Abstract
Breast cancer is the most frequently diagnosed cancer among women and the second-leading cause of cancer death in United States women. African Americans and other minorities in the United States suffer lower survival and worse prognosis than European Americans despite European Americans having a much higher incidence of the disease. Adherence to breast cancer treatment–quality measures is limited, particularly when the data are stratified by race/ethnicity. We aimed to examine breast cancer incidence and mortality trends in South Carolina by race and explore possible racial disparities in the quality of breast cancer treatment received in South Carolina. African Americans have high rates of mammography and clinical breast exam screenings yet suffer lower survival compared to European Americans. For most treatment-quality metrics, South Carolina fairs well in comparison to the United States as a whole; however, South Carolina hospitals overall lag behind SC COC-accredited hospitals for all measured quality indicators including needle biopsy utilization, breast-conserving surgeries, and timely use of radiation therapy. Accreditation may a have a major role in increasing the standard of care related to breast cancer diagnosis and treatment. These descriptive findings may provide significant insight for future interventions and policies aimed at eliminating racial/ethnic disparities in health outcomes. Further risk-reduction approaches are necessary to reduce minority group mortality rates, especially among African-American women.
Introduction
Breast cancer is the most frequently diagnosed cancer among women and the second-leading cause of cancer death in United States (US) women. The Surveillance, Epidemiology, and End Results (SEER) program estimates that over 230,000 new cases of breast cancer will be diagnosed in 2015, and 40,000 women will die from breast cancer in 2014.[1] However, large racial disparities exist within these statistics. African Americans (AAs) and other minorities in the US suffer lower survival rates and worse prognosis than European Americans (EAs), despite EAs having a much higher incidence of the disease.[2] This may be due to genetic differences among racial/ethnic groups in mammography density, breast cancer grade, estrogen and progesterone receptor status.[3] Additionally, there are racial differences in screening practices, diagnosis and treatment. Health systems are now beginning to focus on standardizing quality measures of care to improve breast cancer outcomes in the nation, which may also help reduce racial/ethnic differences in breast cancer outcomes.
South Carolina (SC) provides a unique opportunity to evaluate racial/ethnic differences in breast cancer treatment, as it has some of the largest health disparities in the US, including a drastic difference in breast cancer mortality between AAs and EAs. Despite higher screening rates among AAs compared to EAs in SC, AAs have a lower incidence rate of breast cancer (119 vs. 124 per 100,000) and a higher mortality rate than EAs (30 vs. 21 per 100,000). Despite the substantial racial differences in breast cancer outcomes, the etiology of these differences is unknown.[4] Many studies have shown that the quality of care received has a significant impact on survival [5-7] and with high adherence to quality measures, more commonly diagnosed high-grade/negative ER status breast cancers in AA women may have better prognosis rates.[8] To help ensure quality care across the board, benchmarks have been established. National benchmarks for breast cancer treatment are designed to evaluate the performance of hospitals nationwide and assess accountability, surveillance and quality improvement. These benchmarks have become more prevalent in the past decade, making it easier for women to be more actively involved in their treatment plans and become aware of the standards set forth by the American College of Surgeons, the National Quality Forum (NQF) and other organizations committed to continuously assessing and enriching the standard of care.
Health systems’ adherence to endorsed quality measures may significantly influence health disparities and outcomes. The goals of this study were to 1) examine breast cancer incidence and mortality trends in SC by race and 2) explore possible racial disparities in the quality of breast cancer treatment received in SC compared to the US as a whole.
Methods
Data Sources
To assess quality measures of screening, diagnosis and treatment, we used established guidelines and benchmarks set by the American Cancer Society (ACS), Healthy People 2020 (HP2020), the National Accreditation Program for Breast Centers (NAPBC), the U.S. Preventive Services Task Force (USPSTF), the NQF, the Cancer Program Practice Profile Reports (CP3R) and the South Carolina Cancer Alliance (SCCA) (Table 1).
Table 1.
Epidemiologic and quality of care measures for breast cancer and associated data sources
| Measures | Calculation | Data Source |
|---|---|---|
| (Increase) Yearly mammography screening (age 40+) | Prevalence of self-reported mammography in the past year for ages 40+ | US: BRFSS 2012 SC: NCI State Cancer Profiles 2011 |
| (Increase) Biennial mammography screening (age 40+) | Prevalence of self-reported mammography in the past 2 years for ages 40+ | US: BRFSS 2012 SC: NCI State Cancer Profiles 2011 |
| (Increase) Biennial mammography screening (age 50–74) | Prevalence of self-reported mammography in the past 2 years for ages 50–74 | US/SC: BRFSS 2012 |
| (Increase) Clinical breast exams (CBEs) for women aged 40+ in the past 3 years | Prevalence of self-reported CBE in the past 3 years for ages 40+ | US/SC: BRFSS 2012 |
| (Reduce) Late-stage breast cancer diagnosis rate | Percent of women aged 18+ with regional/distant breast cancer diagnosis | SC: CCR 2007–2011 |
| (Increase) Needle biopsy utilization for breast cancer diagnosis | Percent of women 18+ with first primary breast cancer who received needle biopsy prior to surgical excision/resection for the purpose of breast cancer diagnosis Includes: 18+ at time of diagnosis, known/assumed first or only cancer diagnosis, primary tumors of breast, epithelial invasive malignancy only, surgically treated, diagnosis and all/part of first-course treatment performed at reporting facility |
US/ SC: CP3R SC: CCR 2007–2011 |
| (Increase) Breast-conserving surgery (BCS) and radiation therapy within 1 year of diagnosis (age <70) | Percent of women under age 70 with first primary breast cancer who received BCS and underwent radiation therapy within 1 year of diagnosis Includes: Age 18–69 at time of diagnosis, known/assumed to be first or only cancer diagnosis, primary tumors of breast, epithelial malignancy only, AJCC Stage I–III, surgical treatment by BCS, all/part of first-course treatment performed at reporting facility, known to be alive w/in 1 year of diagnosis |
US/ SC: CP3R SC: CCR 2007–2011 |
| (Increase) Negative breast cancer and chemotherapy administered within 4 months of diagnosis (age <70) | Percent of women with AJCC Stage IC–IIIC hormone receptor–negative breast cancer under age 70 with first primary breast cancer who received combination chemotherapy within 4 months of diagnosis Includes: Age 18–69 at time of diagnosis, known/assumed first or only cancer diagnosis, primary tumors of breast, epithelial invasive malignancy only, AJCC T1cN0M0 or Stage II–III, primary tumor is estrogen receptor–negative and progesterone receptor–negative, all/part of first-course treatment performed at reporting facility, known to be alive w/in 4 months of diagnosis |
US/SC: CP3R SC: CCR 2007–2011 |
| (Increase) Positive breast cancer and tamoxifen/aromatase inhibitor administered within 12 months of diagnosis (age <70) | Percent of women with AJCC Stage IC–IIIC hormone receptor–positive breast cancer aged 18+ who were recommended and/or administered tamoxifen or aromatase inhibitor within 12 months of diagnosis Includes: All female patients age 18+ with Stage IC–IIIC, estrogen receptor– or progesterone receptor–positive breast cancer, age 18–69 at time of diagnosis |
US/SC: CP3R SC: CCR 2007–2011 |
| (Increase) Percentage of patients who are treated with BCS | BCS rate for women with AJCC clinical stage 0, I or II breast cancer Includes: Age 18–69 at time of diagnosis, known/assumed to be first or only cancer diagnosis, primary tumors of breast, epithelial malignancy only, AJCC Stage 0–II |
US/SC: CP3R |
| (Reduce) Age-adjusted invasive breast cancer incidence rate | Age-adjusted breast cancer incidence rate adjusted to the 2000 US standard population | US/ SC: NCI State Cancer Profiles 2007-2011 SC: CCR 2007-2011 |
| (Reduce) Age-adjusted breast cancer death rate | Age-adjusted breast cancer death rate adjusted to the 2000 US standard population | US/ SC: NCI State Cancer Profiles 2007-2011 SC: CCR 2007-2011 |
SCCCR: Central Cancer Registry
AJCC: American Joint Committee on Cancer
BRFSS: Behavioral Risk Factor Surveillance System
NCI: National Cancer Institute
CP3R: Cancer Program Practice Profile Reports
Epidemiologic data on cancer incidence and mortality rates were obtained from the National Cancer Institute (NCI) State Cancer Profiles, which characterize information about screening, risk factors, incidence, prevalence and mortality related to breast cancer at the state level. County-level cancer registry data by race were obtained from the SC Central Cancer Registry (SCCCR). Mammography and clinical breast exam (CBE) utilization data were obtained from the Behavioral Risk Factor Surveillance System (BRFSS), a random-digit-dial telephone survey, and were used to examine and compare the prevalence of breast cancer screenings (mammography and CBEs) among women in SC and the US by race.[9]
Clinical data on needle biopsy use and treatment quality were obtained from Commission on Cancer (CoC)-accredited programs, which report their quality indicators via the CP3R, and from the SCCCR for combined CoC and non-CoC-accredited facilities in SC. The CoC serves more than 70% of cancer patients in the US; SC currently houses 17 CoC-accredited hospitals.
All rates and descriptive statistics were compared with their appropriate benchmarks to provide insight on areas that need further improvement in SC (see Table 1 for calculations). Descriptive statistics were calculated using SAS version 9.3; SAS survey procedures were used for all frequencies related to mammography and CBE utilization because of the complex survey design of the BRFSS.
Results
Incidence and Mortality
The annual incidence rate of female breast cancer in SC for all ages was 123.0 per 100,000. Statewide, the incidence rate for AAs was 118.5 per 100,000 women and the rate for EAs was 124.0 per 100,000 women. A paradoxical relationship exists between incidence and mortality rates in EA and AA populations. Despite a lower incidence of breast cancer, death rates for AA women were much higher across counties and overall (22.3–46.2 vs. 29.8 per 100,000 overall) than those for EA women (15.6–32.0 vs. 21.3 per 100,000 overall; Figure 1). In fact, we observed 11 counties with higher age-adjusted annual death rates for AA women (32.2– 46.2) than the highest county-level death rate for EA women (32.0 deaths per 100,000). Both EA and AA women have higher breast cancer death rates in SC than recommended by HP2020 (Objective C-3: 20.7 per 100,000 women). Figure 1 depicts the current discrepancies in breast cancer incidence and mortality among EA and AA women by SC county. Table 2 also shows a comparison of SC to the US, which follows a similar pattern of disease by race.
Figure 1.
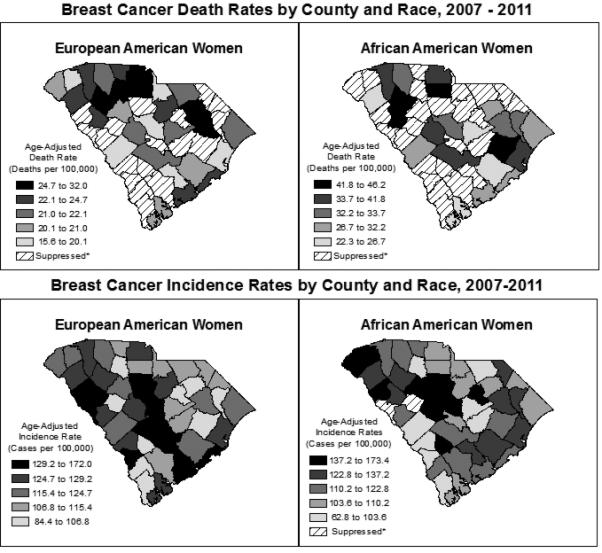
Breast cancer incidence rates by county and race, 2007–2011
Table 2.
Epidemiologic and quality of care metrics for breast cancer in the US and SC, by race
| United States (US) | South Carolina (SC) | Target | |||||
|---|---|---|---|---|---|---|---|
| All | White | Black | All | White | Black | ||
| Yearly Mammography screening (40+) |
69.0% (68.5, 69.4) n=125,841 |
68.8% (68.3, 69.2) n=106,331 |
72.0% (70.5, 73.5) n=12,677 |
64.8% (62.8, 66.7) n=3,496 |
64.3% (62.0, 66.6) n=2,322 |
67.0% (63.3, 70.8) n=1,101 |
N/A |
| Biennial Mammography screening (40+) |
74.3 % (73.9, 74.7) n=158,517 |
74.1 % (73.7, 74.6) n=133,920 |
77.3 % (76.0, 78.6) n=15,725 |
71.4 % (69.6, 73.1) n=4,520 |
70.0 % (67.9, 72.1) n=3,003 |
76.8 % (73.6, 79.7) n=1,412 |
N/A |
| Biennial Mammography screening (50- 74) |
78.7% (78.2, 79.2) n=104,328 |
78.3 % (77.8, 78.8) n=87,948 |
82.2 % (80.6, 83.6) n=10,755 |
75.7 % (73.6, 77.7) n=3,022 |
73.9 % (71.3, 76.3) n=1,596 |
81.9 % (78.3, 85.0) n=984 |
HP2020: 81.1% |
| Clinical Breast Exam (CBE) for women age 40+ in three years |
65.4% (64.9, 65.8) n=124,871 |
64.4% (64.0, 64.9) n=105,735 |
71.9% (70.5, 73.3) n=12,419 |
60.7% (58.8, 62.6) n=3,373 |
59.4% (57.1, 61.6) n=2,279 |
66.0% (62.3, 69.7) n=1,022 |
SCCA: 75.0% |
| Late-stage breast cancer diagnosis rate |
N/A | N/A | N/A | SCCCR: N/A |
SCCCR: 35.0% |
SCCCR: 46.5% |
HP2020: 38.9 per 100,000 |
| Image or palpitation guided- needle biopsy |
CoC: 82.9% (82.7-83.1) n=1341 |
N/A | N/A | SCCCR: 75.5 % CoC: 87.8% (86.5-89.1) |
SCCCR: 76.8 % |
SCCCR: 71.3% |
SCCA: 90.0% |
| Breast- conserving surgery (BCS) and radiation therapy within 1 year of diagnosis (Age < 70) |
CoC: 92.3% (92.1-92.5) n=1326 |
N/A | N/A | SCCCR: 71.1% CoC: 94.8% (93.3-98.7) |
SCCCR: 73.6% |
SCCCR: 64.6% |
SCCA: 73.5% |
| Negative breast cancer and chemo administered within 4 months of diagnosis (Age <70) |
CoC: 92.8% (92.4-93.2) n=1256 |
N/A | N/A | SCCCR: 76.0% CoC: 97.4% (96.3-99.5) |
SCCCR: 76.7% |
SCCCR: 75.3% |
SCCA: 97.2 % |
| Positive breast cancer and tamoxifen/ aromatase inhibitor within 12 months of diagnosis (Age <70) |
CoC: 91.1% (90.9-91.3) n=1331 |
N/A | N/A | SCCCR: 55.8%8 CoC: 92.3% (90.7-93.9) |
SCCCR: 57.0% |
SCCCR: 51.3% |
SCCA: 90.0% |
| Percentage of patients who are treated with BCS |
CoC: 64.0% (63.7-64.3) n=1328 |
N/A | N/A | SCCCR: 62.5% CoC: 64.8% (62.8-66.8) |
SCCCR: 63.0% |
SCCCR: 61.0% |
NAPBC: 50.0% |
| (Reduce) Age- Adjusted Invasive Breast Cancer Incidence Rate |
124.6 per 100,000 |
127.3 per 100,000 |
118.4 per 100,000 |
123 per 100,000 |
124.0 per 100,000 |
118.5 per 100,000 |
N/A |
| (Reduce) Age- Adjusted Breast Cancer Death Rates |
22.6 per 100,000 |
22.7 per 100,000 |
30.8 per 100,000 |
24.0 per 100,000 |
21.3 per 100,000 |
29.8 per 100,000 |
HP 2020: 20.7 per 100,000 |
*Whites and Blacks in these categories include Hispanic populations
*Bolded items signify lower adherence than the target
SCCA: South Carolina Cancer Alliance
HP 2020: Health People 2020
NAPBC: National Accreditation Program for Breast Centers
CoC: Commission on Cancer- accredited cancer programs nationwide
SCCCR: South Carolina Central Cancer Registry
Screening and Diagnosis
Mammogram screening utilization varies between counties and by race. In SC, as in the US, EAs are less likely to have a mammogram and/or CBE than AAs. According to the ACS, women should have yearly mammogram screenings to increase their survival if breast cancer is detected. SC and the US both have less than 70% adherence to this recommendation, and bi-yearly mammogram rates for women ages 50–74 do not reach the recommended HP2020 goal (81.1%). In SC and the US, EAs aged 50–74 fell short of the HP2020 goal, with 74% and 78% adherence, respectively. AA women aged 50–74 in SC (81.9%) and the US (82.2%) achieved the HP2020 benchmark of 81.1% adherence.
The percentage of cases from CoC-accredited programs in SC using image or palpation-guided needle biopsy to establish a diagnosis of breast cancer (87.8%) is higher than in US CoC-accredited programs (82.9%) and in SC hospitals overall (i.e., including both COC- and non–COC-accredited facilities; 75.45%). AA women are less likely to be diagnosed using needle biopsy than EA women (71.3% vs. 76.8%, p<0.0001).
Despite higher rates of breast cancer screening, AAs in SC are much more likely than EAs to be diagnosed with late-stage breast cancer (47% vs. 35%) and EAs have significantly higher odds of early stage cancer diagnosis than AAs, which improves their long-term probability of prognosis and survival. HP2020 aims to reduce late-stage female breast cancer diagnosis from 40.9 to 38.9 new cases per 100,000 to improve the quality of life of US citizens. SC does not currently reach the HP2020 goal, for either EA or AA women.
Treatment
SC hospitals overall have a slightly lower percentage of breast-conserving surgeries performed among women with non-metastatic breast cancer compared to CoC-accredited programs in the state (62.5% vs. 64.8%) and in the US (64.0%); these rates are all above the NAPBC goal of at least 50% surgical resection. However, for timely receipt of breast-conserving surgery and radiation therapy within 1 year of diagnosis, SC hospitals overall (71.1%) fall short of the rates seen in CoC-accredited programs in the state (94.8%) and nationwide (92.3%), particularly for AA women (64.6%).
Adherence to national quality measures varies by hormone receptor type and accreditation status. Overall, the percentage of women under the age of 70 with hormone receptor–negative breast cancer who received chemotherapy within 4 months of diagnosis is similar among SC and US CoC-accredited hospitals but varies for SC hospitals overall (97.4%, 92.8% and 76.0%, respectively). Similarly, women with hormone receptor–positive breast cancer were more likely to be recommended and/or administered tamoxifen or aromatase inhibitor within 12 months of diagnosis in SC and US CoC-accredited hospitals. US and SC CoC-accredited hospitals (91.1% and 92.3%) performed substantially better than SC hospitals overall in this treatment measure (55.8%).
Discussion
The US population faces many challenges in eliminating health disparities, achieving health equity and improving the health of all groups.[10] With a growing proportion of minority populations in the US, it is imperative that the main impairments to better health outcomes among these groups be identified and overcome. An important tool to foster quality improvement in healthcare and elevate the standard of care for all groups is the use of quality measures.[11] Disparities in health are noticeable geographically, biologically and by socioeconomic status. Socioeconomic deprivation may be a significant contributor to the increased risk of breast cancer mortality in AA and Hispanic patients.[12]
Despite high rates of AAs being screened for breast cancer and undergoing CBEs in SC, these women are less likely than EA women to be diagnosed with breast cancer at early stages and to survive after a cancer diagnosis. This may suggest that in addition to screening, other important risk-reduction approaches for minority groups must be identified, including considering more frequent or earlier ages of screening initiation. Important contributors to racial differences among AA and EA women include stage at diagnosis, tumor characteristics, and BMI.[13]
US and SC CoC-accredited programs provide the proper care for women with both hormone receptor–positive and hormone receptor–negative breast cancers more than 90% of the time. However, SC hospitals overall may have difficulty adhering to the treatment guidelines for these two types of breast cancers. Approximately 25% of women do not receive chemotherapy within 4 months of diagnosis of hormone receptor–negative breast cancer, and 50% do not receive the appropriate hormone inhibitor within 12 months of a hormone receptor– positive breast cancer diagnosis. Low adherence to guidelines may be due to a myriad of factors such as acceptance of breast cancer diagnosis, access to care, insurance, delays in care, and poor communication between patient and physician.[14-16]
Racial disparities exist for both hormone receptor-negative and –positive tumors. AAs are more likely to have hormone receptor–negative tumors, which are associated with worse prognosis.[17] This trend may account for a portion of the lower survival rates among AA women. Particularly because of high mortality rates in minority populations, hospitals need to focus on increasing adherence to chemotherapy within 4 months of diagnosis for women with hormone receptor–negative breast cancer. Smaller racial disparities in treatment adherence are seen among EA and AA women with hormone receptor-negative breast cancer (76.7% vs. 75.3%) compared to the statistically significant difference seen among EA and AA women with hormone receptor–positive breast cancer (57.0% vs. 51.3%). Researchers have shown AAs with ER-positive breast cancers were more than twice as likely to die compared to EAs in the first 2 years of diagnosis.[13] Moreover, treatment rates are significantly lower among non-CoC-accredited SC hospitals overall, a fact that requires additional attention. Further research needs to be done to understand these hormone receptor-negative and -positive tumor disparities.
Patients should be informed and thus empowered with the tools to assess their quality of care from diagnosis to survivorship. Many patients presenting with breast cancer do not undergo needle biopsy to establish a diagnosis of breast cancer in SC and the US. Data reporting for needle biopsy utilization prior to breast-conserving surgery is limited. In other studies, race/ethnicity has been a predictor of needle biopsy use. Specifically, minorities are less likely to undergo needle biopsy for breast cancer diagnosis.[18] In SC hospitals overall, EA women are more likely to undergo needle biopsy compared to AA women (76.8% vs. 71.3%). Unless there is a documented reason for open surgical biopsy, 90% of patients should be diagnosed using needle biopsy.[19] Needle biopsy is a high-quality and less-costly diagnostic procedure that can provide a basis for the proper course of treatment with a low likelihood of harm to the patient.
Strengths and Limitations
A major strength of our study includes the rich source of information used to evaluate the performance of health systems vis-à-vis one another and improving healthcare quality for all groups.[11] We included a variety of sources from SC and the US, which adds to the limited amount of research available on quality of care among racial/ethnic groups. Further studies need to compare data from CoC-accredited hospitals in the registry to data from the same CoC-accredited SC hospitals in the national cancer database in order to better understand and address the issue at hand.
An important limitation for measures over an extended time period, such as breast-conserving surgery, is that most of the data sent by larger facilities to the central registry is collected at the ~6-month mark post-surgery, after which extensive deduplication, quality control and quality-control follow up are done. Therefore, SCCCR may have data from before treatment was started and/or completed, even if this treatment was already planned. The numbers for all SC hospitals could reflect missing treatment dates that took place after data submission and do not necessarily indicate a difference in actual treatment.
Conclusion
With proper considerations of quality measures, patients become the focal point of cancer care and can improve a multitude of facets related to their care. These standards for best practices can serve as the foundation for quality improvement. To eliminate health disparities in SC and the US, risk-reduction approaches for minority groups and non–COC-accredited hospitals must be identified. Much research is focused on health outcomes overall, but disparities in health outcomes among AAs despite high rates of mammography screenings and CBEs in the state emphasize the importance of additional research in the field of racial/ethnic groups. Future prevention efforts need to focus on providing racial/ethnic guidelines to improve the overall health of the population.
Acknowledgments
Conflict of Interest: Marsha Samson's participation in this research was supported in part by research training grant T32-GM081740 from the National Institutes of Health, National Institute of General Medical Sciences. SA Adams and JM Eberth were supported in part by grant # R15 CA179355 from the National Institutes of Health, National Cancer Institute.
References
- 1.Surveillance Epidemiology and End Results Program. SEER stat fact sheets: breast cancer. 2012 [Google Scholar]
- 2.Du W, Simon MS. Racial disparities in treatment and survival of women with stage I-III breast cancer at a large academic medical center in metropolitan Detroit. Breast Cancer Res Treat. 2005;91(3):243–248. doi: 10.1007/s10549-005-0324-9. [DOI] [PubMed] [Google Scholar]
- 3.Amend K, Hicks D, Ambrosone CB. Breast cancer in African-American women: differences in tumor biology from European-American women. Cancer Res. 2006;66(17):8327–8330. doi: 10.1158/0008-5472.CAN-06-1927. [DOI] [PubMed] [Google Scholar]
- 4.Smith ER, Adams SA, Das IP, et al. Breast cancer survival among economically disadvantaged women: the influences of delayed diagnosis and treatment on mortality. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2882–2890. doi: 10.1158/1055-9965.EPI-08-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider EC, Zaslavsky AM, Epstein AM. Racial disparities in the quality of care for enrollees in medicare managed care. JAMA. 2002;287(10):1288–1294. doi: 10.1001/jama.287.10.1288. [DOI] [PubMed] [Google Scholar]
- 6.Mandelblatt JS, Edge SB, Meropol NJ, et al. Predictors of long-term outcomes in older breast cancer survivors: perceptions versus patterns of care. J Clin Oncol. 2003;21(5):855–863. doi: 10.1200/JCO.2003.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Nat Cancer Inst. 2005;97(6):439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System. 2015 Available at: http://www.cdc.gov/brfss/
- 10.US Department of Health and Human Services, Office of Disease Prevention and Health Promotion HealthyPeople.gov, Healthy People 2020, Foundation Measures, Disparities. 2014 HealthyPeople.gov Available at: http://www.healthypeople.gov/2020/about/foundation-health-measures/Disparities.
- 11.Families USA. Issue brief: measuring health care quality: an overview of quality measures. 2014 Available at: http://familiesusa.org/sites/default/files/product_documents/HSI%20Quality%20Measurement_Brief_final_web.pdf.
- 12.Maskarinec G, Sen C, Koga K, et al. Ethnic differences in breast cancer survival: status and determinants. Womens Health (Lond Engl) 2011;7(6):677–687. doi: 10.2217/whe.11.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warner ET, Tamimi RM, Hughes ME, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33(20):2254–2261. doi: 10.1200/JCO.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams SA, Smith ER, Hardin J, et al. Racial differences in follow-up of abnormal mammography findings among economically disadvantaged women. Cancer. 2009;115(24):5788–5797. doi: 10.1002/cncr.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams SA, Hébert JR, Bolick S, et al. Breast cancer disparities in South Carolina: early detection, special programs, and descriptive epidemiology. J SC Med Assoc. 2006;102(7):231–239. [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23(Suppl 6):vi7–12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 18.Eberth JM, Xu Y, Smith GL, et al. Surgeon influence on use of needle biopsy in patients with breast cancer: a national medicare study. J Clin Oncol. 2014;32(21):2206–2216. doi: 10.1200/JCO.2013.52.8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Surgeons 2013 Breast Center Standards Manual. 2013 Available at: https://www.facs.org/~/media/files/quality%20programs/cancer/napbc/2013standardsmanual.ashx.


