Brachial as well as lumbosacral plexus avulsion injuries are usually caused by high kinetic traumas, such as car-pedestrian, car and motorcycle accidents or falls from great heights. Traction forces affecting the head and shoulders or extremities pull the spinal nerve sleeves away from the spinal cord and rupture the postganglionic spinal root from the cord. In so called central avulsion injuries, the spinal root is avulsed at the interface between the central and peripheral nervous system (CNS and PNS). This results not only in the disconnection of the root from the cord but also in a longitudinal spinal cord injury. The complexity of the injury leads to degeneration of the spinal root and a marked inflammatory response of the spinal cord followed by the formation of a glial scar (Kachramanoglou et al., 2011).
Over the years, a multitude of studies used disruptions of the dorsal root to study regeneration of sensory axons across the CNS-PNS interface. Typically, sensory axons regenerate readily through the dorsal root and dorsal rootlets but are arrested at the zone of transition between PNS and CNS. This area is usually referred to as the dorsal root transitional zone (DRTZ) or dorsal root entry zone (DREZ). The DRTZ is characterized by CNS astrocytic tissue extending into the central part of the rootlet while its periphery is formed by Schwann cell sheets of the PNS. After injury to the dorsal root, the DRTZ undergoes gliosis resulting in the extension of astrocytic tissue further into the rootlet and the formation of a glial scar (Carlstedt, 2008).
The two commonly used models to analyze sensory axon regeneration across the DRTZ are the dorsal root crush and dorsal root rhizotomy (DRR) model. In dorsal root crush, the root is forcefully squeezed causing the disruption of nerve fibers without interrupting the endoneurial tube. In DRR, the root is completely transected using micro-scissors. In both models, the CNS-PNS interface is left untouched during the procedure. Using the dorsal root crush model, several attempts to overcome the axonal growth inhibiting environment present at the DRTZ succeed to regenerate sensory fibers. Enzymatic digestion of growth inhibiting chrondroitin sulfate proteoglycans (CSPGs) using bacterial chondroitinase ABC supports sensory axon ingrowth, but only when it is combined with growth promoting treatments (Steinmetz et al., 2005). Also infusion with blocking agents aiming at the downstream targets of myelin associated inhibitory protein Nogo, myelin-associated glycoprotein (MAG) and oligodendrocyte myelin glycoprotein (OMgp) lead to the regeneration of myelinated axons (Harvey et al., 2009). Other even more successful approaches use neurotrophic factors to stimulate axonal outgrowth pathways. Intrathecal delivery of nerve growth factor (NGF), neurotrophin-3 or glial derived neurotrophic factor (GDNF), systemic administration of the GDNF family member artemin or viral expression of NGF or fibroblast growth factor-2 induce extension of peptidergic and/or non-peptidergic sensory axons across the site of injury and allow regrowth of sensory axons into the dorsal horn. So far, only systemic delivery of artemin achieves topographically correct projections of both peptidergic and non-peptidergic sensory fibers into the dorsal horn (Smith et al., 2012). Taken together, the dorsal root crush model provides an excellent platform to identify molecules that promote or inhibit sensory regeneration through the reactive DRTZ.
DRR provides an even greater challenge for sensory regeneration due to the complete transection of the root, leaving not even the nerve sheets intact. Using the DRR model, ingrowth of sensory axons into the dorsal horn was first achieved after injection of olfactory ensheathing glia into the DRTZ and dorsal horn and successive micro-suturing of the dorsal root to the cord (Ramón-Cueto and Nieto-Sampedro, 1994). Further developing this approach, olfactory ensheathing cells (OEC) applied to the cut surfaces of dorsal root and spinal cord followed by the application of the tissue adhesive fibrin glue result in the entry of sensory axons into the spinal cord. Interestingly, OECs interact with both CNS astrocytes and Schwann cells to form a growth permissive tissue bridge at the PNS-CNS interface (Li et al., 2004). Partial recovery of sensory and motor functions after DRR was shown after the application of a fibrin sealant alone and was further improved when sealant was applied together with mononuclear cells (Benitez et al., 2014). In conclusion, sensory regeneration after DRR was most successful when cell transplantation was combined with reattachment of the root.
Recently, a new model to study dorsal root injury was introduced. The dorsal root avulsion (DRA) model is characterized by the surgical pulling of individual dorsal roots away from the spinal cord until the complete rupture of the root from the cord. This procedure causes the disruption of the dorsal root and contributing rootlets, the complete disruption of the DRTZ and injury to the dorsal column and horn along the spinal cord segment connected to the avulsed root (Figure 1A). DRA results in a rapid invasion of neutrophils into the dorsal horn followed by a macrophage and microglial response and extensive astrogliosis. All of these events are markedly elevated and prolonged compared to DRR and are not confined to the ipsilateral side. Recovery of vascularization occurs over the first month following DRR but is absent after DRA. Both DRR and DRA result in a loss of spinal cord neurons, but only in DRA a second wave of neurodegeneration occurs two weeks after the injury. Taken together, DRA leads to extensive spinal cord trauma and follows a chronic progression over the first month (Chew et al., 2011). Its close resemblance to the events occurring after central avulsion injuries in patients renders it the ideal model to study sensory regeneration in a clinically relevant perspective.
Figure 1.
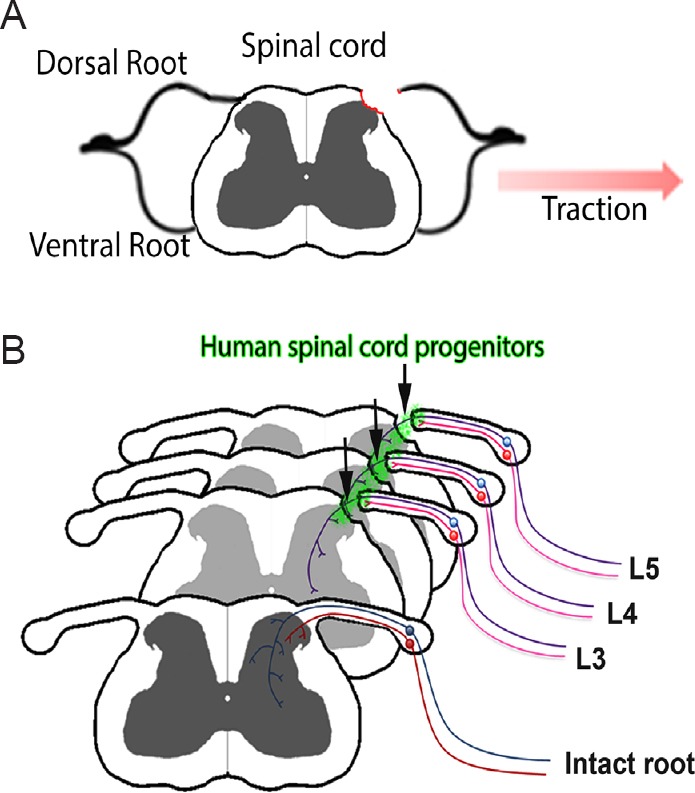
Transplantation of human spinal cord progenitors after dorsal root avulsion injury.
(A) Dorsal root avulsion (DRA) injury results in the disconnection of sensory axons from the spinal cord, disintegration of the dorsal root transitional zone (DRTZ) and a longitudinal spinal cord injury of the dorsal column and horn. (B) Transplantation of human spinal progenitors to the site of L3–5 DRA contributes to the regeneration of a subset of sensory fibres by providing a growth permissive environment. Reprinted by permission from Macmillan Publishers Ltd: [Scientific Reports] (Hoeber et al., 2015), copyright (2015).
We adapted the DRA model to study sensory regeneration in this unique setting. These attempts proved to be especially challenging due to the varying degree of damage to the traumatized dorsal horn, rendering it of great importance to develop ways to create root avulsions in a reproducible manner. In all cases, DRA led to a complete disruption of the DRTZ and extensive glial scarring at the site of injury. DRA was performed along the L3-5 segment of the lumbar spinal cord disrupting the dorsal roots that contain the main contribution to the sciatic nerve in mice. This resulted in a severe reduction of mechanical nociceptive and sensorimotor abilities of the ipsilateral hind paw without affecting the gait of locomotion (Hoeber et al., 2015). In contrast to previous studies that report allodynia after spinal root avulsion of the L5 dorsal and ventral root and after T13 + L1 dorsal root avulsion (Wieseler et al., 2010; Chew et al., 2013), avulsion of the L3–5 dorsal roots did not result in mechanical hypersensitivity (Hoeber et al., 2015).
Our next step was to identify stem cell candidates for transplantation experiments. Suitable candidates should have the potential to act on multiple aspects of the DRA injury while showing a low risk of generating tumorigenic cell types often found in stem cell transplants. In order to achieve this, human embryonic stem cells were restricted to the fate of the target tissue's developmental lineage (Li et al., 2008). The resulting human spinal cord progenitors were grown in suspension as human neural progenitor (hNP) spheres, dense ball-shaped cell conglomerates, and directly placed at the site of injury. In previous dorsal root injury studies, therapeutic cells were usually transplanted as single cell suspension or in combination with cell-carrying matrices or membranes (Kliot et al., 1990; Li et al., 2004; Benitez et al., 2014). Surprisingly, hNP spheres placed between the site of injury and the root stump also provided a substrate to stabilize the root's position without the need of micro-suture or tissue adhesives.
Having found a suitable candidate that can be placed at the site of injury in a controlled manner and that facilitated the reattachment of the root stump, we set out to test the three possible outcomes of this treatment: hNP spheres could provide a substrate for sensory fibers to cross the CNS-PNS interface similar to what has been found after OEC transplantation in rhizotomized dorsal roots (Li et al., 2004); they could form a “synaptic relay” by extending axons into the spinal cord and provide innervation targets for outgrowing sensory axons; or migrate into the dorsal horn and replace lost spinal cord neurons.
Our first observation was that animals receiving hNP spheres performed better in behavioral tests in which they showed sensorimotor deficits before (Hoeber et al., 2015). Mechanical nociceptive sensitivity was consistently improved and after five months animals regained the majority of their ability to hold on to a metal bar using their hind paw. Transganglionic tracing and immunohistochemistry revealed the ingrowth of myelinated sensory axons from the dorsal root stump, through the engrafted hNP transplant and into the dorsal horn gray matter (Figure 1B). In order to confirm that the sensorimotor improvement was in fact caused by ingrowing sensory fibers originating from previously avulsed roots, we performed a second surgery to transect the L3–5 dorsal roots close to the dorsal root ganglion. This surgery caused the complete loss of observed improvements and led us to the conclusion that engrafted hNP spheres act as bridges between the CNS and PNS environment by providing a growth substrate for regenerating sensory fibers.
Engrafted hNP spheres were localized outside of the spinal cord and differentiated primarily into inhibitory neurons and glial cells. They did neither migrate into the dorsal horn gray matter nor extended axons from the site of engraftment into the cord, what renders it unlikely that hNP spheres could act as sensory relays or replace dorsal horn neurons lost to the avulsion injury. Instead, they intermingled with the dorsal root stump and interfered with the astrocytic scar and basal lamina at the DRA injury site. Here, they formed an open “gate” in the glial scar facing the transplant area. Regenerating sensory fibers were found to pass from the transplant area into the dorsal horn through these gates (Hoeber et al., 2015). Future experiments will have to elucidate in greater detail how hNP spheres are able to modify the spinal cord interface to become growth permissive and in this context whether also inhibitory myelin associated proteins and proteoglycans present at the site of dorsal root injury are affected by hNP transplantation. Alternatively, hNP spheres could secret neurotrophic factors that are able to induce growth promoting pathways in sensory neurons or provide an embryonal milieu that might be able to stabilize and protect regenerating axons from an inhibitory environment. Additional transplantation studies with human spinal cord progenitors from fetal sources or induced pluripotent stem cells (iPSC) could help to elucidate whether the regenerative effect observed here is confined to human embryonic stem cell derived neural progenitors. Sensory regeneration after hNP sphere transplantation was limited to myelinated axons. The use of hNP spheres together with already well-established guidance and growth promoting molecules identified in the dorsal root crush model could achieve ingrowth of myelinated as well as unmyelinated axons. A combined approach might also help to direct regenerating sensory axons to specific neuronal populations in the spinal cord and would allow analyzing synergistic effects of multiple treatment regimens (Smith et al., 2012).
Taken together, our study provides the first evidence that sensory regeneration across the CNS-PNS interface can be achieved also in dorsal root avulsion. The mechanism behind the formation of growth permissive gates formed by hNP spheres remains elusive and topographically specific regeneration will most likely require combinatorial approaches that are able to guide sensory axons after entering the spinal cord.
Our research was supported by the Swedish Research Council (Project Nos. 5420 and 20716), Stiftelsen Olle Engkvist Byggmastare and Signhild Engkvist's Stiftelse. I also thank all co-authors that were involved in this project, namely Carl Trolle, Niclas Konig, Zhongwei Du, Alessandro Gallo, Emmanuel Hermans, Håkan Aldskogius, Peter Shortland, Su-Chun Zhang, Ronald Deumens & Elena N. Kozlova. Special thanks to Carl Trolle, Niclas Konig, Håkan Aldskogius and Elena Kozlova for valuable comments on the paper.
References
- Benitez SU, Barbizan R, Spejo AB, Ferreira RS, Barraviera B, Góes AM, de Oliveira AL. Synaptic plasticity and sensory-motor improvement following fibrin sealant dorsal root reimplantation and mononuclear cell therapy. Front Neuroanat. 2014;8:96. doi: 10.3389/fnana.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt T. Root repair review: basic science background and clinical outcome. Restor Neurol Neurosci. 2008;26:225–241. [PubMed] [Google Scholar]
- Chew DJ, Carlstedt T, Shortland PJ. A comparative histological analysis of two models of nerve root avulsion injury in the adult rat. Neuropathol Appl Neurobiol. 2011;37:613–632. doi: 10.1111/j.1365-2990.2011.01176.x. [DOI] [PubMed] [Google Scholar]
- Chew DJ, Murrell K, Carlstedt T, Shortland PJ. Segmental spinal root avulsion in the adult rat: a model to study avulsion injury induced pain. J Neurotrauma. 2013;172:120831034644001. doi: 10.1089/neu.2012.2481. [DOI] [PubMed] [Google Scholar]
- Harvey PA, Lee DHS, Qian F, Weinreb PH, Frank E. Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush. J Neurosci. 2009;29:6285–6295. doi: 10.1523/JNEUROSCI.5885-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeber J, Trolle C, Konig N, Du Z, Gallo A, Hermans E, Aldskogius H, Shortland P, Zhang SC, Deumens R, Kozlova EN. Human embryonic stem cell-derived progenitors assist functional sensory axon regeneration after dorsal root avulsion injury. Sci Rep. 2015;5:10666. doi: 10.1038/srep10666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachramanoglou C, Li D, Andrews P, East C, Carlstedt T, Raisman G, Choi D. Novel strategies in brachial plexus repair after traumatic avulsion. Br J Neurosurg. 2011;25:16–27. doi: 10.3109/02688697.2010.522744. [DOI] [PubMed] [Google Scholar]
- Kliot M, Smith GM, Siegal JD, Silver J. Astrocyte-polymer implants promote regeneration of dorsal root fibers into the adult mammalian spinal cord. Exp Neurol. 1990;109:57–69. doi: 10.1016/s0014-4886(05)80008-1. [DOI] [PubMed] [Google Scholar]
- Li XJ, Hu BY, Jones SA, Zhang Y-S, Lavaute T, Du ZW, Zhang SC. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Carlstedt T, Berthold CH, Raisman G. Interaction of transplanted olfactory-ensheathing cells and host astrocytic processes provides a bridge for axons to regenerate across the dorsal root entry zone. Exp Neurol. 2004;188:300–308. doi: 10.1016/j.expneurol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto a, Nieto-Sampedro M. Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exp Neurol. 1994;127:232–244. doi: 10.1006/exnr.1994.1099. [DOI] [PubMed] [Google Scholar]
- Smith GM, Falone AE, Frank E. Sensory axon regeneration: Rebuilding functional connections in the spinal cord. Trends Neurosci. 2012;35:156–163. doi: 10.1016/j.tins.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz MP, Horn KP, Tom VJ, Miller JH, Busch SA, Nair D, Silver DJ, Silver J. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci. 2005;25:8066–8076. doi: 10.1523/JNEUROSCI.2111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieseler J, Ellis AL, McFadden A, Brown K, Starnes C, Maier SF, Watkins LR, Falci S. Below level central pain induced by discrete dorsal spinal cord injury. J Neurotrauma. 2010;27:1697–1707. doi: 10.1089/neu.2010.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]


