Neurodegeneration is a key aspect of a large number of diseases that come under the umbrella of “neurodegenerative diseases” with the most notable being Parkinson's, Alzheimer's, and Huntington's diseases (AD, PD, HD). They are all incurable and debilitating conditions that result in progressive degeneration and/or death of neurons and are the leading cause of disability in the elderly. The incidence of these diseases is on the rise and yet there is a paucity of effective therapies to treat them.
The etiopathogenesis of these multifactorial diseases is complex and may involve different characteristics like mitochondrial dysfunction, excitotoxicity, abnormal protein aggregation, and inflammation. In particular AD, the most common cause of dementia worldwide, is characterized by an accumulation of extracellular amyloid-β (Aβ) peptide and intracellular neurofibrillary tangles in the cerebral cortex and hippocampus. Reactive oxygen species (ROS) has been suggested to play a pathogenic role in the onset and progression of AD by contributing to the formation and aggregation of Aβ and tau protein hyperphosphorylation (Moneim, 2015). In addition and synergically to oxidative stress, also glycative stress, an overwhelming and unfavourable glycation state with accumulation of glycated proteins, has been reported to have a causative role in AD (Angeloni et al., 2014). Non-enzymatic glycation is a process of post-translational modification of proteins, in which reducing sugars or toxic aldehydes react with amino groups, leading to the formation of a heterogeneous class of compounds called advanced glycation end products (AGEs). AGEs have been strictly linked to neurodegeneration because they accumulate in the brains of AD patients (Krautwald et al., 2011) and co-localize with AD plaques and neurofibrillary tangles. High levels of AGEs have been measured both in neurons and astroglia (Angeloni et al., 2014).
Methylglyoxal (MG), an endogenous α-ketoaldehyde, is the most powerful precursor of AGE production. MG is produced endogenously as an intermediate of the metabolism of carbohydrates, lipids and amino acids under both normal and pathological conditions. The main pathways leading to MG formation are the degradation of the glycolytic triose-phosphate intermediates, acetone metabolism, lipoperoxidation and the catabolism of the amino acids threonine and glycine (Angeloni et al., 2014). MG is detoxified by anti-glycation enzymes that comprise glyoxalase I (GLO1) that catalyzes the production of S-D-lactoylglutathione (SLG) from MG and reduced glutathione (GSH), and glyoxalase II that catalyzes the hydrolysis of SLG to the non-toxic D-lactate. When MG production is increased by high glucose or oxidative stress or when GLO system activity is deranged, glycated proteins accumulate in the brain and lead to glycative stress, playing a fundamental role in the establishment of different neurodegenerative disorders such as AD (Ramasamy et al., 2005).
Since no drugs are available to counteract AD progression, today the research focus has shifted to nutraceutical compounds as an alternative form of prevention/treatment. The isothiocyanate sulforaphane (isothiocyanato-4-(methylsulfinyl)-butane) (SF), abundant in Cruciferous vegetables, has received considerable attention because of its protective activity in different in vitro and in vivo animal models of neurodegeneration (Tarozzi et al., 2013). Moreover, SF bioavailability in the central nervous system (CNS) have been widely demonstrated (Tarozzi et al., 2013). As no studies have been carried out to elucidate the effect of SF in counteracting glycative damage in neurons, we investigated the protective effects of SF against MG-induced glycative stress in neuroblastoma SH-SY5Y cells focusing on different intracellular targets (Angeloni et al., 2015).
First of all we tried to clarify the role of SF in neutralizing MG-induced oxidative stress. Our results indicated that SF counteracts ROS by two possible mechanisms of action: an increase of intracellular GSH levels and an enhancement of MG-detoxification through the up-regulation of the glyoxalase systems. Since GLO1 decreases with the progression of AD (Kuhla et al., 2007), SF ability to strengthen GLO1 expression could represent a new avenue to counteract the deleterious effects of glycation and therefore to prevent the onset of AD. These observations are underpinned by the findings of Xue et al. (2012) that observed that GLO1 up-regulation is mediated by the transcription factor Nrf2 (nuclear factor-erythroid 2 p45 subunit-related factor 2) that binds to a functional ARE (antioxidant-response element) in the 5’-untranslated region of exon 1 of the mammalian GLO1 gene. Nrf2 has been shown to be neuroprotective in many different paradigms of neuronal injury or neurodegeneration. SF has been demonstrated to activate Nrf2 (Hong et al., 2005), and this pathway has been regarded essential for SF neuroprotective effects. We previously demonstrated that SF protects cortical neurons from 5-S-cysteinyl-dopamine-induced neurotoxicity stimulating the Nrf2 pathway of antioxidant gene expression (Vauzour et al., 2010).
Another mechanism by which SF exerts its neuroprotective activity against MG-induced glycative damage is the modulation of mitogen-activated protein kinase (MAPK) signaling pathways involved in apoptotic cell death. MG exposure leads to the activation of extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 MAPK signaling pathways in different cell systems (Zhou et al., 2015). In SH-SY5Y cells, SF-pre-treatment significantly inhibited MG induced phosphorylation of ERK1/2, JNK and p38 suggesting that the protective effect of SF could be also due to the inhibition of the activation of these pro-apoptotic kinases. These results highlight the neuroprotective effect of SF, as all MAPK signaling pathways are activated in AD (Zhu et al., 2001), and their importance as pathological modulators has been widely demonstrated.
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family of growth factors is associated with neuronal survival through its interactions with the tyrosine receptor kinase B (TrkB) and p75 cellular receptors. BDNF expression levels are reduced in the brain of AD patients and the importance of BDNF in AD pathophysiology leads to the proposal of BDNF serum levels as a biomarker of AD risk (Gezen-Ak et al., 2013). In vitro studies showed that this neurotrophin reduces neuronal toxicity induced by Aβ1–42 and Aβ25–35 (Arancibia et al., 2008) and boosts tau protein de-phosphorylation (Elliott et al., 2005). MG exhibits a peculiar modulation of BDNF/TrkB pathway as it leads to an unexpected up-regulation of BDNF whose protective effects are nullified by a strong down-regulation of TrkB (Di Loreto et al., 2008). Our data in SH-SY5H cells confirmed this observation. SF pre-treatment, before MG addition, not only further increased BDNF levels, but significantly induced also TrkB protein levels reverting MG negative effect on this receptor (Angeloni et al., 2015).
Glucose hypometabolism and reduced glucose transport are additional metabolic phenotype characteristics of Alzheimer's brain. MG administration to rats leads to glucose intolerance, reduced GLUT4 and GLUT2 expressions (Dhar et al., 2011), and MG reduced glucose uptake in SH-SY5Y cells (Rizzo et al., 2013). Interestingly, SF totally reverts the reduction of glucose uptake caused by MG exposure, suggesting that SF could also play a role in maintaining glucose availability in the brain of AD patients (Angeloni et al., 2015). This could be particularly important in neuron survival through maintaining their energy status, since it has been demonstrated that MG toxicity in neurons is also related to defects in cellular energy production (de Arriba et al., 2006). On the other hand, glycolysis is not an innocuous metabolic pathway for cells, since it inevitably produces MG, that has been defined has “the dark side of glycolysis” (Allaman et al., 2015). SF is therefore able to both increase glucose influx into neuronal cells and to counteract the highly deleterious effects of one of the most potent glycating agents produced in cells.
In conclusion, SF exerts pleiotropic actions (summarized in Figure 1) on different cellular targets leading to neuron protection against cell death induced by MG exposure. SF action, in fact, could not be ascribed to a simple anti-glycative process, but, considering the “tandem” of free radical and MG and the fall in intracellular GSH concentration, SF can be defined as a multitarget agent modulating different cellular functions leading to a pro-survival frame of particular importance in the prevention/counteraction of multifactorial neurodegenerative diseases like AD.
Figure 1.
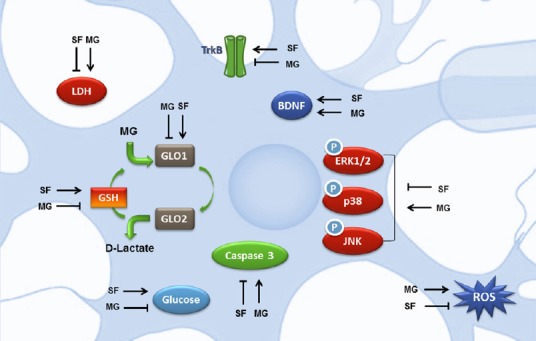
Potential neuroprotective mechanisms exerted by SF against MG-induced injury.
BDNF: Brain-derived neurotrophic factor; ERK1/2: extracellular signal-regulated kinase 1/2; GLO1: glyoxalase I; GSH: glutathione; JNK: c-Jun N-terminal kinase; LDH: lactate dehydrogenase; MG: methylglyoxal; ROS: reactive oxygen species; SF: sulforaphane (isothiocyanato-4-(methylsulfinyl)-butane); TrkB: tyrosine receptor kinase B.
All Authors contributed equally in writing and critically revising the paper. Part of the researches described in this highlight was supported by MIUR-FIRB (project RBAP11HSZS) and Fondazione del Monte di Bologna e Ravenna (ITALY). The authors certify that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter discussed in this paper.
References
- Allaman I, Belanger M, Magistretti PJ. Methylglyoxal, the dark side of glycolysis. Front Neurosci. 2015;9:23. doi: 10.3389/fnins.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeloni C, Zambonin L, Hrelia S. Role of methylglyoxal in Alzheimer's disease. Biomed Res Int 2014. 2014:238485. doi: 10.1155/2014/238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeloni C, Malaguti M, Rizzo B, Barbalace MC, Fabbri D, Hrelia S. Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem Res Toxicol. 2015;28:1234–1245. doi: 10.1021/acs.chemrestox.5b00067. [DOI] [PubMed] [Google Scholar]
- Arancibia S, Silhol M, Mouliere F, Meffre J, Hollinger I, Maurice T, Tapia-Arancibia L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis. 2008;31:316–326. doi: 10.1016/j.nbd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- de Arriba SG, Krugel U, Regenthal R, Vissiennon Z, Verdaguer E, Lewerenz A, Garcia-Jorda E, Pallas M, Camins A, Munch G, Nieber K, Allgaier C. Carbonyl stress and NMDA receptor activation contribute to methylglyoxal neurotoxicity. Free Radic Biol Med. 2006;40:779–790. doi: 10.1016/j.freeradbiomed.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Dhar A, Dhar I, Jiang B, Desai KM, Wu L. Chronic methylglyoxal infusion by minipump causes pancreatic beta-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes. 2011;60:899–908. doi: 10.2337/db10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Loreto S, Zimmitti V, Sebastiani P, Cervelli C, Falone S, Amicarelli F. Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons. Int J Biochem Cell Biol. 2008;40:245–257. doi: 10.1016/j.biocel.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Elliott E, Atlas R, Lange A, Ginzburg I. Brain-derived neurotrophic factor induces a rapid dephosphorylation of tau protein through a PI-3 Kinase signalling mechanism. Eur J Neurosci. 2005;22:1081–1089. doi: 10.1111/j.1460-9568.2005.04290.x. [DOI] [PubMed] [Google Scholar]
- Gezen-Ak D, Dursun E, Hanağası H, Bilgiç B, Lohman E, Araz ÖS, Atasoy IL, Alaylıoğlu M, Önal B, Gürvit H, Yılmazer S. BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer's disease or mild cognitive impairment. J Alzheimers Dis. 2013;37:185–195. doi: 10.3233/JAD-130497. [DOI] [PubMed] [Google Scholar]
- Hong F, Freeman ML, Liebler DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol. 2005;18:1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- Krautwald M, Leech D, Horne S, Steele ML, Forbes J, Rahmadi A, Griffith R, Munch G. The advanced glycation end product-lowering agent ALT-711 is a low-affinity inhibitor of thiamine diphosphokinase. Rejuvenation Res. 2011;14:383–391. doi: 10.1089/rej.2010.1143. [DOI] [PubMed] [Google Scholar]
- Kuhla B, Boeck K, Schmidt A, Ogunlade V, Arendt T, Munch G, Luth HJ. Age- and stage-dependent glyoxalase I expression and its activity in normal and Alzheimer's disease brains. Neurobiol Aging. 2007;28:29–41. doi: 10.1016/j.neurobiolaging.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Moneim AE. Oxidant/Antioxidant imbalance and the risk of Alzheimer's disease. Curr Alzheimer Res. 2015;12:335–349. doi: 10.2174/1567205012666150325182702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- Rizzo B, Zambonin L, Angeloni C, Leoncini E, Dalla Sega FV, Prata C, Fiorentini D, Hrelia S. Steviol glycosides modulate glucose transport in different cell types. Oxid Med Cell Longev 2013. 2013:348169. doi: 10.1155/2013/348169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarozzi A, Angeloni C, Malaguti M, Morroni F, Hrelia S, Hrelia P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid Med Cell Longev 2013. 2013:415078. doi: 10.1155/2013/415078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauzour D, Buonfiglio M, Corona G, Chirafisi J, Vafeiadou K, Angeloni C, Hrelia S, Hrelia P, Spencer JP. Sulforaphane protects cortical neurons against 5-S-cysteinyl-dopamine-induced toxicity through the activation of ERK1/2 Nrf-2 and the upregulation of detoxification enzymes. Mol Nutr Food Res. 2010;54:532–542. doi: 10.1002/mnfr.200900197. [DOI] [PubMed] [Google Scholar]
- Xue M, Rabbani N, Momiji H, Imbasi P, Anwar MM, Kitteringham N, Park BK, Souma T, Moriguchi T, Yamamoto M, Thornalley PJ. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem J. 2012;443:213–222. doi: 10.1042/BJ20111648. [DOI] [PubMed] [Google Scholar]
- Zhou WJ, Gui QF, Wu Y, Yang YM. Tanshinone IIA protects against methylglyoxal-induced injury in human brain microvascular endothelial cells. Int J Clin Exp Med. 2015;8:1985–1992. [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Castellani RJ, Takeda A, Nunomura A, Atwood CS, Perry G, Smith MA. Differential activation of neuronal ERK JNK/SAPK and p38 in Alzheimer disease: the ‘two hit’ hypothesis. Mech Ageing Dev. 2001;123:39–46. doi: 10.1016/s0047-6374(01)00342-6. [DOI] [PubMed] [Google Scholar]


