Spinal cord injury (SCI) with consecutive paralysis below the lesion level is a severe disorder affecting the patient for the rest of his or her life. So far, there is no known fundamental intervention strategy for efficiently helping those patients regain their motor abilities, despite intense research in this area. Thus, effective treatment for those patients is still an open question. A spinal cord injury is accompanied by a primary, severe and irreversible neuronal cell death in the trauma region, followed by a secondary extensive cell necrosis in the lesion surrounding areas. Nevertheless, recent studies indicate that regeneration after spinal cord injury could be possible if three substantial steps are fulfilled: (1) reduction of the inhibitory environment at the SCI lesion site, (2) identification of a neural substrate to establish new spinal circuits, and (3) support of these circuits to form permanent, functional motor, sensory, or autonomic connections (Dru and Hoh, 2015).
In 2014, a study was published on four patients with complete motor paralysis for over two years, two of whom also had complete sensory SCI (Angeli et al., 2014). Previously, it was thought that incomplete sensory SCI was necessary to maintain voluntary movements (Harkema et al., 2011), but Angeli et al. (2014) showed that all of the patients studied were able to perform voluntary movements after intensive epidural spinal cord stimulation. They speculate that epidural spinal cord stimulation neuromodulated the spinal circuitry at sub-threshold motor levels, evoking neuronal activity which accumulated to become supra-threshold. In addition, they showed convincing evidence that the patients were able to make specific voluntary movements of paralysed muscles long after injury. Although this represents a clinical breakthrough for the four patients in the study, the question remains as to the cause of their improvement. One explanation for the observed movements could be the mechanical and electrical stimulation of the skin or the reduction of body weight support (Harkema et al., 2011), or, as claimed by Angeli et al. (2014), the alteration of existing circuitries. A further explanation, which we favor, is that new connections were established within the spinal cord, altering interneuronal networks by acting on commissural spinal cord interneurons with connections to more caudal segments to pass information around the lesion site.
Functional recovery could be credited to synaptic plasticity, recruitment of additional spinal cord interneurons, or plasticity in anatomical circuitries (Flynn et al., 2011). Recently, different subsets of commissural neurons in the spinal cord have been identified and are reported to have an influence on functional recovery (Chedotal, 2014). At least 22 subclasses of such spinal cord interneurons have been found, many of them being gamma-aminobutyric acid (GABA)- or glutamate-positive. The impact of intraspinal networks of interneurons on recovery after incomplete sensory or motor SCI has long been known (Flynn et al., 2011). One of the major factors here is that damaged axons from the motor cortex form new connections with the help of interneurons at the severed level. These interneurons act as an interposition like, for example, a transplant of the surreal nerve that helps to reestablish the function of the ulnar nerve after transection. Via those new connections information from the motor cortex can be transferred to interneurons, which are connected to more caudal segments. This circuitry is a unique property of the spinal cord. Such a newly established pathway can be reinforced, for example, by using epidural electric stimulation (Angeli et al., 2014) and that in turn, could lead to functional recovery.
Within the last year, different embryonic interneuron transplantation studies have been published confirming the inducibility of cortical plasticity (Tang et al., 2014), the recovery of visual cortical function (Tang et al., 2014) and the reduction of neuropathic pain after peripheral nerve injury in murine in vivo models (Braz et al., 2015). Interneuronal precursors used in these publications were dissected from the media ganglionic eminence, a transitory brain structure only present in embryonic and fetal stages, and injected into the region of interest. Promising attempts have also been made using interneurons generated from mouse embryonic stem cells (Brown et al., 2014), olfactory ensheathing cells or bone marrow stromal cells, since spinal interneuronal precursors are rare to extract.
We recently published our improved method for investigating source-specific regeneration of the corticospinal tract into spinal cord slices and intrinsic parenchymal responses (Pohland et al., 2015). Briefly, motor cortices of green fluorescent protein (GFP)-expressing mice P0–3 (postnatal day 0–3) are dissected in coronal sections and co-cultured with wild type spinal cord slices using pups of the same age. We prepared spinal cord slices by cutting the explant perpendicular to the longitudinal axis in order to maintain their ventrodorsal polarity as well as the intrinsic axonal fiber tract. Nevertheless, the rodent corticospinal tract trajectories are different from primates, since most of all nerve fibers are present in the dorsal column (Ni et al., 2014; Rank et al., 2015). We showed outgrowth of motorcortical axons into the spinal cord, demonstrated synaptic connections between both explants, and analyzed migrating GFP-positive cells within the wild type tissue. During the preparation of the motorcortical slices, an artificial transection of the corticospinal tract is caused, since at this time point axons of motorcortical neurons already extend beyond the medulla oblongata and reach the cervical spinal cord (Oishi et al., 2004). Using green fluorescent and non-fluorescent animals allows us to monitor motorcortical regeneration into the dorsal column of the wild type spinal cord slice; even though we cannot rule out that few of the ingrowing fibers may come from previously undamaged axons. In addition, other limiting parameters are that the viability of organotypic slice cultures as well as the potential of regeneration rapidly decreases with older tissue donors, whereas the complexity of explant isolation is increasing (Bonnici and Kapfhammer, 2008; Sypecka et al., 2015). Our co-cultures were prepared until P3, when axons of motorcortical neurons already extend beyond the medulla oblongata and reach the cervical spinal cord (Oishi et al., 2004). Of course one has to keep in mind the limitations of early postnatal slice cultures, due to the age-dependent declination in the regenerative ability of older tissue. Even though postnatal organotypic slice cultures cannot mimic all complex aspects occurring after SCI they can bridge the gap between cell culture and in vivo experiments. They also can provide a convenient approach to cultivate tissue with a maintained cytoarchitecture under controlled and comparable conditions (Bonnici and Kapfhammer, 2008). Additionally, organotypic slice cultures are suitable for long-term incubation allowing an easier, consistent access for instance during microscopically analysis, electrophysiology or treatment (Sypecka et al., 2015).
Applying our improved organotypic slice co-culture model we have published data on axonal outgrowth, regeneration and cell migration. While we have already presented our data on astrocytes, which remain within the motorcortical slice and on migrating neuronal precursors (Pohland et al., 2015), we wanted to further analyze the composition of the entering cell population. Using two different approaches, we were able to verify that migrating interneurons can be studied in our set-up and have already started to differentiate the interneuron population.
On the one hand, we used transgenic mice expressing enhanced GFP under the control of the parvalbumin promotor (Pvalb-EGFP) for our organotypic slice co-cultures. These motor cortex donors have been used before to develop an interneuron network (Bartos et al., 2002). After seven days in vitro we detected, using fluorescent live imaging, Pvalb-EGFP interneurons that migrated up to 300 µm into the wild type tissue (Figure 1A–D). A typical interneuronal soma and dendrite can be seen in Figure 1D. On the other hand, we verified those migrating interneurons with additional immunohistochemical stainings using the primary antibodies mouse anti mouse against glutamic acid decarboxylase isoform 1 (GAD67; 1:250) and rabbit anti mouse against Doublecortin (DCX; 1:200), as well as the secondary antibodies Cy3-conjugated goat anti mouse (1:1,000) and Alexa633-conjugated goat anti rabbit (1:750) (Figure 1E–I). GAD67 is diffusely localized in the cell body and upregulated after CNS traumas, explaining its preparation related high distribution in our slice model (Figure 1G). GABA, which is produced by decarboxylation of glutamic acid with the help of GAD, is one of the major inhibitory neurotransmitters in the adult brain and spinal cord, but has an exhibitory function during embryonic stages, which changes at about P4–5 in rodent spinal cord motorneurons (Allain et al., 2011).
Figure 1.
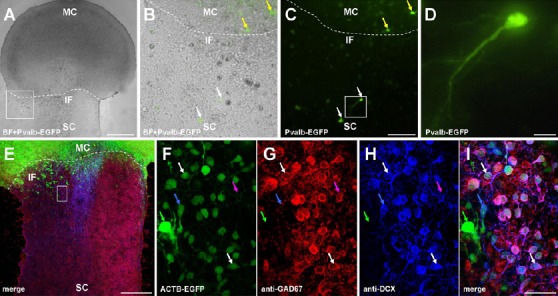
Cortical parvalbumin-positive interneurons and GAD67-positive neuronal precursors migrate into spinal cord.
(A) Overlaid bright field and parvalbumin EGFP (Pvalb-EGFP) images of a representative co-culture at 7 days in vitro (DIV). For this and the following images: MC: Motor cortex; SC: spinal cord; IF: interface between MC and SC (dotted line). Scale bar: 500 μm. (B and C) Sections of (A) at a higher magnification. Overlaid bright field and Pvalb-EGFP images in (B) and Pvalb-EGFP signal in (C) showing green fluorescent cells within the MC (yellow arrows) and the wild type SC (white arrows). Scale bar: 100 μm. (D) Section of (C) at a higher magnification. Scale bar: 20 μm. (E) Confocal image of neuronal precursor (DCX) and glutamic acid decarboxylase isoform 1 (GAD67) staining as well as beta-actin GFP (ACTB-GFP) signal. Scale bar: 300 μm. (F–I) Magnified section of (E) shows in (F) ACTB-GFP, (G) GAD67 and (H) DCX-positive cells. (I) Merge of (F–H) depicting single (green arrow = ACTB-GFP), double (blue arrow: ACTB-GFP and DCX; magenta arrow: GAD67 and DCX), and triple (white arrow: all channels) stained cells. Scale bar: 37.5 μm.
After the proof of principle regarding the migration of motorcortical interneurons, we would like to further specify this very heterogeneous population (Chedotal, 2014). Additionally we want to apply our set-up to investigate the influence of propriospinal interneurons. For this purpose, we want to study organotypic slice co-cultures using motor cortices of red fluorescent protein (RFP)-expressing mice as well as spinal cord tissue of transgenic mice expressing enhanced GFP under the control of the parvalbumin promotor (Pvalb-EGFP). Due to the sagittal longitudinal cutting of the spinal cord during preparation we do expect an appropriate amount of interneurons within our slice, for example in the dorsal horn (Yang, 2015). Our approach is suitable to study if outgrowing corticospinal tract axons can functionally interact with intraspinal networks of interneurons. In addition, if a lesion is placed within the spinal cord slice ingrown motorcortical fibers might be able to surround the scar and allowing the passage of information to segments beyond it by local spinal interneuronal circuits.
It is undeniable that the outcome of organotypic slice culture experiments is not totally comparable to the manifold, complex processes occurring after a spinal cord injury in vivo. But, we think that our co-culture model could help to answer fundamental questions in this field by providing a simple approach to easy analyze propriospinal networks under controlled and comparable conditions. Our method could contribute to better understanding and overcoming the substantial steps that are necessary to induce regeneration after spinal cord injury for instance by different treatments in order to reduce the growth inhibitory environment at the lesion site. Trying to alter the outcome of our model with the focus on local propriospinal interneurons could help to investigate repair strategies in finer detail. And if the dorsal horn interneurons can also form functional connections, receive input from ingrowing corticospinal tract fibers of the motor cortex, the question arises as to whether we can influence those interneurons to promote regeneration after a second spinal cord lesion within the in vitro co-culture.
Further studies will help to address the question of how plasticity of the CNS might be influenced to promote functional recovery. Trying to alter the outcome of a SCI in vitro model using interneuronal precursors or alteration of intraspinal interneuronal networks could help to investigate such a repair strategy in finer detail.
In summary, although two of the patients in the Angeli et al. (2014) study had complete sensory and motor SCI we believe that they still had preserved connections from the rostral to the caudal spinal cord via the network of interneurons. Of course we cannot rule out, that those patients had spared supraspinal fibers such a long time after the injury. In all cases, where the spinal cord has not a complete misalignment, some fibers are preserved. But do to the sheer number of propionalspinal neurons (Flynn et al., 2011), we think, that it is more likely, that propriospinal interneurons and not supraspinal fibers have survived the injury. In addition, recent studies indicate the plasticity of dorsal horn interneurons after SCI as well as the presence of propriospinal interneurons that are located in the mouse upper spinal cord and their long-projecting connections to motor neurons in the lumbar segments (Rank et al., 2015). If those connections can be reinforced by epidural spinal cord stimulation and/or by additional interneurons migrating from the motor cortex into the spinal cord, or by transplantation of neuronal precursors, functional recovery might be possible by amplifying the potential of interneurons to plastic reorganization after SCI.
This study was supported by DFG Grant KFO 213 and the “Else-Kröner-Fresenius-Stiftung” to JG.
References
- Allain AE, Le Corronc H, Delpy A, Cazenave W, Meyrand P, Legendre P, Branchereau P. Maturation of the GABAergic transmission in normal and pathologic motoneurons. Neural Plast 2011. 2011:905624. doi: 10.1155/2011/905624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137:1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici B, Kapfhammer JP. Spontaneous regeneration of intrinsic spinal cord axons in a novel spinal cord slice culture model. Eur J Neurosci. 2008;27:2483–2492. doi: 10.1111/j.1460-9568.2008.06227.x. [DOI] [PubMed] [Google Scholar]
- Braz JM, Wang XD, Guan ZH, Rubenstein JL, Basbaum AI. Transplant-mediated enhancement of spinal cord GABAergic inhibition reverses paclitaxel-induced mechanical and heat hypersensitivity. Pain. 2015;156:1084–1091. doi: 10.1097/j.pain.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR, Butts JC, McCreedy DA, Sakiyama-Elbert SE. Generation of V2a interneurons from mouse embryonic stem cells. Stem Cells Dev. 2014;23:1765–1776. doi: 10.1089/scd.2013.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedotal A. Development and plasticity of commissural circuits: from locomotion to brain repair. Trends Neurosci. 2014;37:551–562. doi: 10.1016/j.tins.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Dru AB, Hoh DJ. Activating spinal interneurons for neural repair after spinal cord injury. World Neurosurg. 2015 doi: 10.1016/j.wneu.2015.09.014. doi: 10.1016/j.wneu.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Flynn JR, Graham BA, Galea MP, Callister RJ. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011;60:809–822. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen YS, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement standing and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–1947. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Nawabi H, Liu X, Yang L, Miyamichi K, Tedeschi A, Xu B, Wall NR, Callaway EM, He Z. Characterization of long descending premotor propriospinal neurons in the spinal cord. J Neurosci. 2014;34:9404–9417. doi: 10.1523/JNEUROSCI.1771-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, Baratta J, Robertson RT, Steward O. Assessment of factors regulating axon growth between the cortex and spinal cord in organotypic co-cultures: effects of age and neurotrophic factors. J Neurotrauma. 2004;21:339–356. doi: 10.1089/089771504322972121. [DOI] [PubMed] [Google Scholar]
- Pohland M, Glumm R, Stoenica L, Holtje M, Kiwit J, Ahnert-Hilger G, Strauss U, Brauer AU, Paul F, Glumm J. Studying axonal outgrowth and regeneration of the corticospinal tract in organotypic slice cultures. J Neurotrauma. 2015;32:1465–1477. doi: 10.1089/neu.2014.3467. [DOI] [PubMed] [Google Scholar]
- Rank MM, Flynn JR, Galea MP, Callister R, Callister RJ. Electrophysiological characterization of spontaneous recovery in deep dorsal horn interneurons after incomplete spinal cord injury. Exp Neuro. 2015;271:468–478. doi: 10.1016/j.expneurol.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Sypecka J, Koniusz S, Kawalec M, Sarnowska A. The organotypic longitudinal spinal cord slice culture for stem cell study. Stem Cells Int 2015. 2015:471216. doi: 10.1155/2015/471216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YS, Stryker MP, Alvarez-Buylla A, Espinosa JS. Cortical plasticity induced by transplantation of embryonic somatostatin or parvalbumin interneurons. Proc Natl Acad Sci U S A. 2014;111:18339–18344. doi: 10.1073/pnas.1421844112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777–791. doi: 10.1016/j.neuron.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


