Abstract
Background:
Bone grafts are required to fill a cavity created after curettage of benign lytic lesions of the bone. To avoid the problems associated at donor site with autologous bone graft, we require allograft or bone graft substitutes. We evaluated the healing of lytic lesions after hydroxyapatite (HA) grafting by serial radiographs.
Materials and Methods:
Forty cases of benign lytic lesions of bone were managed by simple curettage and grafting using HA blocks. Commercially available HA of bovine origin (Surgiwear Ltd., Shahjahanpur, India) was used for this purpose. Mean duration of followup was 34.8 months (range 12–84 months). Mean patient age was 19.05 years (range 3–55 years). Radiological staging of graft incorporation was done as per criteria of Irwin et al. 2001.
Results:
In our series, two cases were in stage I. A total of 11 cases were in stage II and 27 were in stage III. Graft incorporation was radiologically complete by 15 months. Clinical recovery was observed before radiological healing. The average time taken to return to preoperative function was 3 months. Recurrence was observed in giant cell tumor (n = 3) and chondromyxoid fibroma (n = 1). There was no incidence of graft rejection, collapse, growth plate disturbances or antigenic response.
Conclusions:
We conclude that calcium HA is biologically acceptable bone graft substitute in the management of benign lytic lesions of bone.
Keywords: Benign lytic lesions of bone, bone graft substitute, hydroxyapatite crystals
Mesh terms: Grafts, bone neoplasms, hydroxyapatite
INTRODUCTION
Benign lytic lesions of bone include two broad groups - one which does not behave aggressively and the other which does. The first category includes simple bone cyst (SBC), aneurysmal bone cyst (ABC), fibrous dysplasia (FD), nonossifying fibroma, brown's tumor of hyperparathyroidism, etc. The second category of locally aggressive lesions which can be considered to be on the borderline between benign and malignant includes - giant cell tumor (GCT), chondromyxoid fibroma (CMF), chondroblastoma, osteoblastoma and Langerhan's cell histiocytosis. In the treatment of benign lytic lesions of bone by curettage and filling of void by some filler, traditionally autologous bone graft has been used. Filling is done to hasten healing.1,2 Owing to its osteoconductive, osteoinductive and osteogenic potential, the autologous bone graft is considered as the gold standard.3,4
Search for an ideal bone graft substitute has long been in existence because of the problems associated with the gold-standard autologous bone graft and the allografts.1,5,6,7,8 Synthetic bone graft substitutes are considered devoid of such problems. They are not associated with donor site morbidity, prolongation of surgery, immunogenicity, disease transmission, or demand supply mismatch. However, these materials have osteoconductive properties primarily and none is ideal. Calcium based materials have been most commonly used as bone graft substitutes.9,10
Calcium hydroxyapatite (HA) has been shown in a number of series to be a useful biocompatible osteoconductive material, which provide scaffold for bone in growth. Calcium HA can be obtained from natural sources as well as from a synthetic processes. Natural HA may be coral based, obtained from exoskeleton of marine species goniospora or can be of bovine origin. Synthetic HA is formed by the precipitation of calcium nitrate and ammonium-dihydrogen phosphate with a chemical formula Ca10 (PO4)6 (OH)2. Usefulness of HA as a bone graft substitute is determined by its pore diameter and interconnectivity. Ca-P ratio, particle, and pore size vary from product to product. Minimum pore size of 100 μm (preferably 150–200 μm) is optimal for bone in-growth. The pore size of HA is in the similar diameter. We used HA blocks which have a porous structure with interconnected holes. It is derived by sintering the bovine bone at very high temperature of +500°C. At such high temperature, the risk of disease transmission is negligible.
We conducted a study to evaluate the healing of lytic lesions after HA grafting as demonstrated by serial radiographs. The effectiveness of this material in managing the bone voids created after curettage of benign lytic lesions of bone was also studied.
MATERIALS AND METHODS
40 consecutive patients with benign lytic lesions of bone, managed by curettage and filling up of cavity with HA blocks between 2006 and 2012 were included in this prospective study. Inclusion criteria were benign lytic lesions of bone with or without pathological fractures.
The exclusion criteria were: (1) Active infection (2) Suspected or diagnosed malignant lesion (3) Traumatic bone loss (4) Very large tumor volume.
Histopathological examination of curetted material was done routinely. It was done preoperatively in the form of fine needle aspiration cytology (FNAC). But FNAC has its own drawbacks in the form of sampling error, etc. If FNAC was not possible as when cells could not be aspirated, we went for needle core biopsy. To reach a definitive diagnosis, we subjected the curetted material for histopathology postoperatively [Figure 1]. Patients were followed up on the basis of X-rays. Patients with a minimum followup of 6 months were included in the study. Some patients had followup and clinical outcome of more than 7 years. We used HA blocks of following sizes: 1 cm × 0.5 cm × 0.5 cm, 1 cm × 1 cm × 2 cm and 1 cm × 1.5 cm × 2 cm depending on the size of cavity.
Figure 1.
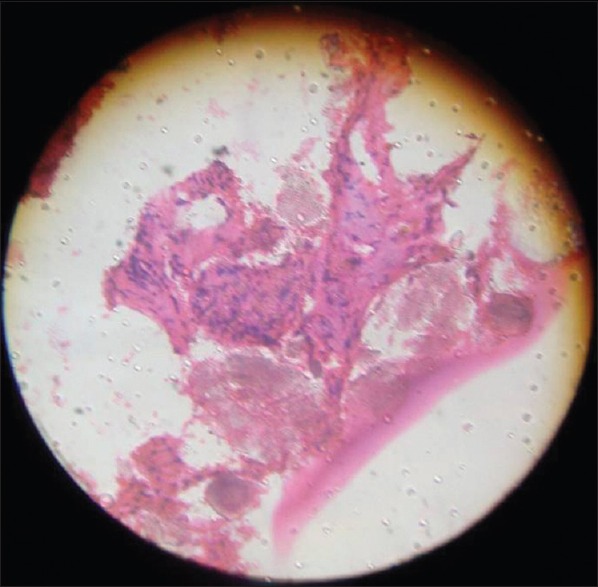
Histopatholgical picture showing new osteoid formation and presence of hydroxyapatite at the grafted area: This also shows ingrowth of osteoid (pink dotted) around hydroxyapatite crystals (dark and dense)
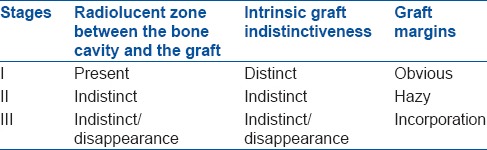
After taking informed written consent, the patient was operated using standard approaches and principles. Cortical window of a size equal to the largest dimension of the lytic lesion was created over the lesion. The cavity and the walls were thoroughly curetted to remove all the tumor tissue. The shape and size of HA blocks were modified according to the need. After curettage, the cavity was treated with hydrogen peroxide,11 thus making curettage an extended one. No other adjuvant12,13 was used to treat the cavity. Defect was packed completely with HA blocks, and the periosteum was opposed. No autologous bone graft or bone marrow aspirate was mixed with HA. Wherever periosteal coverage was not sufficient, the soft tissues were opposed to cover the grafted material. Every attempt was made to prevent granules from spilling into the subcutaneous tissue. Internal fixation was not done for immobilization except in one case. Postoperative immobilization was individualized according to site and size of the lesion in the form of plaster cast or external fixation. All the patients were protected from weight bearing for 6–16 weeks. Regular postoperative followup was done clinically and radiologically at 0, 1, 3, 6, 12, 18 months and annually thereafter. The following observations were made: (a) Healing of fracture site (b) Architecture of amorphous HA blocks which include margin definition (c) Number of surfaces in contact with the surrounding bone (which correlated with osteointegration) (d) Radiological evaluation of graft incorporation was done according to the criterion of Irwin et al. (2001) [Table 1].14
Table 1.
Radiological stages of graft incorporation
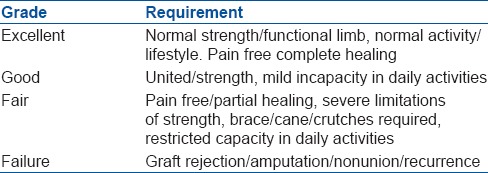
Functional assessment was done on the basis of criteria shown in Table 2.
Table 2.
Functional assessment criteria used in our study
RESULTS
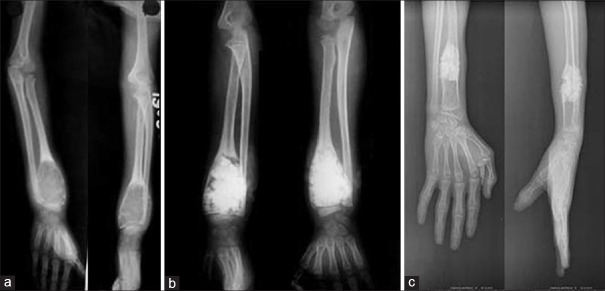
The mean age of patients was 19.05 years (range 3-58 years). The type of lesions were simple bone cyst (n = 12), giant cell tumor (n = 12), aneurysmal bone cyst (n = 7), fibrous dysplasia (n = 6), eosinophilic granuloma (n = 1), chondromyxoid fibroma (n = 1) and osteoblastoma (n = 1) [Figures 2 and 3]. The average period of followup was 34.8 months (range 12 to 90 months) [Table 3].
Figure 2.
X-ray of forearm anteroposterior and lateral views showing (a) Aneurysmal bone cyst of the distal end of left radial metaphysis close to epiphysis in an 8 year male (b) Cavity filled with hydroxyapatite after curettage (c) that growth is evident and intactness of growth plate at 7 years followup
Figure 3A.
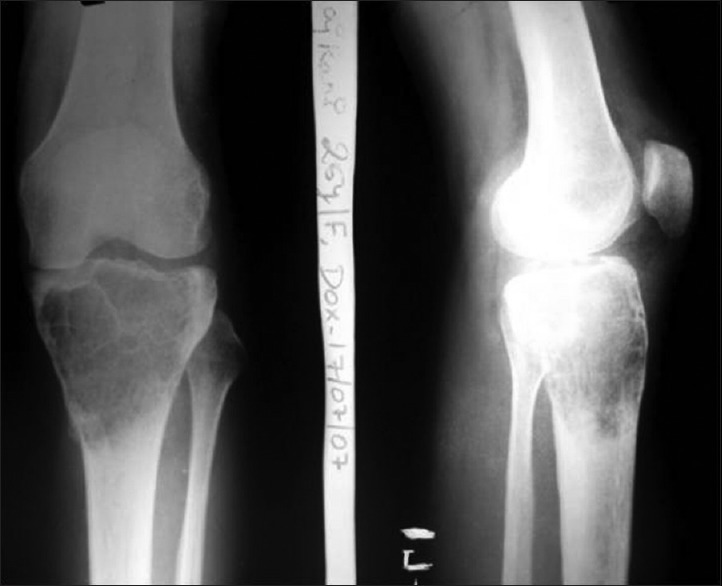
X-ray of knee joint anteroposterior and lateral views showing giant cell tumour of right proximal tibia in a 25 year old female
Table 3.
Clinical details of the patients
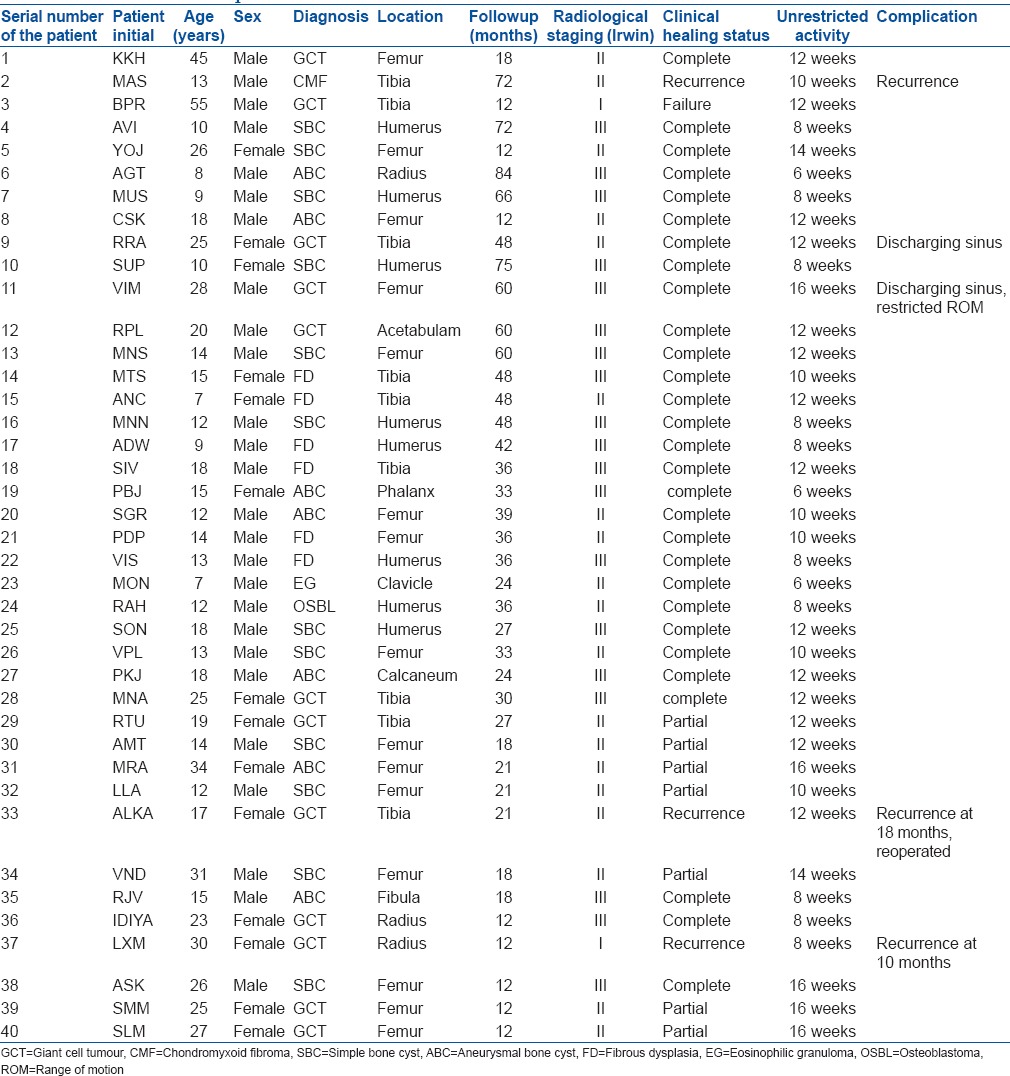
Figure 3B.
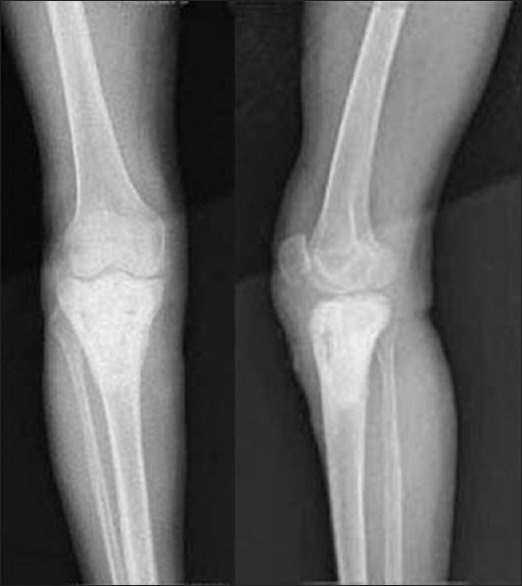
X-ray anteroposterior and lateral views showing followup at 3 years following curettage and grafting by HA crystals
Of our 40 cases, 26 patients were male and 14 were female. Femur (n = 15) was most common site involved followed by tibia (n = 9) and the humerus (n = 8). Radius (n = 3) was the fourth commonest site. There were one lytic lesion each of fibula, clavicle, calcaneum, phalynx and the acetabulum.
All of our cases came with complaints of pain and swelling. Pathological fracture was found in 27.5% (n = 11) cases.
One patient died due to a reason not related to the bone grafting procedure or the disease (myocardial infarction). Recurrences were recorded in four cases; three cases of GCT and 1 of CMF. One patient of GCT had amputation at 12 months followup due to recurrence and progressive enlargement of the affected part. Repeat curettage and HA grafting were done in one case of GCT at 18 months followup, and she is currently doing well. Third patient was lost to followup after 12 months. A total of three patients were lost to followup at 12 months after the operation at which time 2 were asymptomatic while 1 had recurrence. Of the rest 37 cases, five cases were in Irwin stage 1, 13 were in Irwin stage II and 19 were in Irwin stage III [Table 4].
Table 4.
Results of the study at final followup in terms of radiological and functional outcome
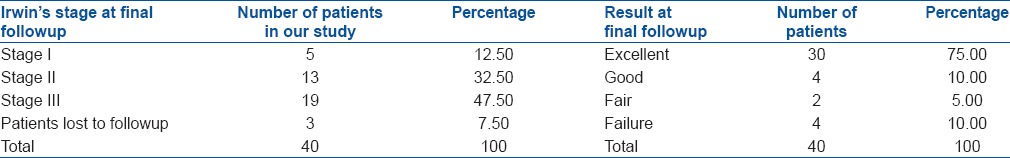
In maximal of 12 months followup, all cases showed very good (combined Irwin grade II and III in different areas) incorporation of HA crystals to host bone.
Local recurrence occurred in 10.0% cases (n = 4). Infection rate was 7.5% (n = 3). Deformity occurred 2.0% cases (n = 1). No case of graft rejection, graft collapse or nonunion was found. Knee joint motion was restricted in 5.0% cases (n = 2). Extravasation of graft occurred in 5.0% (n = 2) cases at the time of operation which ultimately got incorporated with the main graft [Tables 3 and 4].
DISCUSSION
The use of available synthetic bone graft substitutes is rapidly increasing and it is hoped that transplantation of bone from donors will one day become obsolete. HA has low density ultraporous structure with osteoconductive properties. The three dimensional structure provides scaffolding for bone in-growth. The ultrastructure allows migration of osteoblasts, fibroblasts and osteoclasts along with unobstructed flow of nutrients and fluid.
For excellent incorporation to occur, the graft must be guarded from excessive external load until clinical recovery by either internal fixation or external splints. It should be in close opposition to the viable host bone.
Patients treated with HA grafting have bone formation period of 4–6 months. Smaller lesions like SBC of proximal humerus show signs of complete healing clinically and radiologically at 3 months while larger lesions like GCT of the proximal tibia show healing at 12 months. HA blocks could be still traced in X-rays. In the study of Yamamoto et al.,15 mean period for bone formation was 4.2 months. In the study of Reddy and Swamy,16 bone formation was seen in all cases by 4–6 weeks. In cavitary lesions of hand bones, HA incorporation was complete by 3 months, but in larger lesions, there was partial incorporation even after 2–3 years. For patients under 19 years of age, it was 3.2 months. In our study, we observed that in a single case of phalanx of finger, the HA crystals were still surrounded by aneurysmal bone cyst of radiolucent zone at 15 months of followup. However, the interconnection of the HA blocks was found at 6 months followup. In this case, at latest followup at 33 months, the radiolucent zone completely disappeared. Schindler et al.17 found in their study that 3–24 months after operation, bone density increased and tumor demarcation disappeared, indicating osseous integration and consolidation of the graft filled space.
Some lesions like FDs cause weakening of the affected bone and hence use of cortical strut grafts has been described in the literature. In cases of extensive disease, we did use the same for immediate structural strength. We have excluded large lesions of FD in which we had to use structural graft from our study based on the exclusion criteria. Only those lesions which were well contained and relatively small were subjected to bone grafting by HA alone.
In our series, we observed recurrence in giant cell tumors in 3 out of 12 cases while recurrence was noticed in the only case of CMF. All the cases of GCT with recurrence were Campanacci grade II at the time of presentation. In the study of Uchida et al.,8 no local recurrence of tumor was seen. In the study of Yamamoto et al.,15 of 75 patients, there were three recurrences (1 in giant cell tumor, 1 in FD, and 1 in Langerhans cell histiocytoma). Reddy and Swamy16 also did not notice any recurrence. In Schindler et al.17 study of 13 patients, 2 had recurrence. Recurrence is more likely dependent on the aggressiveness of the lesion, thoroughness of the curettage and the effectiveness of any adjuvant agent used prior to implantation of graft material. We did not use any adjuvant other than hydrogen peroxide prior to HA graft implantation.
Of three cases of infection, causative organism was Staphylococcus aureus in 2 cases while Mycobacterium tuberculosis was detected in one case. The infection rate is comparable to the series of Reddy and Swamy (one case),16 Saikia et al. (3 out of 24).18 Chance of infection appear less with HA as compared to allograft. All the cases of infections developed chronic discharging sinus. All three infected cases were of GCT. Similarity between them was that they were large lesion. Reason for postoperative discharge may be inadequate filling of gap after curetting the cavity. The dead space thus formed may provide shelter to infection.
In our series, we did not find any adverse reaction to HA such as excessive postoperative drainage, erythema, immunogenic reaction or other wound problems. Studies of Reddy and Swamy,16 Natarajan et al.,19 Yamamoto et al.15 and Uchida et al.8 supported the fact that there is no reaction to HA material.
Of the three patients in whom restriction in the range of movement was found, the cause appeared to be related to chronic infection in two patients leading to muscle/soft tissue contracture near the joint. In one patient with lesion in phalanx, the range of motion was restricted prior to the treatment and no further deterioration was noted. Yamamoto et al.,16 in their study, found that three patients with lesion in finger had a slight restriction in adjacent joint. No other patient had restriction of movements. Saikia et al.18 in their study found that all the patients attained a range of movement comparable to or better than the preoperative range.
In two of our cases, extravasation of HA material occurred at the time of grafting probably because of inadequate window closure. But in both the cases the extravasated material started disappearing within 6 months postoperatively and disappeared completely from soft tissue surroundings in 1-year followup radiographs. Natarajan et al.19 observed extravasation in 1 of 23 cases in their series.
Clinical recovery occurred in upper limb cases at an average 7.7 months (range 6–12 months) while in lower limb cases at an average 12.3 months (range 8–16 months). The clinical recovery observed before the radiological recovery in our series. All was the patients were able to bear weight without pain at 3 months followup. After 3–5 months, HA graft showed an increase in density with indistinct margins. Our radiological results are comparable to other series.20,21,22,23,24
All the pathological fractures in the vicinity of lytic lesions united in a maximum of 3 months postoperatively. Radiolucent zone around the HA crystals tends to decrease with time. Blocks and granules of HA crystals seem to become attached to each other. These findings were interpreted as evidence of bone regeneration around and within the HA crystals.25,26,27,28 HA crystals resorption and biodegradation is a very slow process. In 6 years followup very little HA crystals were absorbed. Yamaguchi et al.29 also found the degradation-resistant character of synthetic HA blocks.
Though it is difficult to claim excellent results with less number of cases and short followup, the results of this study are comparable to previous studies. From our study, we have concluded that HA is free from the risk of immunogenic reaction, disease transmission or donor site morbidity; complications are very less and if any, can be managed easily. The HA has excellent biocompatibility and provides right scaffolding for in-growth of bone forming tissue and thus ultimately gets well incorporated with the host bone. HA is a safe and convenient alternative to allograft and autograft as an implant material which aids in regeneration of bone in the defects produced by curettage of benign lytic bone lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Greis PE, Hankin FM. Eosinophilic granuloma. The management of solitary lesions of bone. Clin Orthop Relat Res. 1990;257:204–11. [PubMed] [Google Scholar]
- 2.Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol. 2006;29:96–9. doi: 10.1097/01.coc.0000195089.11620.b7. [DOI] [PubMed] [Google Scholar]
- 3.Sponer P, Urban K. Treatment of juvenile bone cysts by curettage and filling of the cavity with BAS-0 bioactive glass-ceramic material. Acta Chir Orthop Traumatol Cech. 2004;71:214–9. [PubMed] [Google Scholar]
- 4.Van Heest A, Swiontkowski M. Bone-graft substitutes. Lancet. 1999;353(Suppl 1):SI28–9. doi: 10.1016/s0140-6736(99)90228-3. [DOI] [PubMed] [Google Scholar]
- 5.Keating JF, McQueen MM. Substitutes for autologous bone graft in orthopaedic trauma. J Bone Joint Surg Br. 2001;83:3–8. doi: 10.1302/0301-620x.83b1.11952. [DOI] [PubMed] [Google Scholar]
- 6.Inoue O, Ibaraki K, Shimabukuro H, Shingaki Y. Packing with high-porosity hydroxyapatite cubes alone for the treatment of simple bone cyst. Clin Orthop Relat Res. 1993;293:287–92. [PubMed] [Google Scholar]
- 7.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–5. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Uchida A, Araki N, Shinto Y, Yoshikawa H, Kurisaki E, Ono K. The use of calcium hydroxyapatite ceramic in bone tumour surgery. J Bone Joint Surg Br. 1990;72:298–302. doi: 10.1302/0301-620X.72B2.2155908. [DOI] [PubMed] [Google Scholar]
- 9.Dreesman H. Uber knochenplombierung. Beitr Klin Chir. 1892;9:804–10. [Google Scholar]
- 10.Peltier LF, Jones RH. Treatment of unicameral bone cysts by curettage and packing with plaster-of-Paris pellets. J Bone Joint Surg Am. 1978;60:820–2. [PubMed] [Google Scholar]
- 11.Nicholson NC, Ramp WK, Kneisl JS, Kaysinger KK. Hydrogen peroxide inhibits giant cell tumor and osteoblast metabolism in vitro . Clin Orthop Relat Res. 1998;347:250–60. [PubMed] [Google Scholar]
- 12.Rock M. Adjuvant management of benign tumors; basic concepts of phenol and cement use. Chir Organi Mov. 1990;75:195–7. [PubMed] [Google Scholar]
- 13.Lane JM. Liquid nitrogen as an adjunct. Chir Organi Mov. 1990;75(Suppl 1):S198–9. [PubMed] [Google Scholar]
- 14.Irwin RB, Bernhard M, Biddinger A. Coralline hydroxyapatite as bone substitute in orthopedic oncology. Am J Orthop (Belle Mead NJ) 2001;30:544–50. [PubMed] [Google Scholar]
- 15.Yamamoto T, Onga T, Marui T, Mizuno K. Use of hydroxyapatite to fill cavities after excision of benign bone tumours. Clinical results. J Bone Joint Surg Br. 2000;82:1117–20. doi: 10.1302/0301-620x.82b8.11194. [DOI] [PubMed] [Google Scholar]
- 16.Reddy R, Swamy M. The use of hydroxyapatite as a bone graft substitute in orthopaedic conditions. Indian J Orthop. 2005;39:52–4. [Google Scholar]
- 17.Schindler OS, Cannon SR, Briggs TW, Blunn GW. Composite ceramic bone graft substitute in the treatment of locally aggressive benign bone tumours. J Orthop Surg (Hong Kong) 2008;16:66–74. doi: 10.1177/230949900801600116. [DOI] [PubMed] [Google Scholar]
- 18.Saikia KC, Bhattacharya TD, Bhuyan SK, Talukdar DJ, Saikia SP, Jitesh P. Calcium phosphate ceramics as bone graft substitutes in filling bone tumor defects. Indian J Orthop. 2008;42:169–72. doi: 10.4103/0019-5413.39588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natarajan M, Dhanapal R, Kumaravel S, Selvaraj R, Uvaraj NR. The use of bovine calcium hydroxyapatite in filling defects following curettage of benign bone tumours. Indian J Orthop. 2003;37:192–4. [Google Scholar]
- 20.Agarwala S, Bhagwat A. Hydroxyapatite as a bone graft substitute: Use in cortical and cancellous bone. Indian J Orthop. 2005;39:254–6. [Google Scholar]
- 21.Agrillo U, Mastronardi L, Puzzilli F. Anterior cervical fusion with carbon fiber cage containing coralline hydroxyapatite: Preliminary observations in 45 consecutive cases of soft-disc herniation. J Neurosurg. 2002;96:273–6. doi: 10.3171/spi.2002.96.3.0273. [DOI] [PubMed] [Google Scholar]
- 22.Bansal S, Chauhan V, Sharma S, Maheshwari R, Juyal A, Raghuvanshi S. Evaluation of hydroxyapatite and beta-tricalcium phosphate mixed with bone marrow aspirate as a bone graft substitute for posterolateral spinal fusion. Indian J Orthop. 2009;43:234–9. doi: 10.4103/0019-5413.49387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucholz RW, Carlton A, Holmes RE. Hydroxyapatite and tricalcium phosphate bone graft substitutes. Orthop Clin North Am. 1987;18:323–34. [PubMed] [Google Scholar]
- 24.Helber MU, Ulrich C. Metaphyseal defect substitute: Hydroxylapatite ceramic. Results of a 3 to 4 year follow up. Unfallchirurg. 2000;103:749–53. doi: 10.1007/s001130050614. [DOI] [PubMed] [Google Scholar]
- 25.Tsai WC, Liao CJ, Wu CT, Liu CY, Lin SC, Young TH, et al. Clinical result of sintered bovine hydroxyapatite bone substitute: Analysis of the interface reaction between tissue and bone substitute. J Orthop Sci. 2010;15:223–32. doi: 10.1007/s00776-009-1441-9. [DOI] [PubMed] [Google Scholar]
- 26.Matsumine A, Myoui A, Kusuzaki K, Araki N, Seto M, Yoshikawa H, et al. Calcium hydroxyapatite ceramic implants in bone tumour surgery. A long term followup study. J Bone Joint Surg Br. 2004;86:719–25. doi: 10.1302/0301-620x.86b5.14242. [DOI] [PubMed] [Google Scholar]
- 27.Parikh SN. Bone graft substitutes: Past, present, future. J Postgrad Med. 2002;48:142–8. [PubMed] [Google Scholar]
- 28.Goto T, Kojima T, Iijima T, Yokokura S, Kawano H, Yamamoto A, et al. Resorption of synthetic porous hydroxyapatite and replacement by newly formed bone. J Orthop Sci. 2001;6:444–7. doi: 10.1007/s007760170013. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi K, Hirano T, Yoshida G, Iwasaki K. Degradation-resistant character of synthetic hydroxyapatite blocks filled in bone defects. Biomaterials. 1995;16:983–5. doi: 10.1016/0142-9612(95)94905-z. [DOI] [PubMed] [Google Scholar]