Abstract
Evidence suggests that interleukin-10 (IL-10) deficiency exacerbates inflammation and worsens the outcome of brain ischemia. In view of the critical role of the single nucleotide polymorphic sites -1082 (A/G) and -819 (C/T) in the promoter region of the IL-10 gene, we hypothesized that they are associated with cerebral infarction morbidity in the Chinese Han population. We genotyped these allelic gene polymorphisms by amplification refractory mutation system-polymerase chain reaction methods in 181 patients with cerebral infarction (cerebral infarction group) and 115 healthy subjects (control group). We identified significant differences in genotype distribution and allele frequency of the IL-10-1082 A/G allele between cerebral infarction and control groups (χ2 = 6.643, P = 0.010). The IL-10-1082 A allele frequency was significantly higher in the cerebral infarction group (92.3%) than in the control group (86.1%) (P = 0.015). Moreover, cerebral infarction risk of the AA genotype was 2-fold higher than with the AG genotype (OR = 2.031, 95%CI: 1.134–3.637). In addition, AA genotype together with hypertension was the independent risk factor of cerebral infarction (OR = 2.073, 95%CI: 1.278–3.364). No statistical difference in genotype distribution or allele frequency of IL-10-819 C/T was found between cerebral infarction and control groups (P > 0.05). These findings suggest that the IL-10-1082 A/G gene polymorphism is involved in cerebral infarction, and increased A allele frequency is closely associated with occurrence of cerebral infarction.
Keywords: neural regeneration, IL-10, promoter, gene polymorphisms, ischemic stroke, genetic susceptibility, inflammation, immune response, ischemia/reperfusion injury, neural regeneration
Introduction
Cerebral infarction is one of the primary causes of long-term functional disability (Liu et al., 2010, 2013b), and results in residual impairment that diminishes a patient's independence and quality of life (Nowak, 2008). Despite advances in medical, thrombolytic, and surgical treatments, the treatment of cerebral infarction still lacks an optimal method (Liu et al., 2011b). Recently, the action of non-specific inflammation has shed light on ischemia/reperfusion injury (Daemen et al., 1999; Collard and Gelman, 2001; Jong et al., 2003; Yellon and Hausenloy, 2007), and is known to play a critical role during cerebral infarction. Cerebral ischemia induces a strong immune response and consequent inflammation, which is characterized by activated cytokines, chemokines, adhesion molecules, and proteolytic enzymes that exacerbate tissue damage (del Zoppo et al., 2000; Emsley and Tyrrell, 2002; Wang et al., 2013; Tu et al., 2014). However, the pathogenesis of cerebral infarction is very complicated (Liu et al., 2013a). Interleukin-10 (IL-10) is a pleiotropic cytokine that can exert anti-inflammatory effects during cerebral ischemia injury. The IL-10 gene promoter is highly polymorphic, with multiple single nucleotide polymorphic sites. The most important three sites are -1082 (A/G), -819 (C/T), and -592 (C/A) (Perrey et al., 1999). In this study, we focused on the relationship between IL-10 gene promoter polymorphisms and cerebral infarction. Accordingly, we used amplification refractory mutation system-polymerase chain reaction (ARMS-PCR) to detect the distribution frequency of -1082 (A/G) and -819 (C/T) in cerebral infarction. Our study supports the notion that IL-10 gene deficiency determines genetic susceptibility to cerebral infarction.
Subjects and Methods
Subjects
The patients included in this study were inpatients of the Department of Neurology in Union Hospital of Fujian Medical University, China from August 2011 to February 2013. In the cerebral infarction group, there were 181 cases, consisting of 123 males and 58 females, aged 66.1 ± 10.5 years. In the control group, there were 115 healthy subjects, consisting of 68 males and 47 females, aged 65.4 ± 11.6 years. There were no differences in age and gender between the groups (P < 0.05).
All cerebral infarction patients met the International Classification of Diseases, 10th revision (ICD-10) cerebral infarction classification criteria (Organization, 2004). All healthy control subjects had received a physical examination in Union Hospital of Fujian Medical University, China.
Patients with any of the following diseases were excluded: cardiac embolism, vasculitis, trauma, thyroid disease, blood disease, use of immunosuppressive agents, cancer, pregnancy, autoimmune diseases, infection, cerebral vascular malformation, or aneurysm.
The study was approved by the Ethics Committee of Fujian Medical University on the basis of the Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. All patients provided written informed consent.
Extraction of genomic DNA
Two mL samples of fasting antecubital venous blood from both groups were placed in EDTA-Na anticoagulant tubes. Genomic DNA extraction was performed according to the instructions of the DNA extraction kit (Promega Corporation, Madison, WI, USA). The final concentration of DNA working solutions was 0.1 g/L. Working solutions were stored at −20°C.
IL-10 promoter polymorphism detection
Genetic testing primers were synthesized by Shanghai Bioengineering Technology Company (Shanghai, China). PCR amplification was performed (MBI Fermentas, Shanghai, China) according to a previously described method (Perrey et al., 1999). The IL-10-1082 A/G and -819 C/T gene loci primers are:
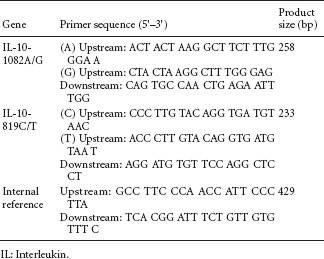
Gene polymorphisms were genotyped by ARMS (Newton et al., 1989), which was performed in 25 μL volumes containing 25-50 ng genomic DNA, 0.25 mM specific primers, and 0.1 mM internal control primers in the presence of 1.5 mM MgCl2, 0.2 mM each dNTP, 500 mM KCl, 200 mM Tris-HCl, 0.001% gelatin, and 0.5 U Taq DNA polymerase. Internal reference primers were used to identify successful PCR amplification, and amplified a conserved region of exon 3 of the HLA-DRB1 gene. ARMS-PCR for amplification of the IL-10-1082 G/A and -819 C/T alleles was performed under the following conditions: amplification with a 1 minute denaturation step at 95°C, then 10 cycles of 15 seconds at 95°C, 50 seconds at 65°C, and 40 seconds at 72°C, and 20 cycles of 20 seconds at 95°C, 50 seconds at 59°C, and 30 seconds at 72°C, followed by cooling to 4°C (Perrey et al., 1999). After PCR amplification, PCR products were resolved by 2% agarose gel electrophoresis and visualized under ultraviolet light, using a 100 bp ladder for sizing. Gene bands from two cases were sequenced by Tiangen Sequencing Biotechnology Co., Ltd. (Beijing, China).
Statistical analysis
Data analysis was performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Allele frequency was calculated as: (2 × homozygotes + heterozygotes)/(2 × the number of patients). Representative samples from each group were analyzed using the Hardy-Weinberg balance method. Categorical data [n(%)] were analyzed by chi-square test. Measurement data (mean ± SD) characterized by normal distributions were analyzed by two-sample t-tests. Non-conditional probability logistic regression analysis was used to eliminate the potential influence of potential and confounding factors. Values of P < 0.05 were considered statistically significant.
Results
Demographics between cerebral infarction and control groups
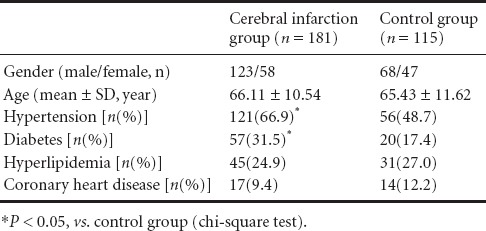
There were no significant differences in age, gender, hyperlipidemia onset, and coronary heart disease onset between cerebral infarction and control groups. The proportion of hypertension and diabetes in the cerebral infarction group was significantly higher than in the control group (P < 0.05; Table 1).
Table 1.
General information for cerebral infarction and control groups
Gene polymorphism analysis of the IL-10-1082 A/G locus
The electrophoretic band at 258 bp represented the homozygote alleles, either G (GG) or A (AA). Using the same two pairs of primers, another electrophoretic band at 258 bp represented the heterozygote allele (AG) (Figure 1). Representative sequencing results are shown in Figure 2.
Figure 1.
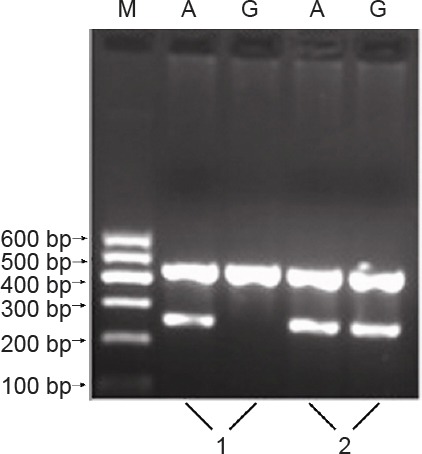
Genotyping electrophoresis pattern for the interleukin (IL)- 10 promoter region -1082 locus.
1: AA genotype; 2: AG genotype; M: marker.
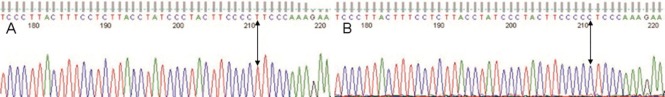
Figure 2.
Reverse sequencing map of the interleukin (IL)-10 promoter region -1082 locus (arrows).
(A) T (as the complementary base of A) was detected at 210 bp; (B) C (as the complementary base of G) was detected at 210 bp.
Gene polymorphism analysis of the IL-10-819 C/T locus
The electrophoretic band at 233 bp represented the homozygote alleles, either C (CC) or T (TT). Using the same two pairs of primers, another electrophoretic band at 233 bp represented the heterozygote allele (CT) (Figure 3). Representative sequencing results are shown in Figure 4.
Figure 3.
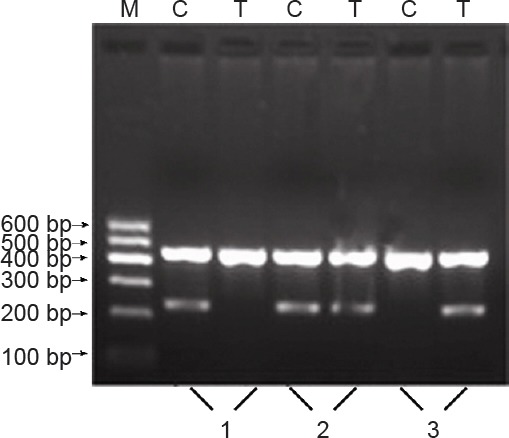
Genotyping electrophoresis pattern for the interleukin (IL)- 10 promoter region -819 locus.
1: CC genotype; 2: CT genotype; 3: TT genotype; M: marker.
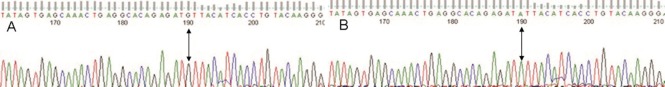
Figure 4.
Reverse sequencing map of the interleukin (IL)-10 promoter region -819 locus (arrows).
(A) G (as the complementary base of C) was detected at 190 bp; (B) A (as the complementary base of T) was detected at 190 bp.
IL-10-1082 A/G and -819 C/T genotype and allele frequencies
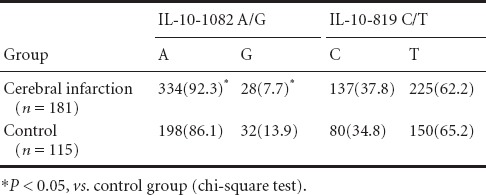
There were significant differences in IL-10-1082 A/G genotype distribution frequency between cerebral infarction and control groups [AA: 153 cases (84.5%) vs. 83 cases (72.2%); AG: 28 cases (15.5%) vs. 32 cases (27.8%) (χ2 = 6.643, P = 0.010)]. For the IL-10-1082 locus, the A allele frequency in the cerebral infarction group was significantly higher than in the control group (92.3% vs. 86.1%; χ2 = 5.894, P = 0.015). There were no significant differences in IL-10-1082 A/G and -819 C/T genotype and allele frequencies between both groups (P < 0.05) (Tables 2 and 3).
Table 2.
Comparison of interleukin (IL)-10-1082 A/G and -819 C/T genotype frequency [n(%)] between cerebral infarction and control groups
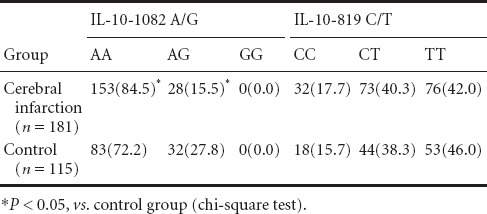
Table 3.
Comparison of interleukin (IL)-10-1082 A/G and -819 C/T allele frequency [n(%)] between cerebral infarction and control groups
Risk factors of cerebral infarction
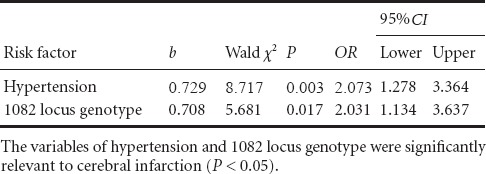
To exclude confounding factors, we analyzed possible factors related to cerebral infarction, specifically, hypertension, diabetes, and IL-10-1082 genotype. Stepwise multiple linear regression and non-conditional logistic regression analyses were performed, in which the three factors served as independent variables and cerebral infarction as the dependent variable. The results were determined using 0.05 and 0.10 as significant standard introducing and eliminating variables, respectively. We found that two independent variables, namely hypertension and 1082 locus genotype, were significantly relevant to cerebral infarction (P < 0.05; Table 4).
Table 4.
Non-conditional logistic regression analysis of risk factors for cerebral infarction
Discussion
Pathogenesis of ischemic brain damage is complicated, with non-specific inflammation mediated by pro-inflammatory cytokines and chemokines and other inflammatory mediators playing an important role (del Zoppo et al., 2000; Emsley and Tyrrell, 2002; Min et al., 2013; Zhou et al., 2014). Interleukin-10, a pleiotropic anti-inflammatory factor, plays a protective role in cerebral ischemia models (Moore et al., 2001; Liu et al., 2009). It prevents downregulation of the anti-apoptotic protein Bcl-2 in ischemic brain tissue, and limits excitotoxic damage to the brain through an activin-dependent mechanism (Liu et al., 2007). Ischemic insult and IL-10 administration augments neurogenesis in the rodent brain (Dietrich et al., 1999; Vila et al., 2003; Woiciechowsky et al., 2004; Liu et al., 2007). Hence, these findings suggest that IL-10 contributes to reduction of infarct volume and neurological improvement after experimental focal cerebral ischemia. The protective mechanism of IL-10 is achieved by downregulating expression of IL-1β, tumor necrosis factor alpha (TNF-α), P-selectin and other pro-inflammatory cytokines, and reducing neuronal apoptosis (Liu et al., 2007; Liu et al., 2009). Previous studies have shown a close association between P-selectin, IL-6 promoter polymorphism, and cerebral infarction (Zee et al., 2004; Tong et al., 2010). However, little is known about the association between IL-10 gene polymorphism and cerebral infarction.
The IL-10 gene promoter region is approximately 5 kb and located within the region upstream of the translation initiation site (Turner et al., 1997; D’Alfonso et al., 2000). The IL-10 gene promoter is highly polymorphic with multiple single nucleotide polymorphic sites. The three most important ones are -1082 (A/G), -819 (C/T), and -592 (C/A) (Perrey et al., 1999). Here, we found that the genotype distribution of the IL-10 promoter in the Chinese Han population is consistent with the results of a previous study (Meenagh et al., 2002). Specifically, the frequency of G allele carriers of the -1082 locus is significantly lower in the Chinese Han population than that in Ireland, Mexico, and other races. Additionally, our data shows that the -1082 locus GG genotype is not detectable in the Chinese Han population, and only the AA and/or AG genotypes are present. These findings indicate that gene polymorphisms of some inflammatory cytokines differ depending upon ethnicity. Based on the finding that IL-10 genetic linkage at the single nucleotide polymorphic sites, -819 and -592, are linked (Perrey et al., 1999), we assumed -592 genotypes from -819 genotypes, instead of directly examining IL-10-592 loci polymorphic sites.
Interleukin-10 gene promoter polymorphisms are closely related to IL-10 expression levels in vivo. Studies indicate that -1082G, -819C, and -592C are highly expressed alleles compared with -1082A, -819T, and -592A (Hoffmann et al., 2001). In the presence of allele -1082A, stimulation of lymphocytes with concanavalin A results in lower IL-10 production than in allele -1082A-negative cells (Turner et al., 1997; Koch et al., 2001). These studies also found that protein production is independent of alleles -819 and -592, suggesting that IL-10 levels are determined mainly by the -1082 genotype. Here, we found a statistical difference in distribution of the IL-10-1082 locus between cerebral infarction and control groups, suggesting that cerebral infarction risk is 2-fold higher with the AA genotype than the AG genotype. Therefore, the AA genotype together with hypertension is the independent risk factor of cerebral infarction. Interestingly, our results show that the A allelic gene frequency in cerebral infarction patients (92.3%) is higher than normal controls (86.1%). This was not observed for IL-10-819 C/T and-592 C/A genotype distributions and allele frequencies, suggesting that the incidence of cerebral infarction is related to increased frequency of the IL-10-(-1082 A/G) A allele.
Cerebral infarction is associated with an extremely high rate of disability (Liu et al., 2011a). For a long time, clinical experts have formed a consensus on its clinical risk factors, i.e., hypertension, diabetes, and hyperlipidemia. Generally, the incidence of cerebral infarction can be significantly reduced through effective interventions to these risk factors. However, these known risk factors are not found in a considerable proportion of patients, even with a thorough medical history and physical examination. Thus, we found significant correlation between the IL-10-1082 gene polymorphism and cerebral infarction. Further studies regarding the IL-10-1082 gene polymorphism are needed, with consideration of a different experimental design. Detection of cerebral infarction susceptibility genes is conducive to identifying populations at disease risk, and improving the effectiveness of primary prevention.
Acknowledgments
We would like to thank our colleague Xiao-dong Pan for language revision.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81171867; a grant from the Key Research Program of Fujian Department of Science and Technology of China, No. 2011Y0027.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded, stringently reviewed by international expert reviewers.
Copyedited by James R, Raye W, Wang J, Li CH, Song LP, Zhao M
References
- Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133–1138. doi: 10.1097/00000542-200106000-00030. [DOI] [PubMed] [Google Scholar]
- D’Alfonso S, Rampi M, Rolando V, Giordano M, Momigliano-Richiardi P. New polymorphisms in the IL-10 promoter region. Genes Immun. 2000;1:231–233. doi: 10.1038/sj.gene.6363666. [DOI] [PubMed] [Google Scholar]
- Daemen MA, van ‘t Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, Buurman WA. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Brain Pathol. Vol. 10. Zurich, Switzerland: 2000. Inflammation and stroke: putative role for cytokines adhesion molecules and iNOS in brain response to ischemia; pp. 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Busto R, Bethea JR. Postischemic hypothermia and IL-10 treatment provide long-lasting neuroprotection of CA1 hippocampus following transient global ischemia in rats. Exp Neurol. 1999;158:444–450. doi: 10.1006/exnr.1999.7115. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 2002;22:1399–1419. doi: 10.1097/01.WCB.0000037880.62590.28. [DOI] [PubMed] [Google Scholar]
- Hoffmann SC, Stanley EM, Darrin Cox E, Craighead N, DiMercurio BS, Koziol DE, Harlan DM, Kirk AD, Blair PJ. Association of cytokine polymorphic inheritance and in vitro cytokine production in anti-CD3/CD28-stimulated peripheral blood lymphocytes. Transplantation. 2001;72:1444–1450. doi: 10.1097/00007890-200110270-00019. [DOI] [PubMed] [Google Scholar]
- Jong WM, Reitsma PH, ten Cate H, de Winter RJ. Modified two-step model for studying the inflammatory response during myocardial ischemia and reperfusion in mice. Comp Med. 2003;53:522–526. [PubMed] [Google Scholar]
- Koch W, Kastrati A, Bottiger C, Mehilli J, von Beckerath N, Schomig A. Interleukin-10 and tumor necrosis factor gene polymorphisms and risk of coronary artery disease and myocardial infarction. Atherosclerosis. 2001;159:137–144. doi: 10.1016/s0021-9150(01)00467-1. [DOI] [PubMed] [Google Scholar]
- Liu N, Du HW, Chen RH, Zheng A, Huang HP, Wu ZH. The effect of interleukin-10 on neurocyte apoptosis in cerebral ischemia in rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2007;23:498–500. [PubMed] [Google Scholar]
- Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J. Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol. 2009;6:207–213. doi: 10.1038/cmi.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Huang H, Lin F, Chen A, Zhang Y, Chen R, Du H. Effects of treadmill exercise on the expression of netrin-1 and its receptors in rat brain after cerebral ischemia. Neuroscience. 2011a;194:349–358. doi: 10.1016/j.neuroscience.2011.07.037. [DOI] [PubMed] [Google Scholar]
- Liu N, Zhang Y, Fan L, Yuan M, Du H, Cheng R, Liu D, Lin F. Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by survivin on experimental stroke in rats. J Transl Med. 2011b;9:105. doi: 10.1186/1479-5876-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Mei Z, Qian J, Zeng Y, Wang M. Puerarin partly counteracts the inflammatory response after cerebral ischemia/reperfusion via activating the cholinergic anti-inflammatory pathway. Neural Regen Res. 2013a;8:3203–3215. doi: 10.3969/j.issn.1673-5374.2013.34.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang Y, Lin L, Lin F, Li T, Du H, Chen R, Zheng W, Liu N. Effects of bone marrow-derived mesenchymal stem cells on the axonal outgrowth through activation of PI3K/AKT signaling in primary cortical neurons followed oxygen-glucose deprivation injury. PloS One. 2013b;8:e78514. doi: 10.1371/journal.pone.0078514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, Savant-Bhonsale S, Chopp M. Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab. 2010;30:1288–1295. doi: 10.1038/jcbfm.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meenagh A, Williams F, Ross OA, Patterson C, Gorodezky C, Hammond M, Leheny WA, Middleton D. Frequency of cytokine polymorphisms in populations from western Europe, Africa, Asia, the Middle East and South America. Hum Immunol. 2002;63:1055–1061. doi: 10.1016/s0198-8859(02)00440-8. [DOI] [PubMed] [Google Scholar]
- Min L, Shao S, Wu X, Cong L, Liu P, Zhao H, Luo Y. Anti-inflammatory and anti-thrombogenic effects of atorvastatin in acute ischemic stroke. Neural Regen Res. 2013;8:2144–2154. doi: 10.3969/j.issn.1673-5374.2013.23.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Ann Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Newton CR, Graham A, Heptinstall LE, Powell SJ, Summers C, Kalsheker N, Smith JC, Markham AF. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS) Nucleic Acids Res. 1989;17:2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DA. The impact of stroke on the performance of grasping: usefulness of kinetic and kinematic motion analysis. Neurosci Biobehav Rev. 2008;32:1439–1450. doi: 10.1016/j.neubiorev.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Perrey C, Turner SJ, Pravica V, Howell WM, Hutchinson IV. ARMS-PCR methodologies to determine IL-10 TNF-alpha TNF-beta and TGF-beta 1 gene polymorphisms. Transpl Immunol. 1999;7:127–128. doi: 10.1016/s0966-3274(99)80030-6. [DOI] [PubMed] [Google Scholar]
- Tong Y, Wang Z, Geng Y, Liu J, Zhang R, Lin Q, Li X, Huang D, Gao S, Hu D, Li Y, Cheng J, Lu Z. The association of functional polymorphisms of IL-6 gene promoter with ischemic stroke: analysis in two Chinese populations. Biochem Biophys Res Commun. 2010;391:481–485. doi: 10.1016/j.bbrc.2009.11.084. [DOI] [PubMed] [Google Scholar]
- Tu Q, Cao H, Zhong W, Ding B, Tang X. Atorvastatin protects against cerebral ischemia/reperfusion injury through anti-inflammatory and antioxidant effects. Neural Regen Res. 2014;9:268–275. doi: 10.4103/1673-5374.128220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Vila N, Castillo J, Davalos A, Esteve A, Planas AM, Chamorro A. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke. 2003;34:671–675. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- Wang H, Guo W, Liu H, Zeng R, Lu M, Chen Z, Xiao Q. Inhibition of inflammatory mediator release from microglia can treat ischemic/hypoxic brain injury. Neural Regen Res. 2013;8:1157–1168. doi: 10.3969/j.issn.1673-5374.2013.13.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woiciechowsky C, Schoning B, Stoltenburg-Didinger G, Stockhammer F, Volk HD. Brain-IL-1 beta triggers astrogliosis through induction of IL-6: inhibition by propranolol and IL-10. Med Sci Monit. 2004;10:BR325–330. [PubMed] [Google Scholar]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, Liu JS, Youssef AA, Chang HW. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- Zee RY, Cook NR, Cheng S, Reynolds R, Erlich HA, Lindpaintner K, Ridker PM. Polymorphism in the P-selectin and interleukin-4 genes as determinants of stroke: a population-based prospective genetic analysis. Hum Mol Genet. 2004;13:389–396. doi: 10.1093/hmg/ddh039. [DOI] [PubMed] [Google Scholar]
- Zhou F, Wang L, Liu P, Hu W, Zhu X, Shen H, Yao Y. Puerarin protects brain tissue against cerebral ischemia/reperfusion injury by inhibiting the inflammatory response. Neural Regen Res. 2014;9:2074–2080. doi: 10.4103/1673-5374.147934. [DOI] [PMC free article] [PubMed] [Google Scholar]