The greatest risk factor for neurodegeneration is aging. However genetics at birth only contributes 20–25% to the determinants of lifespan, so we actually have around 75% control over how well individuals age in body and brain. Health is the real wealth and that everyone can significantly control/achieve sustainable health and quality of life through lifestyle choices needs to be better promoted. Dementia is predominantly a disease of aging with millions of people suffering from dementia and Alzheimer's disease (AD) and in recent years little has happened to change and improve the cognitive functions of elderly people (2015 Alzheimer's disease facts and figures). Every 4 seconds there is a new case of dementia in the world and with 7.7 million cases annually, the estimated global cost of AD and dementia disease is $604 billion.
Diet has a dynamic molecular impact on human health. The nature and quantity of dietary intake profoundly influences cellular functions, epigenetic alterations and mechanisms that control gene expression. Food technology has provided an over-supply of processed food for the western diet and has profoundly changed our eating habits and choices. In modern times our appetites have outgrown our energy needs and this has impacted negatively on our minds and bodies (Ulijaszek et al., 2012). The diet related chronic diseases of modern society are now the single largest cause of death encompassing diabetes, cardiovascular disease, hypertension, obesity and cognitive decline (Scheme). To sustain healthy aging requires dietary restraint, a reduction of the consumption of processed foods and fatty diets, with negative nutritional attributes such as high energy refined sugars, saturated fats, high sodium content and an increasing affinity and tendency to consume those with positive health attributes including nutraceuticals, phytochemicals and micronutrient rich foods. Carbohydrates, lipids and proteins are the primary dietary fuels that yield metabolic energy providing body function and performance, whereas dietary phytochemicals and herbal medicines rich in polyphenols (Hügel, 2015) are associated with a decreased risk of several human chronic diseases, sustain the cellular molecular machinery, preventing the development of disorders, gain of toxic function and disease conditions. For example, by stimulating lipid metabolism in rats, the green tea flavanol epigallocatechin gallate (EGCG) reversed the high-fat diet induced hypercholesterolemic levels in rats and provided protection for the cardiovascular system. The enzymatic and non-enzymatic antioxidant levels were improved, activated sirtuin 1, endothelial nitric oxide synthase and adenosine monophosphate-activated protein kinase α, are all indicators that the protective effect of EGCG and other catechins in green tea act as strong activating agents through stimulation of the metabolism of high-level fats that may lead to a lower risk of developing heart disease (Zhong et al., 2015).
Scheme.
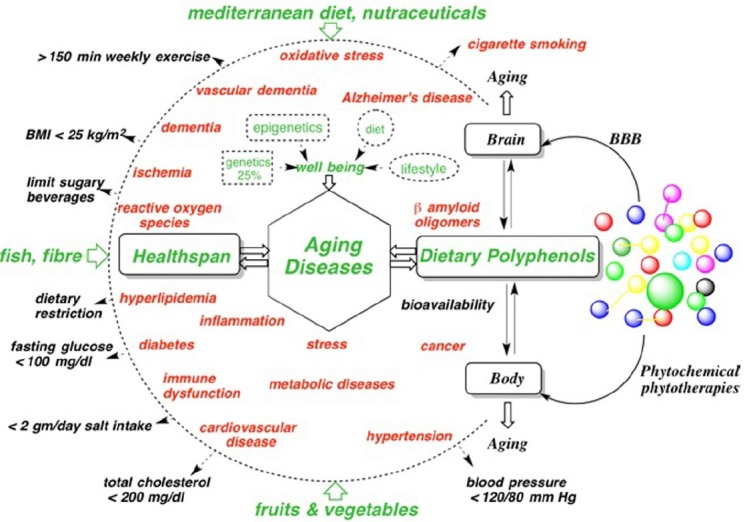
Snapshot of strategies to counter mental and physical human aging diseases.
Summarily body and brain aging diseases and dysfunctions are major health risk factors. The red colors are health risks and potential problems to be minimized; peripheral inward green arrows, and black outward arrows represent contributions to wellbeing, dietary metrics for minimizing dietary related aging diseases and the health (Wong, 2014) risk factors and chronic problems.
Natural products utilized in folk medicine have demonstrated safety profiles since they have already been utilized for decades for the treatment of disease in humans and animals, we use them as templates for the generation of analogues for the development of therapeutic compounds, and probing molecular mechanisms underlying cellular dysfunction. The major liabilities of herbal medicinal products have poor biocompatibility, pharmacokinetic profiles and BBB permeability.
Alkaloids, terpenes, polyphenolic compounds represent the most prevalent classes of herbal constituents with anti-dementia benefit. It is unclear to what extent many of these bioactive phytochemicals, utilized in single or herbal formulae doses can reach the brain in sufficient concentrations and in a biologically active form to exert their neuroprotective effects (Hügel and Jackson, 2014). For AD therapy, herbal products offer a wider range of brain-targets, nutritional benefits, safer dosage, long-term applications and efficacious treatment of AD pathology. For in vivo and large epidemiological studies, the quality assurance of herbal bioactives and production of mass quantities is another challenge for the translation of natural products into therapeutic agents. The majority of herbs are consumed as aqueous extracts so their formulation has to provide increased bioavailability and blood brain barrier permeability. Strategies for enhancing polyphenol bioavailability include encapsulation as phospholipid nanoparticles; incorporation with biodegradable polymers; use of bioactive analogues; modifications to improve pharmacokinetics, use of adjuvants as absorption enhancers.
Dementia is a multifactorial disease, linked to aging, environmental impacts and is different in each patient. Herbs and food supplements are readily available so it is imperative that the molecular mechanisms of their significant health benefits are determined so herbs or formulations are able to complement approved drugs and provide the best therapeutic treatment against Aβ toxicity.
Polyphenols are found in a wide variety of foodstuffs and beverages and the high intake of fruits, vegetables, herbs and many plant foods is inversely related to the incidence of several degenerative diseases, highlighting the increased consumer attention to the importance of a balanced diet in relation to human health. It has been estimated that a balanced diet may provide around 1 g of polyphenols daily. Polyphenols are able to (a) react with free radicals blocking their activity, (b) modulate the expression of genes [epigenetics] involved in metabolism, act as signaling molecules increasing antioxidant defense, and (c) protect and repair DNA damage. Our research efforts focus on the molecular mechanisms that correlate the health benefits of polyphenols against the most common diseases related to oxidative stress driven pathologies, including neurodegenerative, cancer, cardiovascular diseases, inflammation, type II diabetes and metabolic syndrome diseases.
Another characteristic feature of polyphenols is their interactions with peptides and proteins. Animal and human studies have demonstrated that dietary flavonoids from chocolate, including (-)epicatechin, promote cardiovascular health, the result of antioxidant and antithrombotic mechanisms. The consumption of dark chocolate increases blood plasma (-)epicatechin, these effects are diminished when consumed with milk/milk chocolate. This indicates not only milk proteins, but also other dietary foods may interact/impair/reduce bioavailability and the absorption of flavonoids from chocolate in vivo negating the potential health benefits from dark chocolate. A high cocoa flavanol intervention enhances dentate gyrus function, improving cognition in older adults, most likely by the improved vascular function of (-)-epicatechin. Fortunately the ubiquitous polyphenol-protein interactions also have beneficial effects. The propensity of certain natural polyphenols to interact with Aβ42 monomers, blocks their rapid self-association to form low molecular weight oligomers, enables polyphenols to function as Aβ42 inhibitors. One of our challenges in designing Aβ42 inhibitors is to find the chemical ‘Polyphenol Lipinskinisation’ changes necessary, that is, to modify polyphenol structures to improve their pharmacokinetics and efficacy. Aβ42 peptides misfold into soluble oligomers and protofibrils associated with AD. The mechanism of Aβ inhibition is driven by hydrophobic interactions that involve π-π bonding between the planar faces of the polyphenol structure and the aromatic residues of Aβ42. Additionally, hydrogen bonding occurs between the peptide and the phenolic hydroxyl groups. The polyphenols intercede/impose between two β42-amyloid aromatic residues prevents their ϖ-ϖ stacking, blocking the amyloid self-assembly-β-oligomer-sheet-fibril formation and gain of toxic function. Herbal polyphenols are known to also modulate Aβ production by stimulating the α-secretase and inhibiting the β-site amyloid precursor protein cleaving enzyme-1 (BACE1), γ-protease pathways. (−)-Epicatechin, epigallocatechin are potent inhibitors of amyloid precursor protein processing (APP). Some phenolics show both, a strong inhibition of APP-Aβ generation and anti-amyloidogenic binding (Hügel and Jackson, 2012). The flavonoids quercetin and myricetin (Figure) inhibit BACE1 activity, dose-dependently inhibit amyloid fibril formation with myricetin > quercetin > catechin = epicatechin. Similarly, EGCG, resveratrol, curcumin, oleuropein, pentagalloylglucose inhibit β-amyloid misfolding and aggregation by forming nontoxic complexes with the peptide, they also have other benefits against the onset of neurodegeneration. EGCG directly interacts with β-sheet structures in amyloid fibrils leading to an decrease in the binding of Aβ to the fluorescent dye thioflavin T and promotes the assembly of large, spherical oligomersintosafe species, unable to seed fibrillogenesis; remodels Aβ mature fibrils into smaller, amorphous protein aggregates by direct binding to the β-sheet-rich aggregates and mediating a conformational change without generating potentially toxic oligomers (Ehrnhoefer et al., 2008). Curcumin is the main constituent of the spice turmeric, whose extensive use apparently accounts for the lower prevalence of AD in the Indian population. In vitro curcumin inhibits fibril formation and also destabilizes preformed fibrils, binds to plaques and reduces amyloid levels in vivo. Curcumin and resveratrol bind to the N-terminus (residues 5−20) of Aβ42 monomers. Many in vitro studies have demonstrated the multiple potential therapeutic effects of resveratrol, found in herbs, red wine, but its in vivo efficacy is controversial. The beneficial effects of resveratrol may contribute to its protective effects on cognitive function, however the volume of red wine to be consumed for resveratrol therapy is not practicable. Danshen constituents, salvianolic acid B, rosmarinic acid, tanshinones inhibit Aβ42, disaggregate fibrils, and protect cultured cells. Oleuropein in olive oilpreventsformation of β-amyloid oligomers, and dietary oleuropein aglycone improves the cognitive performance of young/middle-aged mice.
Figure.
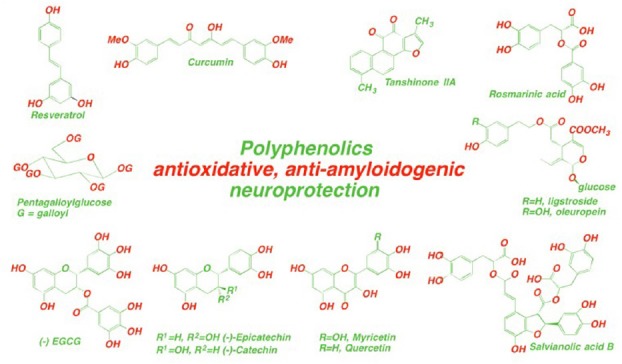
Structures of polyphenols found in widely consumed plant foods and herbs with cognitive enhancement and neurological protection properties against dementia.
Key points
The global population is growing, therefore also aging and dementia increases in almost all parts of the world.
Studies related to autosomal dominant AD indicate that the disease process begins around 20 years prior to onset of dementia (Bateman et al., 2012).
Recent research provides evidence that life style factors and environmental stresses that increase blood pressure may also increase the risk of AD through Angiotensin II-angiotensin type1a pathway (Liu et al., 2015).
There is a need to generate more healthy consumer food products, and with public passion, encourage early adoption of healthy lifestyle — better diets and regular exercise as preventive strategies to reduce cognitive impairment.
To explain the extended AD pathogenic process over 2 decades, amyloidosis, the incremental neuronal damage caused by non-sequestered β-oligomers is the early pathological event and accumulates over time eventually resulting in neurodegeneration (hypometabolism) and then widespread cognitive impairment (Narayan et al., 2014; Yau et al., 2015).
(−)-Epicatechin and other flavonoid inhibitors of BACE1 proteolysis/cleavage of APP could be protective against early amyloidosis events of AD provided they are included in the diet. Studies suggest the efficacy of orally delivered (−)-epicatechin in a transgenic model of AD in reducing Aβ42 production and pathology via modulation of BACE1 as a risk reduction strategy, supporting the positive effects of flavonoid rich diets against the development of cognitive impairment (Cox et al., 2015).
The increase intake of flavonoids and polyphenols is a dietary preventative strategy to (a) reduce β-amyloid formation and (b) competitively prevent β-amyloid misfolding and toxicity against development of AD. A key question is: can these findings translate into preventative benefit for healthy humans?
The protective effects of flavonoid rich diets against the development of dementia needs to be translated into clinical trials to directly test their efficacy in at risk individuals or those with mild cognitive impairment.
In conclusion, despite extensive knowledge about how diet and nutrition has advanced beyond understanding cellular energy status, diet related chronic diseases of modern society are now the single largest cause of death. Epidemiological investigations indicate that nutrition and dietary patterns are modifiable risk factors that can help limit and prevent chronic diseases, enabling the achievement of the overall objective in slowing human aging diseases such as AD and thereby improving the quality of healthspan of everyone.
For the opportunity to perform AD and dementia chemical science research, the authors thank RMIT University, School of Applied Sciences.
References
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CJ, Choudhry F, Peacey E, Perkinton MS, Richardson JC, Howlett DR, Lichtenthalter SF, Francis PT, Williams RJ. Dietary (−)-epicatechin as a potent inhibitor of βã-secretase amyloid precursor protein processing. Neurobiol Aging. 2015;36:178–187. doi: 10.1016/j.neurobiolaging.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE. EGCG redirects amyloidogenic polypeptides into unstructured off-pathway oligomers. Nat Struct Mol Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- Hügel HM. Brain food for AD-free ageing: focus on herbal medicines. Natural compounds as therapeutic agents for amyloidogenic disease. Adv Exp Med Biol. 2015;863:95–116. doi: 10.1007/978-3-319-18365-7_5. [DOI] [PubMed] [Google Scholar]
- Hügel HM, Jackson N. Danshen diversity defeating dementia. Bioorg Med Chem Lett. 2014;24:708–716. doi: 10.1016/j.bmcl.2013.12.042. [DOI] [PubMed] [Google Scholar]
- Hügel HM, Jackson N. Herbs and Dementia: a focus on dietary herbs and antioxidants. Diet and Nutrition in Dementia and Cognitive Decline, Academic Press. 2014 [Google Scholar]
- Liu J, Liu S, Matsumoto Y, Murakami S, Sugakawa Y, Kami A, Tanabe C, Maeda T, Michikawa M, Komano H, Zou K. Angiotensin type 1a receptor deficiency decreases amyloid β-protein generation and ameliorates brain amyloid pathology. Sci Rep. 2015;5:12059. doi: 10.1038/srep12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P, Holmström KM, Kim DH, Whitcomb DJ, Wilson MR, St. Georg-Hyslop P, Wood NW, Dobson CM, Cho K, Abramov AY, Klenerman D. Rare individual amyloid-β oligomers act on astrocytes to initiate neuronal damage. Biochem. 2014;53:2442–2453. doi: 10.1021/bi401606f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulijaszek SJ, Mann N, Elton S. Cambridge University Press; 2012. Evolving human nutrition: implications for public health. [Google Scholar]
- Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11:276–289. doi: 10.1038/nrcardio.2014.26. [DOI] [PubMed] [Google Scholar]
- Yau WY, Tudorascu DL, McDade EM, Ikonomovic S, James JA, Minhas D, Mowrey W, Sheu LK, Snitz BE, Weissfeld L, Gianaros PJ, Aizenstein HJ, Price JC, Mathis CA, Lopez OL, Klunk WE. Longitudinal assessment of neuroimaging and clinical markers in autosomal dominant Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2015;14:804–813. doi: 10.1016/S1474-4422(15)00135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Huan XD, Cao Q, Yang J. Cardioprotective effect of epigallocatechin-3-gallate against myocardial infarction in hypercholesterolemic rats. Exp Ther Med. 2015;9:405–410. doi: 10.3892/etm.2014.2135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Alzheimer's disease facts and figures (2015) Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]


