Abstract
Context
There are concerns about the effects of in utero exposure to antiretroviral drugs (ARVs) on the development of HIV exposed but uninfected (HEU) children.
Objectives
To evaluate whether in utero exposure to ARVs is associated with lower birth weight/height and reduced growth during the first two years of life.
Design
Cohort study of HEU infants.
Setting
Tertiary children's hospital in Rio de Janeiro, Brazil.
Study population
HEU infants born 1996-2010.
Main outcome measures
Weight measured by mechanical scale, height measured by measuring board. Z-scores for weight-for-age (WAZ), length-for-age (LAZ) and weight-for-length (WLZ) were calculated. We modeled trajectories by mixed-effects models and adjusted for mother's age, CD4 cell count, viral load, year of birth and family income.
Results
A total of 588 HEU infants were included of whom 155 (26%) were not exposed to ARVs, 114 (19%) were exposed early (first trimester) and 319 (54%) later. WAZ were lower among infants exposed early compared to infants exposed later: adjusted differences were −0.52 (95% CI −0.99 to −0.04, P=0.02) at birth and −0.22 (95% CI −0.47 to 0.04, P=0.10) during follow-up. LAZ were lower during follow-up: −0.35 (95% CI −0.63 to −0.08, P=0.01). There were no differences in WLZ scores. Z-scores of infants exposed late during pregnancy were similar to unexposed infants.
Conclusions
In HEU children early exposure to ARVs was associated with lower WAZ at birth and lower LAZ up to 2 years of life. Growth of HEU children needs to be monitored closely.
Keywords: Antiretrovirals, HIV-exposed but uninfected children, birth weight, birth length, weight-for-age, length-for-age, Brazil
Antiretroviral drug regimens for the prevention of mother-to-child transmission (PMTCT) of HIV have evolved in recent years.1 In 2010, the World Health Organization (WHO) published revised guidelines which recommended life-long ARVs for all persons, including pregnant women, with a CD4+ cell count ≤350 cells/mm3 or the presence of WHO Stage III or IV disease.2 For pregnant women with CD4+ cell counts >350 cells/mm3 and without WHO Stage III or IV disease, WHO recommended either Option A or Option B. Option A consists of maternal zidovudine during pregnancy, maternal single dose nevirapine at delivery and the combination of maternal zidovudine and lamivudine from delivery to one week postpartum, as well as infant nevirapine throughout breastfeeding. Option B involves the maternal use of triple combination antiretroviral therapy (cART) during pregnancy and breastfeeding. In April 2012, WHO added Option B+ which advocates life-long cART for all pregnant women, independent of CD4+ cell count or clinical stage. Of note, Option A ceased to be recommended in 2013.3
Although antiretroviral drugs (ARVs) in general and cART in particular virtually abolish mother-to-child transmission of HIV and the benefits clearly outweigh potential risks, there are concerns about the effects of in utero exposure to ARVs on birth outcomes, postnatal growth and development of infants born to mothers with HIV infection.4–7A study from Botswana showed that exposure to cART during pregnancy (initiated before conceptionor started during pregnancy) was associated with low birth weight and other adverse birth outcomes compared with ZDV monotherapy started later during pregnancy.8 Growth trajectories after birth are also of concern. A study of infants born to mothers with HIV infection in Tanzania found that ART during pregnancy was associated with an increased risk of wasting during follow-up, after adjusting for HIV-infection, CD4 cell count and other potential confounding factors.9 A recent systematic literature review concluded that it remains unknown whether in utero exposure to ARVs has alasting impact on infant growth.7
In Brazil a large-scale public PMTCT program was introduced in 1996,10 and the Option B+ strategy was adopted in 2013. We previously analyzed infants born to women with HIV infection followed in a HIV reference center in Rio de Janeiro. We found that infants born to women who were receiving ART before pregnancy were more likely to be born pre-term and have low birth weight than infants born to women treated after the first trimester.6 In the present study we aimed to evaluate whether exposure to ARV in the first trimester affects growth in the first two years of life in HIV-exposed but uninfected (HEU) infants born to the mothers of the Rio de Janeiro cohort.
METHODS
Setting and definitions
We analyzed data collected prospectively at the Instituto de Puericultura e Pediatria Martagao Gesteira at Universidade Federal do Rio de Janeiro (UFRJ), a tertiary care pediatric hospital in Rio de Janeiro City, with a pediatric HIV clinic that cares for more than 1,000 HEU infants and more than 400 HIV-infected children. HIV-infected mothers are counselled not to breastfeed; replacement feeding is provided free of charge in the first 12months. HIV-exposed infants receive zidovudine until six weeks of life, and afterwards cotrimoxazole prophylaxis until the infant has had two undetectable viral load measurements. For this analysis, one undetectable viral load measurement at least 6 weeks after birth was required to exclude HIV infection. The Roche Amplicor HIV-1 Monitor Test was used to determine viral load, with a detection limit of 400 copies/mL.
Women were classified as having received no ARVs, zidovudine monotherapy, dual therapy or cART during pregnancy. If a woman changed regimens, the regimen with the larger number of drugs was used in the analysis. If she switched between non-nucleoside reverse transcriptase inhibitor (NNRTI)-based cART and protease inhibitor (PI)-based cART, the regimen received for longer was used in the analysis.
Inclusion criteria and follow-up visits
All HEU singleton births after 1995 with at least one follow-up visit were included in the analysis. Gestational age was based on newborn examination using the method proposed by Capurro et al.11 The gestational and perinatal history was extracted from the child's immunization card and the mother's antenatal card. Infants with congenital abnormalities were excluded. For each eligible HEU child a structured questionnaire was completed at care initiation and subsequent clinic visits. The data covered maternal age, HIV status, use and type of ART and the results of laboratory tests, including syphilis screening, CD4 cell count, viral load, hemoglobin and urine tests. All measurements were done at the first ANC visit. Data on birth weight, birth length, gestational age at birth, mode of delivery, Apgar scores, and use of ARVs during labor and delivery were obtained. Children were scheduled to be followed in monthly intervals in the first year, and every three months afterwards. At each visit clinical and laboratory data, prophylactic zidovudine use, and other treatments and immunizations were recorded.
Measurements of weight and length
At each visit, a trained assistant nurse measured weight with a mechanical weighing scale that was calibrated daily. Recumbent length was measured using a measuring board. Standard normal deviates (z-scores) for weight-for-age (WAZ), length-for-age (LAZ) and weight-for-length (WLZ) were calculated using the 2003 Fenton preterm growth charts (until 50 weeks of gestational age), and the 2006 WHO child growth standards for infants born at term.12, 13
Statistical analysis
We analyzed LAZ, WAZ and WLZ scores at birth and up to the age of 2 years. The data were collected from January 1996 to September 2010. We compared scores between children whose mothers had been exposed to ARVs in the first trimester (early ARV group), children whose mothers were exposed in the second or third trimester (late ARV group), and children whose mothers were not exposed to ARVs during pregnancy (no ARV group). We compared characteristics of children at birth and of mothers at their first ANC visit and at delivery. Continuous variables were described using medians and interquartile ranges (IQR); Kruskal Wallis tests were used to test the null hypothesis of no difference across the three groups. Categorical variables are presented as numbers and percentages, with differences tested by Fisher exact tests.
We used linear regression to evaluate differences in z-scores at birth between comparison groups, and multilevel mixed-effects linear regression models to examine the evolution of anthropometric measurements over time, with the random effect on the child and fixed effects on intercept and independent variables. Since growth is not linear over time we introduced splines with knots at 4.5 and 9.6 months to improve the fit of the model.14 We included mother's age (younger than 25 years or older), maternal CD4 cell count at ANC initiation (square root transformed), maternal viral load at ANC initiation (undetectable or detectable), year of birth and family income (as continuous variables) in multivariable models. We also examined the evolution of anthropometric measurements restricting the analysis to children exposed to cART during pregnancy, defined as use of at least three drugs, from at least two classes. Numbers were too small to allow further subgroup analyses.
Results from regression models are presented as differences in z-scores with 95% confidence intervals (CI). P values <0.05 were considered significant. STATA 13.0 (Stata Corp., College Station, TX, USA) was used for all analyses.
RESULTS
Characteristics of mothers and infants
Of 609 HEU children followed in this cohort, eight pairs of twins, four children with missing date of birth and one infant with trisomy 21 were excluded, resulting in a study population of 588 HEU children. One hundred and fifty-five (26.4%) children were not exposed to ARVs in utero, 114 (19.4%) were exposed in the first trimester (early ARV exposure) and 319 (54.2%) were exposed in the second or third trimester (later exposure). Forty (35.1%) of the 114 women on ARVs in the first trimester started therapy before conception.
Mothers exposed to ARV during pregnancy had higher CD4 cell counts and lower viral loads at their first visit to ANC than mothers not exposed to ARV (Table 1). Mothers of infants exposed early in pregnancy were slightly older than mothers exposed later or not exposed to ARVs. The median family income was US$ 592 in all three groups, corresponding to two minimum Brazilian wages of the year 201015 Most of the 114 children exposed early in pregnancy were born to mothers who received cART: 53 (46%) were exposed to PIs nelfinavir or lopinavir/ritonavir and 32 (28%) to nevirapine or efavirenz. The majority of children exposed later in pregnancy had been born to mothers who received zidovudine monotherapy or dual therapy (Table 1).
Table 1.
Characteristics of pregnant women and their HIV exposed but uninfected infants by exposure to antiretroviral drugs during pregnancy.
| Characteristic (Number of children with values) | No ARV* in pregnancy (N=155) | ARV* in the first trimester (N=114) | ARV* in second or third trimester (N=319) | P-value## |
|---|---|---|---|---|
| Mothers | ||||
| CD4 cell count at first visit, cells/μL (N=415) | 288 (205 to 486) | 371 (333 to 525) | 444 (307 to 597) | 0.0004 |
| Viral load at first visit, copies/mL (N=376) | 7332 (1070 to 30325) | 1608 (0 to 26750) | 6272 (1300 to 24000) | 0.03 |
| Age at delivery, years (N=569) | 27.0 (23 to 32) | 28.5 (25 to 34) | 26.0 (22 to 30) | 0.0007 |
| ARV*s during pregnancy: | 0.0001 | |||
| None | 155 (100%) | |||
| ZDV@ monotherapy | 18 (16%) | 134 (42%) | ||
| Dual therapy | 11 (10%) | 55 (17%) | ||
| NNRTI#-based cART* | 32 (28%) | 37 (12%) | ||
| PI$-based cART* | 53 (46%) | 93 (29%) | ||
| Family income, minimum wages& (N=407) | 2 (1 to 3) | 2 (1 to 3) | 2 (1 to 3) | 0.11 |
| Infants | ||||
| Gender, female | 68 (44%) | 55 (48%) | 152 (48%) | 0.69 |
| Year of birth | ||||
| 1996-2000 | 17 (11%) | 10 (9%) | 29 (9%) | 0.94 |
| 2001-2005 | 98 (63%) | 71 (62%) | 197 (62%) | |
| 2006-2010 | 40 (26%) | 33 (29%) | 93 (29%) | |
| Gestational age at birth, weeks (N=422) | 38 (38 to 39) | 38 (37 to 39) | 39 (38 to 39) | 0.12 |
| Prematurity+ (N=428) | 4 (7.7%) | 13 (13.2%) | 36 (12.9%) | 0.55 |
| Small for gestational age (N=432) | 4 (7.1%) | 14 (14.3%) | 16 (5.8%) | 0.03 |
| Birth weight, kg (N=575) | 2.97 (2.61 to 3.29) | 2.92 (2.6 to 3.3) | 3.04 (2.78 to 3.39) | 0.02 |
| Birth length, cm (N=531) | 48 (46 to 50) | 48 (47 to 50) | 49 (47 to 51) | 0.07 |
| Birth WAZ** (N=575) | −0.65 (−1.47 to 0.10) | −0.78 (−1.59 to −0.02) | −0.53 (−1.13 to 0.24) | 0.04 |
| Birth LAZ*** (N=531) | −0.62 (−1.69 to 0.36) | −0.81 (−0.52 to 0.46) | −0.47 (−1.52 to 0.47) | 0.07 |
| Birth WLZ**** (N=531) | −0.41 (−1.30-0.68) | −0.37 (−1.19-0.31) | −0.38 (−1.28-0.64) | 0.65 |
| Length of follow-up, months (N=588) | 12.6 (7.0 to 17.4) | 12.7 (6.6 to 17.5) | 13.3 (8.2 to 17.3) | 0.36 |
| Number of visits, months | ||||
| 0-6 | 690 (26%) | 500 (19%) | 1423 (54%) | 0.10 |
| 7-12 | 333 (26%) | 247 (19%) | 734 (55%) | |
| 13-18 | 156 (26%) | 156 (19%) | 333 (55%) | |
| 19-24 | 72 (23%) | 58 (18%) | 189 (59%) | |
| Number of children in follow-up, months | ||||
| 0-6 | 155 (100%) | 114 (100%) | 319 (100%) | 0.54 |
| 7-12 | 114 (74% ) | 90 (79%) | 257 (81%) | |
| 13-18 | 88 (57%) | 71 (62%) | 202 (63%) | |
| 19-24 | 52 (34%) | 42 (37%) | 133 (42%) |
Median (interquartile ranges) and percentages are shown.
ARV, antiretroviral drugs;
ZDV, zidovudine;
NNRTI, non-nucleoside reverse transcriptase inhibitor;
PI, protease inhibitor;
WAZ, weight-for-age z-score
LAZ, length-for-age z-score
WLH, weight for length z-score
P values from Kruskal Wallis tests (continuous variables) and Fisher exact tests (categorical variables).
1 Brazilian minimum wage = 296 US$ in 2010 (17).
Gestational age<37 weeks at birth
A total of 275 (46.7%) infants were female (Table 1). The median gestational age at birth was 38 weeks in all three groups; however, birth weight was lower in infants exposed to ARV in the first trimester (2.92 kg) compared to infants not exposed to ARV (2.97 kg) or infants exposed in the second or third trimester (3.04 kg; P=0.02), with corresponding differences in WAZ scores (Table 1). Differences in birth length and LAZ scores failed to reach conventional levels of statistical significance (P=0.07). Four children died during follow up, one in the no ARV group, and three in the late ARV group. One child died of presumed meningococcal disease and three children died of pneumonia. One-hundred-eighty-one (30.8%) children were followed for at least two years; the median follow-up was about 13 months in all groups.
Determinants of WAZ, LAZ and WLZ at birth and up to 2 years
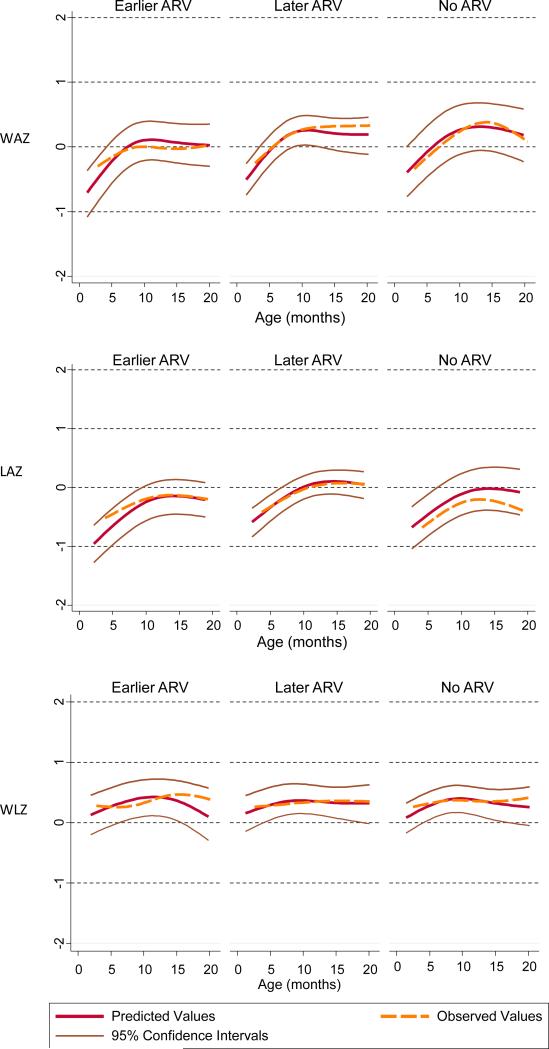
A total of 2107 weights and 2091 lengths were measured up to 2 years of age. Seven weight and 17 length measurements during follow-up resulted in z-scores below −4 or above +4 and were excluded. The median number (interquartile range) of measurements per child was 7.0 (2-13) in the no ARV group, 6.1 (1-14) in the early and 6.2 (1-13) in the late ARV groups (P=0.10). The number of children included in the multivariate analyses ranged from 319 children (54.3%; LAZ at birth) to 359 children (61.1%; WAZ over time). The remaining children were excluded due to missing values. The characteristics of children included in the multivariate analyses were similar to the characteristics of all children. The results for WAZ at birth and its evolution over time are shown in Table 2. The difference in WAZ between infants exposed early in pregnancy and infants exposed later, adjusted for mother's age, CD4 cell count, viral load, calendar year of birth and family income was −0.52 (95% CI-0.99 to −0.04; P=0.02). The corresponding estimate for the evolution of WAZ up to 2 years of age was −0.22 (95% CI −0.47 to 0.04; P=0.10). The evolution of LAZ over time, but not at birth, was less favorable in children exposed to ARVs in the first trimester (Table 3): the adjusted difference compared to infants exposed later in pregnancy was −0.35 (95% CI −0.63 to −0.08; P=0.01). There was a difference at birth for WLZ (adjusted difference −0.40; 96% CI −0.80 to 0.003, P=0.05) but no difference during follow-up (Table 4). The observed and the predicted evolution of WAZ, LAZ and WLZ from birth to age 2 years from the mixed-effects regression models are shown in Figure 1.
Table 2.
Regression analyses of weight-for-age z-scores (WAZ) at birth and their evolution over time by exposure to antiretroviral drugs (ARV).
| Univariable linear regression of WAZ at birth N=575 |
Multivariable linear regression of WAZ at birth* N=359 |
|||
|---|---|---|---|---|
| Difference in WAZ (95%CI**) | P-value | Difference in WAZ (95%CI**) | P-value | |
| ARVs in the first trimester | −0.30 (−0.55 to −0.06) | 0.02 | −0.52 (−0.99 to −0.04) | 0.02 |
| No ARVs during pregnancy | −0.20 (−0.43 to 0.02) | 0.07 | 0.30 (−0.08 to 0.69) | 0.12 |
| ARVs in the second or third trimester | 0 (reference) | - | 0 (reference) | - |
| Univariable mixed-effects regression of WAZ over time (N=582) |
Multivariable mixed-effects regression of WAZ over time* (N=358) |
|||
|---|---|---|---|---|
| Difference in WAZ (95%CI**) | P-value | Difference in WAZ (95%CI**) | P-value | |
| ARVs in the first trimester | −0.17 (−0.40 to 0.06) | 0.15 | −0.22 (−0.47 to 0.04) | 0.10 |
| No ARVs during pregnancy | −0.19 (−0.40 to 0.01) | 0.07 | −0.01 (−0.34 to 0.36) | 0.96 |
| ARVs in the second or third trimester | 0 (reference) | - | 0 (reference) | - |
CI, confidence interval; ARV, antiretroviral drugs; WAZ, weight-for-age z-score
Adjusted for mother's age (younger than 25 years or older), CD4 cell count (square root transformed), viral load (undetectable or detectable), year of birth, and family income. N=358 children
Table 3.
Regression analyses of length-for-age z-scores (LAZ) at birth and their evolution over time by exposure to antiretroviral drugs (ARV).
| Univariable linear regression of LAZ at birth N=531 |
Multivariable linear regression of LAZ at birth* N=319 |
|||
|---|---|---|---|---|
| Difference in LAZ (95%CI**) | P-value | Difference in LAZ (95%CI**) | P-value | |
| ARVs in the first trimester | −0.56 (−0.25 to 0.06) | 0.11 | −0.05 (−0.41 to 0.30) | 0.76 |
| No ARVs during pregnancy | −0.21 (−0.50 to 0.08) | 0.15 | 0.26 (−0.23 to 0.75) | 0.30 |
| ARVs in the second or third trimester | 0 (reference) | - | 0 (reference) | - |
| Univariable mixed-effects regression of LAZ over time (N=577) |
Multivariable mixed-effects regression of LAZ over time (N=356) |
|||
|---|---|---|---|---|
| Difference in LAZ (95%CI**) | P-value | Difference in LAZ (95%CI**) | P-value | |
| ARVs in the first trimester | −0.27 (−0.52 to −0.01) | 0.04 | −0.35 (−0.63 to −0.08) | 0.01 |
| No ARVs during pregnancy | −0.23 (−0.45 to −0.001) | 0.05 | 0.12 (−0.25 to 0.49) | 0.53 |
| ARVs in the second or third trimester | 0 (reference) | - | 0 (reference) | - |
CI, confidence interval; ARV, antiretroviral therapy; LAZ, length-for-age z-score
Adjusted for mother's age (younger than 25 years or older), CD4 cell count (square root transformed), viral load (undetectable or detectable), year of birth, and family income.N=356 children
Table 4.
Regression analyses of weight-for-length z-scores (WLZ) at birth and their evolution over time by exposure to antiretroviral drugs (ARV).
| Univariable linear regression of WLZ at birth N=501 |
Multivariable linear regression of WLZ at birth* N=300 |
|||
|---|---|---|---|---|
| Difference in WLZ (95%CI) | P-value | Difference in WLZ (95%CI) | P-value | |
| ARVs in the first trimester | −0.18 (−0.53 to 0.16) | 0.29 | −0.40 (−0.80 to 0.003) | 0.05 |
| No ARVs during pregnancy | 0.04 (−0.36 to 0.29) | 0.83 | 0.24 (−0.34 to 0.82) | 0.42 |
| ARVs in the second or third trimester | 0 (reference) | - | 0 (reference) | - |
| Univariable mixed-effects regression of WLZ over time (N=577) |
Multivariable mixed-effects regression of WLZ over time (N=340) |
|||
|---|---|---|---|---|
| Difference in WLZ (95%CI) | P-value | Difference in WLZ (95%CI) | P-value | |
| ARVs in the first trimester | 0.01 (−0.19 to 0.22) | 0.91 | 0.03 (−0.20 to 0.26) | 0.80 |
| No ARVs during pregnancy | −0.01 (−0.20 to 0.17) | 0.88 | −0.12 (−0.42 to 0.18) | 0.43 |
| ARVs in the second or third trimester | 0 (reference) | - | 0 (reference) | - |
**CI, confidence interval; ARV, antiretroviral therapy; WLZ, weight-to-length-for-age z-score
Adjusted for mother's age (younger than 25 years or older), CD4 cell count (square root transformed), viral load (undetectable or detectable), year of birth, and family income.N=356 children
Figure 1.
Weight-for-age z-scores (WAZ), length-for-age z-scores (LAZ) and weight-for-length z-scores (WLZ) up to 24 months of life by antiretroviral drug (ARV) exposure during pregnancy: first trimester (earlier ARV), second or third trimester (later ARV) and no ARV.
Results from mixed-effects linear regression models based on 358 (WAZ), 356 (LAZ) and 340 (WLZ) children; splines were introduced with knots at 4.5 and 9.6 months.
The evolution of WAZ and LAZ was similar when we restricted the analysis to children exposed to cART: the WAZ and LAZ differences between infants exposed earlier and later in pregnancy were −0.21 (95% CI −0.50 to 0.09; P=0.17) and −0.31 (95% CI −0.62 to 0.00; P=0.05), respectively. There were no statistically significant differences for WAZ (adjusted difference 0.15, 95% CI −0.61 to 0.92), LAZ (−0.49, 95% CI −1.14 to 0.15), or WLZ (0.53, 95%CI −0.13 to 1.18) between the 85 infants exposed to tenofovir and tenofovir-unexposed infants.
DISCUSSION
In this cohort of HEU newborns in Rio de Janeiro, we found that birth weights were lower among infants exposed to ARVs during the first trimester compared to infants exposed to ARVs later during pregnancy or not exposed in utero. Differences in WAZ scores were statistically significant at birth, even after adjusting for mother's age, CD4 cell count, family income and other potential confounders. The differences in WAZ scores became less important during the first two years of life. In contrast, there was no statistically significant difference in length at birth, but LAZ scores were lower throughout the second year of life in boys and girls exposed to ARVs early in pregnancy, corresponding to a difference in length of 1-2 centimeters at two years.
Previous studies of birth weight and length have generally compared women on cART for their own health with women receiving short-term zidovudine courses later in pregnancy.8,16–18 The time of exposure to ARVs therefore correlated with the CD4 cell count in the mother, and the number of drugs the unborn child was exposed to, which made it difficult to disentangle effects. The study by van der Merwe et al18 is an exception as it examined the effect of cART on the risk of low birth weight in immuno compromised South African women who received cART either early (before 28 weeks of pregnancy) or later in pregnancy. The authors concluded that in utero exposure to cART was not associated with low birth weight; however, the association was also confounded by maternal CD4 counts.18 In contrast, a study from Botswana showed a higher risk of small for gestational age births in mothers who started cART before conception, compared to mothers who started cART during pregnancy.8
Few studies from resource-limited settings have examined the association of in utero exposure to ARVs on growth after birth.9,19,20 A large cohort of over 2,000 HEU infants followed up for 2 years in Tanzania showed that the risk of wasting was increased among HEU infants whose mothers were taking cART during pregnancy, even after adjusting for maternal CD4 cell counts.9 Unfortunately, the CD4 cell counts in mothers and gestational week of starting cART were not reported. A follow-up study of 1,076 children born to mothers enrolled in a PMTCT trial in Thailand found that children exposed to zidovudine for more than 7.5 weeks had slightly lower birth weights (z-score difference −0.08) than children exposed for a shorter period, but evolution of weight from 6 weeks to 18 months of agewas similar in the two groups.19 An analysis of the Mashi and MmaBana PMTCT studies from southern Botswana compared growth in 1,059 HEU children born to women who either received a short course of zidovudine at 34 weeks of gestation or initiated cART between 18 and 34 weeks of gestation.20 Similar to our study the authors found that differences in WAZ disappeared during follow-up whereas LAZ remained lower in cART-exposed infants. Finally, a follow-up of 182 children born to mothers enrolled in the Development of AntiRetroviral Therapy in Africa (DART) trial found little evidence that in utero exposure totenofovir affected growth after 2 years.21 Although other studies, demonstrated lower LAZ and head-circumference-adjusted for age in one year infants in utero exposed to tenofovir,22 and lower WAZ at six months, for infants late in utero (second and third trimester) exposed to tenofovir. 23
Strengths and limitations
The relatively high maternal CD4 counts, which makes confounding by immune status unlikely, and the follow-up to 2 years of age are important strengths of our study. Another strength is that exposure included zidovudine monotherapy, dual therapy and cART and that we could distinguish between first trimester and later trimester exposure to ARVs. Previous studies did not generally examine the time of in utero exposure to ARVs but simply compared exposed with unexposed HEU children,4, 16-18 which may explain some of the discrepant and inconsistent results observed in these studies. Furthermore, follow-up was often relatively short, 6 to 18 months.19-20 Finally, growth trajectories over the first few years of life are meaningful predictors of height in later childhood and of final height.24
Our study has several limitations. We analyzed weight and length measurements taken during routine care rather than measurements taken in a standardized manner for research purposes. Although this is unlikely to have biased our results, it will have introduced additional observer and instrument variability and reduced the power of our study. Also, intervals between visits and duration of follow-up varied, as expected in routine clinical practice, but our statistical models accounted for differences in intervals and loss to follow-up. The differences in growth observed in our study are not proof of a causal relationship between in utero exposure to ARVs and reduced growth. This was an observational study and the characteristics of the groups differed. For example, the children exposed in utero early during pregnancy tended to be exposed to cART, whereas most of the children exposed later were exposed to zidovudine monotherapy or dual therapy.
However, results were similar when excluding PI-based cART. Due to the limited sample size no further stratification was possible. Other variables that could confound associations were well balanced between groups, including gestational age and median family income. Nevertheless we cannot exclude that residual confounding or confounding by variables not measured in this study could have distorted results. For example, we could not adjust for maternal height and weight or tobacco and illicit drug use.
Possible pathways and mechanisms
Several mechanisms that could explain reduced intrauterine and postnatal growth in HEU children exposed to ARVs in utero exist, but further research is needed to clarify their respective role. For example, studies in rhesus macaques exposed in utero to high doses of tenofovir showed reduced levels of insulin-like growth factor-1 (IGF-1) and reduced intrauterine growth.7
Mitochondrial toxicity is another possible pathway.7 In a Canadian cohort of HEU infants exposed to cART in utero plasma lactate levels were increased during the first 6 months of life,25 possibly as a consequence of mitochondrial toxicity that may persist for several years.26 Many pathways of intermediary metabolism, including fatty acid oxidation, organic acid metabolism and amino acid metabolism, depend on normal oxidative phosphorylation and could therefore be affected by mitochondrial toxicity.27 Interestingly, a comparison of acylcarnitine levels in HEU infants exposed and not exposed to ARVs in utero, based on New York state newborn screening data, showed that abnormal acylcarnitine levels were more frequent in ARV-exposed infants.28
Implications and further research
Our study indicates that HEU children who experienced first trimester in utero exposure to ARVs are shorter than their unexposed peers and may face similar problems with social stigma as HIV-infected children and children of short stature in general.29-32 We agree with Owor and colleagues that HEU children should be identified early, their growth should be carefully monitored, and early nutritional interventions should be provided to infants and children with poor growth. Cotrimoxazole prophylaxis in the first year of life and the timely treatment of diarrhea and respiratory infections are also important in this context.33 Finally, in light of the roll-out of the Option B+ strategy for PMTCT, further studies of childhood growth and development, with detailed data on the timing and type of in utero exposure to ARVs, are needed. Collaborative studies combining data from different settings would be a logical next step, for example within the framework of the NICHD International Site Development Initiative (NISDI) in Latin America34 or the International epidemiological Databases to Evaluate AIDS (IeDEA) in sub-Saharan Africa.35
Acknowledgments
Supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Allergy and Infectious Diseases (Grant 2U01AI069924-06) and International epidemiological Databases to Evaluate AIDS (Grant1U01AI069924–01). CBH received funding from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES, Programa Ciências sem Fronteiras, Processo: 8760-13-6, Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro – Jovem Cientista do Nosso Estado – Cristina Barroso Hofer- 2008. This study was also supported by the Ministry of Health/Secretariat of Health Surveillance/National STD and Aids Programme (MOH/SHS/NAP) through cooperative project AD/BRA/03/H34 between the Brazilian Government and the United Nations Office on Drugs and Crime – UNODC.OK was supported by a Swiss National Science fellowship (Ambizione/Prosper Grant 32333B_131629).
Cristina Barroso-Hofer conceived the idea, and was primarily responsible for the analysis and writing. Drs Sepulveda Lustosa, Cisne Frota, Hugo de Oliveira, Abreu, Weber Carvalho and Evangelista Araujo are pediatricians or obstetricians who contributed to data collection, review of drafts, and interpretation of data. Drs Keiser, Zwahlen and Egger contributed to the design of the study, the statistical analyses, writing and interpretation of data.
REFERENCES
- 1.Dao H, Mofenson LM, Ekpini R, et al. International recommendations on antiretroviral drugs for treatment of HIV-infected women and prevention of mother-to-child HIV transmission in resource-limited settings: 2006 update. Am J Obstet Gynecol 2007. 197:S42–55. doi: 10.1016/j.ajog.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach. World Health Organization; Geneva: 2010. [PubMed] [Google Scholar]
- 3.World Health Organization . Recommendations for a public health approach. World Health Organization; Geneva: 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV. [PubMed] [Google Scholar]
- 4.Darak S, Darak T, Kulkarni S, et al. Effect of highly active antiretroviral treatment (HAART) during pregnancy on pregnancy outcomes: experiences from a PMTCT program in western India. AIDS Patient Care STDs. 2013;27:163–70. doi: 10.1089/apc.2012.0401. [DOI] [PubMed] [Google Scholar]
- 5.Ekouevi DK, Coffie PA, Becquet R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Côte d'Ivoire. AIDS Lond Engl. 2008;22:1815–20. doi: 10.1097/QAD.0b013e32830b8ab9. [DOI] [PubMed] [Google Scholar]
- 6.Machado ES, Hofer CB, Costa TT, et al. Pregnancy outcome in women infected with HIV-1 receiving combination antiretroviral therapy before versus after conception. Sex Transm Infect. 2009;85:82–7. doi: 10.1136/sti.2008.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jao J, Abrams EJ. Metabolic Complications of in utero Maternal HIV and Antiretroviral Exposure in HIV-Exposed Infants. Pediatr Infect Dis J. 2013 doi: 10.1097/INF.0000000000000224. doi:10.1097/INF.0000000000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206:1695–705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald CM, Kupka R, Manji KP, et al. Predictors of stunting, wasting and underweight among Tanzanian children born to HIV-infected women. Eur J Clin Nutr. 2012;66:1265–76. doi: 10.1038/ejcn.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recomendações para Profilaxia da Transmissão Vertical do HIV e Terapia Antirretroviral em Gestantes. Ministério da Saúde do Brasil; Brasilia: 2010. [Google Scholar]
- 11.Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R. A simplified method for diagnosis of gestational age in the newborn infant. J Pediatr. 1978;93:120–2. doi: 10.1016/s0022-3476(78)80621-0. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . WHO child growth standards length/height-for-age, weight-forage, weight-for-length, weight-for-height and body mass index-for-age: methods and development. World Health Organization; Geneva: 2006. [20 Mar 2014]. Available at http://www.who.int/childgrowth/standards/Technical_report.pdf. [Google Scholar]
- 13.Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003 Dec 16;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairley L, Petherick ES, Howe LD, et al. Describing differences in weight and length growth trajectories between white and Pakistani infants in the UK: analysis of the Born in Bradford birth cohort study using multilevel linear spline models. Arch Dis Child. 2013;98:274–9. doi: 10.1136/archdischild-2012-302778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira D. Salário minímo de 2014 convertido em dólares é o menor desde 2010. http://extra.globo.com/noticias/economia/salario-minimo-de-2014-convertido-em-dolares-omenor-desde-2010-11153536.html.
- 16.Szyld EG, Warley EM, Freimanis L, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS Lond Engl. 2006;20:2345–53. doi: 10.1097/01.aids.0000253362.01696.9d. [DOI] [PubMed] [Google Scholar]
- 17.Watts DH, Williams PL, Kacanek D, et al. Combination antiretroviral use and preterm birth. J Infect Dis. 2013;207:612–21. doi: 10.1093/infdis/jis728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van der Merwe K, Hoffman R, Black V, Chersich M, Coovadia A, Rees H. Birth outcomes in South African women receiving highly active antiretroviral therapy: a retrospective observational study. J Int AIDS Soc. 2011;14:42. doi: 10.1186/1758-2652-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briand N, Le Coeur S, Traisathit P, et al. Growth of human immunodeficiency virus-uninfected children exposed to perinatal zidovudine for the prevention of mother-to-child human immunodeficiency virus transmission. Pediatr Infect Dis J. 2006;25:325–32. doi: 10.1097/01.inf.0000207398.10466.0d. [DOI] [PubMed] [Google Scholar]
- 20.Powis KM, Smeaton L, Ogwu A, et al. Effects of in utero antiretroviral exposure on longitudinal growth of HIV-exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr 1999. 2011;56:131–8. doi: 10.1097/QAI.0b013e3181ffa4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibb DM, Kizito H, Russell EC, et al. Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial. PLoS Med. 2012;9:e1001217. doi: 10.1371/journal.pmed.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siberry GK, 1, Williams PL, Mendez H, Seage GR, 3rd, Jacobson DL, Hazra R. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26:1151–9. doi: 10.1097/QAD.0b013e328352d135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ransom CE, Huo Y, Patel K, Scott GB, Watts HD, Williams P, Siberry GK, Livingston EG. P1025 Team of the International Maternal Pediatric Adolescent AIDS Clinical Trials Group.Infant growth outcomes after maternal tenofovir disoproxil fumarate use during pregnancy. J Acquir Immune Defic Syndr. 2013;64:374–81. doi: 10.1097/QAI.0b013e3182a7adb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Onis M, Garza C, Onyango AW, Rolland-Cachera M-F. le Comité de nutrition de la Société française de pédiatrie. [WHO growth standards for infants and young children]. Arch Pédiatrie Organe Off Sociéte Fr Pédiatrie. 2009;16:47–53. doi: 10.1016/j.arcped.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Alimenti A, Burdge DR, Ogilvie GS, Money DM, Forbes JC. Lactic acidemia in human immunodeficiency virus-uninfected infants exposed to perinatal antiretroviral therapy. Pediatr Infect Dis J. 2003;22:782–9. doi: 10.1097/01.inf.0000086400.93257.74. [DOI] [PubMed] [Google Scholar]
- 26.Poirier MC, Divi RL, Al-Harthi L, et al. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr 1999. 2003;33:175–83. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 27.McComsey G, Leonard E. Metabolic complications of HIV therapy in children. AIDS. 2004;18:1753–68. doi: 10.1097/00002030-200409030-00004. [DOI] [PubMed] [Google Scholar]
- 28.Kirmse B, Hobbs CV, Peter I, et al. Abnormal newborn screens and acylcarnitines in HIV-exposed and ARV-exposed infants. Pediatr Infect Dis J. 2013;32:146–50. doi: 10.1097/INF.0b013e31827030a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buonora S, Nogueira S, Pone MV, Aloé M, Oliveira RH, Hofer C. Growth parameters in HIV-vertically-infected adolescents on antiretroviral therapy in Rio de Janeiro, Brazil. Ann Trop Paediatr. 2008;28:59–64. doi: 10.1179/146532808X270699. [DOI] [PubMed] [Google Scholar]
- 30.Al-Uzri A, Matheson M, Gipson DS, et al. The impact of short stature on health-related quality of life in children with chronic kidney disease. J Pediatr. 2013;163:736–741. e1. doi: 10.1016/j.jpeds.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noeker M. Psychological functioning in idiopathic short stature. Horm Res Paediatr. 2011;76(Suppl 3):52–6. doi: 10.1159/000330163. [DOI] [PubMed] [Google Scholar]
- 32.Christensen TL, Djurhuus CB, Clayton P, Christiansen JS. An evaluation of the relationship between adult height and health-related quality of life in the general UK population. Clin Endocrinol. 2007 Sep;67(3):407–12. doi: 10.1111/j.1365-2265.2007.02901.x. [DOI] [PubMed] [Google Scholar]
- 33.Owor M, Mwatha A, Donnell D, et al. Long-term follow-up of children in the HIVNET 012 perinatal HIV prevention trial: five-year growth and survival. J Acquir Immune Defic Syndr. 2013;64:464–71. doi: 10.1097/QAI.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazra R, Stoszek SK, Freimanis Hance L, et al. Cohort Profile: NICHD International Site Development Initiative (NISDI): a prospective, observational study of HIV-exposed and HIV-infected children at clinical sites in Latin American and Caribbean countries. Int J Epidemiol. 2009;38:1207–14. doi: 10.1093/ije/dyn239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egger M, Ekouevi DK, Williams C, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41:1256–64. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]