Abstract
Background:
Surgical reconstruction of segmental skeletal defects represents a true challenge for the orthopedic surgeons. Recently, Masquelet et al. described a two-stage technique for reconstruction of bone defects, known as the induced membrane technique. The aim of this study is to assess the results of the induced membrane technique in the management of segmental skeletal defects resulting from debridement of bone infection.
Materials and Methods:
Seventeen patients with segmental skeletal defects were treated in our institution by the induced membrane technique. The average age of the patients was 43 years (range 26- 58 years). The causes of the defects were infected gap nonunion in 12 cases and debridement of osteomyelitis in 5 cases. The defects were located in the tibia (n = 13) and the femur (n = 4). The mean defect was 7 cm (range 4 cm - 11 cm). All cases were treated by the induced membrane technique in two-stages.
Results:
Bone union happened in 14 patients. The limb length discrepancy did not exceed 2.5 cm in the healed cases. The mean time of healing was 10 months (range 6-19 months). The complications included nonunion of the graft in five cases, failure of graft maturation in two cases, reactivation of infection in two cases and refracture after removal of the frame in one case. These complications were managed during the course of treatment and they did not affect the final outcome in all patients except three.
Conclusion:
The induced membrane technique is a valid option for the management of segmental skeletal defects. It is a simple and straight forward procedure, but the time required for growth and maturation of the graft is relatively long.
Keywords: Bone defects, bone infection, induced membrane technique
Mesh terms: Bone diseases, infections, bone cements, membranes
INTRODUCTION
Segmental skeletal defects may result from high energy trauma, after debridement of osteomyelitis or tumor resection. Surgical reconstruction of these defects is one of the most difficult problems in orthopedic surgery. Many options are available for the management of such problem with varying degrees of success and failure.1,2,3
Recently, Masquelet et al.4,5 described a two-stage technique for reconstruction of bone defects. In the first stage, a cement spacer is placed in the bone defect. Its presence induces the formation of specialized tissue or membrane around it. In the second stage, the cement spacer is removed, and bone graft is placed within the tube of the induced membrane. This membrane has been found to be impermeable, hypervascular and biologically active as it secretes certain growth factors such as vascular endothelial growth factor and transforming growth factor-beta-1 and osteoinductive factors as bone morphogenetic protein-2. Thus, it prevents bone resorption and favors growth and maturation of the bone graft.6
It has been used in the management of posttraumatic and post tumor resection bone defects with good results.5,6,7,8
The aim of the present study is to assess the results of the induced membrane technique in the management of bone defects resulting from debridement of osteomyelitis and infected nonunion (INU).
MATERIALS AND METHODS
17 patients with segmental skeletal defects treated by the induced membrane technique between March 2009 and May 2012 were included in this prospective study. The inclusion criteria included all patients with INU or chronic osteomyelitis in which the bone defect is expected to be more than 4 cm after debridement. The exclusion criteria included: Limb length discrepancy (LLD) of more than 3 cm, patients with deficient soft tissue coverage and patients with reflex sympathetic dystrophy. The average age was 43 years (range 26-58 years). There were fifteen males and two females. The causes of the defects were infected nonunion in 12 cases and debridement of osteomyelitis in five cases. Of the 12 cases with INU 9 cases had infected implants and 3 cases had INU without internal fixation. The defects were located in the tibia in 13 cases and the femur in four cases. This study has been approved by the appropriate ethical committee and has therefore been performed in accordance with the pertinent ethical guidelines. All patients gave their informed consent prior to surgery. All cases were treated by the induced membrane technique in two-stages. In the first stage, the infected implants were removed if present and all the infected and necrotic bones were excised down to a healthy bleeding surface. Parts of the infected tissues were sent for culture and sensitivity in all patients. After radical debridement, the defects ranged from 4 cm to 11 cm with a mean of 7 cm. The resultant defects were filled with bone cement. The cement was applied in its doughy stage and it was not allowed to go inside the medulla to facilitate its removal later on, but it was allowed to cover the outer surface of bone ends for about half cm on each side [Figure 1a and b]. The skin and soft tissues were then closed over the cement spacer. No antibiotics were added to the bone cement. The bone was stabilized by Ilizarov external fixator in 13 cases and unilateral external fixator in four cases. The bone cement was left in place for an average of 2.5 months (range: 1–4 months) to induce the formation of a biological membrane around it. Antibiotics were given for 3 weeks according to the results of culture and sensitivity (1-week intravenous and 2 weeks oral). Laboratory investigations in the form of erythrocyte sedimentation rate, C-reactive protein and white blood cell count were done and the second stage surgery was done once they were within normal range.
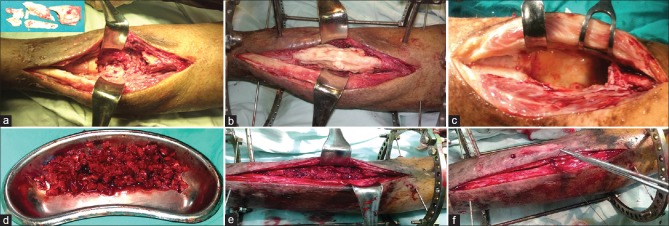
Figure 1.
Peroperative photographs showing (a) All the infected and necrotic bones are excised down to a healthy bleeding surface. (b) The resultant defect is filled with bone cement. (c) In the second stage of surgery, the cement spacer is removed and the bone ends are further debrided. (d) The graft is harvested and divided into small chips. (e) The gap is filled with the cancellus bone graft. (f) The tube of the membrane is closed over the graft
In the second stage, the bone cement spacer was removed and swab was taken from around the cement for culture and sensitivity. The bone ends were further debrided, the medullary canal was opened on each side and the gap was filled with cancellous bone graft. The graft was harvested from the iliac crest (unilateral in 9 cases and bilateral in 8 cases). Large amounts of the graft were taken according to the standard technique, was divided into small chips and put in the defect. Then the tube of the membrane was closed over it [Figure 1c–f]. Some adjustments of the frames were done in five cases to increase the stability of fixation. Any angular or rotational deformities were corrected before definitive fixation. Prophylactic antibiotics were started just before induction of anesthesia and continued for 1-week after surgery according to the results of the previous cultures for each patient. The patients were allowed for partial weight bearing from the second postoperative day. They were discharged from the hospital on an average of 6 days after surgery and followed up regularly in the out-patient clinic. During followup, each patient was examined clinically for any wound problem or pin tract infection (PTI) and radiologically for the growth and maturation of the graft. Variation of compression and distraction was done in three cases to stimulate bone healing. The external fixator was removed after complete healing of the graft under general anesthesia. The leg was protected in cast for 3 weeks after removal of the frame.
RESULTS
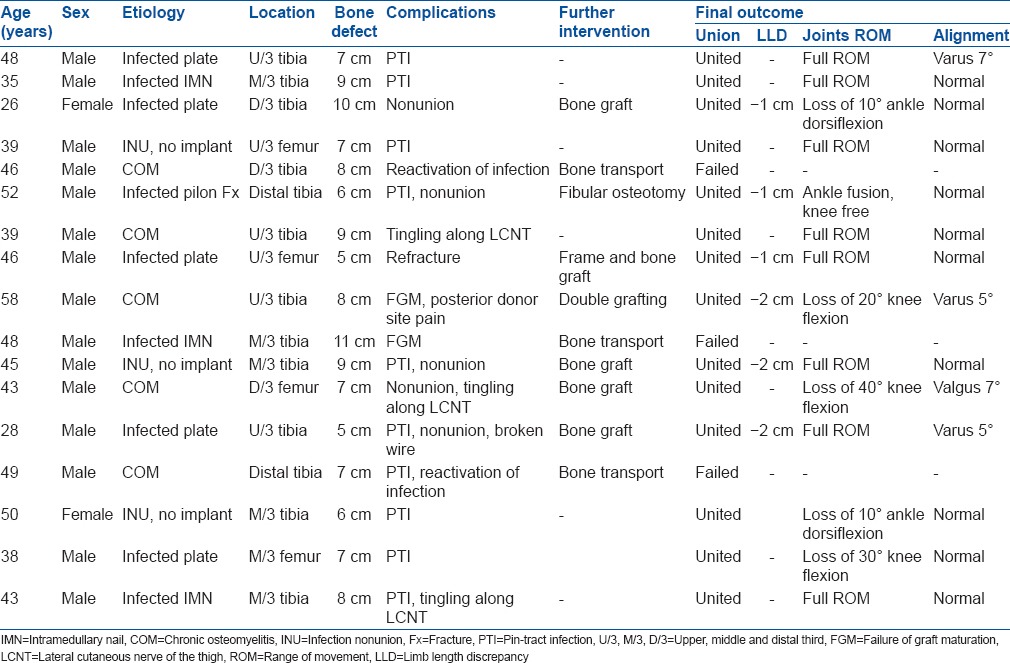
The hospital stay ranged from 1 to 5 days with an average of 3 days for the first stage and from 4 to 13 days with an average of 6 days for the second stage of surgery. The average followup was 23 months (range 14-38 months). Intraoperative tissue cultures taken during the first stage were positive in all patients [(Staphylococcus aureus (n = 5), Staphylococcus epidermidis (n = 4) and mixed polymicrobial infection (n = 8)]. Infection was controlled after the first stage of surgery and the intra-operative tissue cultures taken during the second stage of surgery were negative in all patients. The mean time of healing was 10 months, calculated from the second stage of surgery (range 6-19 months). The bigger the defect, the longer the time of treatment. The external fixation index which is the time of external fixation in months (calculated from the first stage of surgery) per each cm of the defect ranged from 1.6 to 2.1 with an average of 1.8 months/cm. The time to full weight bearing with the frame ranged from 1-month to 4 months with an average of 1.5 months. By the end of treatment 14 cases (82%) united and three cases (18%) failed to unite due to reactivation of infection in 2 cases and failure of graft maturation in one case. Of these 14 cases; eight healed without additional surgical procedures after the second stage while six cases required additional procedures to complete the process of bone healing [Figures 2 and 3]. This included regrafting (n = 5) and osteotomy of the fibula (n = 1).
Figure 2.
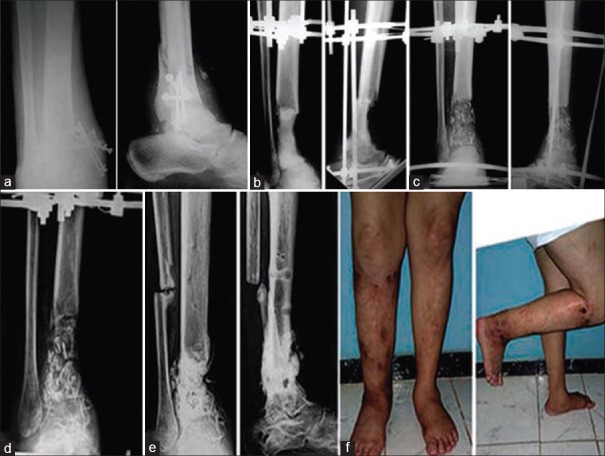
(a) Radiograph of thigh anteroposterior view after the first stage of surgery in 38-year-old male patient with infected non united fracture of the shaft femur (b) The radiograph after the second stage (c) During healing of the graft (d) After removal of the frame with good healing of the graft (e) The patient with good alignment and good range of knee movements
Figure 3.
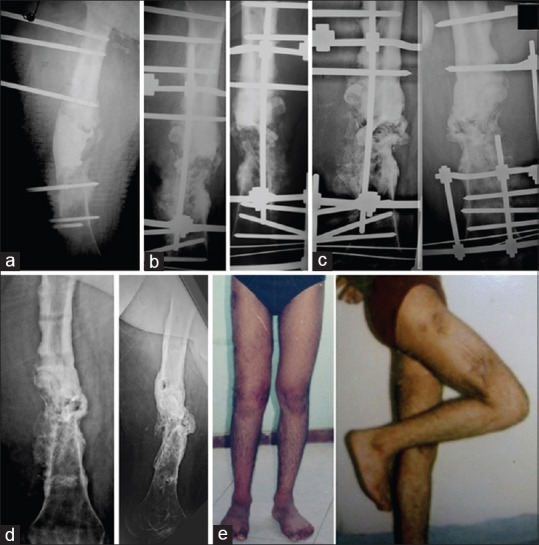
(a) Radiograph of leg bones with ankle anteroposterior and lateral views of a 52-year-old male patient with infected non united pilon fracture with implant failure. (b) The radiograph after debridement and bone cement spacer. (c) The early postoperative radiograph after the second stage of surgery. (d) Radiograph 7 months after the second stage, the graft united up and down but failed to unite at its middle so, osteotomy of the fibula was decided. (e) After removal of the frame with good healing of the graft. (f) The patient with good alignment and stable limb
The LLD did not exceed 2.5 cm and the angular deformity did not exceed 10° of angulation in the healed cases. Ankle fusion was intended in 2 cases due to distal tibial affection. Two cases lost about 10° of ankle dorsiflexion and 3 cases lost the last 20–40° of knee flexion as compared to the normal side. The hip range of movement was not affected in any of our patients. The complications were divided into two groups: Fixator related complications and graft related complications. The fixator related complications included PTI in 10 cases (were treated by local care and systemic antibiotics) and breakage of the wire in one case (was treated by removal of the broken wire). The graft related complications included nonunion of the graft in five cases (three at the middle of the bone graft, one at the upper end and one at the lower end of the graft), failure of graft maturation in two cases, resorption of the graft due to reactivation of infection in two cases and refracture after removal of the frame in one case. The donor site morbidities included transient tingling along the course of the lateral cutaneous nerve of the thigh in 2 cases and chronic pain at the site of the posterior iliac bone graft in one case. Most of these complications were managed during the course of treatment and they did not affect the final outcome in all patients except three [Table 1]. No cases were complicated by reflex sympathetic dystrophy.
Table 1.
Data of the patients
DISCUSSION
Surgical reconstruction of skeletal defects represents a true challenge for the orthopedic surgeons. Autogenous bone graft is considered to be the hallmark in management of segmental skeletal defects, but it carries the risk of certain disadvantages like graft rejection and the donor site morbidity in the form of pain and infection.1 Distraction osteogenesis developed by Ilizarov in the fifties of the last century has been used for treatment of bone defects. It is considered to be the gold standard for the management of bone defects, but, it requires specialized equipments, has a steep learning curve, and is plagued by many complications such as PTI, nonunion, joint stiffness, chronic edema and refractures after frame removal.2,9,10 The rapid advances in microvascular technology have allowed reconstruction of large bone defects with vascularized bone graft, but the procedure is technically demanding, time consuming and may be associated with major complications.3
The induced membrane technique is a relatively new technique described by Masquele et al.4 used for management of bone defects with good results. The membrane provides a biological chamber that prevents resorption of the graft, acts as a barrier to outward diffusion of the growth and osteoinductive factors and provides a source of stem cells and vascular cells that support revascularization and osseous consolidation.4,6,11
In our study, we used the induced membrane technique to manage segmental skeletal defects resulting from debridement of bone infection and we used the external fixator to stabilize the bone segments. Thorough debridement and short course of antibiotics were sufficient to control the infection after the first stage of surgery in all patients. We did not add antibiotics to the bone cement according to the recommendation of Masquelet et al.4 in order not to mask mild infection. There is a controversy about the timing of the second stage of surgery. In our study, the time between the first and second stage ranged from 1 to 4 months with an average of 2.5 months. We were guided by the negative results of the infection profile. Aho et al.12 in a recent study found that the membrane becomes well formed and reaches the top of its biological activity after 1 month from implantation of the cement spacer.
Autogenous bone graft was taken from the iliac crest either unilateral or bilateral and it was sufficient to fill the defects in all of our cases. In big defects allograft or synthetic bone graft may be required to augment the autograft.7,8 The time of bone healing in our study ranged from 6 to 19 months with a mean of 10 months. This is relatively longer than the mean time to bone union in the series reported by Chotel et al.8 who used the same technique for the treatment of bone defects after tumor resection with a mean time to bone union of 4.8 months. This may be related to the difference in the age group between their patients (children) and our patients (adults). The average external fixation index in our study was 1.8 months per each cm of the defect. This is relatively longer than the average external fixation index in cases managed by distraction osteogenesis that was reported to be less than 1.5 months/cm in most of the literatures.13,14,15 Although the long external fixation time, the induced membrane has some advantages over the distraction osteogenesis, which include: The Ilizarov frame is used in the induced membrane technique as an external fixator only and just to stabilize the bone segment. There is no lengthening so, there is no pain from distraction and no risk of joint contracture. No turning of the nuts that require special patient's compliance. Also, a simple frame could be used instead of the complex frame required for bone transport.
The rate of union in our study was 82% (14 out of 17). Eight patients healed without additional surgical procedures after the second stage. Additional procedures were required mainly in patients with big defects (7 cm or more) and in patients with a relatively old age (more than 50 years old). The main cause of failure was the reactivation of infection. Three cases with tibial bone defects failed to unite due to reactivation of infection and massive resorption of the graft in two patients and failure of graft maturation in the third case. A plan of treatment was changed in those three patients into bone transport by Ilizarov external fixator.
Some authors used internal fixation in the induced membrane technique. It has the advantages of being an easy surgery, and it is more comfortable for the patient, but it carries the risk of reactivation of infection and implant failure.16,17 Apard et al.17 reported on 12 cases treated by the induced membrane technique and fixed by intramedullary nailing. Five cases were complicated by infection (41%) and the nail was broken in another case. In our study, we used the external fixator to stabilize the bone for many reasons. First, to reduce the possibility of reactivation of infection as all of our cases were previously infected. Second, to allow for variation of compression and distraction to stimulate bone healing. Third, to allow for early weight bearing with the frame. Also, the use of external fixator could allow for easy change of the plan of treatment in case of graft resorption into bone transport by the same frame or with some modifications.
The complications in our study are numerous because we are dealing with a very difficult group of patients in orthopedic surgery (infection with bone loss). Most of these complications were managed during the course of treatment. Nonunion of the graft was the most common graft related complications in our study. It occurred in five of our cases (29%). Four of them were treated by freshening of the bone ends and regrafting of the nonunion site, and the other case was treated by osteotomy of the fibula and walking with the dynamized frame. On the second look during regrafting the membrane was not well seen, but the active biological chamber was evident with high vascularity and areas of new bone formation [Figure 4]. Failure of graft maturation was considered when there was no progress on the serial radiographs for 3 successive months. This happened in 2 of our cases. One of them required double grafting and the other one was treated by corticotomy of the tibia and segment transport to compress the graft gradually and stimulate its healing. Refracture after frame removal occurred in one of our cases. It took place at the middle third of the graft about 4 months after frame removal due to exposure to mild trauma. He was managed by reapplication of the frame and bone graft.
Figure 4.
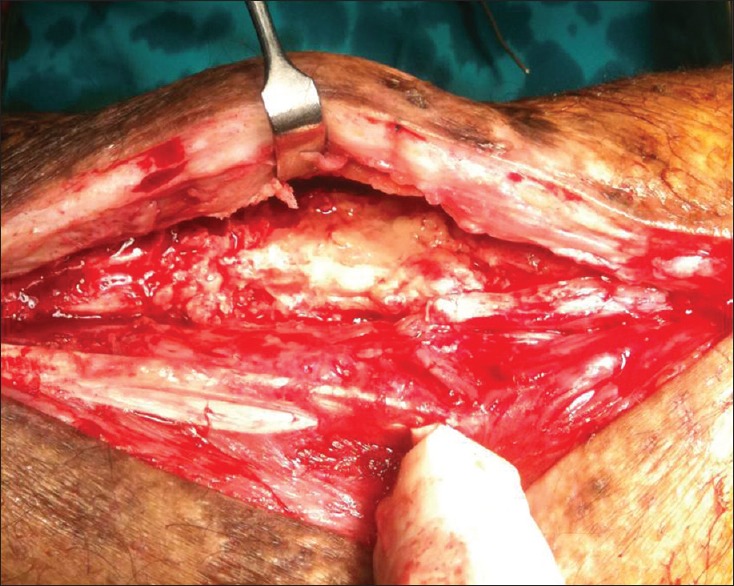
On the second look, the active biological chamber is evident with high vascularity and areas of new bone formation
The time required for growth and maturation of the graft is relatively long. Nonunion may develop at the middle or at each end of the graft. Multiple procedures may be required to complete the process of bone healing. Alteration of the grafting technique may be required to overcome these problems. A limitation in our study is the small number of cases.
In conclusion the induced membrane technique is a valid option for the management of bone defects after debridement of bone infection. It is a simple and straightforward procedure.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.DeCoster TA, Gehlert RJ, Mikola EA, Pirela-Cruz MA. Management of posttraumatic segmental bone defects. J Am Acad Orthop Surg. 2004;12:28–38. doi: 10.5435/00124635-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Ilizarov GA, Ledyaev VI. The replacement of long tubular bone defects by lengthening distraction osteotomy of one of the fragments 1969. Clin Orthop Relat Res. 1992:7–10. [PubMed] [Google Scholar]
- 3.Levin LS. Vascularized fibula graft for the traumatically induced long-bone defect. J Am Acad Orthop Surg. 2006;14:S175–6. doi: 10.5435/00124635-200600001-00038. [DOI] [PubMed] [Google Scholar]
- 4.Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet. 2000;45:346–53. [PubMed] [Google Scholar]
- 5.Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41:27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Pelissier P, Masquelet AC, Bareille R, Pelissier SM, Amedee J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res. 2004;22:73–9. doi: 10.1016/S0736-0266(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 7.Karger C, Kishi T, Schneider L, Fitoussi F, Masquelet AC. French Society of Orthopaedic Surgery and Traumatology (SoFCOT). Treatment of posttraumatic bone defects by the induced membrane technique. Orthop Traumatol Surg Res. 2012;98:97–102. doi: 10.1016/j.otsr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Chotel F, Nguiabanda L, Braillon P, Kohler R, Bérard J, Abelin-Genevois K. Induced membrane technique for reconstruction after bone tumor resection in children: A preliminary study. Orthop Traumatol Surg Res. 2012;98:301–8. doi: 10.1016/j.otsr.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Dahl MT, Gulli B, Berg T. Complications of limb lengthening. A learning curve. Clin Orthop Relat Res. 1994:10–8. [PubMed] [Google Scholar]
- 10.Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990:81–104. [PubMed] [Google Scholar]
- 11.Viateau V, Guillemin G, Calando Y, Logeart D, Oudina K, Sedel L, et al. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defects: An ovine model. Vet Surg. 2006;35:445–52. doi: 10.1111/j.1532-950X.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 12.Aho OM, Lehenkari P, Ristiniemi J, Lehtonen S, Risteli J, Leskelä HV. The mechanism of action of induced membranes in bone repair. J Bone Joint Surg Am. 2013;95:597–604. doi: 10.2106/JBJS.L.00310. [DOI] [PubMed] [Google Scholar]
- 13.Polyzois D, Papachristou G, Kotsiopoulos K, Plessas S. Treatment of tibial and femoral bone loss by distraction osteogenesis. Experience in 28 infected and 14 clean cases. Acta Orthop Scand Suppl. 1997;275:84–8. doi: 10.1080/17453674.1997.11744753. [DOI] [PubMed] [Google Scholar]
- 14.Song HR, Cho SH, Koo KH, Jeong ST, Park YJ, Ko JH. Tibial bone defects treated by internal bone transport using the Ilizarov method. Int Orthop. 1998;22:293–7. doi: 10.1007/s002640050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Megas P, Saridis A, Kouzelis A, Kallivokas A, Mylonas S, Tyllianakis M. The treatment of infected nonunion of the tibia following intramedullary nailing by the Ilizarov method. Injury. 2010;41:294–9. doi: 10.1016/j.injury.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Woon CY, Chong KW, Wong MK. Induced membranes – A staged technique of bone-grafting for segmental bone loss: A report of two cases and a literature review. J Bone Joint Surg Am. 2010;92:196–201. doi: 10.2106/JBJS.I.00273. [DOI] [PubMed] [Google Scholar]
- 17.Apard T, Bigorre N, Cronier P, Duteille F, Bizot P, Massin P. Two-stage reconstruction of posttraumatic segmental tibia bone loss with nailing. Orthop Traumatol Surg Res. 2010;96:549–53. doi: 10.1016/j.otsr.2010.02.010. [DOI] [PubMed] [Google Scholar]