The cingulum, the neural tract connecting the orbitofrontal cortex with the medial temporal lobe, plays an important role in cognition (Bush et al., 2000). It is also important in memory because it provides cholinergic innervations to the cerebral cortex after obtaining innervation from the medial septal nucleus, the vertical nucleus of the diagonal band, and the nucleus basalis of Meynert via the medial cholinergic pathway (Nieuwenhuys et al., 2008; Naidich and Duvernoy, 2009; Hong and Jang, 2010a).
Before introduction of diffusion tensor imaging (DTI), precise assessment of the cingulum in the live human brain has been limited due to its anatomical characteristics and no discriminability with adjacent neural structures on brain MRI. Diffusion tensor tractography (DTT), which is reconstructed from DTI, has recently allowed reconstruction of the cingulum (Concha et al., 2005). Many studies using DTT have reported injury of the cingulum in traumatic brain injury (TBI) and stroke, however, little is known about mild TBI (Brandstack et al., 2013; Jang et al., 2013; Kurki et al., 2014; Kwon et al., 2014).
In this study, using DTT, we report three patients who had severe bilateral anterior cingulum injury after mild TBI.
Three patients (two males and one female, aged 39–59 years with the mean age of 51.3 ± 10.8 years) with mild TBI and 10 normal control subjects (six males and four females, aged 46–56 years with the mean age of 50.4 ± 3.4 years) were included in this study. All these subjects had no history of neurological, physical, or psychiatric illness. Patients were recruited according to the following inclusion criteria: (1) loss of consciousness for < 30 minutes, post-traumatic amnesia for ≤ 24 hours, and initial Glasgow Coma Scale score of 13–15 (Mild Traumatic Brain Injury Committee, 1993; Alexander, 1995), (2) no abnormality on brain MRI, (3) bilateral discontinuations of the anterior cingulum on DTT, and (4) no history of head trauma, neurologic or psychiatric disease. The patients provided signed, informed consent and our hospital review board approved the study protocol.
The Wechsler Intelligence Scale (WAIS) and Memory Assessment Scale (MAS) were employed for evaluation of cognition. We conducted the clinical evaluation at the time of DTI scanning. The reliability and validity of these evaluation tools have been demonstrated by previous studies (Williams, 1981, 1991). DTI data were obtained using a 6-channel head coil on a 1.5 T scanner (Gyroscan Intera; Philips Medical Systems, Best, the Netherlands) with single-shot echo-planar imaging at 1.67 ± 1.53 months after the onset of TBI. Sixty contiguous slices (repetition time 10,726 ms; echo time 76 ms; b 1,000 s/mm2; number of excitations 1; and thickness 2.5 mm) were acquired. DTT was reconstructed by FACT algorithm (Mori et al., 1999). A seed region of interest (ROI) was drawn on the middle portion of the cingulum, and the target ROI was drawn on the posterior portion of the cingulum. DTT was performed across the neural tract passing through both ROIs, and the termination criteria included fractional anisotropy (FA) < 0.15 and angle < 27o (Hong and Jang, 2010b). The FA and fiber number (FN) were measured for each cingulum.
The demographic, clinical, and DTT data of three patients and 10 control subjects are shown in Table 1. In the evaluation of cognition, all three patients showed memory impairment on the MAS, although total cognition was within normal range on the WAIS. On DTT for the cingulum, no discontinuation was observed between the anterior cingulum and the basal forebrain in normal subjects. By contrast, on DTTs of all three patients, bilateral discontinuations were observed between the anterior cingulum and the basal forebrain around the genu of the corpus callosum (right and left side DTT parameters of the cingulum: patient 1, FA: 0.41/0.38 and FN: 1,359/2,084; patient 2, FA: 0.41/0.39 and 768/1,990; patient 3, FA: 0.40/0.37 and FN: 1,058/1,410) (Figure 1). In addition, three cingulums were narrowing (patient 2–right cingulum and patient 3–both cingulums). Compared to the normal control subjects (FA: 0.41 ± 0.02; FN: 2,319.45 ± 414.34), the FN values of the right cingulum in patients 1 and 2 and of both cingulums in patient 3 were lower by more than two standard deviations (SDs) above those of control subjects (Table 1). However, the FA values of six cingulums in three patients were within two SDs of those of control subjects.
Table 1.
Demographic, clinical, and DTT data of the patients and control subjects
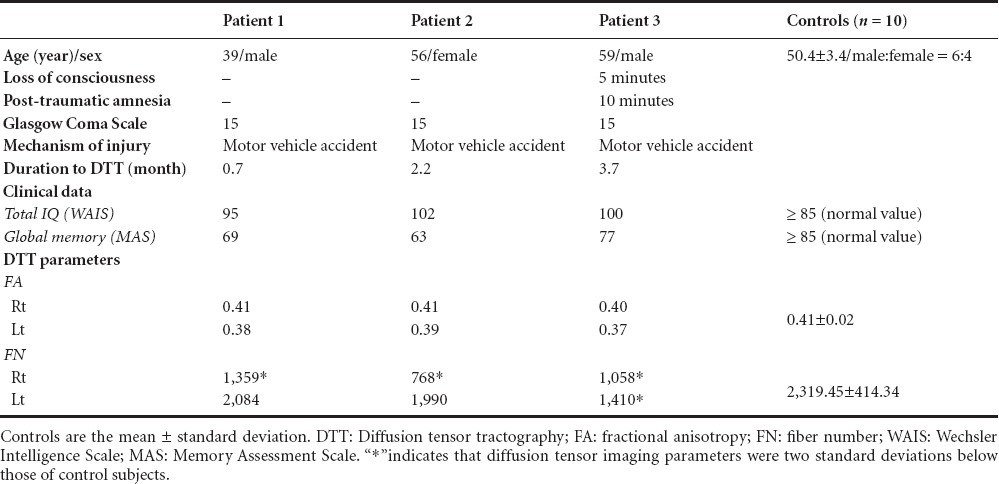
Figure 1.
Brain magnetic resonance imaging (MRI) and diffusion tensor tractography (DTT) images of three patients with mild traumatic brain injury and three normal controls.
(A) No abnormality was detected on brain MRI. (B) On DTT images of the cingulum, bilateral discontinuations between the anterior cingulum and the basal forebrain were observed in all three patients (blue arrows) and narrowing was also observed in three cingulums (green arrows). (C) DTT images of the cingulum in three normal subjects.
In this study, we examined injury of the cingulum in three patients with mild TBI caused by traffic accidents. Our results in terms of cognitive function, DTT configuration, and DTT parameters are as follows: 1) cognitive function – memory impairment with normal total intelligence; 2) DTT configuration – discontinuation of the bilateral anterior cingulums around the genu of the corpus callosum was observed in all three patients, in addition, narrowing of three remaining cingulums was observed in two patients; and 3) DTT parameters – the FN value of four cingulums in three patients was decreased and the FA value of all six cingulums was not significantly changed.
Because discontinuation of the cingulum was not observed in all normal subjects, so discontinuation of the anterior cingulum indicates definite injury of the cingulum. These results observed on DTT configuration appeared to be consistent with the memory impairment in all three patients. DTT parameters such as the FA and FN reflect the state of the neural tract (Neil, 2008; Kwak et al., 2010). FA value indicates the degree of directionality of water diffusion, representing white matter organizatiion (Neil, 2008). FN value means the number of included voxels in a neural tract (Kwak et al., 2010). Decreased FN value without significant change of FA value indicates injury of the cingulum. These results are consistent with the discontinuations observed on DTT configurations. No abnormality was detected on brain MRI, and the injury of bilateral cingulums in three patients occurred because of traumatic axonal injury (Povlishock, 1992; Alexander, 1995; Povlishock and Christman, 1995). However, FN values of three cingulums were within normal range despite discontinuation on DTT, which appeared to be attributed to the fact that a partial injury of the cingulum cannot result in an abnormality of DTT parameters because the cingulum is a large and long neural tract. This result suggests that DTT configuration might be superior to DTT parameters in diagnosis of an injury of the cingulum. Regarding the injury location, Rutgers et al. (2008) reported that the genu of the corpus callosum was injured in mild TBI patients whereas both the genu and splenium were injured in moderate and severe TBI patients (Rutgers et al., 2008a). Therefore, we think that discontinuation of the anterior cingulum around the genu of the corpus callosum might be a typical finding of cingulum injury in patients with mild TBI. In addition, thinning of three cingulums might indicate degeneration after discontinuation of the anterior cingulum (Hong and Jang, 2010b).
In conclusion, we demonstrated severe bilateral anterior cingulum injury with memory impairment following mild TBI due to a traffic accident. Further studies are necessary to clarify other findings of injured cingulum after mild TBI.
Since the development of DTI, many studies have reported on injury of the cingulum by measuring DTI parameters in patients with mild TBI (Rutgers et al., 2008b; Wu et al., 2010; Shenton et al., 2012).
Regarding DTT study, as far as we are aware, this is the first study to report severe anterior cingulum injury following mild TBI. However, limitations should be considered. First, it is a case report; therefore, further studies involving a larger number of patients are encouraged. Second, DTT can cause false negative result due to crossing fibers and partial volume effect (Parker and Alexander, 2005). In addition, DTT show changeable data based on patient's age and duration from TBI onset to DTT conduction.
This work was supported by the National Research Foundation (NRF) of Korea Grant funded by the Korean Government (MSIP), No. 2015R1A2A2A01004073.
References
- Alexander MP. Mild traumatic brain injury: pathophysiology, natural history and clinical management. Neurology. 1995;45:1253–1260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- Brandstack N, Kurki T, Tenovuo O. Quantitative diffusion-tensor tractography of long association tracts in patients with traumatic brain injury without associated findings at routine MR imaging. Radiology. 2013;267:231–239. doi: 10.1148/radiol.12112570. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol. 2005;26:2267–2274. [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Jang SH. Neural pathway from nucleus basalis of Meynert passing through the cingulum in the human brain. Brain Res. 2010a;1346:190–194. doi: 10.1016/j.brainres.2010.05.088. [DOI] [PubMed] [Google Scholar]
- Hong JH, Jang SH. Degeneration of cingulum and fornix in a patient with traumatic brain injury: diffuse tensor tractography study. J Rehabil Med. 2010b;42:979–981. doi: 10.2340/16501977-0603. [DOI] [PubMed] [Google Scholar]
- Jang SH, Kim SH, Kim OR, Byun WM, Kim MS, Seo JP, Chang MC. Cingulum injury in patients with diffuse axonal injury: a diffusion tensor imaging study. Neurosci Lett. 2013;543:47–51. doi: 10.1016/j.neulet.2013.02.058. [DOI] [PubMed] [Google Scholar]
- Kurki T, Himanen L, Vuorinen E, Myllyniemi A, Saarenketo AR, Kauko T, Brandstack N, Tenovuo O. Diffusion tensor tractography-based analysis of the cingulum: clinical utility and findings in traumatic brain injury with chronic sequels. Neuroradiology. 2014;56:833–841. doi: 10.1007/s00234-014-1410-7. [DOI] [PubMed] [Google Scholar]
- Kwak SY, Yeo SS, Choi BY, Chang CH, Jang SH. Corticospinal tract change in the unaffected hemisphere at the early stage of intracerebral hemorrhage: a diffusion tensor tractography study. Eur Neurol. 2010;63:149–153. doi: 10.1159/000281108. [DOI] [PubMed] [Google Scholar]
- Kwon HG, Choi BY, Kim SH, Chang CH, Jung YJ, Lee HD, Jang SH. Injury of the cingulum in patients with putaminal hemorrhage: a diffusion tensor tractography study. Front Hum Neurosci. 2014;8:366. doi: 10.3389/fnhum.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mild Traumatic Brain Injury Committee (1993) Definition of mild traumatic brain injury. J Head Trauma Rehabil. 8:86–87. [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Naidich TP, Duvernoy HM. New York: Springer; 2009. Duvernoy's atlas of the human brain stem and cerebellum: high-field MRI: surface anatomy, internal structure, vascularization and 3D sectional anatomy. [Google Scholar]
- Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008;27:1–7. doi: 10.1002/jmri.21087. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, Huijzen C. 4th Edition. New York: Springer; 2008. The human central nervous system. [Google Scholar]
- Parker GJ, Alexander DC. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci. 2005;360:893–902. doi: 10.1098/rstb.2005.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12. [PubMed] [Google Scholar]
- Povlishock JT, Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma. 1995;12:555–564. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- Rutgers DR, Fillard P, Paradot G, Tadie M, Lasjaunias P, Ducreux D. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am J Neuroradiol. 2008a;29:1730–1735. doi: 10.3174/ajnr.A1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. Am J Neuroradiol. 2008b;29:514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, Vu MA, Purohit MP, Helmer K, Koerte I, Lin AP, Westin CF, Kikinis R, Kubicki M, Stern RA, Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, Mori S. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001;14:723–735. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- Williams JM. New York: Psychological Corporation; 1981. Wechsler Adult Intelligence Scale-Revised. Test manual. [Google Scholar]
- Williams JM. Odessa, Fla: Psychological Assessment Resources; 1991. MAS: Memory Assessment Scales: professional manual. [Google Scholar]
- Wu TC, Wilde EA, Bigler ED, Yallampalli R, McCauley SR, Troyanskaya M, Chu ZL, Li XQ, Hanten G, Hunter JV, Levin HS. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J Neurotrauma. 2010;27:303–307. doi: 10.1089/neu.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]