Abstract
The developmental trajectories of theta band (4-7 Hz) event-related oscillations (EROs), a key neurophysiological constituent of the P3 response, were assessed in 2170 adolescents and young adults ages 12 to 25. The theta EROs occurring in the P3 response, important indicators of neurocognitive function, were elicited during the evaluation of task-relevant target stimuli in visual and auditory oddball tasks. These tasks call upon attentional and working memory resources. Large differences in developmental rates between males and females were found; scalp location and task modality (visual or auditory) differences within males and females were small compared to gender differences. Trajectories of interregional and intermodal correlations between ERO power values exhibited increases with age in both genders, but showed a divergence in development between auditory and visual systems during ages 16 to 21. These results are consistent with previous electrophysiological and imaging studies and provide additional temporal detail about the development of neurophysiological indices of cognitive activity. Since measures of the P3 response has been found to be a useful endophenotypes for the study of a number of clinical and behavioral disorders, studies of its development in adolescents and young adults may illuminate neurophysiological factors contributing to the onset of these conditions.
Keywords: ERO, P3, Adolescent, Development
1. Introduction
Brain development in adolescents and young adults occurs on neuronal, structural, and functional levels. One important indicator of neurocognitive function is the P3 (or P300) response, evidenced by the production of a large positive waveform with a peak between 300 ms. and 700 ms. after the presentation of a target stimulus. The P3 response is elicited by infrequently presented target stimuli in a stream of more frequently occurring non-target stimuli in auditory and visual target detection (oddball) tasks, which call for the subject to respond to only the target stimulus. The P3 response has been proposed to index attentional and working memory resources (Polich, 2007). It has been associated with several anatomical loci (locus coeruleus, anterior cingulate cortex (ACC), insula, and the right-lateralized frontal and temporoparietal regions of the ventral attention network) which may be part of a distributed circuit (Polich and Criado, 2006; Mantini et al., 2009; Sara and Bouret, 2012; Walz et al., 2014). Studies of visual and auditory target detection tasks using functional magnetic resonance imaging (fMRI) suggest that common, supramodal functional systems are involved as well as modality-specific systems (Walz et al., 2013; Linden et al., 1999). Frequency domain analysis suggests that the theta band event related oscillation (ERO) is a major constituent of the P3 response (Karakas et al., 2000a,b; Yordanova et al., 2003; Jones et al., 2006a,b; Rangaswamy et al., 2007). Theta EROs are important for processes underlying frontal inhibitory control, conscious awareness, recognition memory and episodic retrieval, as shown in a number of experimental contexts (Gevins et al., 1998; Jacobs et al., 2006; Klimesch et al., 1994, 2001, 2008; Vertes, 2005).
There are many changes related to brain development during adolescence that may effect theta ERO power. On the neuronal level, there is a decrease in gray matter density and cortical thickness in adolescence, probably reflecting synaptic pruning and myeli-nation, and an increase in white matter (Sowell et al., 2004; Toga et al., 2006). On the structural/anatomical level, trajectories of brain volumes of different regions and tissue types, as well as other features of cortical anatomy, exhibit curvilinear properties which vary between regions (Lenroot et al., 2007; Shaw et al., 2008; Giedd et al., 2010; Raznahan et al., 2011; Sullivan et al., 2011) and between genders (Lenroot and Giedd, 2010; Lenroot et al., 2007; Peper et al., 2011; Koolschijn and Crone, 2013), as determined by magnetic resonance imaging (MRI) of subjects between the ages of 8 and 20. Gender differences are also present in functional MRI studies of the development of task-related brain activity in adolescents and young adults in a number of different tasks (Rubia et al., 2006; Christakou et al., 2009; Rubia et al., 2010, 2013; Rubia, 2013). Brain networks develop from a pattern of local connectivity to more global patterns of connectivity (Fair et al., 2008, 2009; Power et al., 2010; Supekar et al., 2009; Uddin et al., 2011; Vogel et al., 2010; Zielinski et al., 2010; Menon, 2013; Wu et al., 2013). Systematic changes of the electrophysiology of brain activity occur with age, both in the resting state and in a variety of task related conditions (Segalowitz et al., 2010; Sturman and Moghaddam, 2011). Among the most prominent are decrease in power in oscillatory activity in both resting state and task related activity (Yordanova and Kolev, 1997; Whitford et al., 2007). Gender differences in development have also been observed in task related activity (Nanova et al., 2008, 2011). These factors suggest that a general decrease in power should be found in both genders and modalities, that trajectories may have nonlinear characteristics, and that strong gender differences and increased correlation with age between locations will be found.
The theta ERO occurring in the P3 response to target stimuli in the visual oddball experiment has been shown to have significant genetic associations with genetic variants of several different genes encoding neurophysiologically significant factors (Jones et al., 2006b; Chen et al., 2009; Zlojutro et al., 2011; Kang et al., 2012). No other neurophysiological measure has been found to have this span of genetic association. This suggests that a developmental study of the theta ERO would be a useful preliminary to any study of the genetics of the development of neurophysiological function. The development of theta band EROs during adolescence have not previously been studied, although previous studies have examined the pattern of the development of visual and auditory P3 peak amplitude in adolescents (Katsanis et al., 1996; Hill et al., 1999a; Stige et al., 2007; Nanova et al., 2008; van Beijsterveldt et al., 1998, 2001; Carlson and Iacono, 2006; Sumich et al., 2012). No developmental studies of neurophysiological function have attempted to characterize trajectories of any measure of task-related activity in the temporal detail provided here.
The primary goal of this study was to determine the developmental trajectories of the measures of the power of theta EROs obtained in the visual and auditory target detection tasks, and the trajectories of the correlations between them. The study focuses on elucidating gender, modality, and regional differences in these developmental trajectories. The trajectory of the correlations of power values provide a measure of the supraregional and supramodal characteristics of brain development of factors affecting theta ERO generation. In preliminary analyses, theta and delta band EROs in both total and evoked measures were examined. No significant differences in trajectories were found among these measures, so the analysis was restricted to the total power in the theta band in the three midline electrodes the interest of the simplicity of interpretation and consistency with prior studies (Jones et al., 2006a,b; Stige et al., 2007; Nanova et al., 2008). In subsequent studies, the developmental trajectories of the associations between genetic variants (SNPs) from a number of genes associated with neurophysiological factors and the measures of the power of theta EROs employed in this study will be determined using the same data set and similar methodologies.
2. Methods and Materials
2.1. Subjects
The sample comprised 2170 adolescents and young adults from the Prospective Study of the Collaborative Study on the Genetics of Alcoholism (COGA), a multisite collaboration designed to study the genetics of alcoholism (Begleiter et al., 1995), examined within the age range of 12 to 25 years. The Prospective Study began in 2004 as a prospective study of adolescents and young adults from pedigrees ascertained in previous phases of COGA, which contained members from alcoholic families (recruited through a proband in treatment) and a set of community (comparison) families, randomly ascertained to be representative of the general population. These families were recruited during the years 1990 to 2000. Although over 80% of the subjects are from families originally recruited through an alcoholic proband, fewer than 25% of the sample are first degree relatives of the probands, and many are only distantly related to the probands.
Participants in the study were reassessed at approximately two year intervals. Subjects were excluded from neurophysiological assessment if they had any of the following: (1) recent substance or alcohol use (i.e., positive breath-analyzer test and/or urine screen), (2) hepatic encephalopathy/cirrhosis of the liver, (3) history of head injury, seizures or neurosurgery, (4) uncorrected sensory deficits, (5) use of medication known to influence brain functioning, (6) history/symptoms of psychoses, (7) positive test for human immunodeficiency virus, (8) other acute/chronic medical illnesses that affects brain function and (9) and a score of less than 25 on the Mini Mental State Examination. This sample comprised subjects with one or more neurophysiological assessments: 475 had 1 assessment, 583 had 2, 576 had 3, 494 had 4, and 42 had 5 assessments. Data from six collection sites have been included in this study: SUNY Downstate Medical Center; University of Connecticut Health Science Center; Washington University School of Medicine in St. Louis; University of California at San Diego; University of Iowa, and Indiana University School of Medicine. Recruitment and assessment procedures have been described elsewhere (Reich, 1996; Begleiter et al., 1998; Edenberg et al., 2005), and are also available at this website: https://zork5.wustl.edu/niaaa/coga_instruments/resources.html. The experimental protocols were approved by each site's institutional review board, and informed consent (for those over eighteen years of age) or assent (for those under eighteen years of age) was obtained from all participants.
2.2. Electrophysiology
Two oddball tasks, one visual, the other auditory, which have been used in the COGA neurophysiology battery from the inception of the project (Cohen et al., 1994; Alexander et al., 1994) were used for this study. In each task, subjects were verbally instructed to suppress their eye blinks and to sit as still as possible. They were asked to respond to the target with a button press as quickly as possible, but not at the expense of accuracy, and not to respond to other stimuli.
2.2.1. Visual oddball task
A three-stimulus visual oddball task was employed with 280 visual stimuli of three different types: 35 targets (rarely occurring letter ‘X’) to which the subjects responded quickly and accurately with a button press, 210 non-targets (frequently occurring white squares) and 35 novels (rarely occurring random colored geometric figures) (probabilities of occurrence of 0.125, 0.750 and 0.125 respectively). Stimuli subtended a visual angle of 2.5 degrees with stimulus durations of 60 ms. and inter-stimulus intervals of 1625 ms. The stimuli were presented pseudo-randomly with the only constraint that a target or novel stimulus never preceded a target or novel stimulus.
2.2.2. Auditory oddball task
Subjects were presented binaurally with two tones of different frequencies. One stimulus was a low tone (600 Hz) and the other a high tone (1600 Hz) produced by a tone generator. Each stimulus had a 60 ms. duration (10 ms. rise and fall times and 40 ms. plateau) and an intensity level of 60 dB. A computer initiated the stimulus. The probabilities of the rare and the frequent tones were 0.125 and 0.875 respectively. The use of the low or high tone as the rare (target) tone was alternated across the subjects. The auditory stimuli were presented through headphones (model ER-3A Tubephone Insert Earphones, 50 W impedance; Etymotic Research, Elk Grove Village, IL); the earpiece and a short length of the Tubephone were fitted under the electrode cap, and the individual left and right transducer cases were situated on either side of the neck. Subjects received a maximum of 400 trials with a uniform interstimulus interval of 1500 ms.
2.2.3. Event-related potential recording
All six collaborating sites used identical experimental procedures and EEG acquisition hardware and software. Subjects were seated comfortably 1 m from a monitor in a dimly lit sound-attenuated RF-shielded booth (Industrial Acoustics Company, Bronx, NY, USA), and wore an electrode cap (Electro-Cap International, Inc., Eaton, OH, USA) as specified by the International 10-20 System for Electrode Placement (Fig. S2). The nose served as reference and the forehead served as ground. Electrode impedences were maintained below 5kW. Electrical activity was amplified 10,000 times using Neuroscan amplifiers and was recorded continuously over a bandwidth of 0.02-100.0 Hz on a Neuroscan system (Versions 4.1-4.5; Neurosoft, Inc., El Paso, TX, USA) at sampling rates of 256, 500 and 512 Hz, depending on the Neuroscan version, and stored for further analysis. During analysis all signals were re-sampled to 256 Hz and bandpass filtered between 0.05 and 55.0 Hz. Artifact rejection threshold was set at 100 μV. A minimum of 20 trials of 100 ms. pre-stimulus to 750 ms. post-stimulus artifact-free data for each stimulus was required for analysis.
2.2.4. ERO energy estimation
Estimates of localized power of non-stationary evoked potential time series were obtained using the S-transform, a time-frequency representation method developed by Stockwell (Stockwell et al., 1996). This method has been previously described and implemented in our laboratory to evaluate event-related signals in the time-frequency domain (Jones et al., 2006a,b; Kamarajan et al., 2006; Rangaswamy et al., 2007).
Event-related electrophysiological data for the target stimulus from the visual oddball task and the auditory oddball task were analyzed. The amplitude envelope of the S-transform time-frequency region was averaged across single trials, per individual, to obtain estimates of event-related total power. Mean power was calculated for each electrode within time-frequency regions of interest that were defined by frequency band range and time intervals. The measures used in this analysis were the total power in the theta band (3.0-7.0 Hz) oscillation at the frontal (Fz), central (Cz), and parietal (Pz) midline electrodes extracted from the 300-700 ms. time window, which corresponds to the window of the P3 component in the event-related waveforms. The time window and frequency band were chosen to capture relevant data from both tasks at the cost of being overly wide in both frequency and time ranges for each task individually, and used for reporting previous theta ERO results from our laboratory (Kang et al., 2012). The electrodes were chosen because of previous use in studies from our own laboratory (Jones et al., 2006a,b) and other developmental studies (Stige et al., 2007; Nanova et al., 2008). Correlations between measures were estimated using a method described in section 2.3.2.
2.3. Statistical Methodology
2.3.1. Developmental Models
In examining the trajectory of a neurophysiological index, the interest is both in determining the value of that index at any particular age, and in determining how fast and in what direction it is changing. The value of the index observed at a particular age represents the cumulative effect of developmental changes up to the age at which the observation was made. The time course of this cumulative effect is the trajectory of the developmental process. The instantaneous state of the developmental process at any specific age is given by the rate of change of the trajectory (developmental rate) at the age of interest. In this perspective, the state of the developmental process is not directly observable, but can only be estimated from the observable cumulative effects of the developmental process. Although there is a direct mathematical relation between the process and its cumulative effects, results are presented on both the process and its cumulative effects for a more comprehensive view of the phenomena studied. In addition, to understand the relation between the neurophysiological indices studied, the trajectories of their correlations are also determined, and results presented.
If the instantaneous state of the developmental process is represented as g(t), then the measured cumulative value (developmental trajectory) s(t) equals . In order to obtain the developmental rate curve (velocity curve) g(t) the time derivative of s(t) must be estimated. Since there is no explicit biological model for the development process, no parametric form is attributed to g(t) or s(t). In the varying coefficient model (Fan and Zhang, 2008) used in this study, the dependent variable, a point on the developmental trajectory, is an implicit (non-parametric) function of age, and the effects of the independent variables (covariates) on the developmental trajectory can vary with age. (In this study, the developmental trajectories are those of the natural logarithm of theta ERO power values from the scalp locations mentioned above. The logarithmic transformation of the data was used to eliminate the skewed distribution found at every age. In the paper all uses of the word “log” as short for “logarithm” mean natural logarithm, including all text on figures.) The mean of the developmental trajectory s(t) and its time derivative is estimated by a local linear regression calculation (Wu and Zhang, 2002; Wang, 2003; Hoover et al., 1998). The local linear regression calculation is a sequence of regression calculations each centered at a specific age in which the intercept of the regression line is the mean of the trajectory and the slope of the regression line the time derivative at the age at which the regression is centered. That is, a separate calculation is carried out to determine the mean and slope of the theta ERO power values for each central age, which ranges from ages 12 to 25 in one-tenth year intervals, using age-invariant covariates. Each calculation contains data extending before and after the central age (except for the very first and last in the sequence); models centered one year apart share at least 86% of their data and models two years apart share at least 75%, so that there is considerable overlap in the data used for calculation in nearby ages. The regression is weighted so that data near the center age counts more in determining the result than data further away from the center. Each of the six dependent variables, the theta ERO power values at each electrode and each modality, is modeled separately in order to simplify the interpretation of the regression analysis. This methodology is adapted from the work of Cleveland (1979); Cleveland and Devlin (1988); Gasser et al. (1984a,b); Ramsay and Silverman (2002); Gasser et al. (2004); Molinari and Gasser (2004).
The model for each age tk,k = 1...131, is represented by the following equation, in which yij is the dependent variable for subject i at observation j at age tij, Wh(t) the kernel weighting for the local linear regression model, a function of the bandwidth (fraction of data included) h, β0,n,k the parameter to be estimated for covariate n for its effect on the intercept and β1,n,k the parameter to be estimated for covariate n for its effect on the slope, and Xi,j,m the value of covariate m of subject i at observation j. Different sets of covariates may be used for the estimation of the slope and the intercept. The weight Wh(tij −tk) will be non-zero only for an interval near tk and diminishes rapidly as the distance between tij and tk increases. Note that the beta values are indexed to emphasize that each is the product of a separate estimation for each age. (In the equation, intercept terms appear on the first line and slope terms on the second line. The summations are over the repeated indices in the covariates and betas.)
In the statistical model used to estimate gender effects the covariates for the intercept are gender, family type (Alcoholic or Community; see section 2.1), and the first two principal components from the stratification analysis of the genetic data from these subjects; the only covariate for the slope is gender. The principal components from the stratification analysis (Astle and Balding, 2009) were used to ensure the greatest comparability with the genotypic model used in a forthcoming study of the trajectories of the association of genetic variants with the theta EROs. Weights for individuals were adjusted to account for multiple observations on single individuals and co-presence of sibs in each of the local linear regression calculations. The bandwidth for the kernel (Epanechnikov) was taken as 0.6 to minimize the mean squared error, although the variation in the size of the error was less than 2% over the range of bandwidths from 0.4 to 0.8.
As there are 5555 observations spread over a 13 year age-range, there is sufficient data to provide estimates of the means of the variables at one-tenth year intervals and to provide estimates of the mean rates of change of the variables as well. Since 90% of the successive observations of individuals have an interval of greater than 1.75 years, no longitudinal modeling at the shorter time scale (6 months) at which significant changes in the measured variables can occur is possible. (The estimate for the time scale is derived from Sullivan et al. (2011)). Results are not independent between models for different ages since the data in each of the 131 regression models has considerable overlap with the data used in the models for nearby ages. Significance levels and effect sizes were obtained from the regression calculations and corroborated using a non-parametric bootstrap method with 1000 resamplings.
2.3.2. Power correlations
Age-centered and weighted correlations between theta ERO measures both within (intramodal) and between (intermodal) visual and auditory modalities for all pairs of locations were calculated using an approach similar to that employed in the regression analysis, using the same age intervals and same weights as used in the regression analysis. This calculation produces a sequence of 131 correlations for each pair of measures, resulting in 6 intramodal sequences and 9 intermodal sequences per gender. One statistical test was planned on the individual sequences of correlations: whether there was an increase in correlation from age 12 to age 25 for each correlation sequence, using a bootstrap-normal method with the same 1000 resamplings as used for the developmental model.
After examination of the data, which revealed a pattern of decrease followed by increase in some sequences of correlations, an additional statistical calculation was carried out for each sequence of correlations: using a randomization test, test whether the difference between the minimum value, taken over the entire age range, and the value at age 25 in the observed sequence of values could be the result of random fluctuations around the trend from age 12 to age 25. For testing whether the increase in correlation from the minimum value to that at age 25 was the result of random fluctuations around the trend, the null hypothesis was that the sequence of correlation values was like a random walk, with the sequence of increments (differences between successive values) between successive correlation estimates randomly drawn from the realized increments, with the resulting difference between the minimum value, taken over the entire age range, and the value at age 25. The statistical characteristics of the null hypothesis were determined by cumulative summation of each of 1000 permutations of the realized increments to provide 1000 randomized sequences of correlations, determining the difference between the minimum value and the value at age 25 for each randomized sequence, and then finding the mean and standard deviation of the 1000 resulting differences. The realized difference was then represented in terms of its distance in standard deviations from the mean as determined by the randomization process, and its significance estimated accordingly.
3. Results
Both males and females exhibit a general decrease in theta ERO power from ages 12 to 25. Pervasive differences between male and female growth patterns during this age range are the most striking feature of our results. (See Figure 1). In contrast to the decrease in power with age, the correlations between the power values increased from age 12 to age 25 for correlations within each modality between locations (intramodal), and increased from some age between 17 and 20 to age 25 for correlations between modalities at both the same and different locations (intermodal), with the trajectories of intramodal correlations having a different pattern than the trajectories of the intermodal correlations. The differences between males and females in the trajectories of the correlations of the power values is much less than in the trajectories of the power values themselves.
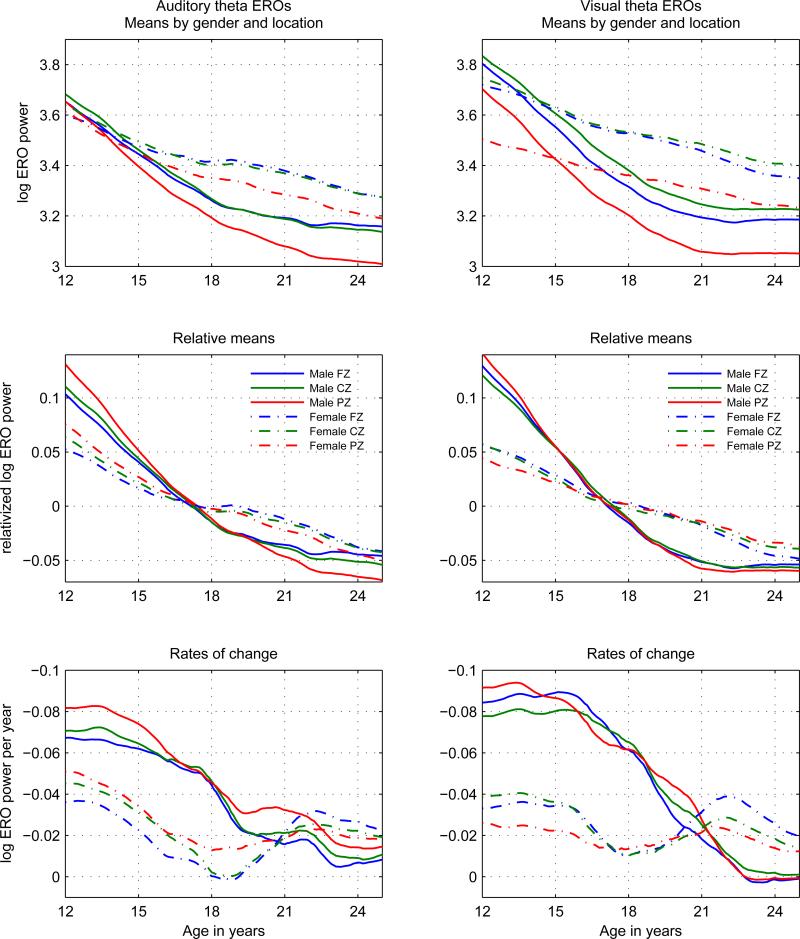
Figure 1.
Theta ERO total power trajectory means (s(t)) in auditory (left column) and visual (right column) modalities presented in three views: Top row: Development curves: log(s(t)). Middle row: Relative development curves:s̃(t) = (log(s(t)) − mean(log(s(t))))/mean(log(s(t))). Values are shown as relative to the mean value of the trajectory over the entire age range of 12 to 25. Plots of relative values emphasize similarities and differences in overall shape, rather than in values. Bottom row: Rates of change of development curves: dlog(s(t))/dt = (ds(t)/dt)/s(t). Each line in this graph represents the slope of the corresponding line in the graph in the top row at the corresponding age. The y-axis is inverted in order to more clearly illustrate the decrease in absolute value of the slopes with time. All line styles and colors of the graphs follow the legends in the middle row.
3.1. ERO power values
To present fully the characteristics of the developmental trajectories, as mentioned at the beginning of Section 2.3.1, three different visual representations are employed in Figure 1: in the top row the values are plotted to compare the trajectories themselves; in the middle row the relative values are plotted to compare the shapes of the trajectories without regard to levels of value; in the bottom row the rates of change of the trajectories are plotted to explicitly represent the size of the changes in the trajectories. The top row of Figure 1 shows the estimated means of the log transformed ERO power. Although male and female trajectories are different, the relation between electrode locations does not differ between males and females: the difference between the posterior channel (Pz) compared to central (Cz) and frontal (Fz) channels is similar in males and females. To emphasize the difference in the shapes, in contrast to the sizes, of the trajectories of males and females the data is also presented in the form of relative values in the middle row: each trajectory is rescaled and recentered in terms of the ratio of each of its values to the mean of the values of the trajectory taken over the entire age range of 12 to 25. The difference between the values at the beginning and at the end of the age range is the developmental change as a proportion of the mean value of the quantity represented, rather than as an absolute difference as shown in the top row. The steeper slopes of males in the relative values of theta ERO trajectories show clearly that males decrease by a much larger proportion of their mean values than females over this age range. The slopes of the male and female trajectories are explicitly shown in the bottom row, a plot of the rates of change of the mean values (developmental rates) in both the visual and auditory modalities. The male slopes are much larger in absolute value than the female slopes until about age 20. In order to emphasize the decrease in absolute values of the slopes, the y-axis has been inverted. Within genders the rates of change of the mean values exhibit only slight differences between modality. Family type was only sporadically statistically significant in an irregular temporal pattern.
3.1.1. Trajectories of Power Values
In the auditory task, theta power in females was greater than males at age 25 at all locations, with the difference between male and female power between 35% and 45% of the combined mean decrease from age 12 to age 25. In the visual task, theta power in females was greater than males at age 25 at all locations, with the difference between male and female power about 35% of the overall decrease from age 12 to age 25.
In both modalities and all locations, differences between males and female became statistically significant by age 16 and continued to age 25. Effect sizes of gender difference when significant range from 0.1 to 0.6. From age 16 onward p-values for age specific gender differences ranged from p < 4 × 10−4 to p < 10−15. Gender differences in the amount of decrease of the mean values from age 12 to age 25 were significant when evaluated over all 6 modality/location variables, obtained from bootstrapping. (See Table 1 for the values of the decreases.) It should be emphasized that there is considerable overlap between the distributions of power values for males and females. The large sample size implies that differences between the means of males and females can be quite significant without being large in terms of the variance within the groups.
Table 1.
Decreases from age 12 to age 25 in all theta ERO power values (log-transformed). The significance for differences between male and female values was calculated by bootstrapping.
| Decrease from age 12 to age 25 | ||||||
|---|---|---|---|---|---|---|
| Auditory | Visual | |||||
| Fz | Cz | Pz | Fz | Cz | Pz | |
| Male | 0.495 | 0.546 | 0.645 | 0.618 | 0.609 | 0.652 |
| Female | 0.322 | 0.364 | 0.423 | 0.371 | 0.343 | 0.272 |
|
P-value for male-female difference | ||||||
| 2.15 × 10–3 | 4.99 × 10–4 | 2.72 × 10–5 | 2.87 × 10–5 | 4.02 × 10–7 | 2.68 × 10–12 | |
3.1.2. Developmental Rates
In section 2.3.1, it was observed that the trajectory, s(t), represented the cumulative effect of the developmental process, and that the state of the process at a particular age was the rate of change (time derivative) of s(t). The trajectories were presented as log(s(t)) in the top row of Figure 1. Since the derivative of log(s(t)) is (ds(t)/dt)/s(t), the bottom row of Figure 1 shows the relative (or fractional) rate of change of the trajectory of the power values or equivalently the rate of change of the trajectory of the log transformed power values. The results are described in terms of the relative rates of change. Theta power in males shows a relatively constant rate of decrease of about 8% to 9% per year from 12 to 16 years of age, then the rate of decrease diminishes in a fairly uniform way before stability is reached at about age 23. Theta power in females shows a relatively constant rate of decrease of about 2.5% to 4% per year from 12 to 16 years of age, then the rate of decrease diminishes until about age 18, remains stable until age 20, and then increases to its previous level of between 2.5% to 4% at age 22 before stabilizing at about 2% around age 25. Note that male and female velocity curves are roughly parallel to each other until the age of 18, when their patterns sharply diverge. Differences greater than 0.015 (1.5%) between values at different ages are statistically significant at the p < 0.05 level, as determined by bootstrapping. Differences in slopes between males and females are statistically significant at the p < 0.05 level from ages 12 to 19.
3.2. ERO power correlations
As mentioned in the introduction, a measure of the development of regional and functional integration of brain activity is provided by the trajectories of intramodal and intermodal correlations between ERO power values. Intramodal correlations measure regional integration within each modality, either through connectivity or commonality of the neurophysiology of the ERO generators. Although volume conduction effects serve to inflate correlation coefficients as a function of distance, these effects are age independent. Thus changes in intramodal correlations with age are not affected by volume conduction. Intermodal correlations measure functional integration between the auditory and visual systems, an index of the development of the supramodal characteristics of the P3 response found in adults (see the second paragraph of section 1). Intermodal correlations may be between power values at the same locations or between power values at different locations. Visual representations of the trajectories of both the intramodal and intermodal correlations of the EROs are provided in Figure 2. Since significant results have gender differences, only correlations of the separate male and female samples are described here. Tables 2 and 3 provide details of the statistical results reported.
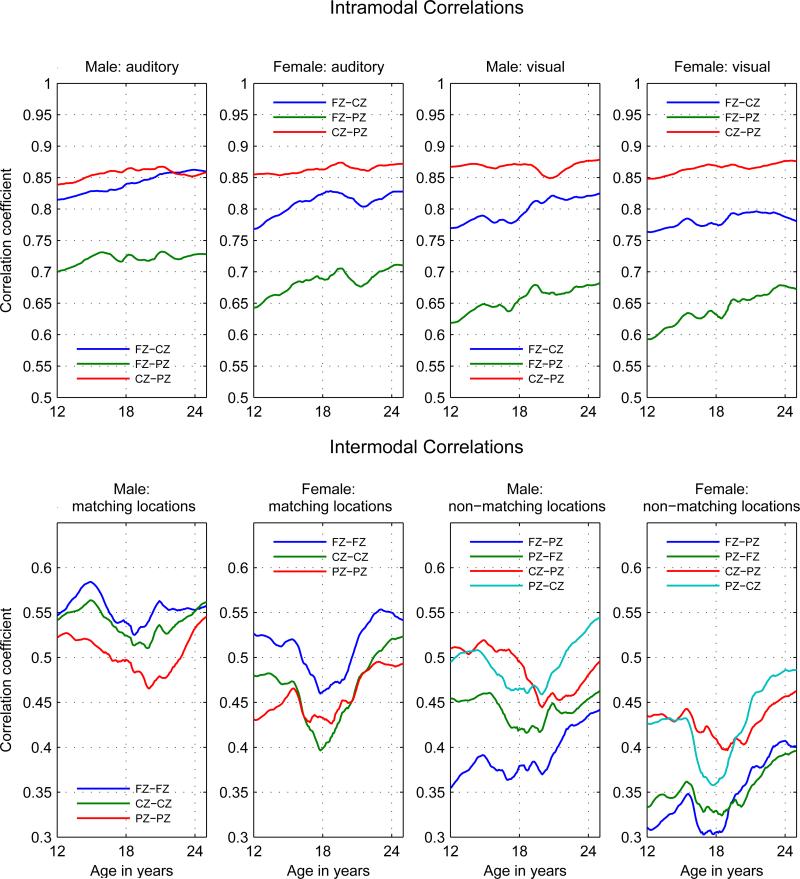
Figure 2.
Intramodal and Intermodal Phenotypic Correlations. Intramodal correlations in top panel: Left Columns: Auditory correlations; Right Columns: Visual Correlations. Intermodal correlations in bottom panel: Left Columns: Matching locations; Right Columns: Non-matching locations. (For non-matching locations the first electrode is the auditory, the second the visual.) Note that the scales are different between in the top and bottom panels.
Table 2.
Power Correlations of Intramodal pairs: Relative increases in correlation from value at age 12 to value age 25 for intramodal pairs. The relative increase is the difference between the value at age 25 and the value at age 12, divided by the mean over the entire age range. Significance calculated by bootstrap-normal methods.
| Relative increase from value at age 12 to value at age 25 | ||||
|---|---|---|---|---|
| Increase | p-value | |||
| Locations | Male | Female | Male | Female |
| Auditory (Intramodal) | ||||
| Fz-Cz | **0.054 | **0.069 | 0.003 | 0.002 |
| Fz-Pz | 0.036 | **0.094 | 0.198 | 0.009 |
| Cz-Pz | 0.019 | 0.018 | 0.292 | 0.283 |
|
Visual (Intramodal) | ||||
| Fz-Cz | **0.065 | 0.025 | 0.008 | 0.288 |
| Fz-Pz | *0.090 | *0.125 | 0.022 | 0.018 |
| Cz-Pz | 0.011 | *0.032 | 0.170 | 0.049 |
Significance levels:
0.01 < p < 0.05
p < 0.01
Table 3.
Power Correlations of Intermodal pairs: Relative increases in correlation from minimum value (over entire age range) to value at age 25 for Intermodal pairs (bottom two sections). The relative increase is the difference between the value at age 25 and the minimum value regardless of age, divided by the mean over the entire age range. Significance calculated by randomization test.
| Relative increase from minimum value to value at age 25 | ||||
|---|---|---|---|---|
| Increase | p-value | |||
| Sites | Male | Female | Male | Female |
| Auditory - Visual (Intermodal – matching sites) | ||||
| Fz-Fz | *0.0544 | **0.1659 | 0.0023 | 10–15 |
| Cz-Cz | **0.0885 | **0.2690 | 10–15 | 10–15 |
| Pz-Pz | **0.1489 | 0.1419 | 10–15 | 0.2125 |
|
Auditory - Visual (Intermodal – non-matching sites) | ||||
| Fz-Cz | 0.083 | **0.222 | 0.772 | 10–15 |
| Fz-Pz | 0.216 | 0.286 | 0.584 | 0.058 |
| Cz-Pz | **0.166 | **0.299 | 10–15 | 10–15 |
| Cz-Fz | **0.124 | **0.262 | 10–15 | 10–15 |
| Pz-Fz | **0.097 | *0.198 | 10–15 | 0.007 |
| Pz-Cz | **0.092 | **0.146 | 10–15 | 10–15 |
Significance levels:
10–6 < p < 0.05
p < 10–6
3.2.1. Intramodal correlations
Intramodal (interregional) power correlations between theta EROs exhibit gradual increases from age 12 to 25; the correlations between anterior and central or posterior theta EROs exhibit the largest increase. Correlations range from 0.59 to 0.87, with Cz-Pz being consistently the largest and Fz-Pz the smallest. Increases from age 12 to age 25 range from 3% to 13% of the mean value of the correlations, with the increase in Fz-Pz the largest in each modality in females and in the male visual modality; the increase in male auditory Fz-Cz was greater than the Fz-Pz increase. The increases at seven of the twelve correlation pairs (three for males and four for females) were significant at the p < 0.05 level as determined by bootstrapping. (See the top panel of Figure 2 and Table 2.)
3.2.2. Intermodal correlations
In contrast to the intramodal correlations, most intermodal correlations did not have a nearly monotonic increase from age 12 to 25, but were punctuated by a sharp decrease during ages 15 to 18 in females and ages 15 to 20 in males, followed by a recovery (see the bottom panel of Figure 2). This was the case in both correlations between modalities at the same location and correlations between modalities at different locations. Intermodal power correlations between theta EROs exhibit increases from age 12 to 25 but the only statistical significant result is for auditory Fz with visual Pz (20%) in males.
The pattern of decrease and recovery is somewhat more pronounced in females than in males. To characterize this pattern, increases from the minimum value of the correlation to the value at age 25 were calculated. Increases from minimum to final values in intermodal correlations ranges between 2% and 30% of the mean values for females and between 2% and 17% of the mean values for males. As described in the final paragraph of section 2.3.2, an additional statistical test was used to evaluate the significance of these increases. Increases in four of the nine intermodal correlations: Auditory Cz – Visual Cz, Auditory Cz – Visual Fz, Auditory Pz – Visual Cz, Auditory Cz – Visual Pz; had p-values less than 10−6 for both genders. Differences in four of the other intermodal correlations were significant in one gender only at a 10−6 level: Auditory Fz – Visual Fz, Auditory Fz – Visual Cz, Auditory Pz – Visual Fz, Auditory Pz – Visual Pz. (See Table 3.)
In summary, the pattern of increase of correlations between ERO power measures was different in intramodal correlations compared to intermodal correlations. For the intramodal correlations the increase is from age 12 to age 25 which occurs in 5 of the 6 intramodal pairs in one (3 pairs) or both (2 pairs) of the genders but in only one of the 9 intermodal pairs in one gender. For the intermodal correlations the increase is not from age 12 but begins at some age between 17 and 20, when a minimum value occurs, to age 25 in 8 of the 9 intermodal pairs; this pattern occurs in one (2 pairs) or both (6 pairs) of the genders in the intermodal pairs but in only one of the intramodal pairs. The uniformity within both the intramodal correlation trajectories and the intermodal correlation trajectories combined with the difference between intramodal and intermodal correlation trajectories suggest a significant divergence in developmental pattern between the auditory and visual systems from age 16 to age 20 followed by a reconvergence. (We note the somewhat anomalous behavior of intramodal visual Cz – Pz and intermodal auditory Fz – visual Cz). Differences between male and female trajectories were small compared to those in the power trajectories.
4. Discussion
There are four significant findings regarding the development of theta EROs in adolescents and young adults reported in this study:
Male and female developmental trajectories of theta ERO power were significantly different in their temporal characteristics, with more rapid decreases with age in males than in females during the ages of 12 to 25. The change in the rate of decrease with age was nearly monotonic in males, with greater fluctuations in females.
Male and female developmental trajectories of power were not significantly different in their regional characteristics. Relations between power at different locations were similar between males and females and the shapes of trajectories did not depend on location.
Both males and females exhibited increasing supraregional (global) functional integration with age in each modality as manifested by increasing intramodal power correlations, with correlations between more distant locations showing the greatest proportional increase over the course of the ages of 12 to 25. Intramodal correlations were always larger than intermodal correlations.
Both males and females exhibited a decrease followed by a sharp increase in supramodal functional integration in a span centered at age 18 and ranging from ages 16 to 21, with the increase persisting to age 25, as manifested by the trajectories of intermodal correlations. This, combined with the nearly monotonic increase within the intramodal power correlations indicates a divergence followed by a reconvergence in the developmental characteristics of the auditory and visual systems during the period from ages 16 to 21.
Non-parametric regression methods made it possible to obtain the detailed trajectories and rates of change of theta EROs and the effects of gender on them, as well as age-specific power correlations. Parametric models in longitudinal data analysis are useful when the conformity or deviation of the observations to a known or hypothesized process is of primary interest, or where the interest is to summarize the large-scale characteristics of a data set in terms of its general shape. The large number of observations with precise ages, and the fact that there are no mathematical models of the biological processes governing the development of brain function provides a strong rationale for the use of non-parametric methods, which enable the estimation of a trajectory that takes into account the non-unformity of the developmental process.
4.1. ERO power trajectories
P3 studies
One study of the development of theta EROs associated with the P3 response is available (Yordanova and Kolev, 1997); however it provides little detail on development in adolescents. Since a parallel between P3 amplitude results and theta EROs may be expected (Jones et al., 2006a; Andrew and Fein, 2010), comparable decreases in P3 peak amplitudes with age in adolescents have been reported in several studies (Katsanis et al., 1996; Hill et al., 1999a; Carlson and Iacono, 2006; Stige et al., 2007; Carlson and Iacono, 2008). Gender difference in the visual P3 response measured by peak amplitudes has been recorded in adults (Hoffman and Polich, 1999; Hill et al., 1999b) and adolescents (Hill et al., 1999a). Decreases in theta EROs are consistent with studies which report decreases in resting EEG power (Whitford et al., 2007).
Structural and anatomical studies
There are two characteristic features of brain development during adolescence: a decrease in gray matter density and cortical thickness, likely reflecting synaptic pruning and myelination; and an increase in white matter (Sowell et al., 2004; Toga et al., 2006). These processes are not uniform across different brain regions (Sowell et al., 2002; Sullivan et al., 2011). Adolescent males exhibit steeper developmental slopes in gray matter reduction and white matter increase than females (De Bellis et al., 2001; Lenroot et al., 2007; Lenroot and Giedd, 2010; Raznahan et al., 2011). The observed decrease in theta ERO power in our study is consistent with the decrease in gray matter density and cortical thickness; perhaps the steeper trajectories of theta ERO power loss in males are related to their steeper trajectory of gray matter reduction. Trajectories of brain volumes of different regions and tissue types, as well as other features of cortical anatomy in pre-adolescents and adolescents, exhibit curvilinear properties which vary between regions (Lenroot and Giedd, 2008; Shaw et al., 2008; Giedd et al., 2010; Raznahan et al., 2011; Sullivan et al., 2011). These variations are possibly connected to the varying rates of decrease of power as well as age related differences in topography not discussed in this study. Relations between global and regional brain anatomical features shown in Lenroot et al. (2007); Raznahan et al. (2011) have no apparent gender specificity.
Functional studies
Gender differences in trajectories across this age range have been reported for functional neuroimaging brain development variables (Rubia et al., 2006; Christakou et al., 2009; Rubia et al., 2010; Rubia, 2013; Rubia et al., 2013), but since trajectories have been reported parametrically, or compared between distinct age ranges, the temporal detail identified here has not previously been reported. Furthermore, gender differences in rates of change across this age range have not previously been reported for brain development variables, nor specifically modeled. Preliminary studies of other P3 ERO related variables from this data set, which differ from those in this study in frequency band, ERO measure, and task condition, show similar gender differences in trajectories as those reported here.
A functional study of a somewhat similar oddball task using fMRI (Rubia et al., 2010) reports increases of activation in some brain regions and decrease of activations in others with development, corresponding to increased relative frontal activity (Rubia et al., 2010, Table 4). This, as well as additional results of the fMRI studies mentioned above, suggest that the spatial distribution of the generators of theta EROs changes with age; this may be responsible for the topographical changes in the relative strengths of the power at the midline electrodes studied (not illustrated here).
4.2. ERO power correlations
Intramodal correlations
The observed increase with age in intramodal power correlations, with the largest relative increase between the most distant electrodes, is consistent with the increase in white matter and increased functional integration with age during adolescence (Rubia, 2013, pp. 84, 86). A trend from local connectivity to more global patterns of connectivity has been shown by studies of the development of brain networks using MRI methods (Fair et al., 2008, 2009; Power et al., 2010; Supekar et al., 2009; Uddin et al., 2011; Vogel et al., 2010; Zielinski et al., 2010). Since data from only three midline electrodes has been analyzed at this time, the topographical information required for a more detailed comparison with the MRI data is not available. However, data has been recorded from a dense array of electrodes across the scalp and will be subject to further analysis.
Intermodal correlations
The trajectories of intermodal correlations and of ERO power development suggest that there is a developmental transition span centered at age 18 and ranging from ages 16 to 21, marked by a pattern of divergent development in the auditory and visual systems, particularly in females. As discussed above (see section 3.2.2 and Figure 2), there is a decrease in intermodal power correlation from ages 15 to 18, followed by recovery beyond previous levels from ages 18 to 25, particularly large in females. All but one of the twelve gender specific differences between minimum values and values at age 25 which are significant at the p < 10−6 level occur in intermodal correlations. Since intramodal power correlations have no such decrease and recovery pattern, it can be supposed that there is a transition between distinct regimes in supramodal development not found in supraregional development, which accounts for the divergence between auditory and visual development. The study by Walz et al. (2013) found that common brain regions in the same individuals were activated in independent auditory and visual oddball tasks. However, the supramodal features of the P3 response were found only during one-fifth of the entire duration of the response, which suggests that our long duration measures are inadequate to fully determine the trajectory of supramodal development, and its relation to development of modality-specific features. We know of no previous observation of divergent development between modality related systems. However, these results may be related to the decrease and subsequent increase of neural synchrony in adolescents in the same age range reported in Uhlhaas et al. (2009) and Uhlhaas and Singer (2011).
4.3. Summary
Given the large number of electrophysiological observations available in the COGA Prospective study, it became possible to estimate mean rates of change of development with considerable accuracy. This enabled the identification of striking gender differences in the age-specific developmental patterns of theta band EROs, but relatively little gender modulation of spatial differences. Preliminary studies of other P3 related measures derived from the same subjects with the same methodology show similar patterns of gender differences in mean rates of change. In addition, the trajectory of correlations between the six electrophysiological variables examined in this study indicated a developmental divergence and reconvergence between auditory and visual systems. This suggests that relatively large neurophysiological systems influenced by multiple genetic factors may experience coordinated developmental patterns regulated by gender and modality specific effects. In a forthcoming study, the developmental trajectories of the associations between genetic variants (SNPs) from a number of genes associated with neurophysiological functions and the measures of the power of theta EROs are studied using the same data set and similar methodologies. Since measures of the P3 response have been found to be useful endophenotypes for the study of substance use disorders (Euser et al., 2012; Iacono and Malone, 2011; Rangaswamy and Porjesz, 2008), externalizing psychopathology (Gilmore et al., 2012), schizophrenia (Decoster et al., 2012; Johannesen et al., 2013), and ADHD (Szuromi et al., 2011), and is highly heritable (van Beijsterveldt and van Baal, 2002), studies of P3 development in adolescents and young adults may illuminate neurophysiological and neuroanatomical factors contributing to the onset of these conditions.
Research Highlights.
There are large differences in developmental rates between males and females.
Locational and and modality differences are small compared to gender differences.
Development diverges between auditory and visual systems during ages 16 to 21.
Acknowledgements
The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, H. Edenberg, L. Bierut, includes eleven different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., T. Foroud); University of Iowa (S. Kuperman, J. Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, A. Brooks); University of Texas Health Science Center at San Antonio (L. Almasy), Virginia Commonwealth University (D. Dick), Icahn School of Medicine at Mount Sinai (A. Goate), and Howard University (R. Taylor). Other COGA collaborators include: L. Bauer (University of Connecticut); D. Koller, J. McClintick, S. O'Connor, L. Wetherill, X. Xuei, Y. Liu (Indiana University); G. Chan (University of Iowa; University of Connecticut); D. Chorlian, N. Manz, C. Kamarajan, A. Pandey (SUNY Downstate); J.-C. Wang, M. Kapoor (Icahn School of Medicine at Mount Sinai) and F. Aliev (Virginia Commonwealth University). A. Parsian and M. Reilly are the NIAAA Staff Collaborators.
We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, currently a consultant with COGA, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David B. Chorlian, Henri Begleiter Neurodynamics Laboratory, Department of Psychiatry, SUNY Downstate Medical Center, Brooklyn, NY.
Madhavi Rangaswamy, Department of Psychology,Christ University, Bangalore, India.
Niklas Manz, Henri Begleiter Neurodynamics Laboratory, Department of Psychiatry, SUNY Downstate Medical Center, Brooklyn, NY.
Chella Kamarajan, Henri Begleiter Neurodynamics Laboratory, Department of Psychiatry, SUNY Downstate Medical Center, Brooklyn, NY.
Ashwini K. Pandey, Henri Begleiter Neurodynamics Laboratory, Department of Psychiatry, SUNY Downstate Medical Center, Brooklyn, NY
Howard Edenberg, Indiana University School of Medicine, Indianapolis, IN, USA.
Samuel Kuperman, University of Iowa School of Medicine, Ames, IA, USA.
Bernice Porjesz, Henri Begleiter Neurodynamics Laboratory, Department of Psychiatry, SUNY Downstate Medical Center, Brooklyn, NY.
References
- Alexander JE, Polich J, Bloom FE, Bauer LO, Kuperman S, Rohrbaugh J, Morzorati S, O'Connor SJ, Porjesz B, Begleiter H. P300 from an auditory oddball task: inter-laboratory consistency. Int J Psychophysiol. 1994;17:35–46. doi: 10.1016/0167-8760(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Andrew C, Fein G. Event-related oscillations versus event-related potentials in a P300 task as biomarkers for alcoholism. Alcohol Clin Exp Res. 2010;34(4):669–80. doi: 10.1111/j.1530-0277.2009.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astle W, Balding DJ. Population Structure and Cryptic Relatedness in Genetic Association Studies. 2009 http://arxiv.org/pdf/1010.4681.
- Begleiter H, Reich T, Hesselbrock VM, Porjesz B, Li TK, Schuckit MA, et al. The Collaborative Study on the Genetics of Alcoholism. Alcohol Health Res. World. 1995;19:228–236. [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, Edenberg HJ, Goate A, Blangero J, et al. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalogr. Clin. Neurophysiol. 1998;108(3):244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG. Heritability of P300 amplitude development from adolescence to adulthood. Psychophysiology. 2006;43(5):470–80. doi: 10.1111/j.1469-8986.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG. Deviant P300 amplitude development in males is associated with paternal externalizing psychopathology. J Abnorm Psychol. 2008;117(4):910–23. doi: 10.1037/a0013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Tang Y, Rangaswamy M, Wang JC, Almasy L, Foroud T, Edenberg HJ, Hesselbrock V, Nurnberger J, Jr, Kuperman S, O'Connor SJ, Schuckit MA, Bauer LO, Tischfield J, Rice JP, Bierut L, Goate A, Porjesz B. Association of single nucleotide polymorphisms in a glutamate receptor gene (GRM8) with theta power of event-related oscillations and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):359–68. doi: 10.1002/ajmg.b.30818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. Neuroimage. 2009;48(1):223–36. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. JASA. 1979;74(368):829–836. [Google Scholar]
- Cleveland WS, Devlin SJ. Locally-Weighted Regression: An Approach to Regression Analysis by Local Fitting. JASA. 1988;83(403):596–610. [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11(6):552–7. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Decoster J, De Hert M, Viechtbauer W, Nagels G, Myin-Germeys I, Peuskens J, van Os J, van Winkel R. Genetic association study of the P300 endophenotype in schizophrenia. Schizophr Res. 2012;141(1):54–9. doi: 10.1016/j.schres.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Wang W, Porjesz B, Bauer L, Kuperman S, O'Connor SJ, Rohrbaugh J, Begleiter H. Visual P300: an interlaboratory consistency study. Alcohol. 1994;11:583–587. doi: 10.1016/0741-8329(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Bierut LJ, Boyce P, Cao M, Cawley S, Chiles R, Doheny KF, Hansen M, Hinrichs T, Jones K, Kelleher M, Kennedy GC, Liu G, Marcus G, McBride C, Murray SS, Oliphant A, Pettengill J, Porjesz B, Pugh EW, Rice JP, Rubano T, Shannon S, Steeke R, Tischfield JA, Tsai YY, Zhang C, Begleiter H. Description of the data from the Collaborative Study on the Genetics of Alcoholism (COGA) and single-nucleotide polymorphism genotyping for Genetic Analysis Workshop 14. BMC Genet. 6 Suppl. 2005;1:S2. doi: 10.1186/1471-2156-6-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, Desikan A, Phillips E, Havstad J. Decreases in energy and increases in phase locking of event-related oscillations to auditory stimuli occur during adolescence in human and rodent brain. Dev Neurosci. 2014;36(3-4):175–95. doi: 10.1159/000358484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euser AS, Arends LR, Evans BE, Greaves-Lord K, Huizink AC, Franken IH. The P300 event-related brain potential as a neurobiological endophenotype for substance use disorders: a meta-analytic investigation. Neurosci Biobehav Rev. 2012;36(1):572–603. doi: 10.1016/j.neubiorev.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105(10):4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a ”local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Zhang W. Statistical methods with varying coefficient models. Statistics and its Interface. 2008;1:179–195. doi: 10.4310/sii.2008.v1.n1.a15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl-Bauch T, Bottlender R, Hegerl U. Neurochemical substrates and neuroanatomical generators of the event-related P300. Neuropsychobiology. 1999;40(2):86–94. doi: 10.1159/000026603. [DOI] [PubMed] [Google Scholar]
- Gasser T, Mller HG, Köhler W, Molinari L, Prader A. Nonparametric regression analysis of growth curves. Ann Stat. 1984a;12(1):210–229. [Google Scholar]
- Gasser T, Köhler W, Mller HG, Kneip A, Largo R, Molinari L, Prader A. Velocity and acceleration of height growth using kernel estimation. Ann Hum Biol. 1984 Sep-Oct. 1984b;11(5):397–411. doi: 10.1080/03014468400007311. [DOI] [PubMed] [Google Scholar]
- Gasser T, Gervini D, Molinari L. Kernel estimation, shape-invariant modeling and structural analysis. In: Hauspie R, Cameron N, Molinari L, editors. In Methods in Human Growth Research. Cambridge University Press; 2004. pp. 179–204. [Google Scholar]
- Gevins A, Smith ME, Leong H, McEvoy L, Whitfield S, Du R, Rush G. Monitoring working memory load during computer-based tasks with EEG pattern recognition methods. Hum Factors. 1998;40(1):79–91. doi: 10.1518/001872098779480578. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Stockman M, Weddle C, Liverpool M, Alexander-Bloch A, Wallace GL, Lee NR, Lalonde F, Lenroot RK. Anatomic magnetic resonance imaging of the developing child and adolescent brain and effects of genetic variation. Neuropsychol Rev. 2010;20(4):349–61. doi: 10.1007/s11065-010-9151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Iacono WG. Brain electrophysiological endophenotypes for externalizing psychopathology: a multivariate approach. Behav Genet. 2010;40(2):186–200. doi: 10.1007/s10519-010-9343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol Psychiatry. 1999a;46(7):970–81. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Locke J, Steinhauer SR. Absence of visual and auditory P300 reduction in nondepressed male and female alcoholics. Biol Psychiatry. 1999b;46(7):982–9. doi: 10.1016/s0006-3223(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Hill SY, Jones BL, Holmes B, Steinhauer SR, Zezza N, Stiffler S. Cholinergic receptor gene (CHRM2) variation and familial loading for alcohol dependence predict childhood developmental trajectories of P300. Psychiatry Res. 2013;209(3):504–11. doi: 10.1016/j.psychres.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LD, Polich J. P300, handedness, and corpus callosal size: gender, modality, and task. Int J Psychophysiol. 1999;31(2):163–74. doi: 10.1016/s0167-8760(98)00050-6. [DOI] [PubMed] [Google Scholar]
- Hoover DR, Rice JA, Wu CO, Yang LP. Nonparametric smoothing estimates of time-varying coefficient models with longitudinal data. Biometrika. 1998;85(4):809–822. [Google Scholar]
- Iacono WG, Malone SM. Developmental Endophenotypes: Indexing Genetic Risk for Substance Abuse with the P300 Brain Event-Related Potential. Child Dev Perspect. 2011;5(4):239–247. doi: 10.1111/j.1750-8606.2011.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: theta correlates of memory retrieval and decision making. Neuroimage. 2006;32:978–987. doi: 10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, O'Donnell BF, Shekhar A, McGrew JH, Hetrick WP. Diagnostic specificity of neurophysiological endophenotypes in schizophrenia and bipolar disorder. Schizophr Bull. 2013;39(6):1219–2. doi: 10.1093/schbul/sbs093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, Stimus A, Begleiter H. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol. 2006a;117(10):2128–43. doi: 10.1016/j.clinph.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Dick D, Goate A, Hinrichs A, Rice JP, Wang JC, Bauer LO, Crowe R, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O'Connor SJ, Rohrbaugh J, Schuckit MA, Tischfield J, Edenberg HJ, Begleiter H. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behav Genet. 2006b;36(5):627–39. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59(7):625–34. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Rangaswamy M, Manz N, Wang JC, Wetherill L, Hinrichs T, Almasy L, Brooks A, Chorlian DB, Dick D, Hesselbrock V, Kramer J, Kuperman S, Nurnberger J, Jr, Rice J, Schuckit M, Tischfield J, Bierut LJ, Edenberg HJ, Goate A, Foroud T, Porjesz B. Family-based genome-wide association study of frontal theta oscillations identifies potassium channel gene KCNJ6. Genes Brain Behav. 2012;11(6):712–9. doi: 10.1111/j.1601-183X.2012.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neurophysiol. 2000a;111(10):1719–32. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neurosci Lett. 2000b;285(1):45–8. doi: 10.1016/s0304-3940(00)01022-3. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK. The association between P300 and age from preadolescence to early adulthood. Int J Psychophysiol. 1996;24(3):213–221. doi: 10.1016/s0167-8760(96)00063-3. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK, Carlson SR. P300 event-related potential heritability in monozygotic and dizygotic twins. Psychophysiology. 1997;34(1):47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll NE, Lazzara M, Rohm D, Gruber W. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Brain Res Cogn Brain Res. 2001;12:33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R, Sauseng P, Gruber W. A short review of slow phase synchronization and memory: evidence for control processes in different memory systems? Brain Res. 2008;1235:31–44. doi: 10.1016/j.brainres.2008.06.049. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, Crone EA. Sex differences and structural brain maturation from childhood to early adulthood. Dev Cogn Neurosci. 2013;5:106–18. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. The changing impact of genes and environment on brain development during childhood and adolescence: initial findings from a neuroimaging study of pediatric twins. Dev Psy chopathol. 2008;20(4):1161–75. doi: 10.1017/S0954579408000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72(1):46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–73. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DE, Prvulovic D, Formisano E, Vallinger M, Zanella FE, Goebel R, Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex. 1999;9(8):815–23. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Perrucci MG, Romani GL, Del Gratta C. Large-scale brain networks account for sustained and transient activity during target detection. Neuroimage. 2009;44(1):265–74. doi: 10.1016/j.neuroimage.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Developmental pathways to functional brain networks: emerging principles. Trends Cogn Sci. 2013;17(12):627–40. doi: 10.1016/j.tics.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Molinari L, Gasser T. The human growth curve: distance, velocity and acceleration. In: Hauspie R, Cameron N, Molinari L, editors. Methods in Human Growth Research. Cambridge University Press; 2004. pp. 27–54. [Google Scholar]
- Nanova P, Lyamova L, Hadjigeorgieva M, Kolev V, Yordanova J. Gender-specific development of auditory information processing in children: an ERP study. Clin Neurophysiol. 2008;119(9):1992–2003. doi: 10.1016/j.clinph.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Nanova P, Kolev V, Yordanova J. Developmental gender differences in the synchronization of auditory event-related oscillations. Clin Neurophysiol. 2011;122(5):907–15. doi: 10.1016/j.clinph.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;2191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45(4):1055–66. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Pogarell O, Padberg F, Karch S, Segmiller F, Juckel G, Mulert C, Hegerl U, Tatsch K, Koch W. Dopaminergic mechanisms of target detection - P300 event related potential and striatal dopamine. Psychiatry Res. 2011;194(3):212–8. doi: 10.1016/j.pscychresns.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60(2):172–85. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67(5):735–48. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay JO, Silverman BW. Applied Functional Data Analysis. Springer-Verlag; New York: 2002. [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O'Connor SJ, Bauer LO, Schuckit MA, Begleiter H. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol. 2007;63(1):3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Uncovering genes for cognitive(dys)function and predisposition for alcoholism spectrum disorders: A review of human brain oscillations as effective endophenotypes. Brain Res. 2008;1235:153–71. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. How does your cortex grow? J Neurosci. 2011;31(19):7174–7. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich T. A genomic survey of alcohol dependence and related phenotypes: results from the Collaborative Study on the Genetics of Alcoholism (COGA). Alcohol Clin Exp Res. 1996;20:A133–137. doi: 10.1111/j.1530-0277.1996.tb01763.x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27(12):973–93. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Hyde Z, Halari R, Giampietro V, Smith A. Effects of age and sex on developmental neural networks of visual-spatial attention allocation. Neuroimage. 2010;51(2):817–27. doi: 10.1016/j.neuroimage.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur Child Adolesc Psychiatry. 2013;22(12):719–31. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Lim L, Ecker C, Halari R, Giampietro V, Simmons A, Brammer M, Smith A. Effects of age and gender on neural networks of motor response inhibition: From adolescence to mid-adulthood. Neuroimage. 2013;83C:690–703. doi: 10.1016/j.neuroimage.2013.06.078. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76(1):130–41. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Jetha MK. Electrophysiological changes during adolescence: a review. Brain Cogn. 2010;72(1):86–100. doi: 10.1016/j.bandc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28(14):3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Stige S, Fjell AM, Smith L, Lindgren M, Walhovd KB. The development of visual P3a and P3b. Developmental Neuropsychology. 2007;32(1):563–584. doi: 10.1080/87565640701361096. [DOI] [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S transform. IEEE Trans Signal Process. 1996;44:998–1001. [Google Scholar]
- Sturman DA, Moghaddam B. The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev. 2011;35(8):1704–12. doi: 10.1016/j.neubiorev.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Rohlfing T, Baker FC, Padilla ML, Colrain IM. Developmental change in regional brain structure over 7 months in early adolescence: comparison of approaches for longitudinal atlas-based parcellation. Neuroimage. 2011;57(1):214–24. doi: 10.1016/j.neuroimage.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumich AL, Sarkar S, Hermens DF, Ibrahimovic A, Kelesidi K, Wilson D, Rubia K. Sex differences in brain maturation as measured using event-related potentials. Dev Neuropsychol. 2012;37(5):415–33. doi: 10.1080/87565641.2011.653461. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuromi B, Czobor P, Kömlosi S, Bitter I. P300 deficits in adults with attention deficit hyperactivity disorder: a meta-analysis. Psychol Med. 2011;41(7):1529–38. doi: 10.1017/S0033291710001996. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci U S A. 2009;106(24):9866–71. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull. 2011;37(3):514–23. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31(50):18578–89. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Individual differences in P300 amplitude: a genetic study in adolescent twins. Biol Psychol. 1998;47(2):97–120. doi: 10.1016/s0301-0511(97)00025-2. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC, Molenaar PC, Boomsma DI, de Geus EJ. Stability of genetic and environmental influences on P300 amplitude: a longitudinal study in adolescent twins. Behav Genet. 2001;31(6):533–43. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol Psychol. 2002;61(1-2):111–38. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Power JD, Petersen SE, Schlaggar BL. Development of the brain's functional network architecture. Neuropsychol Rev. 2010;20(4):362–75. doi: 10.1007/s11065-010-9145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz JM, Goldman RI, Carapezza M, Muraskin J, Brown TR, Sajda P. Simultaneous EEG-fMRI Reveals Temporal Evolution of Coupling between Supramodal Cortical Attention Networks and the Brainstem. J Neurosci. 2013;33(49):19212–22. doi: 10.1523/JNEUROSCI.2649-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz JM, Goldman RI, Carapezza M, Muraskin J, Brown TR, Sajda P. Simultaneous EEG-fMRI reveals a temporal cascade of task-related and default-mode activations during a simple target detection task. Neuroimage. 2014;102(1):229–39. doi: 10.1016/j.neuroimage.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL. Nonparametric Regression Analysis of Longitudinal Data. 2003 http://anson.ucdavis.edu/~wang/paper/EOB3.pdf.
- Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp. 2007;28(3):228–37. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HL, Zhang JT. Local polynomial mixed-effects models for longitudinal data. JASA. 2002;97(459):883–897. [Google Scholar]
- Wu K, Taki Y, Sato K, Hashizume H, Sassa Y, Takeuchi H, Thyreau B, He Y, Evans AC, Li X, Kawashima R, Fukuda H. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PLoS One. 2013;8(2):e55347. doi: 10.1371/journal.pone.0055347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Developmental changes in the event-related EEG theta response and P300. Electroencephalogr Clin Neurophysiol. 1996;104(5):418–30. doi: 10.1016/s0168-5597(97)00054-3. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Rosso OA, Kolev V. A transient dominance of theta event-related brain potential component characterizes stimulus processing in an auditory oddball task. 2003 doi: 10.1016/s1388-2457(02)00415-7. [DOI] [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proc Natl Acad Sci U S A. 2010;107(42):18191–6. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlojutro M, Manz N, Rangaswamy M, Xuei X, Flury-Wetherill L, Koller D, Bierut LJ, Goate A, Hessel-brock V, Kuperman S, Nurnberger J, Jr, Rice JP, Schuckit MA, Foroud T, Edenberg HJ, Porjesz B, Almasy L. Genome-wide association study of theta band event-related oscillations identifies serotonin receptor gene HTR7 influencing risk of alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(1):44–58. doi: 10.1002/ajmg.b.31136. [DOI] [PMC free article] [PubMed] [Google Scholar]