Abstract
Background:
Osteoarthritis (OA) is the most frequent chronic joint disease causing pain and disability. Recent reports have shown that statin may have the potential to inhibit osteoarthritis. This study of early stage OA developed in an experimental rabbit model, aimed to evaluate the chondroprotective effects of intraarticularly applied atorvastatin on cartilage tissue macroscopically and histopathologically by examining intracellular and extracellular changes by light and electron microscope.
Materials and Methods:
The experimental knee OA model was created by cutting the anterior cruciate ligament of the 20 mature New Zealand rabbits. The rabbits were randomly allocated into two groups of 10. Study group: The group that received intraarticular statin therapy; Control group: The group that did not receive any intraarticular statin therapy. The control group received an intraarticular administration of saline and the study group atorvastatin from the 1st week postoperatively, once a week for 3 weeks. The knee joints were removed including the femoral and tibial joint surfaces for light and electron microscopic studies of articular cartilages.
Results:
The mean total points obtained from the evaluation of the lesions that developed in the medial femoral condyle were 11.33 ± 0.667 for the control group and 1.5 ± 0.687 for the study group. The mean total points obtained from the evaluation of the lesions that developed in medial tibial plateau cartilage tissue were 11.56 ± 0.709 for the control group and 1.40 ± 0.618 for the study group. Electron microscopic evaluation revealed healthy cartilage tissue with appropriate chondrocyte and matrix structure in study group and impaired cartilage tissue in control group.
Conclusion:
Chondroprotective effect of statin on cartilage tissue was determined in this experimental OA model evaluated macroscopically and by light and electron microscope. There are some evidences to believe that the chondroprotective effect of the statin is that, by protecting the structure of the endoplasmic reticulum and the Golgi complex.
Keywords: Chondroprotective effect, electron microscope, experimental osteoarthritis, statin
Mesh terms: Osteoarthritis drug evaluation preclinical, osteoarthritis, knee
INTRODUCTION
The pathogenic mechanism of osteoarthritis (OA) is suggested as wearing and destruction of cartilage tissue due to ageing. However, it is described as an active and dynamic process with destruction and repair triggered by biochemical and mechanical factors, nowadays.1
The treatment of OA requires a combination of pharmacological and nonpharmacological therapy.2 The existing pharmacological methods are mostly acting as symptomatically but do not stop the progression of OA. Surgical techniques, such as arthroplasty and osteotomy, provide encouraging results when appropriate patients are selected and good surgical techniques are applied. However, as there are controversies, there are lot of studies for developing cartilage protecting agents for slowing the progress of OA or even reverse it on the cases where there are no surgical indications or in early-mid-stage primary OA. These studies are directed toward providing the balance between the formation and destruction of the chondrocyte and extracellular matrix of cartilage.3
Several epidemiological studies have shown that statins reduce cholesterol synthesis in hypercholesterolemia treatment by inhibiting the 3-hydroxy-3-methylglutaryl coenzyme-A enzyme in the liver and also reduce cardiovascular morbidity and mortality. Besides these effects, independent of the reduction in cholesterol, there is also a positive effect on endothelial functions, thrombocyte functions, vascular inflammation, and the formation of atherosclerotic plaque. All these effects are said to be pleiotropic effects. In recent years, these effects of statins have been the subject of many clinical and preclinical research studies.
We reviewed the literature and identified that the chondroprotective effects of statins using light microscopy.4,5 However, to the best of our knowledge, there are no studies examining cartilage protective effects using an electron microscopy. This study evaluates the cartilage protective effects of statins by examining intracellular and extracellular changes by light and electron microscope in a rabbit experimental knee OA model.
MATERIALS AND METHODS
Uludag University Animal Experiments Ethics Committee approved this study. The study was performed on 20 mature New Zealand rabbits weighing between 2500 and 4000 grams obtained from the Experimental Animal Breeding and Research Centre.
Study plan-operative procedure
The experimental knee OA model was created by cutting the anterior cruciate ligament (ACL) of the rabbits as described by Yoshioka et al.6 Anesthesia was administered intramuscularly at dosages of 2% xylocaine hydrochloride 8 mg/kg and ketamine hydrochloride 100 mg/kg. After a longitudinal anterior midline skin incision over knee joint, an adequate opening was obtained by medial parapatellar arthrotomy with the patella dislocated laterally. The ACL was cut with the knee in full flexion. The anterior drawer test confirmed that the ACL had been completely cut. The joint cavity was washed with saline and the joint capsule sutured with 3/0 absorbable sutures and the skin was closed with 3/0 nonabsorbable sutures. Postoperative analgesia was administered as 1–2 mg/kg 100 ml paracetamol in the drinking water then the rabbits were left active in cages with standard feed.
The 20 rabbits were randomly allocated into two groups of 10. Study group: The group that received intraarticular statin therapy. We added 40 mg atorvastatin into the 100 ml saline as we had 0.4 mg atorvastatin per 1 ml. We applied 1 ml/kg saline atorvastatin concentration. Control group: The group that have not received any intraarticular statin therapy. The control group received an intraarticular administration of saline and the study group 0.4 mg/ml/kg saline atorvastatin concentration from the 1st week postoperatively, once a week for 3 weeks.
All the animals were sacrificed by decapitation 12 weeks after the final injection. The knee joints in which the ACL was cut to develop the experimental OA model were removed including the femoral and tibial joint surfaces for light microscopy. The degenerative changes on the medial femoral condyle and medial tibia plateau were observed as macroscopically after arthrotomy. At the same time, a tissue sample for electron microscopy examination was taken from the degenerative area of the medial joint cartilage (the cartilage in femur medial condyle and medial tibial plateau) using a 16 gauge bone marrow biopsy needle.
Histopathological evaluation
Using the morphological stages classification described by Pelletier et al.,7 cartilage tissues from the knee joint medial compartment (medial femoral condyle and medial tibia plateau) were evaluated for the extent of erosion and degenerative changes, according to the presence of minimal fibrillation, surface and mid-layer erosion, deep layer erosion and subchondral bone erosion.
Histological and histochemical changes in the cartilage tissue were evaluated according to Mankin et al.,8 histological grading system, whereas structural, cellular changes and safranin-O involvement were evaluated according to the integrity of the tidemark structure. According to the Mankin histopathological classification, the points expected for normal joint cartilage were accepted as 0–1 and mild, moderate, severe osteoarthritic changes as 2–5, 6–9, 10–14 respectively.9,10
Statistical analyses
The obtained data were recorded on computer using statistical package for the social sciences (SPSS Inc., Chicago) for Windows 13.0 and the variables related to the macroscopic and microscopic evaluation were given as mean and standard error. The Mann–Whitney U-test was used for comparison between values according to the results.
Tissue preparation
The operated knee joints including the femoral and tibial joint surfaces were removed by cutting at a distance of 2 cm from the joint. The joint cavity was reached by medial parapatellar arthrotomy. After removal from the surrounding soft tissue, the knee joint medial compartments were kept in a 10% formaldehyde solution for 5 days to provide tissue fixation for histopathological examination. Then the bone tissue was kept in a 10% formic acid solution until decalcification. After dehydration, the samples were embedded in paraffin blocks. Sagittal slices of 5 micron thickness were taken after routine procedures and stained with hematoxylin-eosin and safranin-O for histopathological evaluation.
At the same time, samples of joint cartilage were taken from the same area with a 16-gauge biopsy needle for electron microscopy. These samples were fixed for 4 h with 5% glutaraldehyde (0.13 M, pH: 7.2 phosphate pad). The tissues were washed in the pad solution twice for 1 h and decalcified with EDTA. Complete decalcification was confirmed by the ammonium oxalate test, the tissues were washed again with the pad and second fixation was made with 1% osmium tetroxide (1 h at + 4°C). The tissue samples were dehydrated by passing through alcohol of increasing strength from 70%, to 90%, 95% and 100%. After application of propylene oxide, they were embedded in Epon and kept in an incubator for 48 h at 60°C for polymerization. Semi-fine sections (1 µm) taken from the obtained blocks with a Reichert Supernova Ultramicrotome were stained with toluidine blue for joint cartilage to be examined by light microscope. After minimizing this area, fine sections of 70 Å thickness were taken onto a copper grid. The contrast was made with uranyl acetate and lead citrate solution. The samples were examined by permeable JEOL 100SX electron microscope (JEOL Ltd., Tokyo, Japan) and photographed.
RESULTS
In the control group, 1 rabbit died due to septic arthritis thus 9 rabbits in the control group and 10 rabbits in the study group were evaluated.
Macroscopic results
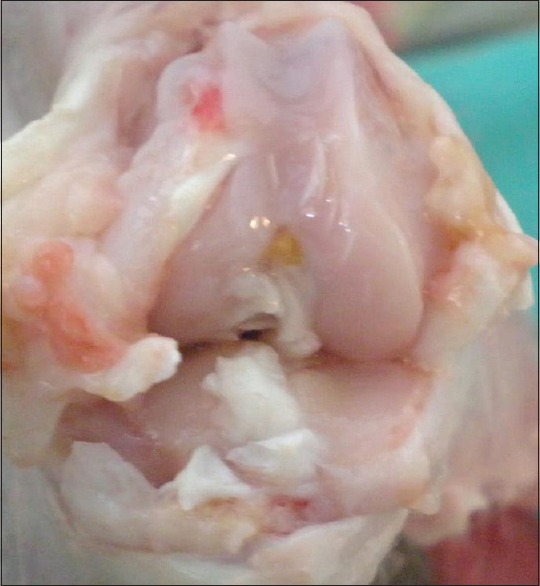
The cartilage tissue of the medial compartment of the knee joint was macroscopically evaluated according to the Pelletier classification system.7 The study group rabbits that had received statin were determined as 4 at stage 0 (40%), 4 at stage 1 (40%), and 2 at stage 2 (20%). The control group rabbits were determined as 2 at stage 3 (33%), and 7 at stage 4 (77%) [Table 1, Figures 1 and 2]. When these results were evaluated statistically, the difference between the statin group and the control group was found to be statistically significant P < 0.001).
Table 1.
Distribution of articular cartilage lesions to the groups according to Pelletier classification system
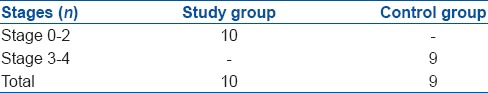
Figure 1.
Macroscopic view of a knee joint in study group (Stage 0)
Figure 2.
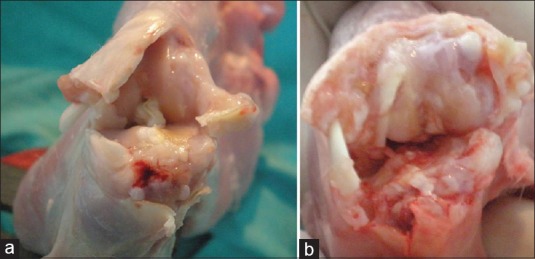
(a and b) Macroscopic view of a knee joint in control group (Stage 4)
Light microscopy results
According to the Mankin histopathological classification, cartilage structure and cellular evaluation of the medial joint surfaces of the rabbit right knees were examined microscopically by considering the extent of safranin-O staining of the matrix and tidemark structure.
Medial tibia plateau cartilage tissue
In the evaluation of cartilage structure of the study group rabbits, 4 (40%) were determined as normal [Figure 3], 4 (40%) showed surface irregularity and 2 (20%) had cracks extending to the mid-layer. In the control group, total disorganization was observed in 3 (33%) rabbits [Figure 4], cracks extending to the calcified layer in 4 (44%) and cracks in the deep layer in 2 (22%).
Figure 3.
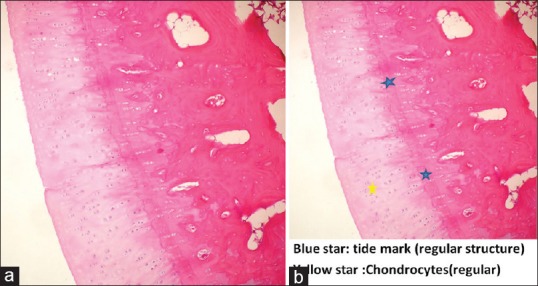
Histopathological picture showing. (a and b) Normal joint cartilage structure and intact Tide-Mark (Study Group) (H and E, ×100)
Figure 4.
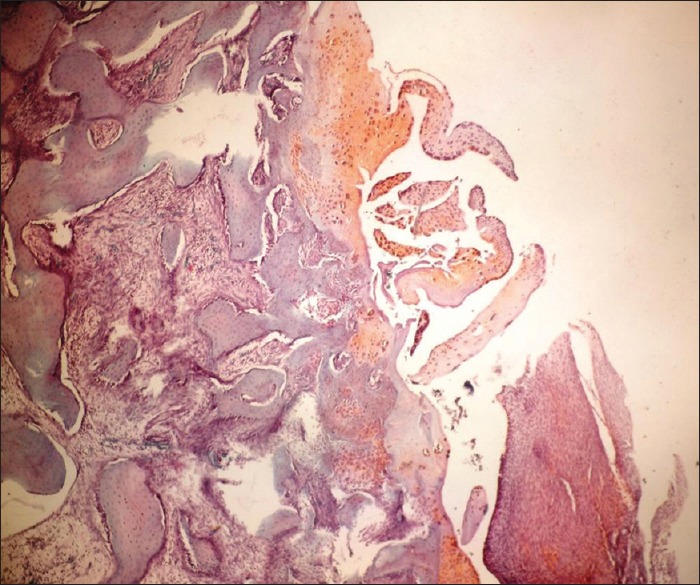
Histopathological picture showing. Impaired joint cartilage structure (control group) (Safranin-O, ×100)
In the cellular evaluation of the study group rabbits, 8 (80%) were determined as normal and 2 (20%) had widespread hypercellularity. The control group were observed as 5 (60%) with hypercellularity and 4 (40%) with cloning.
In the evaluation of safranin-O staining, 8 (80%) of the study group rabbits were determined as normal and 2 (20%) with a low level of staining. In the control group, no staining was seen in 3 (30%) rabbits and a low level of staining in 6 (70%) rabbits.
Tidemark integrity was determined as intact in 10 (100%) rabbits in the study group. In the control group, there was transfer to the veins in 5 (60%) rabbits and intact integrity in 4 (40%) rabbits.
Medial femoral condyle cartilage tissue
In the evaluation of cartilage structure of the study group rabbits, 4 (40%) were determined as normal, 4 (40%) showed surface irregularity and 2 (20%) had cracks extending to the mid-layer. In the control group, total disorganization was observed in 2 (22%) rabbits, cracks extending to the calcified layer in 4 (44%) and cracks in the deep layer in 3 (33%).
In the cellular evaluation of the study group rabbits, 8 (80%) were determined as normal and 2 (20%) had widespread hypercellularity. The control group were observed as 4 (40%) with hypercellualrity and 5 (60%) with cloning.
In the evaluation of safranin-O staining, 8 (80%) of the study group rabbits were determined as normal, 1 (10%) with a slightly low level of staining and 1 (10%) with a moderately low level. In the control group, no staining was seen in 2 (22%) rabbits and an extremely low level of staining in 7 (80%) rabbits.
Tidemark integrity was determined as intact in 10 (100%) rabbits in the study group. In the control group, there was transfer to the veins in 5 (60%) rabbits and intact integrity in 4 (40%) rabbits.
According to the Mankin classification system used in the histological and histochemical classification of the lesions of the knee joint medial compartment cartilage tissue, the results of examination of the cartilage structure, cellular changes, safranin-O involvement and impairment of the tidemark structure were determined as total points obtained from the evaluation of lesions which developed in the medial femoral condyle as mean 11.33 ± 0.667 in the control group and mean 1.5 ± 0.687 in the study group. The total points obtained from the evaluation of lesions that developed in the tibial medial condyle were determined as mean 11.56 ± 0.709 in the control group and mean 1.40 ± 0.618 in the study group. When the points obtained from the medial femoral condyle and medial tibial plateau cartilage tissue were statistically compared, a significant difference was determined between the control group and the study group P < 0.001).
Electron microscopy results
The mid-layer of the cartilage tissue of the knee joint medial compartment of the study group was examined at the cellular level by electron microscope. The collagen fibrils were regular and the structure was homogenous. Typical round chondrocytes surrounded by territorial matrix formed from proteoglycan particles and fibrils were observed. There was a structure of granulous endoplasmic reticulum similar to healthy cartilage tissue. The Golgi complex was regular [Figures 5 and 6].
Figure 5.
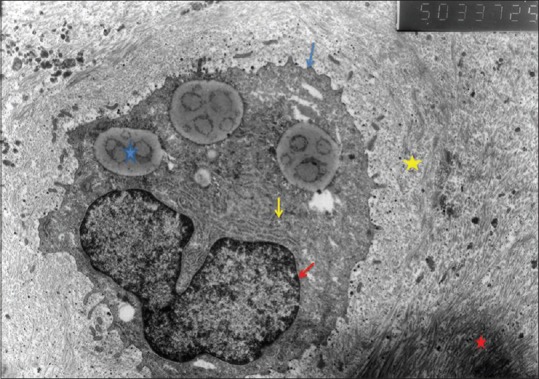
Study group chondrocyte (blue star: Fat granule; yellow star: Territorial matrix; Red star: Interterritorial matrix; blue arrow: Chondrocyte membrane; yellow arrow: Granulated endoplasmic retinaculum; red arrow: Nuclear membrane), (×5000)
Figure 6.
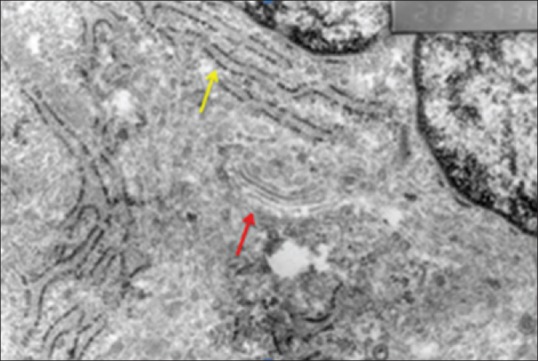
Study group electron microscopic view (yellow arrow: Endoplasmic retinaculum; red arrow: Golgi complex), (×20,000)
In the examination of the mid-layer of the cartilage tissue of the knee joint medial compartment of the control group, irregular and fragmented collagen fibers were observed. The number of chondrocytes were significantly reduced. There was a halo image in the pericellular area and pyknotic nuclei were observed [Figure 7]. The Golgi complex could not be seen. Saccular structures were seen in the granular endoplasmic reticulum of several chondrocytes [Figure 8].
Figure 7.
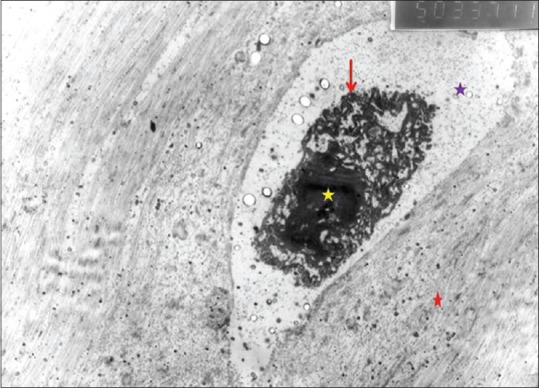
Control group electron microscopic view (purple star: Pericellular halo; yellow star: Pyknotic nucleus; red star: Territorial matrix; red arrow: Chondrocyte membrane), (×5000)
Figure 8.
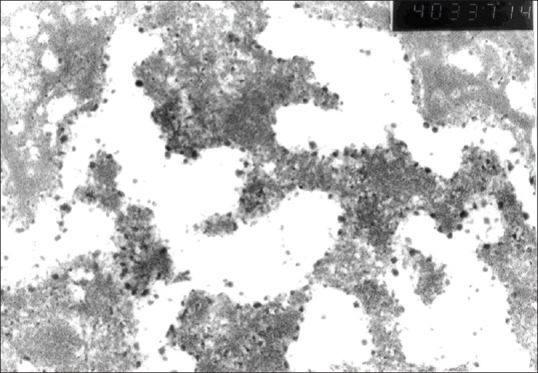
Control group electron microscopic view (saccular appearance of granulated endoplasmic retinaculum), (×40,000)
DISCUSSION and Conclusion
An examination of the literature showed studies where light microscopy had been used to research the chondroprotective effects of statins, which are effective in the destruction of proteoglycans by inhibiting inflammatory mediators and matrix metalloproteinase (MMPs).2,4,5 However, no studies were found where cartilage protective effects had been examined by electron microscope. We evaluated the results of light microscopy and electron microscopy together too. We searched the protective effect of statins on the Golgi complex and endoplasmic reticulum level detailed on the cell level with the aid of electron microscopy and predicted that this study can direct the other future studies even though MMP and interleukin levels were not evaluated in the current study. The cartilage protective effects were evaluated in an experimental OA model, by examining intracellular and extracellular changes by light and electron microscopy.
We predicted to decrease the side effects of oral statins by applying it locally (intraarticularly) as their bioavailability would be different and systemic side effects would be much more. We decided the minimum number of experimental animals to get the statistically significant result.
The Mann–Whitney U-test is the best test to be used instead of importance test of the difference between two averages if the data do not supply the parametric test hypothesis. We have used Mann–Whitney U-test in our study since parametric test hypothesis would be wrong due to <10 number of samples.
In the current study, in the macroscopic evaluation of cartilage tissue lesions from the knee joint medial compartment, no advanced stage OA was determined in the study group, whereas in the control group all the rabbits were determined as having advanced or moderate stage OA P < 0.001). In the histological evaluation, when a comparison was made of the points obtained for the medial femoral condyle and medial tibial plateau cartilage tissue, a statistically significant difference was determined between the study and the control group P < 0.001).
According to the expected points ranges for the knee joint medial compartment cartilage tissue of the study group, 80% were found to be normal and a mild degree of osteoarthritic changes were observed in 20%. In the control group, osteoarthritic changes at a moderate level were observed in 28% and at an advanced level in 72%. These results determined that statins were effective to a significant degree in the histological and histochemical evaluation.
The amount of proteoglycan in the intracellular matrix can be evaluated histologically by examining the prepared samples stained with safranin-O. Lorenz et al.11 determined that OA was revealed by loss of metachromasia. In the current study, we determined a highly significant level of metachromasia loss in the control group using safranin-O staining P < 0.001).
In a study on black rats by Yamamoto et al.,12 it was reported that the degeneration of cartilage tissue does not start in the surface layer, but that the first changes are seen in the mid-layer. Therefore in the current study, the mid-layer of cartilage tissue was examined at the cellular level by electron microscopy. We identify healthy cartilage tissue with appropriate chondrocyte and matrix structures with electron microscopy in the study group whereas pyknotic nuclei and irregular collagen fibers were determined in the control group.
Shakibaei et al.13 examined the effects of fluoroquinolone group medications on cartilage tissue using electron microscope and reported that the pericellular halo image confirmed with cartilage matrix degeneration. In the current study, in the electron microscope evaluation, the pericellular images were examined in addition to the chondrocyte cell structure. While no pericellular halo image was determined on the sections examined from the study group, a halo image around chondrocytes was determined in the control group.
Kouri et al.,14 in a study using electron microscopy on an experimental OA rat model, identified reduction in the Golgi complex intensity as an immune marker of the progression of OA. In the electron microscope evaluation of the current study, although immune staining was not used, the Golgi complex was observed on the sections examined from the study group but not on those of the control group.
In a study on C57 black rats, Yamamoto et al.12 determined heterogenous regularity and reduced density of collagen fibers in cartilage tissue examined by electron microscope. They also observed saccular structures full of protein granules in the endoplasmic reticulum of the chondrocyte cytoplasm. Although this situation shows active protein synthesis, it also shows impairment in the regular protein transport from the endoplasmic reticulum to the Golgi complex. Impairing formation and expression of glycosaminoglycans cause OA. In the current study, although saccular structures were seen in the granular endoplasmic reticulum in the control group sections examined in the electron microscope evaluation, no saccular structures were observed in the granular endoplasmic reticulum of the sections evaluated from the study group.
It can be concluded that in the macroscopic, light and electron microscopy evaluation of cartilage tissue in an experimental OA model, it was determined that there was a chondroprotective effect of statin on the cartilage tissue. It would be much better if our study were supported with immunohistochemical stains. According to the findings obtained from electron microscope examination at the cellular level, the protective effect of statin is shown to be that by protecting the Golgi complex structure and granular endoplasmic reticulum, thus it allows the continuity of the formation and expression of proteoglycans.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sarıdoğan ME. Cerrahpaşa Medical Faculty, Continuing Medical Education Activities Rheumatic diseases Symposium Series. 2003;34:11–8. [Google Scholar]
- 2.Baker JF, Walsh P, Mulhall KJ. Statins: A potential role in the management of osteoarthritis? Joint Bone Spine. 2011;78:31–4. doi: 10.1016/j.jbspin.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 3.Verbruggen G. Chondroprotective drugs in degenerative joint diseases. Rheumatology (Oxford) 2006;45:129–38. doi: 10.1093/rheumatology/kei171. [DOI] [PubMed] [Google Scholar]
- 4.Lazzerini PE, Capecchi PL, Nerucci F, Fioravanti A, Chellini F, Piccini M, et al. Simvastatin reduces MMP-3 level in interleukin 1beta stimulated human chondrocyte culture. Ann Rheum Dis. 2004;63:867–9. doi: 10.1136/ard.2003.009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akasaki Y, Matsuda S, Nakayama K, Fukagawa S, Miura H, Iwamoto Y. Mevastatin reduces cartilage degradation in rabbit experimental osteoarthritis through inhibition of synovial inflammation. Osteoarthritis Cartilage. 2009;17:235–43. doi: 10.1016/j.joca.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Yoshioka M, Coutts RD, Amiel D, Hacker SA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1996;4:87–98. doi: 10.1016/s1063-4584(05)80318-8. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier JP, Fernandes JC, Brunet J, Moldovan F, Schrier D, Flory C, et al. In vivo selective inhibition of mitogen-activated protein kinase kinase 1/2 in rabbit experimental osteoarthritis is associated with a reduction in t he devel opment of structural changes. Arthritis Rheum. 2003;48:1582–93. doi: 10.1002/art.11014. [DOI] [PubMed] [Google Scholar]
- 8.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–37. [PubMed] [Google Scholar]
- 9.Ostergaard K, Andersen CB, Petersen J, Bendtzen K, Salter DM. Validity of histopathological grading of articular cartilage from osteoarthritic knee joints. Ann Rheum Dis. 1999;58:208–13. doi: 10.1136/ard.58.4.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60:2694–703. doi: 10.1002/art.24774. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz H, Wenz W, Ivancic M, Steck E, Richter W. Early and stable upregulation of collagen type II, collagen type I and YKL40 expression levels in cartilage during early experimental osteoarthritis occurs independent of joint location and histological grading. Arthritis Res Ther. 2005;7:R156–65. doi: 10.1186/ar1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto K, Shishido T, Masaoka T, Imakiire A. Morphological studies on the ageing and osteoarthritis of the articular cartilage in C57 black mice. J Orthop Surg (Hong Kong) 2005;13:8–18. doi: 10.1177/230949900501300103. [DOI] [PubMed] [Google Scholar]
- 13.Shakibaei M, Stahlmann R. Changes in cartilage of rats after treatment with Quinolone and in Magnesium-deficient diet. Acta Med Iran. 2002;40:171–6. [Google Scholar]
- 14.Kourí JB, Rojas L, Pérez E, Abbud-Lozoya KA. Modifications of Golgi complex in chondrocytes from osteoarthrotic (OA) rat cartilage. J Histochem Cytochem. 2002;50:1333–40. doi: 10.1177/002215540205001006. [DOI] [PubMed] [Google Scholar]


