect is mediated by CAR-CAT T cells toward nonmodified T and NK cells. (A) T cells were labeled with CellROX for both a short-term and long-term staining, and CellROX MFI on CAR− T cells was determined after cells were acquired by FACS. (B) Healthy donor autologous T cells were CFSE labeled and admixed with transduced T cells followed by staining with Abs and CellROX and acquired by flow cytometry. (C) CAR-CAT and CAR− cells were labeled with maleimide and surface thiols were evaluated. (D) Supernatants were collected from transduced and nontransduced T cells and tested for catalase activity. (E) H2O2 was used to induce oxidative stress in T cell culture for 2 h prior to staining. Cells (2 × 105) were stained for CD3ζ using FITC-conjugated anti-CD3ζ after permeabilization with 0.25% PFA and digitonin. Cells were acquired by FACS, and change in CD3ζ MFI was calculated by: CD3ζ MFI(xμM H2O2) − CD3ζ MFI(0μM H2O2) gated on the CAR− fraction. (F) NK cells were cocultured with engineered T cells at a ratio of 2:1 CAR T cell/NK cell overnight under oxidative stress induced by different concentrations H2O2. K562 target cells were loaded with [51Cr] and added to the NK/T cell mix after H2O2 coculture at a ratio of 1:1 NK cell/K562 cell. Supernatant (25 μl) was transferred to LumaPlates and read out on MicroBeta. Data are presented as means ± SD. *p < 0.05 by two-way ANOVA using GraphPad Prism 5 for (C) between CAR and CAR-CAT.
Intracellular oxidative stress, induced directly by the cancer cells or by immune-suppressive cells infiltrating the tumor lesion, can regulate T cell functions by reducing the CD3ζ, making TCR-mediated T cell activation less efficient (22, 32). We therefore studied whether the CAR-CAT T cells, in a bystander fashion, could protect the nontransduced T cells from this detrimental repression. To simulate the situation, transduced and nontransduced T cells were cocultured, subjected to H2O2 insult, and then stained for CD3ζ. Exposure to H2O2 decreased CD3ζ levels in the nonmodified T cells when cocultured with nonmodified or CAR-modified T cells. In contrast, coincubation with CAR-CAT T cells maintained CD3ζ levels of bystander T cells (Fig. 4E). In the third donor, no decrease in CD3ζ was detected in bystander T cells (data not shown).
TILs also include NK cells, which are sensitive to H2O2-induced inactivation (33). To address whether the CAR-CAT T cell–mediated bystander effect is protective for NK cells in the near vicinity of the transduced T cells, NK cells were admixed with transduced T cells at a ratio of two transduced T cells to one NK cell and the cytolytic capability of the NK cells was assessed against K562 target cells under increasing concentrations of H2O2. In the absence of oxidative stress, there was no difference in NK cell–mediated cytotoxicity against K562 cocultured with CAR-CAT, CAR, or Ctrl-CAR T cells (Fig. 4F). In contrast, when exposed to increasing levels of H2O2, the NK cells coincubated with CAR-CAT T cells were consistently more efficient in killing the K562 cells than were NK cells cocultured with CAR, Ctrl-CAR–transduced, or nontransduced T cells. We conclude that CAR-CAT T cells protect in trans both T and NK cells from oxidative stress–mediated repression.
Discussion
Adoptive therapy with CAR-modified T cells offers a powerful therapy for a variety of malignant entities. This has been realized for the treatment of hematological tumors such as chronic lymphocytic leukemia and acute lymphoblastic leukemia (9, 31); treatment of solid tumors, however, faces additional hurdles and needs further optimization (6). The stroma of solid tumors constitutes a barrier that actively suppresses the function of the adoptively transferred T cells by various immune suppressive mechanisms, including mediators such as ROS, arginase, IDO, and PGE2 (34, 35).
In this study we demonstrate that redirected T cells, engineered to target tumor cells by a CAR specific for a cell surface Ag, can be protected from ROS-induced oxidative stress by coexpressing catalase. The CARs used in this study are specific for Her2 and CEA and have been shown to eliminate tumor cells with the respective targets in a specific fashion (28, 36). The Her2 and CEA are highly expressed in a variety of breast cancer and colorectal cancer (CRC) lesions, respectively (37, 38), both of which have increased ROS production and local oxidative stress (15, 39). In CRC, oxidative stress promotes proliferation of tumor cells while being insufficient to cause cell death (40). Carcinoma-infiltrating lymphocytes have experienced high levels of oxidative stress as measured by 8-hydroxy-2′-deoxyguanosine staining (39). Also, breast cancers have high levels of oxidative stress, being a driving factor in breast cancer progression. Accordingly, lymphocytes from breast cancer patients exhibit increased oxidized DNA levels as compared with healthy donor lymphocytes (41). Interestingly, when reducing oxidative stress in aggressive breast cancer, tumors are sensitized to chemotherapy (15). This ROS feature of both cancer types provides the rationale for a potentially beneficial effect of coexpressing catalase in CAR-transduced T cells homing to these tumors.
We revealed that coexpressing catalase in CAR T cells allowed for a reduced oxidative state in engineered T cells, an effect that remained when cells were activated by TCR/CD3 engagement or while cocultured with tumor cells. A reduced oxidative state is essential for maintaining T cell function in the long-term. This is particularly of clinical relevance in the setting of adoptive cell therapy where the transplanted T cells are thousand fold expanded ex vivo prior to transfer and entering the tumor tissues. T cell subsets are differentially affected by ROS, with CD8+ T effector memory cells being particularly more susceptible to ROS-induced cell death and loss in function than are their naive T cell counterparts (21). These memory cells are essential for providing a better clinical outcome in CRC patients (42).
The tumor tissue is infiltrated with a large number of immune cells, most of which are of myeloid origin, not of lymphoid origin. These macrophages, monocytes, granulocytes, and MDSCs produce ROS and thus suppress the lymphoid antitumor immune response. Activated infiltrating granulocytes in particular inactivate T cells, and the addition of ROS scavengers was able to rescue their function (18). MDSCs are potent producers of ROS, mainly through the activation of the NOX2 pathway leading to the production of superoxide, and they have been shown to exert some of their suppressive function through this pathway (17). High catalase activity in those tumor-targeting lymphoid cells upon transgenic catalase expression provides a strategy to resist ROS-mediated repression in the tumor tissue.
In patients, CAR-transduced T cells are able to clear large tumor burdens, sometimes leading to tumor lysis syndrome (8). We confirmed the high efficacy of the CAR-transduced T cells in cytotoxicity assays. CAR-CAT T cells retained their ability to lyse Her2+ tumor cells under conditions of oxidative stress, whereas this ability was lost in control CAR T cells. Furthermore, 50 μM H2O2 affected the capacity of control T cells and CAR T cells to proliferate in response to a strong proliferative stimulus whereas CAR-CAT–transduced T cells retained proliferative capacity (Fig. 3B). We conclude that genetically modified T cells that overexpress catalase resist oxidative stress to certain levels which may be sufficient to remain functional upon entering the tumor stroma.
Protecting tumor-infiltrating T and NK cells from ROS-mediated inactivation would maintain their antitumor activity, with the latter cells attacking those cancer cells that lack the particular tumor-associated Ag recognized by redirected T cells. In line with this concept, we found that CAR-CAT T cells were capable of reducing the oxidative state of bystander T cells. Under these conditions NK cells are enabled to execute their antitumor response. This bystander effect was likely mediated by the catalase present in the supernatant of CAR-CAT T cells. We showed that T cells engineered with CAR and catalase preserved CD3ζ levels of the bystander T cells (Fig. 4E). This may result in more efficient tumor elimination, including of cancer cells lacking CAR-targeted Ags. Protecting bystander immune cells in trans by catalase engineered T cells may thereby indirectly provide a benefit in the therapy of solid tumors. In addition to CAR-redirected T cells themselves, TILs, which are inactivated by ROS-producing stroma cells, may become reactivated when ROS-induced immunosuppression is removed. Increased oxidative stress decreases TCR/CD3ζ expression in T cells, thus inhibiting their TCR-mediated effector functions. In cancer patients CD3ζ is often downregulated in tumor infiltrating T cells, accompanied by loss of cytolytic activity as well as loss of proliferative potential (23, 32, 43). In gastric carcinoma, the 5-y survival of patients was significantly improved when TILs maintained normal levels of CD3ζ expression (44), underlining the therapeutic potential in sustaining function of infiltrating immune cells in a ROS-mediated immune repressive environment.
Owing to their high sensitivity toward oxidative stress, intratumor activity of NK cells is likely compromised (45), particularly of the cytotoxic CD56dim NK cell subset (33). We found that data indicate that the bystander effect of CAR-CAT T cells extended also to NK cells and rescued their cytolytic ability at high H2O2 concentrations (Fig. 4F). Beyond this, CAR-CAT T cells are able to modify the suppressive cells in the tumor tissue. MDSCs require high levels of ROS to retain their suppressive phenotype; in the absence of ROS, immature MDSCs differentiate into nonsuppressive monocytes (17), which, together with other mechanisms, finally may result in a global change in the immune surveillance of cancer.
The approach of combining tumor-redirected CAR T cells with the transgenic expression of molecules that modulate the oxidative state in the tumor milieu may be extended to several other categories of molecules that counteract T cell function such as arginase-1, IDO, or iNOS. Additionally, other strategies to reduce tumor immunosuppression, for example by coengineering T cells with TGF-β dominant negative receptor, have shown impressive results (46) and suggest that targeting these suppressive mechanisms may be essential to improving T cell–based therapies (6). Our data imply that the strategy to target ROS may improve both the Ag-specific and Ag-independent tumor elimination, resulting in a more rapid and efficacious tumor elimination, which likely improves the outcome of adoptive T cell therapy of cancer.
Supplementary Material
This work was supported by Swedish Medical Research Council Grant K2011-66X-15387-07-3 VR (to R.K.), Swedish Cancer Society Grant 12 0598 (Cancerfonden), Cancer Society of Stockholm Grant 121103 (Cancerföreningen, Radiumhemmets Forskningsfonder), the Knut and Alice Wallenberg Foundation, and by Stockholm City Council Project Grant 20140036 (ALF Medicine 2015). The H.A. laboratory was supported by the Else Kröner-Fresenius Stiftung, the Sander-Stiftung, and the Deutsche Krebshilfe.
The online version of this article contains supplemental material.
- CAR
- chimeric Ag receptor
- CAR-CAT
- CAR coexpressing catalase
- CRC
- colorectal cancer
- Ctrl-CAR
- control CAR
- DHNQ
- dihydroxynaphthoquinone
- MDSC
- myeloid-derived suppressor cell
- MFI
- mean fluorescence intensity
- ROI
- reactive oxygen intermediary
- ROS
- reactive oxygen species
- TIL
- tumor-infiltrating lymphocyte.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Fridman W.-H., Pagès F., Sautès-Fridman C., Galon J. 2012. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12: 298–306. [DOI] [PubMed] [Google Scholar]
- 2.Büning H., Uckert W., Cichutek K., Hawkins R. E., Abken H. 2010. Do CARs need a driver’s license? Adoptive cell therapy with chimeric antigen receptor-redirected T cells has caused serious adverse events. Hum. Gene Ther. 21: 1039–1042. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg S. A., Yang J. C., Sherry R. M., Kammula U. S., Hughes M. S., Phan G. Q., Citrin D. E., Restifo N. P., Robbins P. F., Wunderlich J. R., et al. 2011. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17: 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott A. M., Wolchok J. D., Old L. J. 2012. Antibody therapy of cancer. Nat. Rev. Cancer 12: 278–287. [DOI] [PubMed] [Google Scholar]
- 5.Hiraki A., Kaneshige T., Kiura K., Ueoka H., Yamane H., Tanaka M., Harada M. 1999. Loss of HLA haplotype in lung cancer cell lines: implications for immunosurveillance of altered HLA class I/II phenotypes in lung cancer. Clin. Cancer Res. 5: 933–936. [PubMed] [Google Scholar]
- 6.Gilham D. E., Debets R., Pule M., Hawkins R. E., Abken H. 2012. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol. Med. 18: 377–384. [DOI] [PubMed] [Google Scholar]
- 7.Davila M. L., Rivière I., Wang X., Bartido S., Park J., Curran K., Chung S. S., Stefanski J., Borquez-Ojeda O., Olszewska M., et al. 2014. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6: 224ra25. doi:10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer J. N., Dudley M. E., Carpenter R. O., Kassim S. H., Rose J. J., Telford W. G., Hakim F. T., Halverson D. C., Fowler D. H., Hardy N. M., et al. 2013. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 122: 4129–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupp S. A., Kalos M., Barrett D., Aplenc R., Porter D. L., Rheingold S. R., Teachey D. T., Chew A., Hauck B., Wright J. F., et al. 2013. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brentjens R. J., Davila M. L., Rivière I., Park J., Wang X., Cowell L. G., Bartido S., Stefanski J., Taylor C., Olszewska M., et al. 2013. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5: 177ra38. doi:10.1126/scitranslmed.3005930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamers C. H. J., Sleijfer S., Vulto A. G., Kruit W. H. J., Kliffen M., Debets R., Gratama J. W., Stoter G., Oosterwijk E. 2006. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 24: e20–e22. [DOI] [PubMed] [Google Scholar]
- 12.Kershaw M. H., Westwood J. A., Parker L. L., Wang G., Eshhar Z., Mavroukakis S. A., White D. E., Wunderlich J. R., Canevari S., Rogers-Freezer L., et al. 2006. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 12: 6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J. R., Digiusto D. L., Slovak M., Wright C., Naranjo A., Wagner J., Meechoovet H. B., Bautista C., Chang W.-C., Ostberg J. R., Jensen M. C. 2007. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 15: 825–833. [DOI] [PubMed] [Google Scholar]
- 14.Toyokuni S., Okamoto K., Yodoi J., Hiai H. 1995. Persistent oxidative stress in cancer. FEBS Lett. 358: 1–3. [DOI] [PubMed] [Google Scholar]
- 15.Brown N. S., Bicknell R. 2001. Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 3: 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poschke I., Kiessling R. 2012. On the armament and appearances of human myeloid-derived suppressor cells. Clin. Immunol. 144: 250–268. [DOI] [PubMed] [Google Scholar]
- 17.Corzo C. A., Cotter M. J., Cheng P., Cheng F., Kusmartsev S., Sotomayor E., Padhya T., McCaffrey T. V., McCaffrey J. C., Gabrilovich D. I. 2009. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182: 5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmielau J., Finn O. J. 2001. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 61: 4756–4760. [PubMed] [Google Scholar]
- 19.Kiessling R., Mao Y., Pico de Coaña Y. 2014. Myeloid suppressors decrease melanoma survival by abating tumor-fighting T cells. Clin. Cancer Res. 20: 1401–1403. [DOI] [PubMed] [Google Scholar]
- 20.Malmberg K.-J., Arulampalam V., Ichihara F., Petersson M., Seki K., Andersson T., Lenkei R., Masucci G., Pettersson S., Kiessling R. 2001. Inhibition of activated/memory (CD45RO+) T cells by oxidative stress associated with block of NF-κB activation. J. Immunol. 167: 2595–2601. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi A., Hanson M. G. V., Norell H. R., Havelka A. M., Kono K., Malmberg K.-J., Kiessling R. V. 2005. Preferential cell death of CD8+ effector memory (CCR7−CD45RA−) T cells by hydrogen peroxide-induced oxidative stress. J. Immunol. 174: 6080–6087. [DOI] [PubMed] [Google Scholar]
- 22.Kono K., Salazar-Onfray F., Petersson M., Hansson J., Masucci G., Wasserman K., Nakazawa T., Anderson P., Kiessling R. 1996. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing ζ molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur. J. Immunol. 26: 1308–1313. [DOI] [PubMed] [Google Scholar]
- 23.Nakagomi H., Petersson M., Magnusson I., Juhlin C., Matsuda M., Mellstedt H., Taupin J. L., Vivier E., Anderson P., Kiessling R. 1993. Decreased expression of the signal-transducing ζ chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 53: 5610–5612. [PubMed] [Google Scholar]
- 24.Mizoguchi H., O’Shea J. J., Longo D. L., Loeffler C. M., McVicar D. W., Ochoa A. C. 1992. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science 258: 1795–1798. [DOI] [PubMed] [Google Scholar]
- 25.Meyer C., Sevko A., Ramacher M., Bazhin A. V., Falk C. S., Osen W., Borrello I., Kato M., Schadendorf D., Baniyash M., Umansky V. 2011. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc. Natl. Acad. Sci. USA 108: 17111–17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ando T., Mimura K., Johansson C. C., Hanson M. G., Mougiakakos D., Larsson C., Martins da Palma T., Sakurai D., Norell H., Li M., et al. 2008. Transduction with the antioxidant enzyme catalase protects human T cells against oxidative stress. J. Immunol. 181: 8382–8390. [DOI] [PubMed] [Google Scholar]
- 27.Hombach A., Sent D., Schneider C., Heuser C., Koch D., Pohl C., Seliger B., Abken H. 2001. T-cell activation by recombinant receptors: CD28 costimulation is required for interleukin 2 secretion and receptor-mediated T-cell proliferation but does not affect receptor-mediated target cell lysis. Cancer Res. 61: 1976–1982. [PubMed] [Google Scholar]
- 28.Chmielewski M., Hombach A. A., Abken H. 2011. CD28 cosignalling does not affect the activation threshold in a chimeric antigen receptor-redirected T-cell attack. Gene Ther. 18: 62–72. [DOI] [PubMed] [Google Scholar]
- 29.Imada I., Sato E. F., Miyamoto M., Ichimori Y., Minamiyama Y., Konaka R., Inoue M. 1999. Analysis of reactive oxygen species generated by neutrophils using a chemiluminescence probe L-012. Anal. Biochem. 271: 53–58. [DOI] [PubMed] [Google Scholar]
- 30.Devadas S., Zaritskaya L., Rhee S. G., Oberley L., Williams M. S. 2002. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 195: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter D. L., Levine B. L., Kalos M., Bagg A., June C. H. 2011. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kono K., Ressing M. E., Brandt R. M., Melief C. J., Potkul R. K., Andersson B., Petersson M., Kast W. M., Kiessling R. 1996. Decreased expression of signal-transducing ζ chain in peripheral T cells and natural killer cells in patients with cervical cancer. Clin. Cancer Res. 2: 1825–1828. [PubMed] [Google Scholar]
- 33.Harlin H., Hanson M., Johansson C. C., Sakurai D., Poschke I., Norell H., Malmberg K.-J., Kiessling R. 2007. The CD16− CD56bright NK cell subset is resistant to reactive oxygen species produced by activated granulocytes and has higher antioxidative capacity than the CD16+ CD56dim subset. J. Immunol. 179: 4513–4519. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber R. D., Old L. J., Smyth M. J. 2011. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
- 35.Mao Y., Poschke I., Kiessling R. 2014. Tumour-induced immune suppression: role of inflammatory mediators released by myelomonocytic cells. J. Intern. Med. 276: 154–170. [DOI] [PubMed] [Google Scholar]
- 36.Hombach A., Schlimper C., Sievers E., Frank S., Schild H. H., Sauerbruch T., Schmidt-Wolf I. G., Abken H. 2006. A recombinant anti-carcinoembryonic antigen immunoreceptor with combined CD3ζ-CD28 signalling targets T cells from colorectal cancer patients against their tumour cells. Gut 55: 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moasser M. M. 2007. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26: 6469–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beauchemin N., Arabzadeh A. 2013. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 32: 643–671. [DOI] [PubMed] [Google Scholar]
- 39.Kondo S., Toyokuni S., Iwasa Y., Tanaka T., Onodera H., Hiai H., Imamura M. 1999. Persistent oxidative stress in human colorectal carcinoma, but not in adenoma. Free Radic. Biol. Med. 27: 401–410. [DOI] [PubMed] [Google Scholar]
- 40.Kang K. A., Kim K. C., Bae S. C., Hyun J. W. 2013. Oxidative stress induces proliferation of colorectal cancer cells by inhibiting RUNX3 and activating the Akt signaling pathway. Int. J. Oncol. 43: 1511–1516. [DOI] [PubMed] [Google Scholar]
- 41.Soliman A. S., Vulimiri S. V., Kleiner H. E., Shen J., Eissa S., Morad M., Taha H., Lukmanji F., Li D., Johnston D. A., et al. 2004. High levels of oxidative DNA damage in lymphocyte DNA of premenopausal breast cancer patients from Egypt. Int. J. Environ. Health Res. 14: 121–134. [DOI] [PubMed] [Google Scholar]
- 42.Pagès F., Berger A., Camus M., Sanchez-Cabo F., Costes A., Molidor R., Mlecnik B., Kirilovsky A., Nilsson M., Damotte D., et al. 2005. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 353: 2654–2666. [DOI] [PubMed] [Google Scholar]
- 43.Lockhart D. C., Chan A. K., Mak S., Joo H.-G., Daust H. A., Carritte A., Douville C. C., Goedegebuure P. S., Eberlein T. J. 2001. Loss of T-cell receptor-CD3ζ and T-cell function in tumor-infiltrating lymphocytes but not in tumor-associated lymphocytes in ovarian carcinoma. Surgery 129: 749–756. [DOI] [PubMed] [Google Scholar]
- 44.Ishigami S., Natsugoe S., Tokuda K., Nakajo A., Higashi H., Iwashige H., Aridome K., Hokita S., Aikou T. 2002. CD3-zetachain expression of intratumoral lymphocytes is closely related to survival in gastric carcinoma patients. Cancer 94: 1437–1442. [DOI] [PubMed] [Google Scholar]
- 45.Seaman W. E., Gindhart T. D., Blackman M. A., Dalal B., Talal N., Werb Z. 1982. Suppression of natural killing in vitro by monocytes and polymorphonuclear leukocytes: requirement for reactive metabolites of oxygen. J. Clin. Invest. 69: 876–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L., Yu Z., Muranski P., Palmer D. C., Restifo N. P., Rosenberg S. A., Morgan R. A. 2013. Inhibition of TGF-β signaling in genetically engineered tumor antigen-reactive T cells significantly enhances tumor treatment efficacy. Gene Ther. 20: 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.