Abstract
Gonadotropin-releasing hormone (GnRH) and its synthetic analog leuprolide acetate, a GnRH agonist, have neurotrophic properties. This study was designed to determine whether administration of leuprolide acetate can improve locomotor behavior, gait, micturition reflex, spinal cord morphology and the amount of microglia in the lesion epicenter after spinal cord injury in rats. Rats with spinal cord compression injury were administered leuprolide acetate or saline solution for 5 weeks. At the 5th week, leuprolide acetate-treated rats showed locomotor activity recovery by 38%, had improvement in kinematic gait and exhibited voiding reflex recovery by 60%, as compared with the 1st week. By contrast, saline solution-treated rats showed locomotor activity recovery only by 7%, but voiding reflex did not recover. More importantly, leuprolide acetate treatment reduced microglial immunological reaction and induced a trend towards greater area of white and gray matter in the spinal cord. Therefore, leuprolide acetate has great potential to repair spinal cord injury.
Keywords: nerve regeneration, spinal cord injury, leuprolide acetate, gonadotropin-releasing hormone, neurotrophic factor, microglia, micturition reflex, gait, inflammation, neural regeneration
Introduction
Spinal cord injury (SCI) causes different and long-term neurological disorders and even morbidity in humans. There have been no effective interventions for any of the main types of neurological disorders after SCI, including motor and sensory deficits, bladder, bowel and sexual dysfunction, chronic pain, and autonomic dysreflexia. SCI results in acute as well as progressive secondary tissue damage, and initiates a number of regenerative and neuroprotective responses within the damaged nervous system. Possible therapies are directed to generate neuroprotection, regeneration or an enhancement in plasticity of the uninjured tissues (Hagg and Oudega, 2006).
There are different experimental approaches to counteracting the effects of SCI, such as the use of neurotrophic factors, Nogo neutralizing antibodies and N-metyl-D-aspartate receptor modulators (Schwab, 2004; Thuret et al., 2006). Neurohormone gonadotropin-releasing hormone (GnRH) has been recently shown to be neurotrophic. In vitro, GnRH administration induced changes in outgrowth, number and length of neurites in rat cortical neurons (Quintanar and Salinas, 2008). The receptor for this decapeptide has been identified in cerebral cortex neurons, spinal cord, bladder and other extra-pituitary tissues (Bahk et al., 2007; Quintanar et al., 2007, 2009). GnRH treatment improved locomotor activity and bladder function, and increased the expression of neurofilaments in spinal cords in rats with SCI (Calderon-Vallejo and Quintanar, 2012).
Leuprolide acetate (LA) is a synthetic agonist analog of GnRH. It is less susceptible to proteolysis and has a greater binding affinity to GnRH receptors than the natural hormone, increasing its biological activity (Periti et al., 2002). LA is used for disorders of the reproductive system such as prostate cancer. However, LA treatment has been shown to decrease the severity of clinical signs related to locomotion of rats with experimental autoimmune encephalomyelitis. It also induces a significant body weight gain together with increases in neurofilament and myelin basic protein expression and axonal diameter in the spinal cord (Guzmán-Soto et al., 2012).
One of the processes that occur in the injured spinal cord area is activation of the immune response. SCI leads to an early infiltration of macrophages/microglia. The initial inflammatory response is followed by an active phase of resolution which is necessary to repair the injury. Inflammatory response has undesirable effects and produces a greater neurological damage and neurodegeneration (Raposo and Schwartz, 2014).
The aim of this study was to determine whether administration of LA in rats with SCI can improve locomotion behavior, kinematic gait and maturation, promote the recovery of morphology of injured spinal cord, and increase the number of microglial cells.
Materials and Methods
Animals
Female Wistar rats, aged 8 months, weighing 250–330 g, from Universidad Autønoma de Aguascalientes, Mexico were included in this study. They were treated according to Institutional Welfare Regulations of The University Autonomous of Aguascalientes. This study was approved by Ethics Committee of Laboratory Animals Care, Universidad Autønoma de Aguascalientes, Mexico. All efforts were made to minimize animal discomfort and reduce the number of animals used. Animals were kept under 12-hour light/dark cycle and at controlled temperature. Purina chow and tap water were provided ad libitum.
Surgery
One month before SCI, both ovaries of rats were surgically removed through a dorsal incision under deep anesthesia with methyl ether, in order to avoid the effects of ovarian hormones. Ovariectomized animals were randomly divided into three groups: Sham SCI (sham; n = 9), SCI treated with physiological saline solution (SS; n = 11) and SCI treated with LA (LA; n = 10). Rat models of spinal cord compression injury were established according to a previously described method (Vanicky et al., 2001). A 2-French Fogarty catheter was inserted at T12 level and then advanced 1 cm cranially to the site where laminectomy was performed. The catheter balloon was inflated with 20 µL of 0.9% sodium chloride for 5 minutes and then deflated and removed. In the sham group, the catheter was introduced without inflating the balloon. From the day of surgery, manual bladder emptying was performed at least twice a day until reflex bladder control was re-established. All animals were injected with penicillin (Penprocilin; 5,000 IU, i.m.) once a day for 7 days and Neomelubrin (15 mg/kg; i.m.) once daily for 3 days. All animals were sacrificed 5 weeks later.
LA administration
Rats were administered LA (Sigma, St. Louis, MO, USA; 10 µg/kg, i.m.) once a day after SCI for 3 consecutive days. Thereafter, only one injection was given at the dose the same as that used in our previous experiments (Guzmán-Soto et al., 2012) every 4 days for 5 weeks. Rats in the SS group were identically given physiological saline solution (0.9% sodium chloride).
Locomotor behavior and kinematic gait evaluation
Locomotor activity evaluation was performed using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale (Basso et al., 1995), beginning 1 day after SCI and later once a week. Six evaluations were performed starting from the 1st day after SCI (week 0). Two independent examiners blinded to therapy rated movement capacities during the tests. On the same days, kinematic gait analysis was also performed. To visualize iliac crest, greater trochanter, knee, external malleolus and fifth metatarsal correctly, rats were marked on these five points with a permanent marker in the left leg. A clear rectangular rigid structure delimited the path of the animals. The walking activity was videotaped. The video was divided according to the principle of 15 pictures per second and the abovementioned five points were identified on every picture. Using MacBiophotonics Image J software (National Institute of Health, MD, USA), these points were transformed into coordinates, which were interpreted by the software developed in our laboratory to establish the step cycle duration and the stance and swing phases. To obtain values for all the variables plotted for step cycle duration, swing, and stance phase, the average of three steps performed for each rat was calculated. In rats that had a complete step cycle, the distance development between each stance phase plotted as stride distance was measured (cm) and the stride speed (cm/sec) was calculated using the stride distance and the swing phase duration. In some rats, SCI caused locomotor inability that restricted them to develop the path. The maximum waiting time to carry out the path was 10 seconds. The rats that did not move were retired, and the step cycle duration was rated as 10 seconds.
Micturition reflex
Data on micturition was obtained from a daily record about the presence or absence of distended bladder and the requirement of manual bladder empty. It was expressed as the average number of days that rats from each group required assistance.
Histology of white and gray matter and microglial cells
Five weeks after SCI, all rats were sacrificed by exposing them to a deep anesthesia with methyl ether. To achieve a positive pressure inside the left ventricle, physiological saline solution was filled through the use of a syringe. The spinal cords were removed, and 1 cm2 of epicenter of the lesioned area was obtained. The harvested tissue was fixed with 10% neutral formalin and then sliced into 5 µm-thick sections on a microtome for morphological and immunohistochemical analysis. For morphological analysis, quantification of white and gray matter of spinal cord was performed using Klüver-Barrera staining (Prophet et al., 1994). Five rats from each group were used for light microscopy examination (Zeiss, Axiostar plus, Carl Zeiss Microscopy, Thornwood, NY, USA) at 4× magnification. The area (mm2) was determined using SigmaScan Pro software (version 4.01, Cranes Software, 512093 Bangalore, Karnataka, India). For microglial cells analysis, immunohistochemistry was performed. Slices were incubated with primary polyclonal antibody against ionized calcium binding adapter molecule 1 (IBA-1; goat polyclonal to IBA-1; Abcam, USA, Ab48004) (marker to microglia) 1:500, in a wet chamber at 4°C overnight. Labeling was made according to the kit instruction (Dako Cytomatio LSAB + System HRP, K0690 Kit, USA). Color was developed by reaction with 3,3’-diaminobenzidine (Sigma, USA). The nuclei were counterstained with hematoxylin. The immunoreactivity was determined using AxioVision40V4.8.2.0. (Carl Zeiss Microimaging, Inc., Thornwood, NY, USA). Microglial cells were quantified as density of immunoreactive cells per 100 µm2 of tissue. The average of microglial cells across three spinal cords of each group was calculated. All microglial cells in one cross-section were evaluated. At least 10 microphotos were taken from each section at 40× magnification for histological observation.
Statistical analysis
One-way analysis of variance (ANOVA) with Dunnett's post hoc test was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). Data are expressed as the mean ± SEM. Statistical significance of locomotor behavior and gait analysis was evaluated at each week during the evaluation period. For microglia, white and gray matter, and micturition reflex, analysis was made at the end of the evaluation period (5th week). A level of P < 0.05 was considered statistically significant.
Results
Locomotor behavior
The rats that showed hind limb paralysis after SCI were included in this study. Initially, there was no significant difference in BBB scores between SS and LA groups. Rats in these two groups exhibited similar locomotion characteristics, with a score close to 0 (Figure 1). However, rats in the sham group showed slightly reduced locomotor activity followed by an immediate and progressive recovery close to level 21 corresponding to normal locomotor activity (Figure 1). A more significant recovery of locomotor activity was observed in the LA group than in the SS group from the 2nd week to the 5th week. During this time period, the locomotor activity in the LA group improved by 38% of that in the 1st week. At the same time, spontaneous recovery was enhanced by 7% in the SS group. Although recovery in the LA group was significantly greater than that in the SS group, it did not reach the level of recovery in the sham group (Figure 1).
Figure 1.
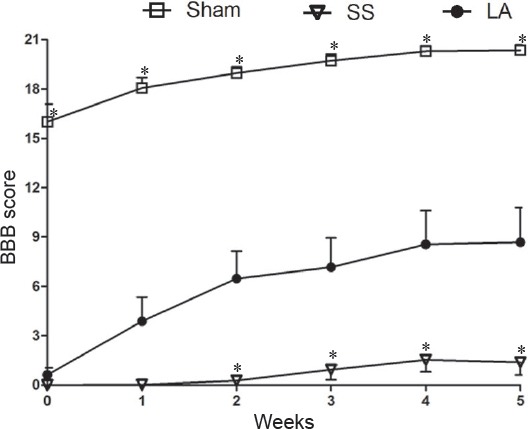
Locomotor ability of rats with spinal cord injury after LA treatment.
Higher BBB scores represent better locomotor function. Every plotted point represents BBB score (mean ± SEM) obtained at each week (n = 9, 11 and 10 for sham, SS and LA groups, respectively). *P < 0.05, vs. LA (one-way analysis of variance with Dunnett's post hoc test). LA: Leuprolide acetate; SS: physiological saline solution; BBB: the Basso, Beattie, and Bresnahan locomotor rating scale.
Kinematic gait
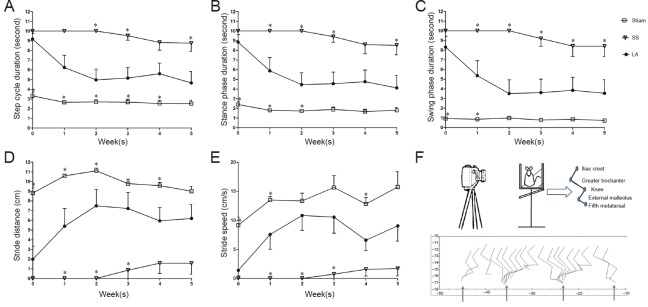
Gait analysis was divided into three sections: step cycle duration, stance phase duration, and swing phase duration. Step cycle duration in the LA group decreased from 9 to 4.6 seconds (initial and final evaluations) and it was significantly different compared with the SS group in the 2nd, 3rd and 5th weeks (Figure 2A).
Figure 2.
Gait of rats with spinal cord injury after LA treatment.
(A) Step cycle duration. (B) Stance phase duration. (C) Swing phase duration. (D) Stride distance. (E) Stride speed. (F) The top scheme reveals the points of reference. The bottom is a representative graphic resulting from these points. Narrows indicate the beginning for every stance phase. X axis is measured distance and Y axis represents angles. Each point of all graphics represents the mean ± SEM (n = 9, 11 and 10 for sham, SS and LA groups, respectively). *P < 0.05, vs. LA (one-way analysis of variance with Dunnett's post hoc test). LA: Leuprolide acetate; SS: physiological saline solution.
In the LA group, a reduction in stance phase duration was observed from 8.8 seconds at initial evaluation to 4.1 seconds at final evaluation. Swing phase duration also diminished in the LA group along weeks, from 8.3 to 3.5 seconds. In the SS group, reduction in stance phase duration was significant at the 1st, 2nd, 3rd and 5th weeks, and as for swing phase duration, significant reduction lasted from the 1st week until the 5th week (Figure 2B and C).
The distance traveled in the LA group was greater in final evaluation than in initial evaluation (6.1 to 1.9 cm respectively) and it was significantly higher than that in the SS group at the 1st, 2nd and 3rd weeks Figure 2D). The stride speed of rats in the LA group increased from 1.3 cm/sec as initial rate to 9.0 cm/sec in final evaluation. The increase of stride speed in the LA group was significantly greater than that in the SS group at the 1st, 2nd and 3rd weeks (Figure 2E). In the sham group, there were no significant differences in these variables even at the beginning of evaluation, and locomotor activity was slightly altered with time.
Micturition control
According to the data daily collected during the experiment, only four out of ten rats in the LA group needed manual bladder emptying at the end of the experiment. In the SS group, all rats required assistance. The number of the days in which rats in each group needed assistance before retrieval of urination reflex in the LA group was significantly less than in the SS group (19 vs. 35 days)(Figure 3).
Figure 3.

The micturition in rats with spinal cord injury after LA treatment.
Data represent the average days ± SEM (n = 9, 11 and 10 for sham, SS and LA groups, respectively) on which the rats required manual bladder emptying. *P < 0.0001, vs. LA (one-way analysis of variance with Dunnett's post hoc test). LA: Leuprolide acetate; SS: physiological saline solution.
Morphology of injured spinal cord
In the LA group, the configuration of white and gray matter of spinal cord was more similar to that in the sham group and it was better than that in the SS group. After LA administration, a trend towards a slight, but not significant, increase in the area of white and gray matter was observed (Figure 4B). Spinal cord sections from the SS group showed many large cavitations without nervous tissue, trabecular structures or cellular infiltrates, and in each cavitation, a small number of motorneurons with poor morphology were observed. However, similar to the sham group, spinal cord sections in the LA group showed fewer cavitations in which a greater number of neurons with better morphology were observed than in the SS group (Figure 4A).
Figure 4.
White and gray matter area in spinal cord of rats with spinal cord injury after LA treatment.
Micrographs show a representative image from each group obtained at the end of evaluation (5th week) (n = 5). (A) The micrographs of spinal cord cross sections stained with the Klüver-Barrera technique from each group at 10× and 40× magnifications respectively. (B) Data shows that both white and gray matter was conserved in all treated rats. Data are expressed as the mean ± SEM. One-way analysis of variance with Dunnett's post hoc test was performed. There was no significant difference in spared area between LA and SS groups. LA: Leuprolide acetate; SS: physiological saline solution.
Microglial immunoreactivity
In order to analyze the amount of microglia present in the epicenter of the lesion, immunohistochemistry was performed and the density of microglial cells was measured. Results showed that the area of microglia in the epicenter lesion in the LA group was significantly minimized than that in the SS group (1,080 ± 270 vs. 2,842 ± 434 µm2), but it was similar to that in the sham group (1,421 ± 101 µm2) (Figure 5).
Figure 5.
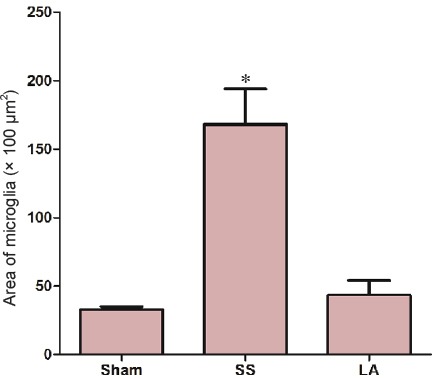
Density of microglial cells in spinal cord of rats with spinal cord injury after LA treatment.
Micrographs from top to bottom show a representative image of sham, SS and LA groups, respectively. Immunoreactivity for ionized calcium binding adapter molecule 1 is shown by arrows. Graphic represents the analysis of values obtained from density quantification (n = 3 by triplicate) at the 5th week. *P < 0.05, vs. LA (n = 9, 11 and 10 for sham, SS and LA groups, respectively).
Discussion
The most common consequence of SCI is the paralysis due to neural circuit interruptions. Although there is some degree of spontaneous recovery, lost connections are hardly restored. It is an option to consider the use of neurotrophic factors to induce nerve regeneration. Neurotrophic factors have a key role in the modulation of neuronal survival, neurite outgrowth, synaptic plasticity and neurotransmission in both intact and injured nervous system.
In this study, we found that LA, a GnRH agonist, significantly regained locomotor activity of SCI rats. Calderon-Vallejo and Quintanar (2012) reported that GnRH treatment recovered locomotor activity with similar findings to ours. Both results are strongly tied, because these effects are probably mediated through activation of GnRH receptors, which have been described in the spinal cord motoneurons (Dolan et al., 2003; Quintanar et al., 2007). In an experimental autoimmune encephalomyelitis model, LA administration produces an increase in the axonal growth and in neurofilaments and myelin basic protein expression (Guzmán-Soto et al., 2012), which could explain the improvement in locomotor activity found in our experiments. The degree of locomotor activity recovery in rats treated with LA was higher than in those treated with GnRH (Calderon-Vallejo and Quintanar, 2012), which suggests that LA has greater potential for clinical application than GnRH.
In kinematic analysis, we observed that LA treatment in SCI rats decreased step cycle, stance phase, and swing phase durations, but it resulted in increases in the stride distance and speed. LA rats spent less time in each step phase, traveled greater distance, and performed faster steps. These results can be considered as a sign of locomotor activity recovery, which were not observed in rats with physiological saline solution treatment. These results are similar to the outcomes by Hamers et al. (2001) who studied gait parameters in two different SCI types, i.e., transection of the dorsal half of the spinal cord and spinal cord contusion.
The locomotor activity recovery in LA-treated animals is due to different mechanisms including remyelination (Guzman-Soto et al., 2012) or increases in microfilament protein expression, neurite outgrowth (Calderon-Vallejo and Quintanar, 2012), and axonal diameter (Quintanar et al., 2011). These results can be explained by reorganization of local networks via propriospinal circuitry. These local networks control the involuntary movements, which do not require cortico- or rubrospinal tracts, proposed as an explanation for the ability of spinal cord injured animals to produce walking movements (Ek et al., 2010). However, supraspinal tracts were likely involved in the control of voluntary movements observed in animals treated with LA. It could be related to reconnections going through injured area originated from supraspinal neurons.
Propiospinal circuits provide an explanation for the restorations of micturition reflex in LA animals, in which the number of days that they need assistance of manual bladder emptying was reduced. This is possible due to the reorganization in reflex pathways inside the spinal cord, as reported by Groat and Yoshimura (2012).
In this study, LA administration modified the gray and white matter areas, decreasing scar area and promoting thte recovery of spared tissue in SCI rats. High BBB scores were positively correlated with spared tissue in the spinal cord lesion (Basso et al., 1995). Structural conformation of the spinal cord is kept by both sufficient numbers of neurons and axons including their myelin. In traumatized spinal cord, only remnants of both gray and white matter exist due to the presence of hypercellularity, inflammation, cavitation, increased extracellular space and a loose fibrous matrix (Totoiu and Keirstead, 2005). Our results showed that in the LA group, spared tissue was increased, BBB scores were higher, faster stride speed and longer stride distance were observed in the LA group than in the sham group. These results reflect the new integration of spared descending and afferent-driven signals, as recovery after contusive SCI has been reported to be identified by changes in gait biomechanics and muscle activation patterns (Hansen et al., 2012).
In the LA group, significantly higher BBB score and improved gait were observed at the 1st and 2nd weeks during the evaluation period compared to the sham group. As a consequence of SCI, microglia considerably increased independently of spared tissue. An early infiltration of macrophages/microglia in traumatic lesions of spinal cords has been observed in a rat model of SCI. The immune cell cascade involves infiltration of neutrophils and activation of resident microglia, followed by subsequent accumulation of monocyte-derived macrophages and the later entry of lymphocytes into the lesion site. The monocytes that infiltrate the injured area acquire a pro-inflammatory/classical profile (Raposo and Schartz, 2014). The reduction in the density of microglial cells observed in the LA group is probably associated with the locomotor activity in these rats.
In conclusion, administration of LA partially improves lotomotor activity, gait, micturition reflex, spinal cord morphology and decreases microglial area in a rat model of SCI. Promotion of neuronal survival by administration of neurotrophic factors and a possible immunomodulation to counteract secondary injury are a promising approach to repair of SCI. Additionally, even LA administered via intramuscular injection is able to cross the blood-spinal cord barrier as observed in previous studies on patients with prostate cancer. LA is a potential alternative treatment of SCI because of its safety and ease in use as well as few side effects.
Acknowledgments
We would like to express our sincere gratitude to Kalman Kovacs and Fabio Rotondo for paper review and Dra. Irene Guzmán Soto, Crhistian Jafet Pérez Ferrer, Biól. Araceli Adabache Ortíz, and Violeta Saraí Jiménez Hernández for methodological support.
Footnotes
Funding: This study was supported by a grant from CONACyT for scholarship 376921/246887.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded, stringently reviewed by international expert reviewers.
Copyedited by Moon SM, Liu L, Zhang M, Li CH, Song LP, Zhao M
References
- Bahk JY, Kim MO, Park MS, Lee HY, Lee JH, Chung BC, Min SK. Gonadotropin-releasing hormone (GnRH) and GnRH receptor in bladder cancer epithelia and GnRH effect on bladder cancer cell proliferation. Urol Int. 2008;80:431–438. doi: 10.1159/000132703. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Calderón-Vallejo D, Quintanar JL. Gonadotropin-releasing hormone treatment improves locomotor activity, urinary function and neurofilament protein expression after spinal cord injury in ovariectomized rats. Neurosci Lett. 2012;515:187–190. doi: 10.1016/j.neulet.2012.03.052. [DOI] [PubMed] [Google Scholar]
- Dolan S, Evans NP, Richter TA, Nolan AM. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor in sheep spinal cord. Neurosci Lett. 2003;346:120–122. doi: 10.1016/s0304-3940(03)00594-9. [DOI] [PubMed] [Google Scholar]
- Ek CJ, Habgood MD, Callaway JK, Dennis R, Dziegielewska KM, Johansson PA, Potter A, Wheaton B, Saunders NR. Spatio-temporal progression of grey and white matter. Damage following contusion injury in rat spinal cord. PLoS One. 2010;5:e12021. doi: 10.1371/journal.pone.0012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012;235:123–132. doi: 10.1016/j.expneurol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Soto I, Salinas E, Hernández-Jasso I, Quintanar JL. Leuprolide acetate, a GnRH agonist, improves experimental autoimmune encephalomyelitis: a possible therapy for multiple sclerosis. Neurochem Res. 2012;37:2190–2197. doi: 10.1007/s11064-012-0842-x. [DOI] [PubMed] [Google Scholar]
- Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006;23:264–280. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- Hamers FP, Lankhorst AJ, Laar TJ, Veldhuis WB, Gispen WH. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J Neurotrauma. 2001;18:187–201. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- Hansen CN, Linklater W, Santiago R, Fisher LC, Moran S, Buford JA, Basso M. Characterization of recovered walking patterns and motor control after contusive spinal cord injury in rats. Brain Behav. 2012;2:541–552. doi: 10.1002/brb3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periti P, Mazzei T, Mini E. Clinical Pharmacokinetics of depot leuprorelin. Clin Pharmacokinet. 2002;41:485–504. doi: 10.2165/00003088-200241070-00003. [DOI] [PubMed] [Google Scholar]
- Prophet EB, Mills B, Arrington JB, Sobin LH. Washington DC. USA: Armed forces institute of pathology; 1994. Laboratory methods in histotechnology. [Google Scholar]
- Quintanar JL, Salinas E, González R. Expression of gonadotropin-releasing hormone receptor in cerebral cortical neurons of embryos and adult rats. Neurosci Lett. 2007;411:22–25. doi: 10.1016/j.neulet.2006.06.077. [DOI] [PubMed] [Google Scholar]
- Quintanar JL, Salinas E. Neurotrophic effects of GnRH on neurite outgrowth and neurofilament protein expression in cultured cerebral cortical neurons of rat embryos. Neurochem Res. 2008;33:1051–1056. doi: 10.1007/s11064-007-9549-9. [DOI] [PubMed] [Google Scholar]
- Quintanar JL, Salinas E, González R. Gonadotropin-releasing hormone receptor in spinal cord neurons of embryos and adult rats. Neurosci Lett. 2009;461:21–24. doi: 10.1016/j.neulet.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Quintanar JL, Salinas E, Quintanar-Stephano A. Gonadotropin-releasing hormone reduces the severity of experimental autoimmune encephalomyelitis, a model of multiple sclerosis. Neuropeptides. 2011;45:43–48. doi: 10.1016/j.npep.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Raposo C, Schwartz M. Glial scar and immune cell involvement in tissue remodeling and repair following acute CNS injuries. Glia. 2014;62:1895–1904. doi: 10.1002/glia.22676. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Vanicky I, Urdzíková L, Saganová K, Cízková D, Gálik J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma. 2001;18:1399–1407. doi: 10.1089/08977150152725687. [DOI] [PubMed] [Google Scholar]