Abstract
Translation generally initiates with the AUG codon. While initiation at GUG and UUG is permitted in prokaryotes (Archaea and Bacteria), cases of CUG initiation were recently reported in human cells. The varying stringency in translation initiation between eukaryotic and prokaryotic domains largely stems from a fundamental problem for the ribosome in recognizing a codon at the peptidyl-tRNA binding site. Initiation factors specific to each domain of life evolved to confer stringent initiation by the ribosome. The mechanistic basis for high accuracy in eukaryotic initiation is described based on recent findings concerning the role of the multifactor complex (MFC) in this process. Also discussed are whether non-AUG initiation plays any role in translational control and whether start codon accuracy is regulated in eukaryotes.
Keywords: translation initiation, start codon selection, initiation factors, non-AUG initiation, bacterial translation, archaeal translation, eukaryotic translation, start codon fidelity, ribosome, multifactor complex
Translation or mRNA-dependent protein synthesis is a crucial process of gene expression that determines the abundance of the cellular proteome. The ribosome that catalyzes this reaction has three tRNA-binding sites, A-site, P-site and E-site, for aminoacyl-, peptidyl- and exit tRNA-binding sites, respectively. During translation initiation, the ribosome binds initiator methionyl-tRNA (Met-tRNAi) and mRNA with the initiation codon (start codon) base-paired to the anticodon of the former in the P-site. During the elongation phase, the ribosome binds aminoacyl-tRNA matching the next codon at the A-site, transfers the methionyl or peptidyl moiety of the P-site tRNA to the N-terminus of the aminoacyl-tRNA in the A-site, and then translocates the A-site tRNA carrying the elongated polypeptide to the P-site, releasing the deacylated tRNA from the E-site.
The fidelity of protein translation is very high during the elongation phase, because the ribosome possesses special mechanisms to achieve this.1-5 Among them is the stabilization of codon:anticodon pairing through two universally conserved adenine residues (A-1492 and A-1493 in E. coli) of the small subunit (SSU) rRNA as well as other rRNA residues and ribosomal protein side chains. However, the fidelity of translation initiation varies widely between different organisms. While eukaryotic translation generally starts with the AUG codon, prokaryotic translation permits frequent GUG and UUG initiation besides AUG. In E. coli (Gram negative), AUG, GUG and UUG start translation of 83, 14 and 3% of proteins encoded by the genome, respectively. In B. subtilis (Gram positive), these codons start translation of 78, 9 and 13% of proteins encoded by the genome.6 Archaea display similar levels of UUG and GUG initiation.7 This inaccuracy largely stems from a fundamental problem for the ribosome to precisely recognize codon:anticodon pairing at the P-site, where the initiator methionyl-tRNA is bound.
This review provides an overview concerning how initiation factors specific to each domain of life evolved to confer stringent initiation by the ribosome with varying accuracies. The mechanistic basis for high accuracy in eukaryotic initiation is described based on recent findings concerning the role of the Eukarya-specific translation initiation multifactor complex (MFC) in this process.8-11 Inspired by recent reports of non-AUG initiation found in the 5`-untranslated region (UTR) of many eukaryotic genes,12,13 conceptual frameworks will be provided to understand how the set of start codons is chosen for each domain of life and how initiation accuracy can be regulated in eukaryotes, thereby changing the cellular proteome translation.
Start Codon Selection in Bacteria
Although bacterial initiation permits initiation from UUG and GUG in addition to AUG, a simpler initiation mechanism operates to achieve this level of fidelity, distinguishing these three codons from other codons. There are examples of complex translational control mechanisms involving GUG codons, raising the possibility that the GUG start codon is maintained to achieve efficient translational control mechanisms.
IF3 is the stand-alone fidelity factor in bacterial translation initiation
As shown in Table 1, there are three bacterial initiation factors, IF1, IF2 and IF3. After ribosome dissociation into the 30S small subunit (SSU) and 50S large subunit (LSU), the former binds GTP-bound IF2 and IF3, priming the subsequent loading of IF1 and formyl-methionyl initiator tRNA (fMet-tRNAifMet) onto the SSU A-site and P-site, respectively.14 mRNA binds to the SSU through base-pairing between the Shine-Dalgarno (SD) sequence of the mRNA located 5′ of the start codon and the anti-SD sequence located at the 3′-end of the 16S SSU rRNA, forming a 30S pre-initiation complex (PIC).14-16 The rate of mRNA binding to the PIC varies substantially depending on the complementarity of the SD sequence to the rRNA anti-SD sequence and the stability of inhibitory secondary structures in the mRNA. Prior to start codon selection, IF3 binds to the subunit-interface side of the SSU, thereby preventing re-association of the SSU with LSU (anti-association function).17 However, the start codon base-pairing to the tRNAifMet anticodon triggers transitioning of the 30S PIC into the 30S initiation complex (IC). This is accompanied by stabilization of fMet-tRNAfMet, mRNA, IF2 and IF1, and strong destabilization of IF3.14 The 30S IC then joins the 50S LSU.18,19 After subunit joining, the LSU stimulates the GTP hydrolysis for IF2, promoting the release of the remaining IFs. As the consequence, the 70S IC forms with tRNAifMet bound to the start codon in the P-site, which is ready for the polypeptide elongation phase.20
Table 1. Translation initiation factors found in bacteria, archaea, and eukaryotes#.
| Bacteria | Archaea | Eukaryotes | # of subunits (name) | Function |
|---|---|---|---|---|
| IF11 | aIF1A1 | eIF1A1 | 1 | Binds SSU A site, blocking misloading of Met-tRNAi/fMet-tRNAf |
| IF21 | aIF5B1 | eIF5B1 | 1 | Binds GTP. GTP-bound form interacts with tRNAi acceptor stem and the SSU subunit interface to mediate the LSU joining. GTP hydrolysis promoted by LSU factor binding center. |
| IF32 | 1 | Anti-subunit re-association. Monitors correct tRNAf pairing with start codon. Bacteria-specific | ||
| aIF13 | eIF1*4 | 1 | Anti-subunit reassociation. Monitors correct tRNAi pairing with start codon | |
| aIF23 | eIF2*4 | 3 (α, β, γ) | Binds GTP and Met-tRNAi. GTP hydrolysis before start codon recognition. This is promoted by eIF5 in eukaryotes. | |
| eIF5*4 | 1 | Activates eIF2 GTPase (GAP) to control start codon recognition. Binds eIF1, eIF2 and eIF3 | ||
| eIF3*4 | Yeast, 6 (a-c, g, i, j) Mammals, 13 (a-m) |
Scaffold of assembly, to bind eIF1, eIF5, eIF4F and the 40S SSU | ||
| eIF2B | 5 (α−ε) | Guanine nucleotide exchange factor (GEF) for eIF2 | ||
| eIF4F | 3 (eIF4E, eIF4G, eIF4A) | Binds mRNA at its 5′ m7G cap structure and at its poly(A) tail. Contains eIF4A. | ||
| eIF4A | 1 | ATP-dependent mRNA helicase | ||
| eIF4B | 1 | Co-activator of eIF4A |
Base-pairing between the start codon and the tRNAifMet anticodon serves as a crucial checkpoint for the translation initiation reaction. IF3 plays a central role in this process. IF3 matures the 30S PIC into the 30S IC, only when the anticodon pairs with AUG, GUG or UUG. When it pairs with other codons, IF3 is believed to promote dissociation of the 30S PIC.21 The precise mechanism by which IF3 discriminates against codons other than AUG, GUG and UUG is currently unknown.
GUG and UUG start codons are recognized by the fMet-tRNAifMet anticodon (5′-CAU-3′) through “wobble” base-pairing at the 1st position of the start codon (NUG). A likely explanation for why Bacteria allow initiation from these codons may concern a unique feature in the anticodon loop of fMet-tRNAifMet. With a single exception, all bacterial and eukaryotic tRNAs that recognize codons with A in the 1st position (ANN codons) have N6-threonylcarbamoyladenosine (t6A) located 3′ of the anticodon U.22,23 This modification has been proposed to enhance the decoding capacity of ANN codons at the ribosomal A-site through strong stacking interactions imposed by this bulky hydrophobic modification.23 The unique exception among the ANN-decoding tRNAs is bacterial initiator tRNA, fMet-tRNAifMet, which lacks this modification. The paucity of this modification is believed to explain, at least in part, why “wobble” in decoding the 1st position of the start codon can occur at moderately high frequency in Bacteria.24 It would be intriguing to understand how the decreased initiation accuracy has evolved in Bacteria in light of the molecular mechanism by which protein translation factors, fMet-tRNAifMet and the ribosome cooperate to specify a start codon in a different atomic environment at the P-site.
The role for non-AUG initiation in bacterial translational control
The classical example of the use of a non-AUG start codon in bacterial translational control is that of the AUU start codon in IF3 mRNA translation.25 When the IF3 abundance decreases, the cellular initiation stringency goes down, thereby allowing AUU initiation of IF3 translation. The fact that the frequency of AUU initiation is increased when the IF3 level is low provides strong (genetic) evidence for the central role that IF3 plays in initiation fidelity.
Although UUG and GUG codons are considered to be normal start codons in bacteria, these sequences are viewed as weaker initiation signals.26,27 Provided that certain mechanistic constraints including the lack of t6A modification in fMet-tRNAifMet explain the translation initiation from these codons, is there any selective advantage associated with starting translation from them? A hint for answering this question might reside in their property that, due to being an intrinsically weak initiation signal, these codons can be completely masked by a relatively weak secondary structure when accompanied with a weak SD sequence.28 The GUG codon is particularly suited for regulation by secondary structures, because two G:C pairs (rather than one G:C pair in the case of AUG and UUG) can directly mask it.
Since Bacteria lack mRNA helicases that act on translation start sites, the rate of PIC binding by a given mRNA is directly proportional to the fraction of the mRNA with the unstructured (vs. structured or masked) SD sequence. Thus, translation of bacterial mRNA can be regulated in a wide range – up to 10,000 fold – through folding and unfolding of the secondary structure that masks translation initiation signals. The quantitative and theoretical framework of this regulation was established by de Smit and van Duin. Using a bacteriophage MS2 coat protein mRNA as a model, they demonstrated that the stability of the secondary structure masking the SD sequence (ΔGfo) linearly correlates with the log of expression from a particular start codon.15 A mere increase of ΔGfo by 1.4 kcal/mol increases MS2 coat gene expression by 10-fold. In other words, the disruption of a hydrogen bond (ΔGfo ~1 kcal/mol) in Watson-Crick base pairs can theoretically lead to as much as a 5-fold translational induction. This allows for a wide range of gene regulation executed at the translational level in Bacteria. However, the linear correlation between ΔGfo and the log of gene expression does not hold at higher ΔGfo for the inhibitory structure, where the SD interaction can directly disrupt it. In other words, the weak initiation signal, including the GUG codon, can be masked substantially by relatively weak structures, that are more suited for regulation by mRNA structure changes.
The GUG start codon is a crucial element in the translational control of plasmid replication
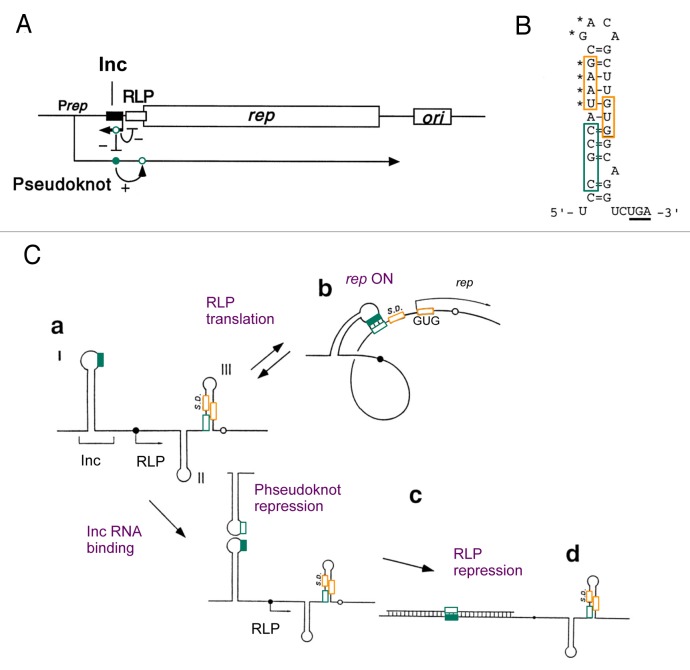
GUG start codons are found in some of the well-characterized mRNAs whose translation is regulated by dynamic structural changes.29-34 One of the best described, instructive examples is found in the translational control of the replication initiator (Rep) protein of the IncIα plasmid Colicin Ib-P9 (ColIb-P9) and related low-copy number plasmids29,32-34 (Fig. 1). In these plasmids, rep translation initiates with the GUG start codon, which is masked by a stable secondary structure along with its weak SD sequence35 (Fig. 1B). Remarkably, the inhibited GUG initiation can be strongly induced by the formation of a mRNA pseudoknot, thereby opening the rep initiation signal (Fig. 1C). By the intricate mechanism described below, the plasmids can achieve Rep translational induction of as much as 3000-fold.36 This translational induction is crucial for plasmid transformation or transfer, and stable copy-number maintenance through the action of the antisense Inc RNA.32 Inc RNA was identified as a genetic element responsible for incompatibility of like plasmids and is encoded ~100-bp upstream of the Rep initiation site (Fig. 1A). Inc RNA efficiently shuts down rep translation, after the plasmid establishes itself in a new host cell or when the plasmid copy number is too high during the host cell cycles.
Figure 1. Control of GUG-initiated translation through dynamic mRNA conformational changes. (A) The replication region of ColIb-P9 plasmid. The horizontal line represents the DNA of the ColIb-P9 replication region. Boxes denote coding regions for Inc, RLP and Rep and the origin of replication (ori). The short leftward arrow below denotes Inc RNA (antisense), while the long rightward arrow denotes Rep mRNA, starting from its promoter, Prep. Green circles on the transcripts denote the GGCG (filled) and CGCC (open) sequences, which interact together during rep gene regulation. The base-pairing between these sequences in Rep mRNA forms a pseudoknot with the Inc target stem-loop, which then allows rep translation (+ sign). The CGCC sequence (green open circle) on Inc RNA binds the GGCG sequence (green filled circle) on Rep mRNA, inhibiting pseudoknot formation (- sign). Inc RNA also represses RLP translation (- sign). (B) Inhibitory secondary structure found in the translation initiation region of the rep genes in ColIb-P9. Oragne boxes, GUG start codon and SD sequence (also with asteriska). Underline, RLP stop codon. Green box, the CGCC sequence forming a pseudoknot. (C) Models for the control of ColIb-P9 rep translation by competition between pseudoknot formation and antisense RNA binding. Panels a-d denote a conformation state of Rep mRNA, with green boxes denoting the GGCG (filled box) and CGCC (open box) sequences with the potential to form a pseudoknot. Bracket indicates the Rep mRNA region complementary to Inc RNA. Orange boxes are the SD and the GUG start codon for rep. Thin arrows on the mRNA indicate the regions that get translated in each state. Black filled and open circles denote the start and stop codons of RLP.
An important trigger of the mRNA conformational change and subsequent Rep translation is the translation and termination of an upstream ORF (uORF) termed the rep leader peptide (RLP) (Fig. 1A). The ribosome stalled at the RLP stop codon is proposed to expose the 5′-CGCC-3′ sequence immediately upstream of the Rep SD sequence, allowing the pseudoknot to form through a long-rage base-paring with the 5′-GGCG-3′ sequence in the loop of the Inc target stem-loop (Fig. 1B and C, panel a).29,36 The pseudoknot thus formed is able to keep the Rep initiation signal (SD and GUG, orange squares in Figure 1B and C) unfolded long enough for the stalled ribosome to bind it and re-initiate at Rep (Fig. 1C, panel b). Inc RNA initially binds the Inc target stem-loop on Rep mRNA through the loop-loop contact termed “kissing interaction” (Fig. 1C, panel c). Because this kissing interaction is competitive with pseudoknot formation, Inc RNA is able to inhibit Rep translation directly and quickly (Fig. 1C, panel c).32,37 Subsequently, Inc RNA forms a more stable complex capable of inhibiting RLP translation through blocking the RLP SD sequence (Fig. 1C, panel d).38
Mutations in the Rep initiation signal, increasing the complementarity to anti-SD or changing the GUG start codon to AUG, generally make Rep expression less dependent on the pseudoknot and less repressible by Inc.28,33-35 Therefore, the weak Rep initiation signal, including the GUG start codon, is an essential component of the sophisticated translational control mechanism involving dynamic RNA structural changes.28
Plasmids play a very important role in the bacterial life cycle, by providing sexual gene transfer, multi-drug resistance, and bacterial toxins including colicins. If the GUG start codon is a crucial element for replication control of certain plasmids, it is entirely possible that the bacterial hosts receive a selective pressure to maintain GUG initiation for their own survival or fitness. Since nearly ~10% of bacterial proteins initiate from GUG, it also is anticipated to find cases of GUG-initiated bacterially coded genes regulated by complex mRNA structural changes, for example, among those regulated by riboswitches.39
Start Codon Selection in Archaea
Archaea form a group (domain) of prokaryotes, previously called archaeabacteria. Phylogenetic analysis of SSU rRNA sequences established that Archaea are more closely related to eukaryotes (Eukarya) than to Bacteria (previously called eubacteria) (three-domain classification).40 In agreement with this idea, archaeal translation initiation factors are more similar to eukaryotic factors than to bacterial factors, as described below. However, archaeal initiation is less stringent than eukaryotic initiation, permitting UUG and GUG initiation besides AUG. This fact suggests that the complexity of the eukaryotic initiation system at least in part evolved to confer stringent AUG initiation.
aIF1 and aIF2 evolved to confer initiation accuracy in Archaea
Archaea contain four initiation factors, aIF1, aIF1A, aIF2, and aIF5B (Table 1). Of these, aIF1A and aIF5B are the orthologs of bacterial IF1 and IF2, respectively, being involved in Met-tRNAiMet loading to the P-site and the 50S LSU joining. aIF1 and aIF2 are homologous to eIF1 and eIF2 found in eukaryotes. In the crenarchaeon Sulfolobus solfataricus, aIF1A and aIF1 bind the 30S SSU, preparing it for binding aIF2-GTP. Met-tRNAiMet then binds to the 30S:aIF1A:aIF1:aIF2 complex through aIF2.41 Archaeal mRNAs fall into two distinct classes: leaderless mRNAs starting immediately from the start codon and bacterial-type (and often polycistronic) mRNAs with typical SD sequences.7,42,43 Bacterial-type mRNAs bind the 30S SSU, depending on the SD:Anti-SD interactions. In the absence of aIF1, the 30S:aIF1A:aIF2:Met-tRNAiMet complex is able to bind both AUG and AUU model mRNAs. But it binds only AUG mRNA in its presence, demonstrating the ability of aIF1 to discriminate against AUU.41 It is unclear whether GTP hydrolysis for aIF2 is coupled to start codon selection by the archaeal 30S PIC. However, after the 30S initiation complex is formed, aIF5B most likely promotes subunit joining, as proposed previously,44 because aIF5B is demonstrated to be a ribosome-dependent GTPase and binds the 30S SSU competitively with aIF2.45
Leaderless mRNA binds the 30S SSU, depending on Met-tRNAiMet bound to the 30S SSU,46 similar to bacterial leaderless mRNAs.47 Other than this, the precise pathway for leaderless mRNA translation in Archaea has not been determined.
IF3 and aIF1 appear to bind to similar locations in the SSU decoding site,41 but they are distinct proteins with different folds and domain organizations. Furthermore, the GTP-dependent tRNAi-binding factors, IF2/aIF5B and aIF2, are evolutionarily distinct proteins, because IF2/aIF5B is an ancient translational GTPase superfamily protein that belongs to neither the EF-G or the EF-Tu family. In contrast, aIF2γ (the GTP and tRNAi-binding subunit) clearly belongs to the EF-Tu family.48,49 Thus, aIF1 and aIF2 can be considered as evolutionarily independent entities to confer accuracy of initiation in this group of life (Table 1).
Evolution of non-AUG initiation in Archaea
It is unclear if archaeal Met-tRNAiMet carries the t6A modification in the anticodon loop that is suited for precisely decoding ANN codons during the elongation phase. However, genomics analyses indicate that Archaea permit frequent initiation from UUG and GUG codons,7,42,43 suggesting a decreased accuracy of translation initiation. Since Archaea contain aIF1, whose eukaryotic homolog, eIF1, plays the central role in discriminating against non-AUG codons including UUG and GUG (see below), it seems clear that the interaction between aIF1 and the SSU decoding site alone is not sufficient to confer the level of accuracy found in eukaryotic initiation. It remains to be determined whether the GUG or UUG start codon has any selective advantage toward archaeal translational control, as suggested in some bacterial translational control systems with GUG codons. Because translation of leaderless mRNAs more strongly depends on the start codon: tRNAiMet anticodon interaction, it would be important to determine whether AUG initiation (with complete matching to the anticodon) is favored in leaderless mRNA translation over UUG or GUG initiation. It would be intriguing to learn whether the initiation stringency operates equally well on both types of archaeal mRNAs.
Start Codon Selection in Eukaryotes
The eukaryotic 18S rRNA in the SSU has lost the anti-SD sequence.50,51 Translation generally initiates at the 5′-proximal AUG, irrespective of the length or sequence of the 5′-UTR, implying selection by a process of scanning from the 5′-end. The efficiency of recognition of the 5′-proximal AUG is influenced by a Kozak consensus sequence ([A/G]xxAUGG) in mammals52 or a similar initiation context (AA[A/G]AUG) in fungi including yeast.53 If the context of the 5′-proximal AUG is poor, those ribosomes, which fail to initiate at this site, continue scanning and initiate at the next AUG (leaky scanning). Despite the stringent AUG selection mechanism even discriminating against AUG in a poor context, it has been known that the translation of metazoan translational regulator protein, p97/NAT1/DAP5, or the longer form of mammalian oncogenic transcription factor c-Myc starts from the GUG and CUG codons, respectively (reviewed in Ref54). Recent ribosome profiling studies also suggest that translation of as much as ~30% of genes may start from non-AUG codons in mouse embryonic stem cells.12 In plants, in-frame non-AUG start codons add mitochondria or chloroplast localization signals to change the protein’s location.55 Likewise in humans, an in-frame CUG codon adds an ER signal sequence to the phosphatase PTEN, allowing it to be secreted outside the cell.56 After briefly overviewing the eukaryotic initiation pathway, here I describe the mechanism by which eukaryotic translation initiates strictly from the AUG codon, focusing on the role of the translation initiation multifactor complex (MFC) in this process. Then I discuss whether, or if so how, alterations in MFC formation or its activity in stringent initiation can affect cellular proteome translation.
The MFC represents the evolutionarily distinct entity to confer initiation accuracy
Eukaryotic translation initiation is promoted by as many as 10 eukaryotic initiation factors (eIF)57-59 (Table 1). Like in prokaryotes, the orthologs of IF1 and IF2, termed eIF1A and eIF5B, bind to the conserved sites in the 40S SSU, promoting Met-tRNAiMet loading to the P-site and 60S LSU joining, respectively.60,61 Of the remaining factors, four of them, eIF1, eIF2, eIF3 and eIF5 are considered as an evolutionarily independent entity to confer the accuracy of eukaryotic initiation, because (i) their mutual physical interactions allow them to form a multifactor complex (MFC)62-64 and (ii) earlier yeast genetic data indicate that mutations altering each of the MFC components impair stringent initiation, allowing initiation from UUG65-67 (suppressor of initiation codon mutation or Sui phenotype) (Table 1) (Also see Box 1). In addition to the MFC, the universally conserved factor, eIF1A, evolved to possess N- and C-terminal extensions, playing crucial roles in controlling PIC conformation in response to AUG recognition.11,68-70
Box 1. Is UUG initiation in yeast Sui- mutants analogous to bacterial UUG initiation?
“Interestingly, yeast is but one amino acid substitution (in one gene…..) away from being able to allow initiation at UUG codons, an interaction facilitated by IF3” – Hartz et al., 1990138
In their genetic analyses, the Donahue group used five start codon mutations, AUU, ACG, CUG, ACC and GUG, as parental mutations to screen for extragenic suppressors of the initiation codon mutations (Sui). Sui- mutants isolated from each parent were able to suppress all other start codon mutations. Because the reporter gene (HIS4) was translated from the third codon UUG in these mutants in all the cases examined, the UUG codon, but not GUG or CUG, was determined to be the second optimal start codon used in the Sui- mutants.139,140 As quoted above, these beautiful data tempt us to speculate that UUG is a second “preferred” start codon for a somewhat less stringent system found in Bacteria or yeast Sui- mutants and that this property reflects the fundamental structural requirement or constraint for decoding in the P-site. However, it should be noted that nearly all the studies on non-AUG initiation in the Sui- mutants have used the HIS4 mRNA as a model. The HIS4 3rd codon UUG is the first UUG as found in the HIS4 mRNA leader region. All other potential (near-cognate) non-AUG start codons appear more than once in the HIS4 5′ UTR and most are out of frame to HIS4. Thus, if a suppressor were to be isolated and allowed HIS4 translation from the first non-AUG codon, the mutant probably had to utilize the 3rd codon UUG. Nevertheless, the frequency of in-frame GUG initiation of HIS4 was compared with that of the UUG initiation, by disrupting the sole natural GUG codon in the 5′ UTR. In a SUI4 or SUI5 mutant altering eIF2γ and eIF5, respectively, the frequency of GUG initiation was 39% and 1% compared with that of UUG initiation.66 Thus, UUG is still a preferred start codon even compared with GUG. In addition, the site-directed mutations altering the critical interfaces of eIF1 within MFC in the open PIC allow initiation from UUG-initiated HIS4 allele,106,112 suggesting that, in general, the MFC impairment can cause UUG initiation by destabilizing the open PIC, as far as it is found in the HIS4 mRNA leader. More works using different reporter constructs are needed to determine whether the strong UUG initiation in the suppressor mutants has any general relevance.
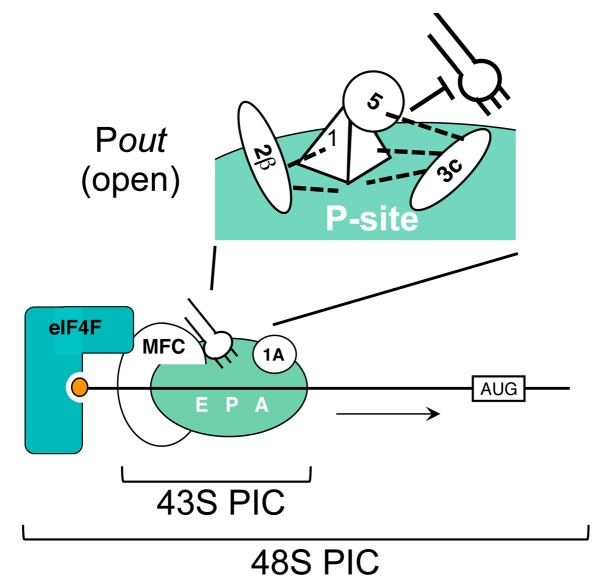
Of the MFC factors, eIF2 binds Met-tRNAiMet in a GTP dependent manner, thereby recruiting it to the 40S SSU in a ternary complex (TC). The C-terminal domain of eIF5 bridges eIF2 and eIF3.62 Thus, MFC-mediated mutual cooperativity between the 40S SSU interactions with eIF2 and eIF3 would promote the formation of the 43S PIC, which is made of the 40S SSU, eIF1A, MFC and Met-tRNAiMet 71,72 (Fig. 2).
Figure 2. Eukaryotic 48S PIC. Schematics on the bottom depict the eukaryotic 48S PIC formed at the 5` end of mRNA (horizontal line) with the AUG codon (box) ahead. The m7G cap, shown by an orange small circle, is bound by eIF4F in teal. The translation initiation multifactor complex (MFC) bridges eIF4F and the 40S SSU, the large pale green circle with while letters indicating E, P, and A-sites. Met-tRNAiMet, shown as a plug, is attached through the eIF2 component of the MFC, but not firmly linked to the P-site during scanning (Pout). Arrow indicates the direction of scanning. The 43S PIC is made of the 40S SSU, MFC, eIF1A, and Met-tRNAiMet. On top, eIF1 (pyramid) bound near the P-site blocks Met-tRNAiMet (plug) binding to the P-site together with other MFC partners, until it base-pairs to the AUG codon. The thick stopped bar indicates the physical block of the P-site formed by the presented part of the MFC. Dotted lines denote the interactions of eIF1 with other MFC partners shown by circles.
eIF4F binds the 5′ cap directly through its eIF4E subunit and the poly(A) tail through an interaction between its eIF4G subunit and the poly(A) binding protein (PABP). The RNA helicase activity carried by the eIF4A subunit unfolds the 5′ proximal region of the mRNA, thereby allowing the 43S PIC to bind to the 5′ end of the mRNA59,73 (Fig. 2). Besides eIF4A,74 various mRNA helicases unwind mRNA structures along the path of the scanning PIC, assisting mRNA base triplet pairing to the tRNAiMet anticodon. The MFC bound to the 40S SSU bridges the eIF4G subunit and the SSU (Fig. 2). The 48S PIC thus formed scans for the first AUG codon of the mRNA, likely through a 5′-to-3′ migration of the PIC along the mRNA (mRNA scanning).
Similar to all ANN-decoding tRNAs, eukaryotic Met-tRNAiMet carries t6A 3′ of the anticodon. However, this modification is not necessary for stringent initiation, because in vitro transcribed Met-tRNAiMet can be used to reconstitute the translation initiation reaction capable of discriminating against non-AUG codons.75,76 Instead, initiation factor proteins that couple GTP hydrolysis to AUG recognition by the PIC play the major role in producing the 80S initiation complex precisely at the AUG codon, as outlined briefly next and described in detail later in this review.
On the 48S PIC formation, eIF5 starts to promote GTP hydrolysis for eIF2, but the products, GDP and Pi, remain bound to eIF2, keeping a scanning-competent conformation. Once the tRNAi anticodon base-pairs to AUG, the Pi is released and the PIC stops scanning. As a consequence, eIF2 adopts the GDP-bound conformation, releasing itself from the PIC.75 As described below, eIF1 leaves the PIC before Pi release and eIF5 leaves the PIC together with eIF2-GDP, allowing the 40S IC to form. eIF5B then binds the 40S IC and promotes 60S LSU joining.77,78 eIF2-GDP is recycled into eIF2-GTP by the action of the guanine nucleotide exchange factor (GEF), eIF2B (Table 1).
In yeast, a substantial fraction of eIF5 associates with eIF2 but lacks Met-tRNAiMet, suggesting that the clearance or recycling of the eIF2-GDP:eIF5 complex is rate-limiting for efficient MFC formation.79,80 eIF5 inhibits GDP dissociation from eIF2 in this complex, thereby serving as a GDP dissociation inhibitor (GDI).81 Importantly, eIF2 phosphorylation increases the abundance of this complex, thereby enhancing the inhibition of guanine nucleotide exchange by eIF2B.81 The significance of the eIF5:eIF2:GDP complex as the sequester of both the factors80 was recently enhanced by the discovery of eIF5 dissociation function carried by eIF2B (GDI dissociation factor or GDF function).82
Conservation and diversity in the mechanism of translation initiation throughout eukaryotes
All factors that interact mainly with the subunit interface of the SSU—eIF1, eIF1A, eIF2, eIF5 and eIF5B—are highly conserved in structure and function throughout eukaryotes. However, much diversity is seen with molecular compositions of eIF3 and eIF4F involved in mRNA recruitment to the ribosome. Remarkably, the subunit composition of the yeast Saccharomyces cerevisiae eIF3 is simpler than mammalian eIF3 (Table 1). Nearly all the 13 subunits of eIF3 are generally well conserved in plants, fungi and metazoans, and the simplified eIF3 is found only in a group of closely related yeasts. However, the anaerobic protozoan Giardia intestinalis (lamblia), the most distantly related and perhaps primitive eukaryote, possesses only four identifiable eIF3 subunits (b, c, g, i) also found in S. cerevisiae eIF3 (KA, personal observations) – an example of convergent evolution. Thus, yeast eIF3 most likely carries a minimal function in various aspects of translation initiation. Likewise, the structure of eIF4G is diverse among eukaryotes, except for the Y(X)4LΦ motif responsible for eIF4E-binding and the MIF4G/HEAT domain responsible for eIF4A-binding.83 There is no conservation in PABP-binding sites between plant, yeast and mammalian homologs.8,84-86 In line with the difference in eIF3 structures, the nature of the interactions within the MFC differ somewhat, at least in strength, between yeast and mammals.64 The eIF4G-binding sites in the MFC are also different between yeast (via eIF5 and eIF1),8,87 and mammals (via eIF3).88-90
In accordance with the diversity in factors involved in mRNA recruitment, Metazoa have evolved many non-redundant mRNA helicases involved in translation initiation,91 including Vasa, a DEAD-box helicase, that binds the 3` UTR, remodeling mRNA for PIC recruitment.92 Mammalian DHX29 and DDX3 (yeast homolog, Ded1p), which unwind secondary structures in the 5′ UTR, also belong to distinct subfamilies of mRNA helicases, and hence arose independently during eukaryotic evolution.93-96
Molecular mechanistics of stringent AUG initiation in eukaryotes
Careful studies of the yeast translation initiation system, coupling in vitro biochemical assays with in vivo phenotypic observations, have reveled the following, intricate mechanism of stringent AUG initiation. The protein motifs and three-dimensional structures involved in this process are highly conserved across the eukaryotic kingdoms, and, in particular, between yeast and mammals. The key features of the mechanistics, including the SSU ribosomal conformational changes, are also being verified in the mammalian system.
eIF1 plays the central role in maintaining the scanning competent state.
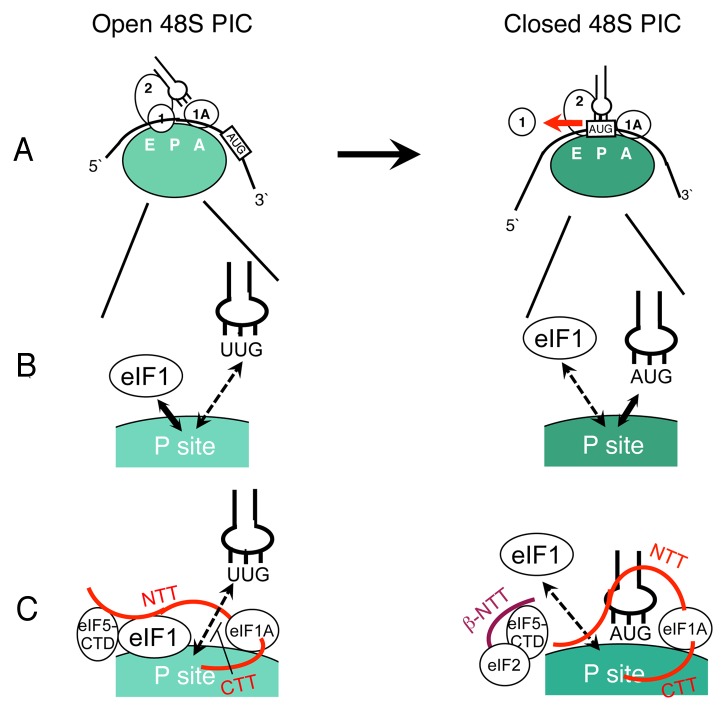
eIF1, the smallest component (12 kDa) of the MFC, plays an important role in stringent AUG initiation in eukaryotes. Accumulating evidence from yeast and mammalian cells indicates that this is the key regulator of ribosome conformation from the “open” scanning-competent state to the “closed” scanning-incompetent state. 97 , 98 During scanning, the PIC allows mRNA sliding with a wider mRNA-binding channel. Met-tRNAiMet is not locked into the P-site at this point (Pout), allowing the tRNAi anticodon to base-pair with base triplets in the mRNA. However, when the anticodon base-pairs to the AUG codon, the PIC stops scanning, accommodating the Met-tRNAi:AUG pair in the P-site (Pin) (Fig. 3A).
Figure 3. Translation factor control of the PIC conformational change, coupled to AUG recognition. (A) The open (left) and closed (right) states of the 48S PIC are depicted with the 40S SSU (large circle with E, P and A-sites, different colors indicating distinct states) bound to initiation factors (small circles with numbers corresponding to each eIF). In the open state (left), the decoding site is “open,” allowing mRNA (curved thick line with 5′ and 3′ ends indicated) to slide along the mRNA-binding channel. Met-tRNAiMet (plug) is tilted to imply that it is out of the P-site. eIF1 (circle numbered 1) prevents Met-tRNAiMet paired with the non-AUG codon from binding the P-site. In the closed state (right), eIF1 is released (thick horizontal arrow), allowing Met-tRNAiMet paired with the AUG codon to bind the P-site. The Met-tRNAiMet is positioned vertically to imply the Pin state (near the P/I configuration18,20). (B) The conformational change is explained by the competition between eIF1 (circle) and the Met-tRNAiMet (plug):base triplet pair (UUG or AUG) for the P-site (labeled white on a part of green circles representing the 40S SSU). (C) MFC partners and eIF1A tails serve to stabilize each state, tightly coupling AUG recognition and commitment to initiation. In the open state (left), indicated MFC partners (circles) and eIF1A-NTT (thick red line) assist eIF1 to block tRNAiMet paired to UUG (representing a non-AUG codon) from binding the P-site. eIF1A-CTT (thick red line) destabilizes tRNAiMet binding to the P-site, contributing to the maintenance of the open state. In right, eIF2β-NTT (thick maroon line) plays the major role in the shift to the closed state by binding eIF5-CTD and disrupting the eIF1 linking to the PIC. These interactions promote eIF1 release. eIF1A-NTT (thick red line) stabilizes tRNAiMet binding to the P-site by direct interaction with tRNAiMet and the 40S P-site.
This “conformational change” model was first proposed as the result of analyses of the in vitro reconstituted initiation system using mammalian factors, identifying eIF1 and eIF1A as crucial elements for mRNA scanning and eIF1 for discriminating against non-AUG initiation.99-101 The cryo-EM structure of the yeast eIF1/eIF1A/40S SSU complex (the open state) displayed a wider mRNA-binding space located between the “head” and “body” of the SSU than the 40S SSU alone (the closed state), in agreement with the mRNA-sliding capacity of the open conformation.102 More recently, comparison between Tetrahymena eIF1/eIF1A/40S (open) complex and the 40S SSU alone (closed) defined conformational changes rather locally restricted to eIF1- and eIF1A-binding sites.103 More work is needed to delineate structural features in the open PIC required for mRNA sliding, including the role of a “latch” formed by interactions of an rRNA helix of the body with another rRNA helix and Rps3 of the head, which clamp around the mRNA.104
Experiments with a partial yeast initiation system (composed of eIF1, eIF1A, eIF2, eIF5, Met-tRNAiMet, mRNA and the 40S SSU) demonstrated that eIF1 release is the key event associated with the conformational change, as detected in the FRET (fluorescence resonance energy transfer) assay,105,106 and precedes Pi release75 (Fig. 3A). Thus, the presence of eIF1 defines the open state of the PIC. Recent studies highlighted the role of eIF1 in maintaining the Pout state of the open PIC (Fig. 3B), as described next.
In a TC binding assay with the yeast eIF1:eIF1A:mRNA:40S complex, eIF1 dramatically increases the off rate of the TC, leading to the idea that eIF1 antagonizes tRNAi binding to the P site before start codon selection (Pout).102 This idea is strongly supported by genetic and biochemical studies, indicating that many yeast eIF1 mutations impairing the interaction with the 40S SSU107 or MFC partners106 (also see below) allow faster release of eIF1, thereby increasing the frequency of UUG initiation (Sui or suppressor of initiation codon mutation phenotype) – a premature change to the closed state at the non-AUG codon.53,106 Recently solved crystal structures of rabbit 40S SSU complexes revealed how eIF1 maintains the Pout conformation: The tRNAi located in the 40S:mRNA:tRNAi:eIF1A (closed) complex clashed with eIF1 in the 40S:eIF1 or 40S:eIF1:eIF1A (open) complex.104 Thus, eIF1 binds near the P-site and physically blocks tRNAi binding to the P-site, until its release caused by tRNAiMet binding to the AUG codon (Fig. 2 and Fig. 3B).
The MFC and eIF1A-terminal tails control initiation accuracy by dynamic rearrangement.
An excessive amount of eIF5 stimulates premature eIF1 release, thereby allowing initiation at UUG. 108,109 Interestingly, mutational studies delimited the part of eIF5 responsible for this activity to the C-terminal domain (CTD), 10 not the N-terminal domain responsible for GTPase activation for eIF2. 9 NMR studies revealed an evolutionarily conserved, overlapping surface of eIF5-CTD responsible for binding to eIF2β and eIF1. A mutation altering the eIF2β-binding site of eIF5-CTD alleviated the ability of eIF5 to promote eIF1 release, indicating that the eIF2β:eIF5-CTD interaction is involved in the shift to the closed state. 9 eIF5-binding sites in eIF2β were located in three lysine-rich segments (K-boxes) in its N-terminal tail (NTT). 110 Mutations altering the mutual binding sites prevented UUG initiation enforced by an eIF Sui- mutation (Ssu, or suppressor of SUI, phenotype), 8,9,111 in agreement with the role for this interaction in stabilizing the closed state of the PIC ( Fig. 3C , right). In contrast, the eIF5-CTD interaction with eIF1 contributes to eIF1 anchoring to the open PIC 112 (Fig. 3C , left). Thus, the eIF2β:eIF5-CTD interaction promotes eIF1 release by disrupting the eIF1 link to the PIC ( Fig. 3C ).
The interaction between the eIF5-CTD and the eIF2β-K-boxes is the major driver of MFC assembly in yeast.62,71,72 How can this interaction also trigger the PIC shift from the open to closed conformation? The eIF2β-K boxes bind RNA in a manner competitive with eIF5-CTD binding, and evidence suggests that the K-boxes mediate mRNA binding to the PIC.8 Thus, it is proposed that the interaction between the eIF5-CTD and eIF2β-K-boxes gets resolved upon 48S PIC formation, such that the K-boxes can stabilize mRNA binding during scanning. Once AUG is recognized, the K-boxes are somehow disengaged from mRNA binding and instead gets committed to close the PIC conformation.
The eukaryote-specific extensions of eIF1A, its NTT and CTT (C-terminal tail), play opposing roles in regulating PIC conformation.69 The eIF1A-NTT stabilizes the closed conformation through a direct interaction with tRNAi in the P-site,103,104,113 while the eIF1A-CTT stabilizes the open conformation by linking tRNAi to the open PIC directly or indirectly through eIF268-70 (Fig. 3C). A recent study also revealed a new interaction between the eIF1A-NTT and eIF5-CTD.11 Similar to eIF2β K-boxes, the eIF1A-NTT is rich in positively charged residues. NMR studies showed that the eIF1A-binding site on eIF5-CTD almost completely overlaps with its eIF2β-binding site. In vivo mutational studies on the charged residues in eIF1A-NTT suggest that the eIF1A-NTT interaction with eIF5 contributes to eIF1 retention of the open PIC. Thus, eIF1A-NTT masks the eIF2β-binding site of eIF5-CTD during the course of scanning and contributes to the retention of eIF1 through eIF5-CTD11 (Fig. 3C).
Translational Control through Inhibition of MFC Formation
uORF-mediated control
Virtually all eukaryotic mRNAs are structurally and functionally monocistronic. If a bicistronic mRNA coding for two full-length proteins is generated and tested in a laboratory, only the 5′-proximal ORF is expressed, because ribosomes are released from the mRNA at the stop codon of this ORF. However, ~45–50% of mammalian mRNAs and ~13% of yeast mRNAs have at least one short upstream ORF (uORF) that either is bypassed by leaky scanning, prevents translation of the downstream ORF, or, alternatively, allows its re-initiation after the translation of the short uORF. 114 An uORF can become permissive to downstream re-initiation, if the uORF is very short and somehow prevents ribosome dissociation at the stop codon, allowing the ribosomes that had anchored to mRNA following uORF translation to resume scanning.
Intriguingly, combination of a short, re-initiation-permissive uORF and an inhibitory uORF (paired uORFs) allows translational control in response to eIF2 inhibition and resulting delayed re-initiation at the main ORF. Examples of regulation by paired uORFs include mammalian ATF4 and yeast GCN4 whose translation is induced by eIF2α phosphorylation.59 Various stress-activated protein kinases, such as GCN2, PKR, PERK and HRI, phosphorylate the conserved Ser-51 residue of the eIF2α subunit. This renders eIF2 a competitive inhibitor of eIF2B, thereby decreasing the eIF2:GTP:Met-tRNAiMet TC level. As a result, TC binding to the PIC that is about to re-initiate at the inhibitor uORF is delayed, allowing it to bypass the uORF and re-initiate at ATF4 or GCN4 instead.
In yeast, eIF5 overxpression decreases TC abundance through eIF2B inhibition (the GDI activity) and slows down TC binding to the PIC through disturbing MFC formation. These effects mimic the effect of eIF2 phosphorylation, thereby allowing the post-uORF PIC to bypass the downstream uORFs and initiate translation of GCN4.97 In line with this observation, overexpression of a new translational inhibitor protein, eIF5-mimic protein 1 (5MP1, also known as BZW2), can inhibit eIF2 through a direct competition with eIF5, thereby inducing ATF4 translation in mouse embryonic fibroblasts with eIF2α Ser 51-to-Ala mutation.115 Thus, GCN4 or ATF4 can be translationally induced through eIF2 inhibition independent of eIF2 phosphorylation.
Interestingly, the structural requirement for re-initiation following translation at an uORF is different between yeast and mammals or perhaps the whole Metazoa. In yeast, an uORF is generally non-permissive or inhibitory to downstream initiation. An mRNA cis element that binds eIF3 (via the a subunit) is responsible for rendering GCN4 uORF1 to being permissive.116 In mammals, the permissiveness of an uORF is primarily determined by its size.114 A short uORF (of ~2–3 amino acids) is generally permissive, likely because eIF3 and/or eIF4G remain(s) anchored more stably to the ribosome that has just finished its translation. uORFs longer than 20 amino acids are inhibitory to downstream initiation, likely because these factors dissociate from the ribosome during the time it takes to complete the uORF translation. Longer uORFs are even more inhibitory if they overlap with the main ORF, because the ribosome which has translated the uORF does not migrate backward (in a 3′-to-5′ direction) and therefore is effectively prevented from re-initiating at the main ORF. This difference leads to a marked difference between yeasts and Metazoa in the paired uORF organization required for translational control through eIF2 inhibition.
Competition with cap-independent translation.
Besides the cap-dependent mode, eukaryotic translation can initiate from an internal ribosome-entry site (IRES) often found in RNA viruses, such as poliovirus (PV), encephalomyocarditis virus (EMCV), hepatitis C virus (HCV) and cricket paralysis virus (CrPV) (cap-independent translation). 117 , 118 The MFC-driven cap-dependent pathway competes with cap-independent translation of IRES-carrying mRNAs. Accordingly, virus infection often inactivates cap-dependent translation through eIF4F cleavage and induces eIF2α phosphorylation, resulting in a decrease in eIF2:GTP:Met-tRNAiMet TC levels. 59 This effect renders the IRES to compete more effectively for the MFC-loaded 43S PIC (EMCV), allows other MFC components or the 40S SSU available for IRES-mediated translation (HCV or CrPV), or, alternatively, allows non-canonical initiation factors (eIF2D/LGTN or MCT-1:DNER) promoting eIF2-indendnent Met-tRNAiMet recruitment 119 , 120 to bind the 40S SSU in place of the MFC (HCV). If eIF5 associates with eIF2:GDP and inhibits GDP dissociation from phosphorylated eIF2 (GDI activity) during virus infection, 79 , 81 eIF5 is sequestered effectively from the MFC, thereby enhancing the effect of eIF2 phosphorylation in favor of MFC inhibition and IRES translation.
Translational control through altered initiation stringency in eukaryotes
In mammals, eIF1 also works to discriminate against AUG codons in a poor Kozak context (consensus).100 The AUG start codon of mammalian eIF1 carries a poor Kozak context. When the eIF1 level goes down, a larger proportion of the PIC lacks eIF1, thereby decreasing its ability to discriminate against the AUG codon in a poor Kozak context. As a consequence, eIF1 translation goes up, correcting for the low eIF1 abundance121 – an autoregulatory mechanism similar to AUU-initiated bacterial IF3 regulation. Similarly, the yeast gene encoding eIF1 is translated from an AUG codon in a poor context and is auto-regulated by eIF1 abundance. However, this auto-regulation is only modulatory, since the expression of eIF1 from a high copy plasmid permits its substantial overexpression.53
Ribosome profiling, based on deep sequencing of ribosome-protected mRNA fragments, has been used to identify ribosome “footprints” on all the mRNAs in the cells.122 The drug harringtonine causes the ribosome to accumulate precisely at start codons. Thus, the ribosome profiling of harringtonine-treated cells can potentially reveal all the start codons used in the cells. Analysis of mouse embryonic stem cells revealed frequent non-AUG initiations within 5′ UTRs, which were suppressed by ~25% after differentiation.12 Based on this fact and that the genes displaying frequent non-AUG initiation at their 5′ UTR include transcription factors governing pluripotency, such as c-Myc and Nanog, it was proposed that non-AUG initiation allows translational control characteristic of pluripotency.12
The use of harringtonine to identify functional start codons in mouse ES cells revealed numerous translated uORFs with near-cognate start codons, which outnumbered AUG-initiated uORFs by ~4:1, with CUG the most frequent.12 In yeast the similar ribosome profiling data (but done without harringtonine that cannot diffuse into yeast) suggest that uORFs with near-cognate start codons are roughly equal in number to AUG-initiated uORFs.122 It should be noted that, similar to the prokaryotic cases, the frequencies of initiation from these non-AUG codons might be much more lower than that of initiation from the AUG codon. In a classical work on the yeast CYC7 initiation site, the strongest non-AUG initiation codons were GUG, ACG, AUU and UUG, but their frequency of initiation was 0.4~0.5% compared with that of AUG initiation.24
Provided that non-AUG initiation occurs at a sufficiently high frequency, non-AUG initiation in a 5′ UTR can affect gene expression through generating new uORFs114 or in-frame start codons54 (Fig. 4). If non-AUG start codons initiate translation of short uORFs (of up to 3~5 amino acids), they are expected to be permissive to downstream initiation (Fig. 4B, panel 1). On the other hand, if they initiate translation of long uORFs – especially those overlapping with the main ORF, they are inhibitory to initiation at the main ORF (Fig. 4B, panel 2). In addition, if non-AUG start codons generate a pair of permissive and inhibitory uORFs with appropriate locations (such as the ones found in the ATF4 leader region123,124), 5` UTR translation may allow regulated initiation of the main ORF in response to changes in eIF2 TC levels (Fig. 4B, panel 3). In-frame non-AUG start codons add N-terminal extensions to the protein encoded by the main ORF, which may regulate its enzyme or transcription factor activity (Fig. 4B, panel 4). Alternatively, these N-terminal extensions may contain an intracellular localization signal (ER, mitochondrial or plastid localization signal) (Fig. 4B, panel 4), regulating protein transport.55,56
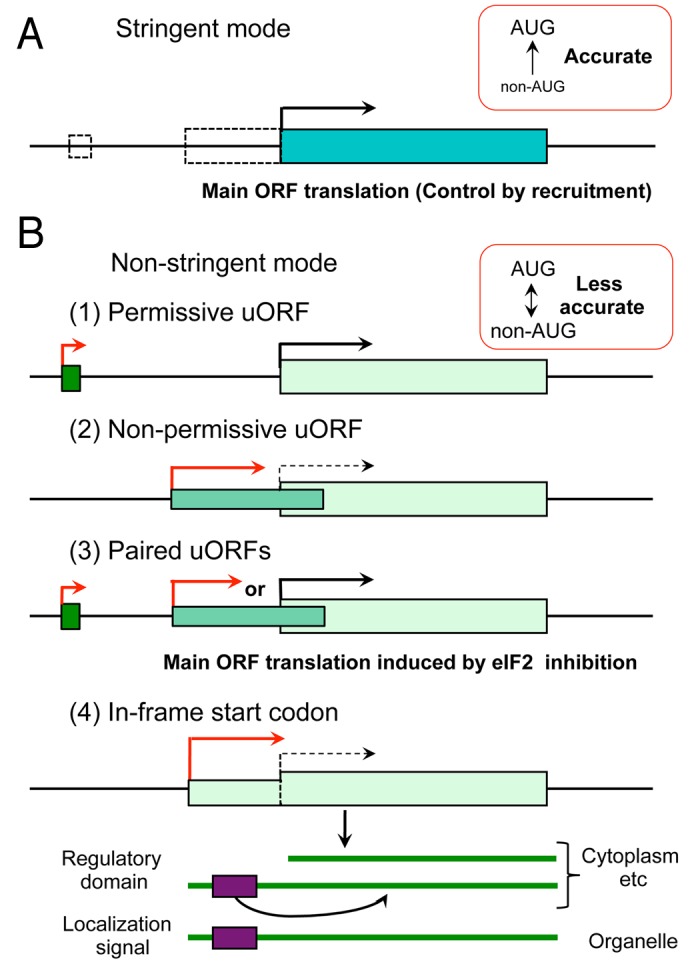
Figure 4. Possible mechanisms of translational control by regulated initiation stringency. Typical coding structures of mRNA (horizontal lines) are depicted with the main ORF starting with the canonical AUG codon (longer box to the right). Black and red arrows indicate AUG- and non-AUG-initiated translation, respectively. mRNA translation patterns in the normal stringent mode (A) and the hypothetical non-stringent mode (B) are depicted. In (A), dotted boxes depict non-AUG-initiated uORFs that are not translated due to the normal, stringent mode of initiation. The main ORF is translated predominantly. In (B), panels 1–4 describe four patterns of regulation achieved by non-AUG-initiation. In panel 1, non-AUG-initiated uORF is permissive to downstream re-initiation, allowing the main ORF translation. In panel 2, non-AUG-initiated uORF is non-permissive to downstream re-initiation. The translation of the main ORF is only possible by the ribosome that had leaky-scanned the uORF (dotted line). In panel 3, the upstream permissive uORF allows downstream re-initiation of the second non-permissive uORF “or” the main ORF. When eIF2 TC abundance and recruitment are normal, the second uORF is re-initiated, inhibiting the main ORF translation. When eIF2 TC is limited or its ribosome binding is inhibited, the re-initiation is delayed and occurs at the main ORF. In panel 4, thick lines below mRNA schematics denote proteins encoding by the mRNA, with boxes indicating N-terminal functional segments added by the in-frame non-AUG initiation. Though not depicted here, there are myriads of mRNAs with AUG-initiated uORFs that are translated in the stringent mode, playing important roles in translational control. See text for details.
Is CUG initiation mediated by a Leu-tRNA initiator?
Much of the non-AUG initiation observed in the ribosome profiling studies mentioned above starts with CUG or GUG.12Interestingly, CUG initiation was reported to play a specialized role in MHC class I antigen protein synthesis and presentation.13The ribosome engaged in the CUG initiation in rabbit reticulocyte lysates was shown to contain a Leu-tRNA. Gene silencing studies also showed that the CUG initiation is promoted by a non-canonical initiation factor, eIF2A. Evidence is also presented that Leu-tRNA initiates the translation of a CUG-initiated uORF found in the c-Myc leader region.
This study poses important questions concerning the mechanism of non-AUG initiation found in higher eukaryotes. Is it a modification of the canonical cap-dependent, MFC-mediated pathway, a totally new Leu-tRNA-driven pathway, or a combination thereof?
Altered initiation stringency and disease.
If we consider non-AUG initiation in a 5′ “UTR” as the consequence of regulation of the MFC-mediated stringency mechanism, the finding that 5′ UTR translation goes up in embryonic stem cells and gets suppressed upon differentiation is very intriguing. 12 Considering the MFC-mediated mechanism mentioned above, there are two ways to control the frequency of non-AUG initiation without introducing mutations into MFC components. One is to change expression of eIF1 or eIF5, the key regulators of initiation stringency, as demonstrated in mammalian cells. 109 , 121 The other is to modulate interactions between MFC components through phosphorylation or protein inhibitors. In plants, the MFC is the target of frequent protein phosphorylation during active protein synthesis. 63 Similar MFC components are also phosphorylated in mammals and yeasts. 125 - 129 Recently, the eIF5-mimic protein (5MP, also known as BZW or eIF5C) was reported to inhibit general protein synthesis by competing with eIF5 for eIF2. 115 These findings raise an interesting possibility that MFC regulation leads not only to regulation of general translation, but also to controlled stringency of translation initiation.
The cellular pluripotency, whose translation status may be characterized by non-AUG initiation, plays an important role in the generation of cancers. While overexpression or truncation of initiation factors is associated with cancers,130,131 there is no report indicating that the modulation of eIF1 or eIF5 levels is associated with them. However, human 5MP2 (BZW1) was shown to be overexpressed in distinct types of cancers including mucoepidermoid carcinoma (MEC). Since MEC cell lines knocked down for 5MP2 generated smaller tumors in nude mice, 5MP2 is proposed to be an oncogenic protein.132 As mentioned above, 5MP1 (BZW2) was also reported to promote translation of ATF4, the oncogenic transcription factor, in mouse embryonic fibroblast cell lines with the homozygous eIF2α-S51A mutation defective in eIF2α phosphorylation.115 It would be intriguing to determine whether MFC regulation through 5MP can affect cellular stringency of translation initiation and whether mis-programming of this regulation can lead to tumorigenesis.
Another category of disease caused by changes in translation components include “ribosomopathy,” whose founding members are Diamond-Blackfan anemia and 5q- syndromes, caused by mutations in ribosomal proteins.133,134 While most of the symptoms, including macrocytic anemia and cogenital disorders, may be explained by the lack of sufficient protein synthesis required for tissue development due to inappropriate ribosome synthesis, there are many other symptoms associated with ribosomopathies that are not explained by this model. One of them is an increased risk of cancers,133,134 which is also observed in zebrafish lines deficient in ribosomal proteins.135 It is puzzling that that the ribosomal protein mutations cause common phenotypes despite the fact that the altered proteins locate everywhere on the ribosome and that no common functional defect is found. Recently, it was reported that a common phenotype of rather subtle, small and large rRNA mutations in yeast is to increase the accuracy of initiation (Ssu phenotype).78,136,137 An interesting possibility emerges that the ribosomal protein mutations reduce non-AUG initiation in stem cells (even to a small extent), thereby compromising the gene regulation program that sets the stage for later differentiation while suppressing tumorigenesis.
Conclusions and Perspectives
Although the role of the MFC in rapid PIC assembly may not yet have been established in higher eukaryotes, recent studies highlight the mechanism by which eIF2 phosphorylation limits both eIF2 TC and free eIF5 available for efficient MFC formation.81,82 In addition, it seems clear that the MFC is the evolutionarily conserved unit responsible for stringent AUG initiation specific to eukaryotes. Careful NMR interaction studies using both yeast and human proteins have identified conserved mutual interfaces in MFC, and its partner, eIF1A-NTT, responsible for stringent AUG initiation.9,11 The immediate next goal of this line of study is to delineate the precise molecular role of eIF3, the major component of the MFC, and eIF4G, the major mRNA-binding partner of the MFC, in stringent AUG initiation, since the binding sites for both eIF1 and eIF5 were identified in the unstructured segments of the c subunit of eIF3 and eIF4G.8,67
The discovery of non-AUG initiation that is prevalent in the pluripotent cell stage has raised the very important question – how can cell’s stringency in translation initiation be regulated? Whether this involves the control of MFC activity, the new Leu-tRNA-driven pathway or both, the quest for the answer to this question requires the efforts of coming decades, combining all the cutting edge technologies to study the mechanism and regulation of translation initiation. The results of such studies are expected to change the paradigm concerning how we view translational control, diseases caused by its perturbation, and evolution of life on Earth.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
I thank the anonymous reviewers for creative and helpful comments. Thanks are also due to John Hershey for critical reading of the manuscript and Kazuyuki Takai (Ehime University, Japan) and Tsutomu Suzuki (University of Tokyo, Japan) for discussion. I regret that, due to constraints, there are relevant communications that have not been cited here.
References
- 1.Moore PB, Steitz TA.. The involvement of RNA in ribosome function. Nature 2002; 418:229 - 35; http://dx.doi.org/ 10.1038/418229a; PMID: 12110899 [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan V.. Ribosome structure and the mechanism of translation. Cell 2002; 108:557 - 72; http://dx.doi.org/ 10.1016/S0092-8674(02)00619-0; PMID: 11909526 [DOI] [PubMed] [Google Scholar]
- 3.Rodnina MV, Beringer M, Wintermeyer W.. How ribosomes make peptide bonds. Trends Biochem Sci 2007; 32:20 - 6; http://dx.doi.org/ 10.1016/j.tibs.2006.11.007; PMID: 17157507 [DOI] [PubMed] [Google Scholar]
- 4.Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FV 4th, Weir JR, Ramakrishnan V.. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 2009; 326:688 - 94; http://dx.doi.org/ 10.1126/science.1179700; PMID: 19833920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asano K, Ito K. in Encyclopedia of Systems Biology eds Werner Dubitzky, Olaf Wolkenhauser, Kwang-Hyun Cho, & Hiroki Yokota) 2259-2263 (Springer, 2013). [Google Scholar]
- 6.Rocha EPC, Danchin A, Viari A.. Translation in Bacillus subtilis: roles and trends of initiation and termination, insights from a genome analysis. Nucleic Acids Res 1999; 27:3567 - 76; http://dx.doi.org/ 10.1093/nar/27.17.3567; PMID: 10446248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torarinsson E, Klenk HP, Garrett RA.. Divergent transcriptional and translational signals in Archaea. Environ Microbiol 2005; 7:47 - 54; http://dx.doi.org/ 10.1111/j.1462-2920.2004.00674.x; PMID: 15643935 [DOI] [PubMed] [Google Scholar]
- 8.Singh CR, et al.. Sequential eIF5 binding to the charged disordered segments of eIF4G and eIF2β stabilizes the 48S pre-initiation complex and promotes its shift to the initiation mode. Mol Cell Biol 2012; 32:3978 - 89; http://dx.doi.org/ 10.1128/MCB.00376-12; PMID: 22851688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luna, R. E. et al. The C-terminal domain of eukaryotic initiation factor 5 promotes start codon recognition by its dynamic interplay with eIF1 and eIF2β Cell Reports 1, 689–702 (2012). [DOI] [PMC free article] [PubMed]
- 10.Nanda JS, Saini AK, Muñoz AM, Hinnebusch AG, Lorsch JR.. Coordinated movements of eukaryotic translation initiation factors eIF1, eIF1A, and eIF5 trigger phosphate release from eIF2 in response to start codon recognition by the ribosomal preinitiation complex. J Biol Chem 2013; 288:5316 - 29; http://dx.doi.org/ 10.1074/jbc.M112.440693; PMID: 23293029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luna RE, Arthanari H, Hiraishi H, Akabayov B, Tang L, Cox C, Markus MA, Luna LE, Ikeda Y, Watanabe R, et al.. The interaction between eukaryotic initiation factor 1A and eIF5 retains eIF1 within scanning preinitiation complexes. Biochemistry 2013; 52:9510 - 8; http://dx.doi.org/ 10.1021/bi4009775; PMID: 24319994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingolia NT, Lareau LF, Weissman JS.. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell 2011; 147:789 - 802; http://dx.doi.org/ 10.1016/j.cell.2011.10.002; PMID: 22056041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starck SR, Jiang V, Pavon-Eternod M, Prasad S, McCarthy B, Pan T, Shastri N.. Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I. Science 2012; 336:1719 - 23; http://dx.doi.org/ 10.1126/science.1220270; PMID: 22745432 [DOI] [PubMed] [Google Scholar]
- 14.Milón P, Maracci C, Filonava L, Gualerzi CO, Rodnina MV.. Real-time assembly landscape of bacterial 30S translation initiation complex. Nat Struct Mol Biol 2012; 19:609 - 15; http://dx.doi.org/ 10.1038/nsmb.2285; PMID: 22562136 [DOI] [PubMed] [Google Scholar]
- 15.de Smit MH, van Duin J.. Secondary structure of the ribosome binding site determines translational efficiency: a quantitative analysis. Proc Natl Acad Sci U S A 1990; 87:7668 - 72; http://dx.doi.org/ 10.1073/pnas.87.19.7668; PMID: 2217199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzi S, Myasnikov AG, Serganov A, Ehresmann C, Romby P, Yusupov M, Klaholz BP.. Structured mRNAs regulate translation initiation by binding to the platform of the ribosome. Cell 2007; 130:1019 - 31; http://dx.doi.org/ 10.1016/j.cell.2007.07.008; PMID: 17889647 [DOI] [PubMed] [Google Scholar]
- 17.Dallas A, Noller HF.. Interaction of translation initiation factor 3 with the 30S ribosomal subunit. Mol Cell 2001; 8:855 - 64; http://dx.doi.org/ 10.1016/S1097-2765(01)00356-2; PMID: 11684020 [DOI] [PubMed] [Google Scholar]
- 18.Simonetti A, Marzi S, Myasnikov AG, Fabbretti A, Yusupov M, Gualerzi CO, Klaholz BP.. Structure of the 30S translation initiation complex. Nature 2008; 455:416 - 20; http://dx.doi.org/ 10.1038/nature07192; PMID: 18758445 [DOI] [PubMed] [Google Scholar]
- 19.Julián P, Milon P, Agirrezabala X, Lasso G, Gil D, Rodnina MV, Valle M.. The Cryo-EM structure of a complete 30S translation initiation complex from Escherichia coli. PLoS Biol 2011; 9:e1001095; http://dx.doi.org/ 10.1371/journal.pbio.1001095; PMID: 21750663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen GS, Zavialov A, Gursky R, Ehrenberg M, Frank J.. The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell 2005; 121:703 - 12; http://dx.doi.org/ 10.1016/j.cell.2005.03.023; PMID: 15935757 [DOI] [PubMed] [Google Scholar]
- 21.Meinnel T, Sacerdot C, Graffe M, Blanquet S, Springer M.. Discrimination by Escherichia coli initiation factor IF3 against initiation on non-canonical codons relies on complementarity rules. J Mol Biol 1999; 290:825 - 37; http://dx.doi.org/ 10.1006/jmbi.1999.2881; PMID: 10398584 [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan M, Mehta P, Yu Y, Prugar E, Koonin EV, Karzai AW, Sternglanz R.. The highly conserved KEOPS/EKC complex is essential for a universal tRNA modification, t6A. EMBO J 2011; 30:873 - 81; http://dx.doi.org/ 10.1038/emboj.2010.343; PMID: 21183954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyauchi K, Kimura S, Suzuki T.. A cyclic form of N6-threonylcarbamoyladenosine as a widely distributed tRNA hypermodification. Nat Chem Biol 2013; 9:105 - 11; http://dx.doi.org/ 10.1038/nchembio.1137; PMID: 23242255 [DOI] [PubMed] [Google Scholar]
- 24.Clements JM, Laz TM, Sherman F.. Efficiency of translation initiation by non-AUG codons in Saccharomyces cerevisiae. Mol Cell Biol 1988; 8:4533 - 6; PMID: 3141793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler JS, Springer M, Grunberg-Manago M.. AUU-to-AUG mutation in the initiator codon of the translation initiation factor IF3 abolishes translational autocontrol of its own gene (infC) in vivo. Proc Natl Acad Sci U S A 1987; 84:4022 - 5; http://dx.doi.org/ 10.1073/pnas.84.12.4022; PMID: 2954162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozak M.. Regulation of translation via mRNA structure in prokaryotes and eukaryotes. Gene 2005; 361:13 - 37; http://dx.doi.org/ 10.1016/j.gene.2005.06.037; PMID: 16213112 [DOI] [PubMed] [Google Scholar]
- 27.Vellanoweth RL, Rabinowitz JC.. The influence of ribosome-binding-site elements on translational efficiency in Bacillus subtilis and Escherichia coli in vivo. Mol Microbiol 1992; 6:1105 - 14; http://dx.doi.org/ 10.1111/j.1365-2958.1992.tb01548.x; PMID: 1375309 [DOI] [PubMed] [Google Scholar]
- 28.Asano K, Hama C, Inoue S, Moriwaki H, Mizobuchi K.. The plasmid ColIb-P9 antisense Inc RNA controls expression of the RepZ replication protein and its positive regulator repY with different mechanisms. J Biol Chem 1999; 274:17924 - 33; http://dx.doi.org/ 10.1074/jbc.274.25.17924; PMID: 10364239 [DOI] [PubMed] [Google Scholar]
- 29.Asano K, et al.. Positive and negative regulations of plasmid ColIb-P9 repZ gene expression at the translational level. journal of biological. Chemistry 1991; 266:3774 - 81 [PubMed] [Google Scholar]
- 30.Wulczyn FG, Kahmann R.. Translational stimulation: RNA sequence and structure requirements for binding of Com protein. Cell 1991; 65:259 - 69; http://dx.doi.org/ 10.1016/0092-8674(91)90160-Z; PMID: 1826635 [DOI] [PubMed] [Google Scholar]
- 31.Blomberg P, Nordström K, Wagner EG.. Replication control of plasmid R1: RepA synthesis is regulated by CopA RNA through inhibition of leader peptide translation. EMBO J 1992; 11:2675 - 83; PMID: 1378398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asano K, Mizobuchi K.. Copy number control of IncIalpha plasmid ColIb-P9 by competition between pseudoknot formation and antisense RNA binding at a specific RNA site. EMBO J 1998; 17:5201 - 13; http://dx.doi.org/ 10.1093/emboj/17.17.5201; PMID: 9724656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Athanasopoulos V, Praszkier J, Pittard AJ.. Analysis of elements involved in pseudoknot-dependent expression and regulation of the repA gene of an IncL/M plasmid. J Bacteriol 1999; 181:1811 - 9; PMID: 10074073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Praszkier J, Pittard AJ.. Pseudoknot-dependent translational coupling in repBA genes of the IncB plasmid pMU720 involves reinitiation. J Bacteriol 2002; 184:5772 - 80; http://dx.doi.org/ 10.1128/JB.184.20.5772-5780.2002; PMID: 12270836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asano K, Moriwaki H, Mizobuchi K.. An induced mRNA secondary structure enhances repZ translation in plasmid ColIb-P9. J Biol Chem 1991; 266:24549 - 56; PMID: 1722206 [PubMed] [Google Scholar]
- 36.Asano K, Mizobuchi K.. An RNA pseudoknot as the molecular switch for translation of the repZ gene encoding the replication initiator of IncIalpha plasmid ColIb-P9. J Biol Chem 1998; 273:11815 - 25; http://dx.doi.org/ 10.1074/jbc.273.19.11815; PMID: 9565606 [DOI] [PubMed] [Google Scholar]
- 37.Asano K, Niimi T, Yokoyama S, Mizobuchi K.. Structural basis for binding of the plasmid ColIb-P9 antisense Inc RNA to its target RNA with the 5′-rUUGGCG-3′ motif in the loop sequence. J Biol Chem 1998; 273:11826 - 38; http://dx.doi.org/ 10.1074/jbc.273.19.11826; PMID: 9565607 [DOI] [PubMed] [Google Scholar]
- 38.Asano K, Mizobuchi K.. Structural analysis of late intermediate complex formed between plasmid ColIb-P9 Inc RNA and its target RNA. How does a single antisense RNA repress translation of two genes at different rates?. J Biol Chem 2000; 275:1269 - 74; http://dx.doi.org/ 10.1074/jbc.275.2.1269; PMID: 10625672 [DOI] [PubMed] [Google Scholar]
- 39.Mandal M, Breaker RR.. Gene regulation by riboswitches. Nat Rev Mol Cell Biol 2004; 5:451 - 63; http://dx.doi.org/ 10.1038/nrm1403; PMID: 15173824 [DOI] [PubMed] [Google Scholar]
- 40.Woese CR, Kandler O, Wheelis ML.. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A 1990; 87:4576 - 9; http://dx.doi.org/ 10.1073/pnas.87.12.4576; PMID: 2112744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasenöhrl D, Fabbretti A, Londei P, Gualerzi CO, Bläsi U.. Translation initiation complex formation in the crenarchaeon Sulfolobus solfataricus. RNA 2009; 15:2288 - 98; http://dx.doi.org/ 10.1261/rna.1662609; PMID: 19861425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolstrup N, Sensen CW, Garrett RA, Clausen IG.. Two different and highly organized mechanisms of translation initiation in the archaeon Sulfolobus solfataricus. Extremophiles 2000; 4:175 - 9; http://dx.doi.org/ 10.1007/s007920070032; PMID: 10879562 [DOI] [PubMed] [Google Scholar]
- 43.Slupska MM, King AG, Fitz-Gibbon S, Besemer J, Borodovsky M, Miller JH.. Leaderless transcripts of the crenarchaeal hyperthermophile Pyrobaculum aerophilum. J Mol Biol 2001; 309:347 - 60; http://dx.doi.org/ 10.1006/jmbi.2001.4669; PMID: 11371158 [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, Choi SK, Roll-Mecak A, Burley SK, Dever TE.. Universal conservation in translation initiation revealed by human and archaeal homologs of bacterial translation initiation factor IF2. Proc Natl Acad Sci U S A 1999; 96:4342 - 7; http://dx.doi.org/ 10.1073/pnas.96.8.4342; PMID: 10200264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maone E, Di Stefano M, Berardi A, Benelli D, Marzi S, La Teana A, Londei P.. Functional analysis of the translation factor aIF2/5B in the thermophilic archaeon Sulfolobus solfataricus. Mol Microbiol 2007; 65:700 - 13; http://dx.doi.org/ 10.1111/j.1365-2958.2007.05820.x; PMID: 17608795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benelli D, Maone E, Londei P.. Two different mechanisms for ribosome/mRNA interaction in archaeal translation initiation. Mol Microbiol 2003; 50:635 - 43; http://dx.doi.org/ 10.1046/j.1365-2958.2003.03721.x; PMID: 14617185 [DOI] [PubMed] [Google Scholar]
- 47.Grill S, Gualerzi CO, Londei P, Bläsi U.. Selective stimulation of translation of leaderless mRNA by initiation factor 2: evolutionary implications for translation. EMBO J 2000; 19:4101 - 10; http://dx.doi.org/ 10.1093/emboj/19.15.4101; PMID: 10921890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito K, Ito K. in Encyclopedia of Systems Biology eds Werner Dubitzky, Olaf Wolkenhauser, Kwang-Hyun Cho, & Hiroki Yokota) 678-682 (Springer, 2013). [Google Scholar]
- 49.Asano K. in Encyclopedia of Systems Biology eds Werner Dubitzky, Olaf Wolkenhauser, Kwang-Hyun Cho, & Hiroki Yokota) 682-687 (Springer, 2013). [Google Scholar]
- 50.Kozak M.. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 1986; 44:283 - 92; http://dx.doi.org/ 10.1016/0092-8674(86)90762-2; PMID: 3943125 [DOI] [PubMed] [Google Scholar]
- 51.Pisarev AV, Kolupaeva VG, Yusupov MM, Hellen CUT, Pestova TV.. Ribosomal position and contacts of mRNA in eukaryotic translation initiation complexes. EMBO J 2008; 27:1609 - 21; http://dx.doi.org/ 10.1038/emboj.2008.90; PMID: 18464793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kozak M.. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem 1991; 266:19867 - 70; PMID: 1939050 [PubMed] [Google Scholar]
- 53.Martin-Marcos P, Cheung Y-N, Hinnebusch AG.. Functional elements in initiation factors 1, 1A, and 2β discriminate against poor AUG context and non-AUG start codons. Mol Cell Biol 2011; 31:4814 - 31; http://dx.doi.org/ 10.1128/MCB.05819-11; PMID: 21930786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivanov IP, Firth AE, Michel AM, Atkins JF, Baranov PV.. Identification of evolutionarily conserved non-AUG-initiated N-terminal extensions in human coding sequences. Nucleic Acids Res 2011; 39:4220 - 34; http://dx.doi.org/ 10.1093/nar/gkr007; PMID: 21266472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wamboldt Y, Mohammed S, Elowsky C, Wittgren C, de Paula WB, Mackenzie SA.. Participation of leaky ribosome scanning in protein dual targeting by alternative translation initiation in higher plants. Plant Cell 2009; 21:157 - 67; http://dx.doi.org/ 10.1105/tpc.108.063644; PMID: 19182105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hopkins BD, Fine B, Steinbach N, Dendy M, Rapp Z, Shaw J, Pappas K, Yu JS, Hodakoski C, Mense S, et al.. A secreted PTEN phosphatase that enters cells to alter signaling and survival. Science 2013; 341:399 - 402; http://dx.doi.org/ 10.1126/science.1234907; PMID: 23744781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pestova TV, Lorsch JR, Hellen CUT. in Translational Control in Biology and Medicine eds M B Mathews, N Sonenberg, & John W. B. Hershey) 87-128 (Cold Spring Harbor Lab Press, 2007). [Google Scholar]
- 58.Hinnebusch AG, Dever TE, Asano K. in Translational Control in Biology and Medicine eds M B Mathews, N Sonenberg, & John W. B. Hershey) 225-268 (Cold Spring Harbor Lab Press, 2007). [Google Scholar]
- 59.Sonenberg N, Hinnebusch AG.. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 2009; 136:731 - 45; http://dx.doi.org/ 10.1016/j.cell.2009.01.042; PMID: 19239892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roll-Mecak A, Cao C, Dever TE, Burley SK.. X-Ray structures of the universal translation initiation factor IF2/eIF5B: conformational changes on GDP and GTP binding. Cell 2000; 103:781 - 92; http://dx.doi.org/ 10.1016/S0092-8674(00)00181-1; PMID: 11114334 [DOI] [PubMed] [Google Scholar]
- 61.Allen GS, Frank J.. Structural insights on the translation initiation complex: ghosts of a universal initiation complex. Mol Microbiol 2007; 63:941 - 50; http://dx.doi.org/ 10.1111/j.1365-2958.2006.05574.x; PMID: 17238926 [DOI] [PubMed] [Google Scholar]
- 62.Asano K, Clayton J, Shalev A, Hinnebusch AG.. A multifactor complex of eukaryotic initiation factors, eIF1, eIF2, eIF3, eIF5, and initiator tRNA(Met) is an important translation initiation intermediate in vivo. Genes Dev 2000; 14:2534 - 46; http://dx.doi.org/ 10.1101/gad.831800; PMID: 11018020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dennis MD, Person MD, Browning KS.. Phosphorylation of plant translation initiation factors by CK2 enhances the in vitro interaction of multifactor complex components. J Biol Chem 2009; 284:20615 - 28; http://dx.doi.org/ 10.1074/jbc.M109.007658; PMID: 19509420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sokabe M, Fraser CS, Hershey JW.. The human translation initiation multi-factor complex promotes methionyl-tRNAi binding to the 40S ribosomal subunit. Nucleic Acids Res 2012; 40:905 - 13; http://dx.doi.org/ 10.1093/nar/gkr772; PMID: 21940399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoon HJ, Donahue TF.. The suil suppressor locus in Saccharomyces cerevisiae encodes a translation factor that functions during tRNA(iMet) recognition of the start codon. Mol Cell Biol 1992; 12:248 - 60; PMID: 1729602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang HK, Yoon H, Hannig EM, Donahue TF.. GTP hydrolysis controls stringent selection of the AUG start codon during translation initiation in Saccharomyces cerevisiae.. Genes Dev 1997; 11:2396 - 413; http://dx.doi.org/ 10.1101/gad.11.18.2396; PMID: 9308967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valásek L, Nielsen KH, Zhang F, Fekete CA, Hinnebusch AG.. Interaction of eIF3 subunit NIP1/c with eIF1 and eIF5 promote preinitiation complex assembly and regulate start codon selection. Mol Cell Biol 2004; 24:9437 - 55; http://dx.doi.org/ 10.1128/MCB.24.21.9437-9455.2004; PMID: 15485912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fekete CA, Applefield DJ, Blakely SA, Shirokikh N, Pestova T, Lorsch JR, Hinnebusch AG.. The eIF1A C-terminal domain promotes initiation complex assembly, scanning and AUG selection in vivo. EMBO J 2005; 24:3588 - 601; http://dx.doi.org/ 10.1038/sj.emboj.7600821; PMID: 16193068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fekete CA, Mitchell SF, Cherkasova VA, Applefield D, Algire MA, Maag D, Saini AK, Lorsch JR, Hinnebusch AG.. N- and C-terminal residues of eIF1A have opposing effects on the fidelity of start codon selection. EMBO J 2007; 26:1602 - 14; http://dx.doi.org/ 10.1038/sj.emboj.7601613; PMID: 17332751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saini AK, Nanda JS, Lorsch JR, Hinnebusch AG.. Regulatory elements in eIF1A control the fidelity of start codon selection by modulating tRNA(i)(Met) binding to the ribosome. Genes Dev 2010; 24:97 - 110; http://dx.doi.org/ 10.1101/gad.1871910; PMID: 20048003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh CR, Yamamoto Y, Asano K.. Physical association of eukaryotic initiation factor (eIF) 5 carboxyl-terminal domain with the lysine-rich eIF2β segment strongly enhances its binding to eIF3. J Biol Chem 2004; 279:49644 - 55; http://dx.doi.org/ 10.1074/jbc.M409609200; PMID: 15377664 [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto Y, Singh CR, Marintchev A, Hall NS, Hannig EM, Wagner G, Asano K.. The eukaryotic initiation factor (eIF) 5 HEAT domain mediates multifactor assembly and scanning with distinct interfaces to eIF1, eIF2, eIF3, and eIF4G. Proc Natl Acad Sci U S A 2005; 102:16164 - 9; http://dx.doi.org/ 10.1073/pnas.0507960102; PMID: 16254050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marintchev A, Edmonds KA, Marintcheva B, Hendrickson E, Oberer M, Suzuki C, Herdy B, Sonenberg N, Wagner G.. Topology and regulation of the human eIF4A/4G/4H helicase complex in translation initiation. Cell 2009; 136:447 - 60; http://dx.doi.org/ 10.1016/j.cell.2009.01.014; PMID: 19203580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watanabe R, Murai MJ, Singh CR, Fox S, Ii M, Asano K.. The eukaryotic initiation factor (eIF) 4G HEAT domain promotes translation re-initiation in yeast both dependent on and independent of eIF4A mRNA helicase. J Biol Chem 2010; 285:21922 - 33; http://dx.doi.org/ 10.1074/jbc.M110.132027; PMID: 20463023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Algire MA, Maag D, Lorsch JR.. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol Cell 2005; 20:251 - 62; http://dx.doi.org/ 10.1016/j.molcel.2005.09.008; PMID: 16246727 [DOI] [PubMed] [Google Scholar]
- 76.Kapp LD, Kolitz SE, Lorsch JR.. Yeast initiator tRNA identity elements cooperate to influence multiple steps of translation initiation. RNA 2006; 12:751 - 64; http://dx.doi.org/ 10.1261/rna.2263906; PMID: 16565414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shin BS, Maag D, Roll-Mecak A, Arefin MS, Burley SK, Lorsch JR, Dever TE.. Uncoupling of initiation factor eIF5B/IF2 GTPase and translational activities by mutations that lower ribosome affinity. Cell 2002; 111:1015 - 25; http://dx.doi.org/ 10.1016/S0092-8674(02)01171-6; PMID: 12507428 [DOI] [PubMed] [Google Scholar]
- 78.Hiraishi H, et al.. Interaction between 25S rRNA A loop and eIF5B promotes subunit joining and ensures stringent AUG selection. Mol Cell Biol 2013; 33:3540 - 8; http://dx.doi.org/ 10.1128/MCB.00771-13; PMID: 23836883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh CR, Lee B, Udagawa T, Mohammad-Qureshi SS, Yamamoto Y, Pavitt GD, Asano K.. An eIF5/eIF2 complex antagonizes guanine nucleotide exchange by eIF2B during translation initiation. EMBO J 2006; 25:4537 - 46; http://dx.doi.org/ 10.1038/sj.emboj.7601339; PMID: 16990799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh CR, Udagawa T, Lee B, Wassink S, He H, Yamamoto Y, Anderson JT, Pavitt GD, Asano K.. Change in nutritional status modulates the abundance of critical pre-initiation intermediate complexes during translation initiation in vivo. J Mol Biol 2007; 370:315 - 30; http://dx.doi.org/ 10.1016/j.jmb.2007.04.034; PMID: 17512538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jennings MD, Pavitt GD.. eIF5 has GDI activity necessary for translational control by eIF2 phosphorylation. Nature 2010; 465:378 - 81; http://dx.doi.org/ 10.1038/nature09003; PMID: 20485439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jennings MD, Zhou Y, Mohammad-Qureshi SS, Bennett D, Pavitt GD.. eIF2B promotes eIF5 dissociation from eIF2*GDP to facilitate guanine nucleotide exchange for translation initiation. Genes Dev 2013; 27:2696 - 707; http://dx.doi.org/ 10.1101/gad.231514.113; PMID: 24352424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marintchev A, Wagner G.. eIF4G and CBP80 share a common origin and similar domain organization: implications for the structure and function of eIF4G. Biochemistry 2005; 44:12265 - 72; http://dx.doi.org/ 10.1021/bi051271v; PMID: 16156639 [DOI] [PubMed] [Google Scholar]
- 84.Park EH, Walker SE, Lee JM, Rothenburg S, Lorsch JR, Hinnebusch AG.. Multiple elements in the eIF4G1 N-terminus promote assembly of eIF4G1•PABP mRNPs in vivo. EMBO J 2011; 30:302 - 16; http://dx.doi.org/ 10.1038/emboj.2010.312; PMID: 21139564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng S, Gallie DR.. Competitive and noncompetitive binding of eIF4B, eIF4A, and the poly(A) binding protein to wheat translation initiation factor eIFiso4G. Biochemistry 2010; 49:8251 - 65; http://dx.doi.org/ 10.1021/bi1008529; PMID: 20795652 [DOI] [PubMed] [Google Scholar]
- 86.Cheng S, Gallie DR.. Eukaryotic initiation factor 4B and the poly(A)-binding protein bind eIF4G competitively. Translation 2013; 1:e24038; http://dx.doi.org/ 10.4161/trla.24038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He H, et al.. The yeast eIF4G HEAT domain interacts with eIF1 and eIF5 and is involved in stringent AUG selection. Mol Cell Biol 2003; 23:5431 - 45; http://dx.doi.org/ 10.1128/MCB.23.15.5431-5445.2003; PMID: 12861028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morino S, Imataka H, Svitkin YV, Pestova TV, Sonenberg N.. Eukaryotic translation initiation factor 4E (eIF4E) binding site and the middle one-third of eIF4GI constitute the core domain for cap-dependent translation, and the C-terminal one-third functions as a modulatory region. Mol Cell Biol 2000; 20:468 - 77; http://dx.doi.org/ 10.1128/MCB.20.2.468-477.2000; PMID: 10611225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.LeFebvre AK, Korneeva NL, Trutschl M, Cvek U, Duzan RD, Bradley CA, Hershey JW, Rhoads RE.. Translation initiation factor eIF4G-1 binds to eIF3 through the eIF3e subunit. J Biol Chem 2006; 281:22917 - 32; http://dx.doi.org/ 10.1074/jbc.M605418200; PMID: 16766523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Villa N, Do A, Hershey JW, Fraser CS.. Human eukaryotic initiation factor 4G (eIF4G) protein binds to eIF3c, -d, and -e to promote mRNA recruitment to the ribosome. J Biol Chem 2013; 288:32932 - 40; http://dx.doi.org/ 10.1074/jbc.M113.517011; PMID: 24092755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parsyan A, Svitkin Y, Shahbazian D, Gkogkas C, Lasko P, Merrick WC, Sonenberg N.. mRNA helicases: the tacticians of translational control. Nat Rev Mol Cell Biol 2011; 12:235 - 45; http://dx.doi.org/ 10.1038/nrm3083; PMID: 21427765 [DOI] [PubMed] [Google Scholar]
- 92.Sengoku T, Nureki O, Nakamura A, Kobayashi S, Yokoyama S.. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell 2006; 125:287 - 300; http://dx.doi.org/ 10.1016/j.cell.2006.01.054; PMID: 16630817 [DOI] [PubMed] [Google Scholar]
- 93.Chuang RY, Weaver PL, Liu Z, Chang TH.. Requirement of the DEAD-Box protein ded1p for messenger RNA translation. Science 1997; 275:1468 - 71; http://dx.doi.org/ 10.1126/science.275.5305.1468; PMID: 9045610 [DOI] [PubMed] [Google Scholar]
- 94.de la Cruz J, Iost I, Kressler D, Linder P.. The p20 and Ded1 proteins have antagonistic roles in eIF4E-dependent translation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 1997; 94:5201 - 6; http://dx.doi.org/ 10.1073/pnas.94.10.5201; PMID: 9144215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pisareva VP, Pisarev AV, Komar AA, Hellen CUT, Pestova TV.. Translation initiation on mammalian mRNAs with structured 5’UTRs requires DExH-box protein DHX29. Cell 2008; 135:1237 - 50; http://dx.doi.org/ 10.1016/j.cell.2008.10.037; PMID: 19109895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soto-Rifo R, Rubilar PS, Limousin T, de Breyne S, Décimo D, Ohlmann T.. DEAD-box protein DDX3 associates with eIF4F to promote translation of selected mRNAs. EMBO J 2012; 31:3745 - 56; http://dx.doi.org/ 10.1038/emboj.2012.220; PMID: 22872150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Asano K, Sachs MS.. Translation factor control of ribosome conformation during start codon selection. Genes Dev 2007; 21:1280 - 7; http://dx.doi.org/ 10.1101/gad.1562707; PMID: 17545463 [DOI] [PubMed] [Google Scholar]
- 98.Hinnebusch AG.. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol Mol Biol Rev 2011; 75:434 - 67; http://dx.doi.org/ 10.1128/MMBR.00008-11; PMID: 21885680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pestova TV, Borukhov SI, Hellen CUT.. Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature 1998; 394:854 - 9; http://dx.doi.org/ 10.1038/29703; PMID: 9732867 [DOI] [PubMed] [Google Scholar]
- 100.Pestova TV, Kolupaeva VG.. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev 2002; 16:2906 - 22; http://dx.doi.org/ 10.1101/gad.1020902; PMID: 12435632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lomakin, I. B., Kolupaeva, V. G., Marintchev, A., Wagner, G. & Pestova, T. V. Position of eukaryotic initiation factor eIF1 on the 40S ribosomal subunit determined by directed hydroxyl radical probing. genes and Development 17, 2786-2797 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Passmore LA, Schmeing TM, Maag D, Applefield DJ, Acker MG, Algire MA, Lorsch JR, Ramakrishnan V.. The eukaryotic translation initiation factors eIF1 and eIF1A induce an open conformation of the 40S ribosome. Mol Cell 2007; 26:41 - 50; http://dx.doi.org/ 10.1016/j.molcel.2007.03.018; PMID: 17434125 [DOI] [PubMed] [Google Scholar]
- 103.Weisser M, Voigts-Hoffmann F, Rabl J, Leibundgut M, Ban N.. The crystal structure of the eukaryotic 40S ribosomal subunit in complex with eIF1 and eIF1A. Nat Struct Mol Biol 2013; 20:1015 - 7; http://dx.doi.org/ 10.1038/nsmb.2622; PMID: 23851459 [DOI] [PubMed] [Google Scholar]
- 104.Lomakin IB, Steitz TA.. The initiation of mammalian protein synthesis and mRNA scanning mechanism. Nature 2013; 500:307 - 11; http://dx.doi.org/ 10.1038/nature12355; PMID: 23873042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maag D, Fekete CA, Gryczynski Z, Lorsch JR.. A conformational change in the eukaryotic translation preinitiation complex and release of eIF1 signal recognition of the start codon. Mol Cell 2005; 17:265 - 75; http://dx.doi.org/ 10.1016/j.molcel.2004.11.051; PMID: 15664195 [DOI] [PubMed] [Google Scholar]
- 106.Cheung Y-N, Maag D, Mitchell SF, Fekete CA, Algire MA, Takacs JE, Shirokikh N, Pestova T, Lorsch JR, Hinnebusch AG.. Dissociation of eIF1 from the 40S ribosomal subunit is a key step in start codon selection in vivo. Genes Dev 2007; 21:1217 - 30; http://dx.doi.org/ 10.1101/gad.1528307; PMID: 17504939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin-Marcos P, et al.. β-hairpin loop of eIF1 mediates 40S ribosome binding to regulate initiator tRNAMet recruitment and accuracy of AUG selection in vivo. J Biol Chem 2013; In press http://dx.doi.org/ 10.1074/jbc.M113.498642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nanda JS, Cheung YN, Takacs JE, Martin-Marcos P, Saini AK, Hinnebusch AG, Lorsch JR.. eIF1 controls multiple steps in start codon recognition during eukaryotic translation initiation. J Mol Biol 2009; 394:268 - 85; http://dx.doi.org/ 10.1016/j.jmb.2009.09.017; PMID: 19751744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Loughran G, Sachs MS, Atkins JF, Ivanov IP.. Stringency of start codon selection modulates autoregulation of translation initiation factor eIF5. Nucleic Acids Res 2012; 40:2898 - 906; http://dx.doi.org/ 10.1093/nar/gkr1192; PMID: 22156057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Asano K, Krishnamoorthy T, Phan L, Pavitt GD, Hinnebusch AG.. Conserved bipartite motifs in yeast eIF5 and eIF2Bepsilon, GTPase-activating and GDP-GTP exchange factors in translation initiation, mediate binding to their common substrate eIF2. EMBO J 1999; 18:1673 - 88; http://dx.doi.org/ 10.1093/emboj/18.6.1673; PMID: 10075937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Laurino JP, Thompson GM, Pacheco E, Castilho BA.. The β subunit of eukaryotic translation initiation factor 2 binds mRNA through the lysine repeats and a region comprising the C2-C2 motif. Mol Cell Biol 1999; 19:173 - 81; PMID: 9858542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reibarkh M, Yamamoto Y, Singh CR, del Rio F, Fahmy A, Lee B, Luna RE, Ii M, Wagner G, Asano K.. Eukaryotic initiation factor (eIF) 1 carries two distinct eIF5-binding faces important for multifactor assembly and AUG selection. J Biol Chem 2008; 283:1094 - 103; http://dx.doi.org/ 10.1074/jbc.M708155200; PMID: 17974565 [DOI] [PubMed] [Google Scholar]
- 113.Yu Y, Marintchev A, Kolupaeva VG, Unbehaun A, Veryasova T, Lai SC, Hong P, Wagner G, Hellen CU, Pestova TV.. Position of eukaryotic translation initiation factor eIF1A on the 40S ribosomal subunit mapped by directed hydroxyl radical probing. Nucleic Acids Res 2009; 37:5167 - 82; http://dx.doi.org/ 10.1093/nar/gkp519; PMID: 19561193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jackson RJ, Hellen CUT, Pestova TV.. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 2010; 11:113 - 27; http://dx.doi.org/ 10.1038/nrm2838; PMID: 20094052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Singh CR, Watanabe R, Zhou D, Jennings MD, Fukao A, Lee B, Ikeda Y, Chiorini JA, Campbell SG, Ashe MP, et al.. Mechanisms of translational regulation by a human eIF5-mimic protein. Nucleic Acids Res 2011; 39:8314 - 28; http://dx.doi.org/ 10.1093/nar/gkr339; PMID: 21745818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Szamecz B, Rutkai E, Cuchalová L, Munzarová V, Herrmannová A, Nielsen KH, Burela L, Hinnebusch AG, Valásek L.. eIF3a cooperates with sequences 5′ of uORF1 to promote resumption of scanning by post-termination ribosomes for reinitiation on GCN4 mRNA. Genes Dev 2008; 22:2414 - 25; http://dx.doi.org/ 10.1101/gad.480508; PMID: 18765792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hellen CUT, Sarnow P.. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev 2001; 15:1593 - 612; http://dx.doi.org/ 10.1101/gad.891101; PMID: 11445534 [DOI] [PubMed] [Google Scholar]
- 118.Hellen CUT.. IRES-induced conformational changes in the ribosome and the mechanism of translation initiation by internal ribosomal entry. Biochim Biophys Acta 2009; 1789:558 - 70; http://dx.doi.org/ 10.1016/j.bbagrm.2009.06.001; PMID: 19539793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Skabkin MA, Skabkina OV, Dhote V, Komar AA, Hellen CU, Pestova TV.. Activities of Ligatin and MCT-1/DENR in eukaryotic translation initiation and ribosomal recycling. Genes Dev 2010; 24:1787 - 801; http://dx.doi.org/ 10.1101/gad.1957510; PMID: 20713520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dmitriev SE, Terenin IM, Andreev DE, Ivanov PA, Dunaevsky JE, Merrick WC, Shatsky IN.. GTP-independent tRNA delivery to the ribosomal P-site by a novel eukaryotic translation factor. J Biol Chem 2010; 285:26779 - 87; http://dx.doi.org/ 10.1074/jbc.M110.119693; PMID: 20566627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ivanov IP, Loughran G, Sachs MS, Atkins JF.. Initiation context modulates autoregulation of eukaryotic translation initiation factor 1 (eIF1). Proc Natl Acad Sci U S A 2010; 107:18056 - 60; http://dx.doi.org/ 10.1073/pnas.1009269107; PMID: 20921384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS.. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 2009; 324:218 - 23; http://dx.doi.org/ 10.1126/science.1168978; PMID: 19213877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D.. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 2000; 6:1099 - 108; http://dx.doi.org/ 10.1016/S1097-2765(00)00108-8; PMID: 11106749 [DOI] [PubMed] [Google Scholar]
- 124.Vattem KM, Wek RC.. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A 2004; 101:11269 - 74; http://dx.doi.org/ 10.1073/pnas.0400541101; PMID: 15277680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feng L, Yoon H, Donahue TF.. Casein kinase II mediates multiple phosphorylation of Saccharomyces cerevisiae eIF-2 α (encoded by SUI2), which is required for optimal eIF-2 function in S. cerevisiae.. Mol Cell Biol 1994; 14:5139 - 53; PMID: 8035796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Majumdar R, Bandyopadhyay A, Deng H, Maitra U.. Phosphorylation of mammalian translation initiation factor 5 (eIF5) in vitro and in vivo. Nucleic Acids Res 2002; 30:1154 - 62; http://dx.doi.org/ 10.1093/nar/30.5.1154; PMID: 11861906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maiti T, Bandyopadhyay A, Maitra U.. Casein kinase II phosphorylates translation initiation factor 5 (eIF5) in Saccharomyces cerevisiae. Yeast 2003; 20:97 - 108; http://dx.doi.org/ 10.1002/yea.937; PMID: 12518314 [DOI] [PubMed] [Google Scholar]
- 128.Homma MK, Wada I, Suzuki T, Yamaki J, Krebs EG, Homma Y.. CK2 phosphorylation of eukaryotic translation initiation factor 5 potentiates cell cycle progression. Proc Natl Acad Sci U S A 2005; 102:15688 - 93; http://dx.doi.org/ 10.1073/pnas.0506791102; PMID: 16227438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Llorens F, Duarri A, Sarró E, Roher N, Plana M, Itarte E.. The N-terminal domain of the human eIF2β subunit and the CK2 phosphorylation sites are required for its function. Biochem J 2006; 394:227 - 36; http://dx.doi.org/ 10.1042/BJ20050605; PMID: 16225457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Silvera D, Formenti SC, Schneider RJ.. Translational control in cancer. Nat Rev Cancer 2010; 10:254 - 66; http://dx.doi.org/ 10.1038/nrc2824; PMID: 20332778 [DOI] [PubMed] [Google Scholar]
- 131.Asano K, Merrick WC, Hershey JWB.. The translation initiation factor eIF3-p48 subunit is encoded by int-6, a site of frequent integration by the mouse mammary tumor virus genome. J Biol Chem 1997; 272:23477 - 80; http://dx.doi.org/ 10.1074/jbc.272.38.23477; PMID: 9295280 [DOI] [PubMed] [Google Scholar]
- 132.Li S, Chai Z, Li Y, Liu D, Bai Z, Li Y, Li Y, Situ Z.. BZW1, a novel proliferation regulator that promotes growth of salivary muocepodermoid carcinoma. Cancer Lett 2009; 284:86 - 94; http://dx.doi.org/ 10.1016/j.canlet.2009.04.019; PMID: 19446954 [DOI] [PubMed] [Google Scholar]
- 133.Narla A, Ebert BL.. Ribosomopathies: human disorders of ribosome dysfunction. Blood 2010; 115:3196 - 205; http://dx.doi.org/ 10.1182/blood-2009-10-178129; PMID: 20194897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ellis SR, Gleizes P-E.. Diamond Blackfan anemia: ribosomal proteins going rogue. Semin Hematol 2011; 48:89 - 96; http://dx.doi.org/ 10.1053/j.seminhematol.2011.02.005; PMID: 21435505 [DOI] [PubMed] [Google Scholar]
- 135.Amsterdam A, Sadler KC, Lai K, Farrington S, Bronson RT, Lees JA, Hopkins N.. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol 2004; 2:E139; http://dx.doi.org/ 10.1371/journal.pbio.0020139; PMID: 15138505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nemoto N, Singh CR, Udagawa T, Wang S, Thorson E, Winter Z, Ohira T, Ii M, Valásek L, Brown SJ, et al.. Yeast 18 S rRNA is directly involved in the ribosomal response to stringent AUG selection during translation initiation. J Biol Chem 2010; 285:32200 - 12; http://dx.doi.org/ 10.1074/jbc.M110.146662; PMID: 20699223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nemoto N, et al.. Random mutagenesis of yeast 25S rRNA identify bases critical for 60S subunit structural integrity and function. Translation 2013; 1:e26402; http://dx.doi.org/ 10.4161/trla.26402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hartz D, Binkley J, Hollingsworth T, Gold L.. Domains of initiator tRNA and initiation codon crucial for initiator tRNA selection by Escherichia coli IF3. Genes Dev 1990; 4:1790 - 800; http://dx.doi.org/ 10.1101/gad.4.10.1790; PMID: 1701151 [DOI] [PubMed] [Google Scholar]
- 139.Castilho-Valavicius B, Yoon H, Donahue TF.. Genetic characterization of the Saccharomyces cerevisiae translational initiation suppressors sui1, sui2 and SUI3 and their effects on HIS4 expression. Genetics 1990; 124:483 - 95; PMID: 2179049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Donahue T. in Translational Control of Gene Expression eds N. Sonenberg, J. W. B. Hershey, & M. B. Mathews) 487-502 (Cold Spring Harbor Laboratory Press, 2000). [Google Scholar]