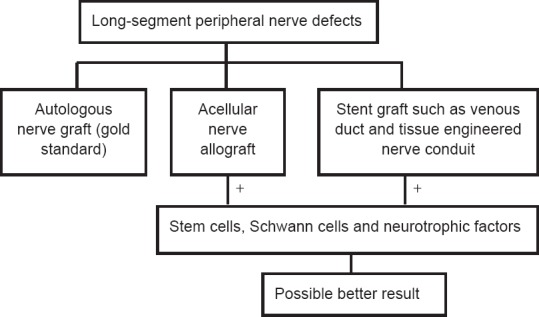
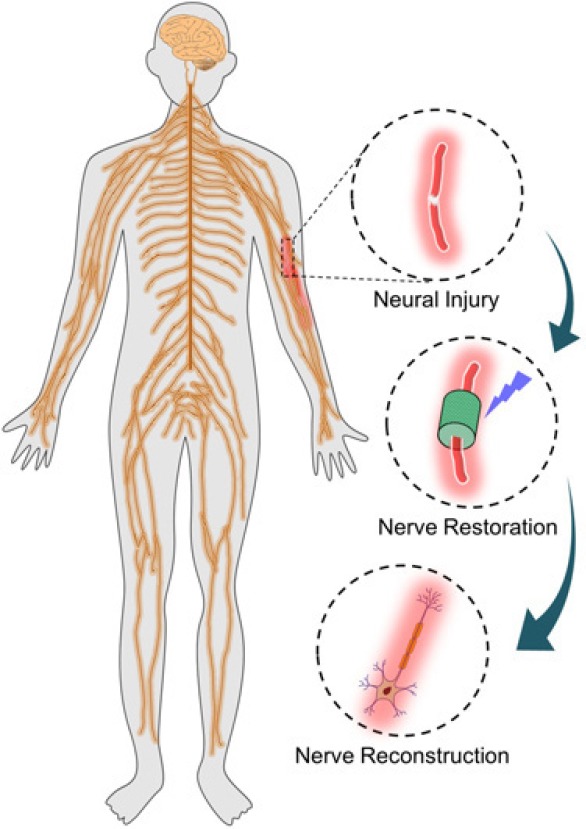
1778 Repair of long-segment peripheral nerve defects
1779 Bionic reconstruction of hand function after adult brachial plexus root avulsion
1780 Optimized design of regeneration material for the treatment of peripheral nerve injury
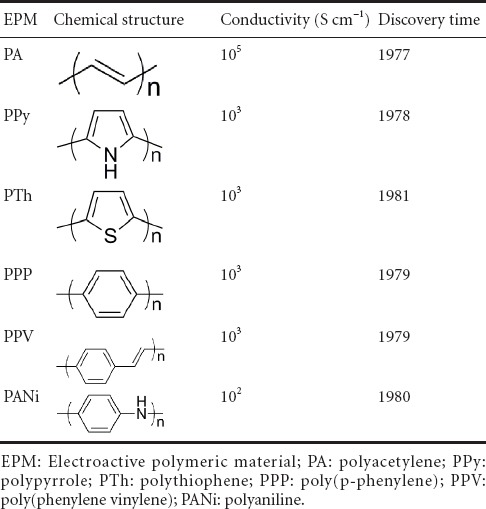
1781 Synergism of electroactive polymeric materials and electrical stimulation promotes peripheral nerve repair
1783 Schwann cell effect on peripheral nerve repair and regeneration
1785 Biomaterials with specific topological structures for the repair and regeneration of injured peripheral nerves
1787 Drug control of Schwann cells during peripheral nerve repair
1788 In vivo transplantation of green fluorescent protein-labeled neural stem cells delays denervation-induced muscle atrophy
1789 Key issues of stem cell therapy for peripheral nerve injury
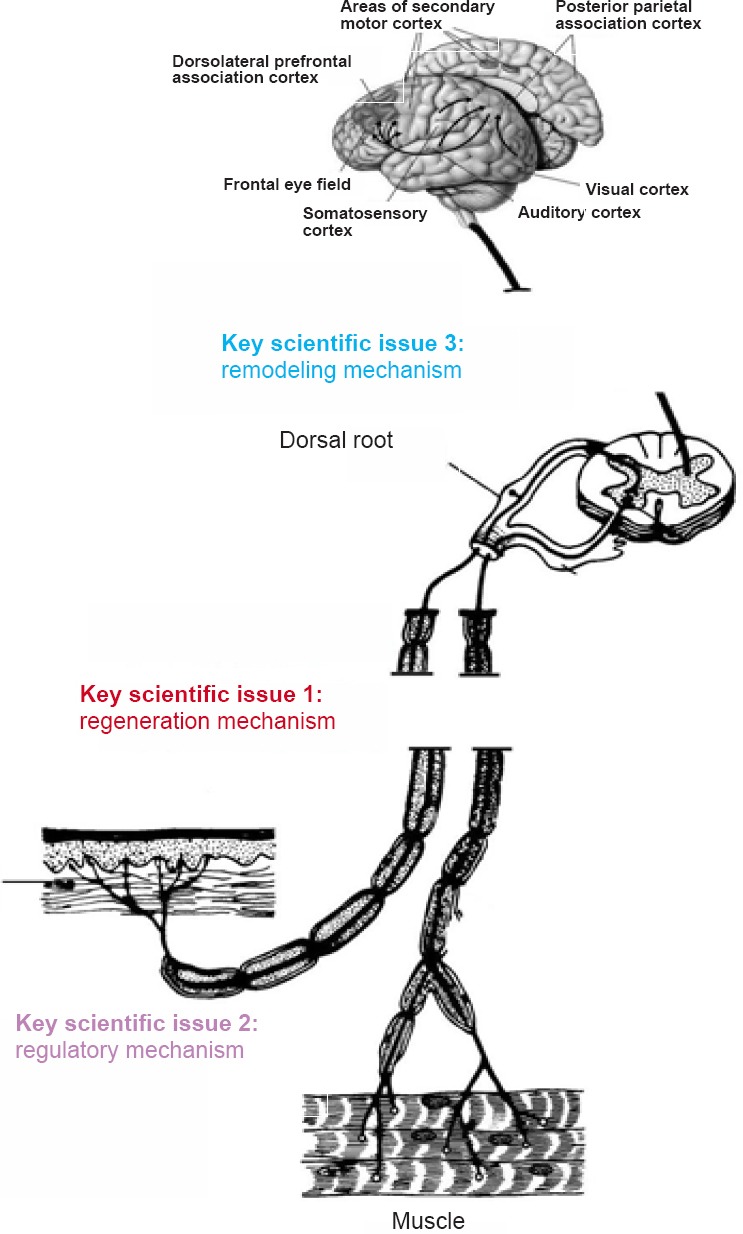
1790 Repair, regeneration and remodeling of injured peripheral nerves
1792 Molecular mechanism of the regulation of neuron-intrinsic regeneration
1793 Neurotropism in peripheral nerve regeneration
1794 Intervention, repair, and protection of nerve injury in carpal tunnel syndrome
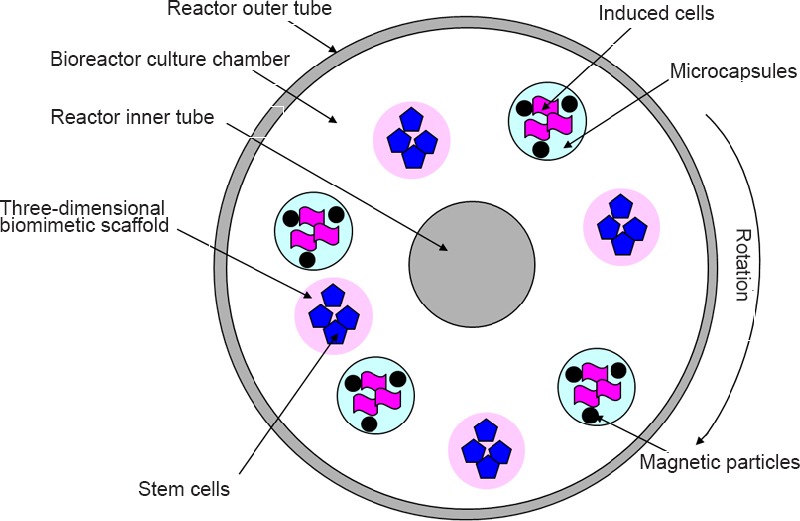
1795 Significance and strategy of promoting seed cell survival in tissue-engineered artificial nerve construction
1796 Challenges for repairing peripheral nerve defects using 3D nerve grafts with nerve tissue-derived extracellular matrix and basement membrane tube-like conduits
First authors and corresponding authors:
Shan-lin Chen* Department of Hand Surgery, Beijing Jishuitan Hospital
Zeng-gan Chen* Department of Orthopedic Surgery, Zhongshan Hospital, Fudan University
Hong-lian Dai#, * State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology; Biomedical Materials and Engineering Research Center of Hubei Province
Jian-xun Ding#, * Key Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences
Jia-song Guo* Department of Histology and Embryology, Southern Medical University; Key Laboratory of Tissue Construction and Detection of Guangdong Province; Institute of Bone Biology, Academy of Orthopedics
Na Han#, * Peking University People's Hospital
Bao-guo Jiang* Peking University People's Hospital
Hua-jun Jiang#, * Department of Orthopedics, First Affiliated Hospital of Dalian Medical University
Juan Li# Department of Orthopedic Surgery, Zhongshan Hospital, Fudan University
Shi-pu Li* State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology; Biomedical Materials and Engineering Research Center of Hubei Province
Wen-jun Li# Department of Hand Surgery, Beijing Jishuitan Hospital
Jing Liu#, * The First Affiliated Hospital of Dalian Medical University
Yang Liu# Laboratory of Biotechnology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences
Jun-xiong Ma# Department of Orthopedics, General Hospital of Shenyang Military Area Command of Chinese PLA, Rescue Center of Severe Wound and Trauma of Chinese PLA
Jiang Peng#, * Institute of Orthopedics, Chinese PLA General Hospital
Yun-dong Shen# Department of Hand Surgery, Huashan Hospital Affiliated to Fudan University
Guang-wei Sun* Laboratory of Biotechnology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences
Pei-fu Tang* Department of Orthopedics, the PLA General Hospital Gu-heng Wang# Department of Hand Surgery, Affiliated Hospital of Nantong University
Xiang-hai Wang# Department of Histology and Embryology, Southern Medical University
Liang-bi Xiang* Department of Orthopedics, General Hospital of Shenyang Military Area Command of Chinese PLA, Rescue Center of Severe Wound and Trauma of Chinese PLA
Ren-guo Xie* Trauma Center, Department of Orthopedic Surgery, Shanghai First People's Hospital, Shanghai Jiao Tong University School of Medicine
Jian-guang Xu* Department of Hand Surgery, Huashan Hospital Affiliated to Fudan University
Bin Yu* Jiangsu Provincial Key Laboratory of Nerve Regeneration of Nantong University, Nerve Regeneration Collaborative Innovation Center
Li-cheng Zhang# Department of Orthopedics, the PLA General Hospital
Pei-xun Zhang#, * Peking University People's Hospital
Song-lin Zhou# Jiangsu Provincial Key Laboratory of Nerve Regeneration of Nantong University, Nerve Regeneration Collaborative Innovation Center
Note: Arranged vertically in alphabetical order by their last name; #First author; *Corresponding author