Abstract
Background:
Autograft from iliac crest is considered as gold standard for augmentation of bone healing in delayed and nonunion of fractures. Bone demineralized with 0.6N hydrochloric acid has shown to retain its osteoinductive capacity. We report the outcome of partially decalcified bone allograft (decal bone) in the treatment of delayed union and atrophic nonunions of bones.
Materials and Methods:
Twenty patients with clinicoradiological diagnosis of delayed union or atrophic nonunion of long bone fractures were included in this retrospective study. Patients at extreme of ages (<18 years and >60 years), pathological fractures, metabolic bone diseases, infected nonunion, hypertrophic nonunion and those having systemic illness like diabetes mellitus and on drugs that impair fracture healing were excluded from the study. Decal bone was prepared in the bone bank and maintained in department of orthopedics. Allografting was done in 20 patients of delayed union (9/20) and atrophic nonunion (11/20) of long bone fractures with mean age of 34 years (range 18–55 years). The bones involved were humerus (8/20), tibia (7/20) and femur (5/20). Fourteen patients underwent treatment in the form of internal fixation and allografting and six patients were operated with osteoperiosteal allografting.
Results:
Nineteen patients achieved union in mean time of 14.9 weeks range (range 8–20 weeks). Eight patients had serous discharge from the operative site that subsided in 11 days (range 4–21 days). One patient had pus discharge that required repeat debridement and antibiotics for 6 weeks. The fracture healed in 16 weeks.
Conclusion:
The partially decalcified bone allograft is an effective modality for augmentation of bone healing without complication associated with autograft like donor site morbidity, increased blood loss and increase in the surgical time.
Keywords: Allograft, augmentation, bone healing, decal bone, partially decalcified, union
Mesh terms: Fractures, bone, grafting bone, allograft
INTRODUCTION
Ability to stimulate fracture repair and heal nonunion is a common goal among all orthopedic surgeons. Of all the fractures of the long bones that occur annually, 5–10% end in delayed or nonunion.1 The standard treatment of delayed union and atrophic nonunion has been freshening and decortications of fracture ends stable surgical fixation and augmentation of bone healing. Autogenos bone graft (autograft) from iliac crest has been considered as the gold standard for augmentation of bone healing. But autograft gives donor site morbidity hence various studies are being done to find a suitable alternative.2 Partially decalcified bone allograft (decal bone) is considered good alternative in benign cystic lesions of bones but their role in augmentation of bone healing is not well defined.3,4 When demineralization is carried out by 0.6N hydrochloric acid (HCl), the matrix retains high levels of bone morphogenic protein (BMP) responsible for osteoinductive property of decal bone.5,6 The present study evaluated the outcome of decal bone in augmentation of bone healing in delayed and atrophic nonunions of long bone fracture.
MATERIALS AND METHODS
Patients with clinicoradiological diagnosis of delayed union or atrophic nonunion of long bone fractures were included in this retrospective study conducted in a tertiary care hospital. A fracture was labeled as nonunion when at least 9 months have elapsed since fracture; there is painless abnormal mobility at the fracture site and there is no radiological progression in healing in last 3 months. A fracture was labeled as delayed union when there is clinical and radiological lag compared to similar fracture in similar bone in similar age group. Patients at extreme of ages (<18 years and >60 years), pathological fractures, metabolic bone diseases, infected nonunion, hypertrophic nonunion and those having systemic illness like diabetes mellitus and patients not willing to receive allograft were excluded from the study. The authors ruled out infective nonunion by clinical examination, erythrocyte sedimentation rate and C-reactive protein. Those patients who had suspicion of infected nonunion were excluded from the study. Of 32 patients with delayed and nonunion of fractures, 22 patients met the inclusion and exclusion criterion. Two patients were lost to follow up. Hence, 20 patients were included in the study and were assessed for the outcome (union and complications, if any). The mean age of the patients was 34 years (range 18–55 years). There were 14 males and 6 females. The bones involved were humerus (n = 8), tibia (n = 7), and femur (n = 5). There were 11 patients with atrophic nonunion and 9 patients with the delayed union [Table 1]. Eleven patients sustained injuries in road traffic accident; three patients had fall from height and six patients had fall from stairs. Ten patients had history of smoking and four patients had history of alcohol abuse.
Table 1.
Clinical details of the patients
Technique
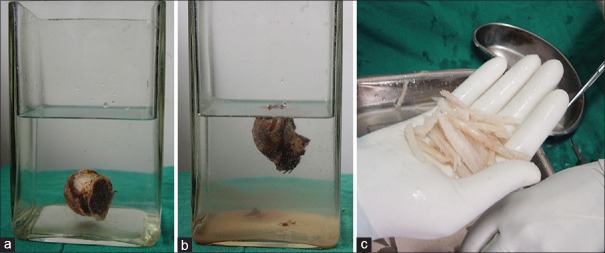
Bones were obtained from the patients undergoing amputations or arthroplasties after taking informed consent. The guidelines of tissue procurement and bone banking were followed.7,8 The patients were screened for allograft transmittable diseases like HIV, Hep B, Hep C before surgery and after 6 months to cover for window period responsible for initial false negative results. After harvesting, bone was thoroughly cleaned of soft tissues and blood. Thereafter, the bone was partially decalcified in freshly prepared solution of 0.6N HCl for 24–48 h [Figure 1a and b]. After decalcification sample of the bone was sent for aerobic, anaerobic and fungal cultures, the allograft was stored in 90% ethanol at 4°C in a domestic refrigerator. The stored allograft was taken out of the container in Operation Theater and was thoroughly washed with normal saline and a piece of allograft was sent for culture. The allogenic bone was cut into 5 mm slivers and bridged across the fracture site [Figure 1c].
Figure 1.
Photographs showing (a) Preserved femoral head at the bottom of jar filled with 0.6N hydrochloric acid (b) After partial decalcification floating femoral head in jar (c) Slivers of decal bone prepared in theatre that are put across the fracture site
Eight patients underwent open reduction and internal fixation with low contact dynamic compression plate and allografting, six patients had open reduction, intramedullay nailing and allografting while another six patients underwent osteoperiosteal allografting [Table 1]. Patients were reviewed at 4 weeks interval for clinical and radiological signs of union. The clinical variables observed were redness, swelling, induration, raised temperature and tenderness or discharge from the operative site to rule out postoperative infection and the radiological parameters to assess status, and time of union of fracture. The fracture was labeled as united when clinically there was no local tenderness at fracture site and radiologically bridging callus across the fracture site was observed in at least three cortices in two orthogonal views.
RESULTS
Nineteen out of twenty fractures showed union in a mean duration of 14.9 weeks (range 8–20 weeks) [Figures 2–4]. The mean time of union was 14 weeks in atrophic nonunions and 16 weeks in delayed unions. There were two major and eight minor complications in the study. One patient had persistent nonunion, and one patient had postoperative wound infection. One patient with atrophic nonunion of humerus shaft did not unite. The failure of the union was attributed to technical error of fixation. Repeat fixation and autologous bone grafting from iliac crest was done and the fracture united after 8 weeks of grafting. One patient developed purulent discharge from the operative site in the immediate postoperative period. Culture from the wound grew Staphylococcus aureus. Despite use of intravenous antibiotics, patient continued to have purulent discharge from the operative site. At 4 weeks, postoperatively patient was taken up for wound debridement and loose pieces of allograft were removed whereas nonloose pieces were left behind. The wound healed subsequently and fracture healed in 16 weeks [Figure 5].
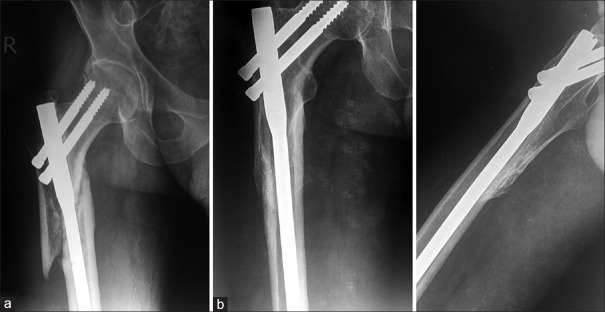
Figure 2.
X-ray right hip joint with thigh (a) anteroposterior view showing surgically intervened delayed union proximal femur (b and c) Followup anteroposterior and lateral radiographs showing union
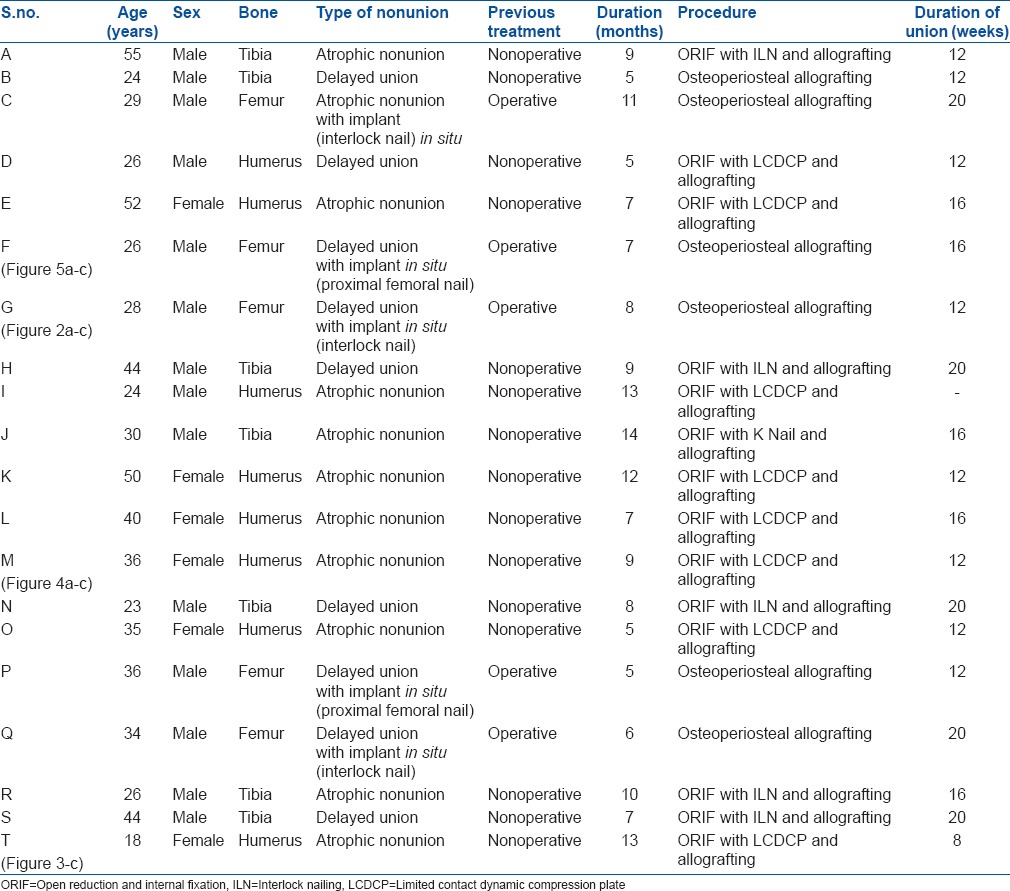
Figure 4.
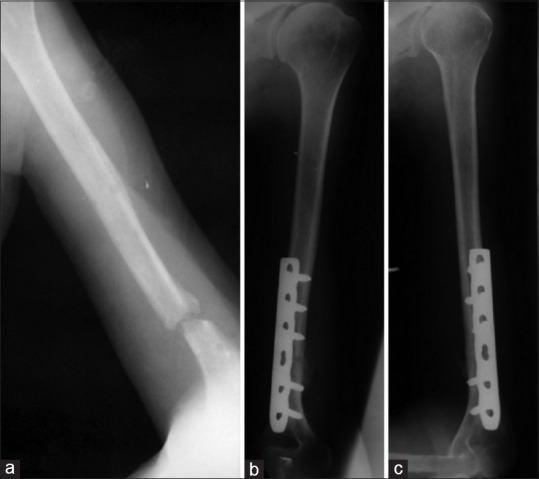
(a) X-ray of arm anteroposterior view showing atrophic nonunion of humerus (b and c) Followup anteroposterior and lateral radiographs showing union
Figure 5.
(a) Surgically intervened delayed union proximal femur. The patient had postoperative infection (b and c) Union despite of infection evident by bridging callus in both anteroposterior and lateral radiographs
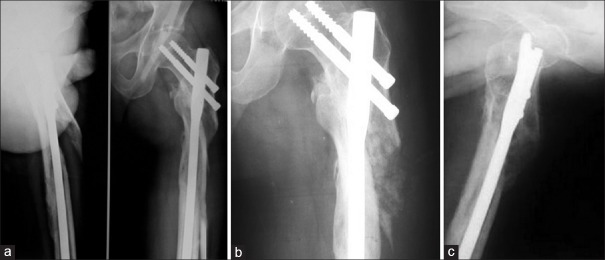
Figure 3.
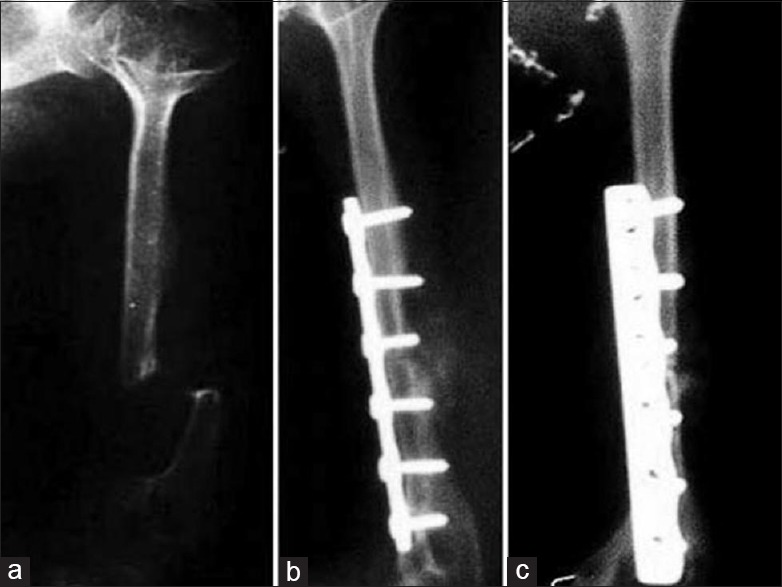
(a) X-ray of arm anteroposterior view showing atrophic nonunion of fracture of humerus (b and c) Followup anteroposterior and lateral radiographs showing union
Minor complication included clear, nonpurulent serous discharge from the operative site in the immediate postoperative period in eight patients (4 patients with delayed union and 4 patients with atophic nonunion). The culture of the discharge was sterile after 48 h of incubation period. The discharge subsided in all the patients in 11 days (range 4–18 days).
DISCUSSION
Autografts are considered as gold standard for delayed/nonunion of fractures grafting. However, autograft present with problems like limited availability of graft, increased surgical time, more blood loss, donor site morbidity and other uncommon complications like fatigue fractures, growth impairment in children, osteomyelitis, peritoneal perforation, massive hematoma and sacroiliac joint instability.2 Due to disadvantages associated with autograft, a lot of research is going on to find a suitable alternative for augmentation of bone healing. Allograft in different forms has been considered as good alternative option. Allogenic bone graft can be freeze dried, deep frozen, or partially decalcified. Freeze dried and deep frozen allograft bones need sophisticated equipment and a bone bank set up which are very expensive to maintain.9,10
Osteoinductive capacity of allogenic decal bone depends on the agents used for demineralizing and extent of demineralization. When bone is demineralized with 0.6 N HCl, it remains osteoinductive and readily osteoconductive material without any appreciable local foreign body reaction.11 Decalcification using EDTA and nitric acid markedly decreases the osteoinductive capacity.12 It has been reported that mineral content should be reduced to at least 40% of normal before a strong osteoinductive response can occur.13
The biological process of incorporation of the partially decalcified bone graft is similar to that of autograft. When graft is placed at the fracture site, there is osteoclastic and phagocytic resorption of calcium hydroxyapatite and cellular debris.12 This makes the graft porous through which neovascularization propogates. Release of osteoinductive material (e.g. BMP) by the autograft leads to conversion of osteoprogenitor cells to osteoblastic cells that lay down new bone on the porous matrix by the process of creeping substitution. In autografts, the mineral and organic material must undergo resorption before creeping substitution starts, whereas in decal bone resorption of demineralized matrix is faster because of the prior removal of nearly half of minerals in the laboratory. Decal bone provides an easily permeable scaffolding structure that permits creeping substitution.
Various experimental studies have proven the osteoinductive potential of decal bone. Reparative role of decal bone has been well established in benign cystic lesions of bones.3,4 However, there is scanty literature on role of decal bone in augmentation of fracture healing in humans. Saraf et al.14 observed that allogenic bone shows high degree of osteoinduction and remodeling when used as bone strips and is less osteoinductive when used in form of bone cylinders or bone powder. Tuli and Gupta reported efficacy of partially decalcified bone in bridging of osteoperiosteal defects.15,16 Zhang et al. used allogeneic decalcified bone graft taken from fresh corpse in seventeen children with nonunion and showed successful outcome in 9 cases whereas 7 cases required two surgeries.17
Like any bone allografts, decal bone transplanted in the recipient evokes an immunogenic reaction. Cancellous bone is considered to be more antigenic than cortical bone that can be attributed to greater cellularity; both osteogenic and hematopoietic. Though the exact mechanism may be unclear, it is well established that allograft bone carries immunogenic potential. Immunogenic reaction to bone (cartilage) allograft is a “low threshold” and may last for a longer period; it is unlike allografting of solid organs where the immunogenic reaction is like a sudden surge or “spike.” Efforts are made to reduce and possibly eliminate antigenicity of bone by processing of the allograft bone. Wang showed immune response to allogenic bone in the sera 32 patients out of 50 patients and complete graft healing in 30 of these patients.18 A study on immunogenicity of decal bone by assessing type of cellularity (CD4 and CD8 cells) in perigraft area by fine-needle aspiration cytology concluded that decal bone did not excite an appreciably significant immunological response and partially decalcified allografts are a good substitute of autogenous bone grafts in clinical practice.19
Our study also had several limitations. Firstly small sample size, secondly, nonunion from upper as well as lower limbs, thirdly, different treatment modalities used for different patients like nailing, plating or phemister allografting and fourthly, adjunctive procedures like reaming the canal, freshening the margins, rose petaling can themselves enhance fracture union and can act as confounding factor.
The present study has given clinical evidence of efficacy of partially decalcified bone as an induction of bone healing in delayed and atrophic nonunion of fractures of long bones but additional focused independent multicentric trials are required to accurately assess the efficacy of partially decalcified bone allograft in the induction of bone healing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Novicoff WM, Manaswi A, Hogan MV, Brubaker SM, Mihalko WM, Saleh KJ. Critical analysis of the evidence for current technologies in bone-healing and repair. J Bone Joint Surg Am. 2008;90(Suppl 1):85–91. doi: 10.2106/JBJS.G.01521. [DOI] [PubMed] [Google Scholar]
- 2.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine (Phila Pa 1976) 1989;14:1324–31. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Sethi A, Agarwal K, Sethi S, Kumar S, Marya SK, Tuli SM. Allograft in the treatment of benign cystic lesions of bone. Arch Orthop Trauma Surg. 1993;112:167–70. doi: 10.1007/BF00662282. [DOI] [PubMed] [Google Scholar]
- 4.Goel SC, Tuli SM, Singh HP, Sharma SV, Saraf SK, Srivastava TP. Allogenic decalbone in the repair of benign cystic lesions of bone. Int Orthop. 1992;16:176–9. doi: 10.1007/BF00180212. [DOI] [PubMed] [Google Scholar]
- 5.Urist MR, Silverman BF, Büring K, Dubuc FL, Rosenberg JM. The bone induction principle. Clin Orthop Relat Res. 1967;53:243–83. [PubMed] [Google Scholar]
- 6.Urist MR. The search for and discovery of bone morphogenetic protein. In: Urist MR, O’Connor BT, Burwell RG, editors. Bone Grafts, Derivatives and Substitutes. Oxford (UK) and Waltham, Massachusetts (United States): Butter Worth Heinemann Ltd; 1994. pp. 315–62. [Google Scholar]
- 7.Friedlaender E, Mankin J. Guidelines for the banking of musculoskeletal tissues. Am Assoc Tissue Banks Newsl. 1979;3:2–4. [Google Scholar]
- 8.Friedlaender E, Mankin HJ. The American Academy of Orthopaedic Surgeons. Vol. 30. St. Louis: C. V. Mosby; 1981. Bone banking: Current methods and suggested guidelines. Instructional Course Lectures; pp. 36–55. [PubMed] [Google Scholar]
- 9.Tomford WW, Doppelt SH, Mankin HJ, Friedlaender GE. 1983 bone bank procedures. Clin Orthop Relat Res. 1983;174:15–21. [PubMed] [Google Scholar]
- 10.Friedlaender GE. Personnel and equipment required for a “complete” tissue bank. Transplant Proc. 1976;8(2 Suppl 1):235–40. [PubMed] [Google Scholar]
- 11.Goel SC, Tuli SM. Use of decal bone in healing of osseous cystic defects. In: Urist MR, O’Connor BT, Burwell RG, editors. Bone Grafts, Derivatives and Substitutes. 1st ed. Oxford (UK) and Waltham, Massachusetts (United States): Butter worth Heinemann Ltd; 1994. pp. 210–9. [Google Scholar]
- 12.Urist MR, Strates BS. Bone formation in implants of partially and wholly demineralized bone matrix. Including observations on acetone-fixed intra and extracellular proteins. Clin Orthop Relat Res. 1970;71:271–8. [PubMed] [Google Scholar]
- 13.Hallfeldt KK, Stützle H, Puhlmann M, Kessler S, Schweiberer L. Sterilization of partially demineralized bone matrix: The effects of different sterilization techniques on osteogenetic properties. J Surg Res. 1995;59:614–20. doi: 10.1006/jsre.1995.1213. [DOI] [PubMed] [Google Scholar]
- 14.Saraf SK, Kumar A, Tuli SM, Khanna S. Effect of size and shape of the allogeneic bone grafts in bridging experimental ulnar gap in rabbits. Indian J Exp Biol. 1994;32:690–3. [PubMed] [Google Scholar]
- 15.Gupta D, Tuli SM. Osteoinductivity of partially decalcified alloimplants in healing of large osteoperiosteal defects. Acta Orthop Scand. 1982;53:857–65. doi: 10.3109/17453678208992839. [DOI] [PubMed] [Google Scholar]
- 16.Tuli SM, Gupta KB. Bridging of large chronic osteoperiosteal gaps by allogeneic decalcified bone matrix implants in rabbits. J Trauma. 1981;21:894–8. doi: 10.1097/00005373-198110000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Liu Z, Li M. Treatment of nonunion in childhood by allogeneic decalcified bone graft. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2000;14:96–8. [PubMed] [Google Scholar]
- 18.Wang H. Study of antibody against protein in human allografts of decalcified bones. Zhonghua Wai Ke Za Zhi. 1993;31:177–80. [PubMed] [Google Scholar]
- 19.Garg M, Dev G, Tuli SM, Kumar S. Immunocellular responses of bone grafts in humans – A Fine Needle Aspiration Study. Indian J Orthop. 2000;34:135–7. [Google Scholar]