Neuroscience, neuroprosthetics and neural regeneration would benefit from more adequate interfacing devices. To understand how neurons communicate, process information and control behavior, researchers need to monitor nerve cell activity with high specifity and high spatio-temporal resolution. Neural prostheses require minimally invasive implantable devices to re-place lost function, and bypass dysfunctional pathways in the nervous system. Devices built to repair damaged nerves have to support and promote regeneration of host neurons through an injured area. Finally, as neuromodulation is being elevated from last resort to first choice treatment for an increasing number of conditions, implantable devices able to perform targeted regulation of neural activity are needed. Recent advances in device miniaturization, materials engineering, and nanotechnology are enabling development of an increasing number of devices that effectively interface with neural circuits. Wireless spinal cord and deep brain stimulators, retinal and cochlear implants, high density electrodes arrays for neural recording have already proven to significantly impact fundamental research in neuroscience, as well as individuals’ quality of life.
The latest developments in the field of neural interfaces led to fabrication of controlling/recording devices based on inorganic semiconductor nanomembranes (NMs) (Scott and Lagally, 2007) on compliant substrates (Kim et al., 2011; Jeong et al., 2014). Crystalline materials with thickness in the nanoscale have mechanical properties significantly different from those of corresponding bulk materials (Scott and Lagally, 2007; Cavallo et al., 2011; Rogers et al., 2011). If that thin sheet or NM is a semiconductor, it retains all its valuable signaling and sensing functions, while bridging the elastic and the geometrical mismatch between devices and neural circuits (Kim et al., 2011, 2015; Jeong et al., 2014). Active flexible arrays of electrodes based on inorganic NMs on compliant substrates have been repeatedly used for in vivo neural recording from multiple neuronal cells, with comparable or better performance than their rigid counterpart (Kim et al., 2011, 2015; Jeong et al., 2014). More recently, stretchable optoelectronic devices have been used as minimally invasive interfaces for optogenetics (Kim et al., 2013; Park et al., 2014).
In this scenario we have successfully strain-engineered Si and SiO2/Si nanocrystals (Si-nc) NMs to pattern 3D scaffolds (or microchannels) on a compliant substrate (Figure 1) (Cavallo et al., 2014a). Scaffolds with cross-sectional openings scalable in a wide range and down to a single axon diameter have been obtained by our approach (Cavallo et al., 2014b). Combining a novel plasma-based approach to coat the inside of the microchannels with poly-D-lysine (PDL), we have shown that single axons of primary cortical neurons are three-dimensionally confined and guided through them (Figure 2) (Cavallo et al., 2014b). Our NM scaffolds are an alternative to commonly used substrates for cell culture, such as glass and polysterene. They are completely made out of silicon and SiO2/Si-nc, both of which are widely studied non-toxic materials with electrical and optical functionality. In contrast to traditional cell culture (e.g., glass, polystyrene) or semicon ductor device materials (e.g., bulk single-crystal Si and SiO2), the fabricated scaffolds are compliant, thereby they enhance material-cell contact, and mimic the mechanical microenvironment of cells/tissues. Compliant semiconductor scaffolds hold the promise to advance several fields.
Figure 1.
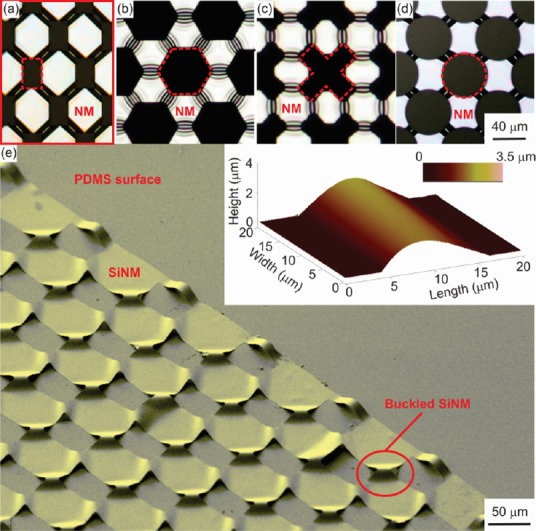
Ordered arrays of compliant semiconductor scaffolds.
(a)–(d) Topview optical images of 2D arrays of ordered buckled channels formed by nanomembrane (NM)/polydimethylsiloxane (PDMS) immersed in acetone for 2 hours, rinsed in isopropyl alcohol (IPA) for 120 seconds and exposed to air for 15 minutes. From left to right buckles of different widths are shown (see inset for definition of length and width). 2D arrays of buckled channels are obtained by patterning the NM in a near-checkerboard pattern with rectangular islands connected by narrow portions of the NM. (e) Tilted scanning electron micrograph (SEM) image showing 72 nm thick SiNMs formed into buckled channels at the interconnects of the near-checkerboard pattern. The inset shows a 3D acomic force microscope (AFM) image of a single buckle-delaminated channel formed by a 72 nm SiNM. Reprinted from Cavallo et al. (2014b).
Figure 2.
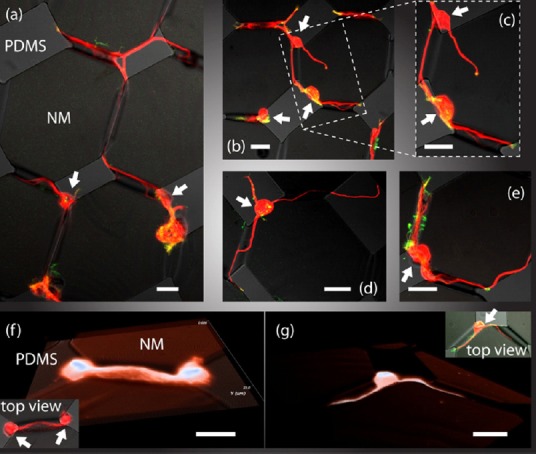
Confocal fluorescence microscope images showing strong guidance of neuronal outgrowths by buckle-delaminated channels, while excluding the neuronal cell body (indicated by arrows).
Neuronal processes have been observed to be guided through up to 5 channels (a), and to form putative networks with each other (b, c), whether the cell is multipolar (d) or bipolar (e). Three-dimensional rendering of confocal images confirms that neuronal processes are confined inside the channel for both putative network (f) and single-cell (g). The channels are formed by 72 nm thick SiNMs on polydimethylsiloxane (PDMS) after immersion in acetone for 2 hours, rinse in isopropyl alcohol for 120 seconds and subsequent 15 minutes exposure to air at room temperature. The measured amplitude of the channels is ~3.5 μm. Actin is labeled in green. Tubulin is labeled in red. Tubulin and actin are labeled to capture neurites and small filopodium processes, respectively. Scale bar in all cases: 20 µm. Reprinted from Cavallo et al. (2014b). NM: Nanomembrane; PDMS: polydimethylsiloxane.
In the field of electrophysiology our fabricated microchannels may enable recording from single neurons with high signal-to-noise ratio (SNR). In general the activity of individual neurons can be recorded by electrodes or field effect transistors (FETs) adjacent to the cell membrane. The amplitudes of action potentials to be measured are very small (~40 µV) and decay rapidly away from the cell, becoming easily overwhelmed by background noise. In an ideal scenario recording devices should be in close contact and compliant with the neuronal membrane. Furthermore, the cell/device interface should be electrically isolated from nearby cells to maximize the SNR. Electrodes or FET integrated on the inner side of the microchannel may be used to record action potentials propagating along the axon confined by the 3D scaffold. Indeed, because scaffolds are made of semiconductor materials, whose processing is well-established, electrical functionality can be easily integrated into the scaffold. Recording of single-neuron activity with high SNR and high spatial resolution will be enabled by the close and conformal contact between the flexible device and the neurite. The SNR may be further enhanced by depositing a dielectric coating on the NM.
Our approach to fabrication of 3D scaffolds on compliant substrates is applicable to any type of NM that can be released and transferred from the original growth substrate to a new host. We have demonstrated fabrication of 3D scaffolds made by SiO2/Si-nc NMs on compliant substrates. SiO2/Si-nc membranes are optically active with a photo- and electroluminescence tunable in the visible spectrum (Creazzo et al., 2010; Shirahata et al., 2010). Genetically, modified neurons can be stimulated by optical signals in the visible range (Fenno et al., 2011; Yizhar et al., 2011). Hence our devices may be used for applications in optogenetics studies. In addition, genetically modified cells may be implanted in individuals with impaired neural functions along with our optically active devices. One may envision a scenario where optical devices are activated by wireless signals to stimulate implanted neurons and restore lost functions. As our approach can be applied to a large variety of thin films, magnetic materials may be also be used to implement control of neural activity.
We have shown that tight seal exists between the cell membrane and the NM formed into a 3D scaffold of the appropriate size (Cavallo et al., 2014b). This property would make our device an attractive candidate to mimic myelin, whose tight enclosure around the axon is crucial to salutatory propagation, a key process that maintains efficient nerve communication. This artificial myelin may find application in in vitro studies and neural prosthesis. The majority of in vitro neural studies are conducted on demyelinated neurites, i.e., neural processes directly exposed to the culture solution. In contrast, neurites in their natural environment are wrapped by a myelin sheath. A 3D compliant NM mimicking a myelin sheath is a step forward towards reproducing in vivo neuron environment in an in vitro culture. In another interesting scenario, dielectric NMs may be used as neural prosthesis in individuals suffering from demyelination, such as patients affected by multiple sclerosis.
Finally, 3D compliant scaffolds have potential application in the field of neural regeneration. Specifically the 3D confinement provided by the scaffolds is commonly believed to increase cytoskeletal tension of the growth cone, a desirable feature to guide axon extension or branching, similar to the native topographic environment (e.g., myelin, packed interstitial space) of CNS. Our fabricated devices may then be used in vitro to stimulate and support neurogenesis. In addition, devices implanted in proximity of damaged nerves may support and promote axon regrowth by providing cytoskeleton tension of the growth cone, as well as by guiding healthy neurons to bridge the injury and rebuild neural circuits.
In conclusion, we have demonstrated that inorganic NMs scaffolds on compliant substrates are a versatile platform with potential application in a variety of fields related to neuroscience, neuroprosthetics and neural regeneration. Our platforms are minimally invasive, lightweight and flexible, thereby they hold the potential to bridge the mismatch between in vitro and in vivo neurons environment from a geometrical, elastic and electrical point of view. These unique characteristics will potentially enable a successful integration between neurons and interfacing devices from a variety of perspectives.
References
- Cavallo F, Turner KT, Lagally MG. Facile fabrication of ordered crystalline-semiconductor microstructures on compliant substrates. Adv Func Mater. 2014a;24:1730–1737. [Google Scholar]
- Cavallo F, Grierson DS, Turner KT, Lagally MG. “Soft Si”: effective stiffness of supported crystalline nanomembranes. ACS Nano. 2011;5:5400–5407. doi: 10.1021/nn200461g. [DOI] [PubMed] [Google Scholar]
- Cavallo F, Huang Y, Dent EW, Williams JC, Lagally MG. Neurite guidance and three-dimensional confinement via compliant semiconductor scaffolds. ACS Nano. 2014b;8:12219–12227. doi: 10.1021/nn503989c. [DOI] [PubMed] [Google Scholar]
- Creazzo T, Redding B, Marchena E, Murakowski J, Prather DW. Tunable photoluminescence and electroluminescence of size-controlled silicon nanocrystals in nanocrystalline-Si/SiO(2) superlattices. J Lumin. 2010;130:631–636. [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Ann Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Kim MK, Cheng H, Yeo WH, Huang X, Liu Y, Zhang Y, Huang Y, Rogers JA. Capacitive epidermal electronics for electrically safe, long-term electrophysiological measurements. Adv Healthc Mater. 2014;3:642–648. doi: 10.1002/adhm.201300334. [DOI] [PubMed] [Google Scholar]
- Kim DH, Lu N, Ma R, Kim YS, Kim RH, Wang S, Wu J, Won SM, Tao H, Islam A, Yu KJ, Kim TI, Chowdhury R, Ying M, Xu L, Li M, Chung HJ, Keum H, McCormick M, Liu P, et al. Epidermal electronics. Science. 2011;333:838–843. doi: 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]
- Kim TI, McCall JG, Jung YH, Huang X, Siuda ER, Li Y, Song J, Song YM, Pao HA, Kim RH, Lu C, Lee SD, Song IS, Shin G, Al-Hasani R, Kim S, Tan MP, Huang Y, Omenetto FG, Rogers JA, et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340:211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DW, Schendel AA, Mikael S, Brodnick SK, Richner TJ, Ness JP, Hayat MR, Atry F, Frye ST, Pashaie R, Thongpang S, Ma Z, Williams JC. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat Commun. 2014;5:5258. doi: 10.1038/ncomms6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, Lagally MG. Elastically strain-sharing nanomembranes: flexible and transferable strained silicon and silicon-germanium alloys. J Phys D. 2007;40:R75. [Google Scholar]
- Shirahata N, Tsuruoka T, Hasegawa T, Sakka Y. Size-Tunable UV-Luminescent Silicon Nanocrystals (vol 6, pg 915, 2010) Small. 2010;6:1075–1075. doi: 10.1002/smll.200902236. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]


