Abstract
Stable neuropsychological deficits may provide a reliable basis for identifying etiological subtypes of schizophrenia. The aim of this study was to identify clusters of individuals with schizophrenia based on dimensions of neuropsychological performance, and to characterize their neural correlates. We acquired neuropsychological data as well as structural and functional magnetic resonance imaging from 129 patients with schizophrenia and 165 healthy controls. We derived eight cognitive dimensions and subsequently applied a cluster analysis to identify possible schizophrenia subtypes. Analyses suggested the following four cognitive clusters of schizophrenia: (1) Diminished Verbal Fluency, (2) Diminished Verbal Memory and Poor Motor Control, (3) Diminished Face Memory and Slowed Processing, and (4) Diminished Intellectual Function. The clusters were characterized by a specific pattern of structural brain changes in areas such as Wernicke's area, lingual gyrus and occipital face area, and hippocampus as well as differences in working memory-elicited neural activity in several fronto-parietal brain regions. Separable measures of cognitive function appear to provide a method for deriving cognitive subtypes meaningfully related to brain structure and function. Because the present study identified brain-based neural correlates of the cognitive clusters, the proposed groups of individuals with schizophrenia have some external validity.
Keywords: Neuropsychological performance, Cluster analysis, Neural correlates, Cortical thickness, Subcortical volume, Working memory, Schizophrenia, Cognitive subtypes
1. Introduction
Despite recent advances in our understanding of the pathophysiology of schizophrenia, the heterogeneity of the illness limits the effectiveness of clinical and biological research to elucidate causes of the disorder. The extraordinary variability of clinical symptoms and cognitive deficits within schizophrenia likely reflects different etiological factors at play. Such variability impedes the search for underlying neurobiological mechanisms of the disorder. Phenotype refinement through classifying individuals into more homogeneous subgroups has been a successful approach in complex disorders such as Parkinson's disease (Dekker et al., 2003) and familial Alzheimer disease (Scott et al., 2003), and it could be a fruitful means of gaining insight into specific and causal pathological processes in schizophrenia.
To address the problem of heterogeneity in schizophrenia, various attempts have been made to define subtypes based on clinical characteristics. About a century ago, Kraepelin defined nine different forms of dementia praecox and Bleuler, who introduced the term “schizophrenia”, spoke of multiple “schizophrenias”. This tradition was continued by Leonhard (1999), by Schneider (1959) and more recently by Crow (1985) and Carpenter (1988), who described schizophrenia subtypes (or groups of symptoms) based on various clinical characteristics.
Although there is a long tradition of using clinical features to define subtypes of schizophrenia, this approach has been criticized repeatedly (Andreasen et al., 1997; Goldberg and Weinberger, 1995) because of a lack of strong theoretical background and the relative absence of neurobiological correlates (Berrios, 1985; Sommers, 1985; Tandon and Greden, 1991; Peralta et al., 1995) as well as the temporal instability of clinical symptoms and their corresponding subtypes. In particular, psychotic symptoms and disorganization have been observed to be highly variable across time (Arndt et al., 1995). Marneros et al. (1992) found that subtypes defined according to four different diagnostic systems (among them DSM-III-R and ICD-10) were markedly unstable and patients frequently changed between subgroups within a 5-year period.
A central aspect of schizophrenia is marked cognitive impairment that is evident across domains measured by standard neuropsychological tests. Meta-analyses have demonstrated that cognitive measures reliably distinguish a majority of schizophrenia patients from healthy controls (Heinrichs and Zakzanis, 1998; Heinrichs, 2005). In addition, cognitive deficits are regarded as the single strongest correlate of real world functioning (Green, 1996). In contrast to the aforementioned clinical characteristics, cognitive and executive functioning has been shown to be remarkably stable over time (Hoff et al., 1999; Heaton et al., 2001), with similar deficits observed during the first episode of psychosis and through the chronic course of the disorder (Sponheim et al., 2010). Furthermore, deficits in multiple cognitive domains seem to predate the onset of clinical symptoms (Lencz et al., 2006; Seidman et al., 2010), and a review over 65 studies (Torrey, 2002) confirmed that neuropsychological impairments are also observable in medication-naïve patients.
Given the trait-like characteristics of cognitive deficits in schizophrenia, they have been used by researchers as intermediate phenotypes in genetic studies (Egan et al., 2001; Bertisch et al., 2010) and seem to be reasonable candidates for delineating clusters of patients with separable cognitive profiles. The study of how subsets of individuals with schizophrenia may cluster together in their pattern of cognitive deficits has identified plausible subtypes of dysfunction. Despite differences in the choice of underlying neuropsychological test, several authors reported four-cluster solutions (Goldstein and Shemansky, 1995; Sautter et al., 1995;Goldstein et al., 1998; Seaton et al., 1999; Allen et al., 2000; Hill et al., 2002) beside an unifactorial solution (Keefe et al., 2006).
Goldstein compared two cluster-analytic approaches in the same relatively large sample - one based on an abstraction battery and another one based on a variety of cognitive abilities. Although different sets of cognitive measures for classification were used, both analyses resulted in four-cluster solutions with strikingly similar characteristics. In a more recent study, Hill et al. (2002) administered a more comprehensive neuropsychological battery, including multiple measures of memory, attention, language, and sensory performance. As with previous studies, cluster analysis identified four neurocognitive clusters. Of note, the identified cognitive clusters of schizophrenia patients did have common elements across studies. First, a cluster of patients with relatively intact cognitive functioning was evident across studies (e.g., second cluster in Goldstein et al., 1998). Second, a cluster of patients with impairment in generalized cognitive functioning was also identified in each of the studies (e.g., fourth cluster in Goldstein and Shemansky, 1995). Third, studies tended to yield a cluster of patients showing impaired motor function as well as poor verbal memory performance (e.g., fourth cluster in Seaton et al., 1999). Finally, although there was some variation, investigations tended to identify a cluster of patients with deficits in nonverbal skills and abstraction/attention/executive functions (e.g., fourth cluster in Hill et al., 2002).
Additionally, studies have provided preliminary evidence supporting the longitudinal stability of cognitive clusters (Heinrichs and Awad, 1993; Heinrichs et al., 1997). Nevertheless, no studies have attempted to validate the identified cognitive clusters in schizophrenia with brain-based correlates by relating subtypes to possible forms of pathophysiology.
Cognitive deficits have been repeatedly associated with reductions of grey matter density and volumes of frontal and temporal lobe structures commonly found in schizophrenia patients (for a review, see Antonova et al., 2004). Due to the putative pathogenetic neurodevelopmental mechanisms proposed to underlie schizophrenia (Weinberger, 1987; Rapoport et al., 2005) cortical thickness may be of even greater etiologic relevance than grey matter volume or density. Cortical thickness measures have been shown to be heritable (Goghari et al., 2007; Gogtay et al., 2007; Goldman et al., 2009; Winkler et al., 2010), suggesting that this aspect of cortical anatomy may represent a reliable intermediate phenotype for schizophrenia (Gottesman and Gould, 2003). Furthermore family studies using structural magnetic resonance imaging (sMRI) studies indicate that, at least for genetic imaging studies, cortical thickness and surface area should be considered separately (while volume is a combination of thickness and surface parameters) since they have different genetic determinants (Panizzon et al., 2009; Winkler et al., 2010). In line with previous work, we recently reported marked reductions of cortical thickness in patients with schizophrenia, as well as circumscribed associations between cortical thickness and cognitive deficits (Hartberg et al., 2010; Ehrlich et al., (2012a)).
Similarly, relationships between cognitive deficits and aberrant neural activity have been documented. Due to the well-replicated deficits in working memory functioning in schizophrenia, this aspect of cognitive dysfunction has attracted particular attention. In contrast to matched healthy controls, schizophrenia patients were shown to recruit more neural resources in prefrontal and parietal brain regions (hyperactivity) at low levels of task difficulty but decreased neural activity (hypoactivity) when task difficulty increased (Manoach et al., 1999; Callicott et al., 2003; Karlsgodt et al., 2007; Potkin et al., 2009). This pattern (which is based on an inverted U-shaped relationship between BOLD response and task difficulty that is shifted in schizophrenia compared with healthy controls) has been termed “neural inefficiency” (Manoach et al., 1999).
Given the evidence for cognitive clusters in schizophrenia and the likely relationships between cognitive deficits and aspects of neural anatomy and function, the aim of this study was to identify clusters of schizophrenia based on their neuropsychological performance and to characterize their structural and functional neural correlates.
2. Methods
2.1. Participants
The Mind Clinical Imaging Consortium (MCIC) study of schizophrenia (Ehrlich et al., 2010; White et al., 2011; Gollub et al., 2013) obtained structural and functional MRI scans on a total of 378 subjects from four participating sites: Massachusetts General Hospital in Boston (MGH) and the Universities of Iowa (UI), Minnesota (UMN) and New Mexico (UNM). After complete description of the study to the participants, written informed consent was obtained. The institutional review boards (IRBs) at each of the four sites approved the study protocol. The patient group included subjects with a DSM-IV diagnosis of schizophrenia, established through administration of structured clinical interviews and review of case files by trained clinicians (see Section 2.2). Healthy controls were included if they had no history of a medical or Axis I psychiatric diagnosis. For further information, e.g,. regarding exclusion criteria, see Supplementary Materials (SM) 1.1. and Ehrlich et al. (2012a). The final sample with complete and acceptable sMRI scans comprised 165 healthy controls and 129 patients. Complete functional MRI (fMRI) data were available for 155 healthy controls and 118 patients. For quality assurance procedures, see below and Sponheim et al. (2010).
2.2. Clinical measures
All study participants underwent an extensive clinical diagnostic assessment that included either a Structured Clinical Interview for DSM disorders (SCID-I/P or NP) (First et al., 2002) or the Comprehensive Assessment of Symptoms and History (CASH (Andreasen et al., 1992)). Severity of positive and negative symptoms was rated using the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983) and the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984). Depressive symptoms were measured with the Calgary Depression Scale for Schizophrenia (CDS) (Addington et al., 1992). Premorbid cognitive achievement was estimated based on the Wide Range Achievement Test (WRAT3 (Wilkinson, 1993)). Parental socioeconomic status (SES) was determined using the Hollingshead index (Hollingshead, 1965). Handedness was measured using the Annett Scale of Hand Preference (Annett, 1970). For further information regarding clinical measures and antipsychotic exposure, see SM 1.2.
Extrapyramidal symptoms were evaluated using the Simpson-Angus Scale (SAS) (Simpson and Angus, 1970) and the Barnes Akathisia Scale (BAS) (Barnes, 1989).
2.3. Neuropsychological measures
For the neuropsychological assessment, instruments were chosen to sample a wide range of functions (for details, see Sponheim et al., 2010). For the current analysis, we chose 18 scales representative of key cognitive functions which are thought to be affected in patients with schizophrenia. See Table 1 for a detailed overview of the 18 scales. The neuropsychological measurements were obtained for all patients and 138 of 165 healthy controls.
Table 1.
Neuropsychological indices used to derive components characterizing cognitive functioning.
| Neuropsychological Index | Test Name | Cognitive Domain | Reference | |
|---|---|---|---|---|
| 1. | Letter Number Sequencing (Total Raw Score) | Wechsler Adult Intelligence Scale (WAIS-III): Letter Number Sequencing, Similarities, Vocabulary, Block Design | Working Memory, Verbal Abstraction, Verbal Knowledge, Spatial Reasoning and Problem Solving | (Wechsler, 1997a) |
| 2. | Similarities (Total Raw Score) | |||
| 3. | Vocabulary (Total Raw Score) | |||
| 4. | Block Design (Total Raw Score) | |||
|
| ||||
| 5. | FAS Items (Total Raw Score) | The Delis–Kaplan Executive Function System (D-KEFS) Verbal Fluency Test: FAS | Verbal Fluency | (Delis et al., 2001) |
|
| ||||
| 6. | Word List Total Items Recalled – Immediate (Raw Score) | Hopkins Verbal Learning Test (HVLT): Immediate/Hopkins Verbal Learning Test (HVLT): Delay | Verbal Learning and Memory (word lists) | (Brandt, 1991) |
| 7. | Word List Total False Alarms – Delay (Raw Score) [log-transformed] | |||
|
| ||||
| 8. | Benton Total Correct Score | Benton Visual Retention Test (BVRT) | Visual Memory and Visual Construction | (Benton, 1962) |
|
| ||||
| 9. | Face Memory Recognition - Immediate (Total Raw Score) | Wechsler Memory Scale-3 (WMS-3): Faces I Wechsler Memory Scale-3 (WMS-3): Faces II-Delay | Visual Learning and Memory | (Wechsler, 1997b) |
| 10. | Face Memory Recognition - Delay (Total Raw Score) | |||
|
| ||||
| 11. | Story Recall -Immediate (Total Raw Score) | Wechsler Memory Scale-3 (WMS-3): Logical Memory I Wechsler Memory Scale-3 (WMS-3): Logical Memory II – Delay | Verbal Learning and Memory (story) | (Wechsler, 1997b) |
| 12. | Story Thematic Recall - Immediate (Total Raw Score) | |||
| 13. | Story Recall - Delay (Total Raw Score) | |||
| 14. | Story Thematic Recall - Delay (Total Raw Score) | |||
|
| ||||
| 15. | Trails A Total Time [log-transformed] | Trail Making Test (TMT) | Speed of processing, Set Shifting | (Reitan, 1958) |
| 16. | Trails B Total Time [log-transformed] | |||
|
| ||||
| 17. | Pegboard Total Time for Both Hands | Grooved Pegboard | Speed of processing, Fine Motor Dexterity | (Ruff and Parker, 1993) |
|
| ||||
| 18. | Working Memory for Five Item Condition – Percent Correct [log-transformed] | Sternberg item recognition paradigm (SIRP) | Working Memory for Numerals | (Sternberg, 1969) |
2.4. Functional imaging task
Participants performed a version of the Sternberg item recognition paradigm (SIRP) (Sternberg, 1969). Stimuli were presented during fMRI, and responses were collected using E-prime software (EPrime v1.1, Psychology Software Tools, Inc., Pittsburgh, PA). The paradigm was administered during six 46-s blocks per run for two 360-s runs. In each block, a memory set, composed of one (load 1), three (load 3), or five (load 5) digits, was presented (two blocks per load condition). The encode phase was followed by a presentation of 14 digits, one at a time (the probe phase) and participants responded to each probe to indicate whether or not the probe digit was in the memory set. The subjects were instructed to respond as quickly and accurately as possible. (For additional details about the paradigm, see Ehrlich et al., 2012b.)
2.5. Structural and functional image acquisition and data processing
Structural MRI data were acquired with either a 1.5T Siemens Sonata (UNM, MGH, UI) or a 3T Siemens Trio (UMN). Also, functional MRI data were acquired with either a 1.5T Siemens Sonata (UNM) or a 3T Siemens Trio (UI, MGH, UMN). For details on image acquisition, see SM Table 1. Structural MRI data from three consecutive volumes were registered, motion corrected, averaged and analyzed in an automated manner with the atlas-based FreeSurfer software suite (http://surfer.nmr.mgh.harvard.edu, Version 4.0.1 for preprocessing and Version 5.0.0 for statistical analysis). This process included volumetric segmentation and cortical surface reconstruction. Functional images were processed using the FBIRN Image Processing Stream (FIPS), a pipeline using the FMRIB Software Library of FSL. A Functional Imaging Linear Model (FILM (Woolrich et al., 2001) was fit to model the Probe phases of each subject's preprocessed functional time series. Based on the extensive literature on working memory deficits in schizophrenia (Goldman-Rakic, 1994; Rapoport et al., 2005), we used a linear Contrast of Parameter Estimate (COPE) specified as load 5 versus load 1. Here we refer to responses to this contrast as “load-dependent” activation. For details on image processing, see SM 1.3.
2.6. Statistical analyses
Neuropsychological data of all schizophrenia patients were subjected to principal component analyses (PCAs) followed by varimax rotation, an approach that has been frequently used in schizophrenia research (Nuechterlein et al., 2004). Before analysis, distributions of all neuropsychological variables were examined for extreme skewness and kurtosis. To improve distributional properties, a base 10 logarithmic transformation was performed on the working memory index from the SIRP and the item discrimination index from the HVLT reflective of false positives during item recognition. Eight principal components (PCs) were extracted, which together summarized 85% of the overall variance after rotation (22%, 13%, 11%, 9%, 9%, 8%, 7%, and 6%). The scree plot depicted an elbow at the 7th component; however, eight components were selected because they better conformed to previously identified dimensions of cognitive functions in schizophrenia (for a review, see Nuechterlein et al., 2004). Neuropsychological indices with absolute PC loadings greater than 0.5 indicated that the first (“Verbal Episodic Memory”), second (“General Intellectual Function”), third (“Face Episodic Memory”), fourth (“Processing Speed”), and sixth (“Working Memory”) agreed with cognitive factors identified by Nuechterlein (2004). The fifth PC had a -0.89 absolute loading of Grooved Pegboard time to completion with 0.52 loadings of Block Design and Benton Visual Retention Test scores and was thus interpreted as representing “Fine Motor Control”. The seventh PC only had a -0.9 loading of false positives from the recognition portion of the Hopkins Verbal Learning Test and was thus called “Signal Detection”. The eighth component had a 0.8 loading of the FAS subtest and was identified as “Fluency”. The additional fifth, seventh, and eighth PCs are likely due to the inclusion of indices not typically considered in other factor-analytic studies, while the absence of the “Problem Solving” factor resulted because such a task was not incorporated in the present work. See SM Table 2 for PC loadings of the eight-PC structure.
PC scores for the schizophrenia patients on the eight cognitive domains were subsequently processed with a k-means cluster analysis using the Hartigan-Wong algorithm (Hartigan and Wong, 1979). Since there is evidence supporting the existence of four distinct neuropsychological clusters of schizophrenia (Hill et al., 2002), we selected k=4 and obtained clusters with sizes of N=38, 26, 21, and 44, which we refer to as cognitive clusters of schizophrenia.
Vertex-wise analyses of cortical thickness over the entire cortex were performed with FreeSurfer using surface-based registration methods and a Gaussian smoothing kernel with a full-width-at-half-maximum of 10 mm. Because of known confounding effects and in line with similar studies, we included age, gender and scanner field-strength into the general linear models as control variables (Kuperberg et al., 2003; Narr et al., 2005, 2007; Walhovd et al., 2006; Goldman et al., 2009; Schultz et al., 2010). All cortical thickness results were corrected for multiple comparisons using a Monte-Carlo simulation. For further details on thickness analyses, see SM 1.5.
Higher level mixed effect analyses of the fMRI data were carried out using FSL. We created Z-statistic images of the contrasts between schizophrenia clusters and between each schizophrenia cluster and healthy controls. The underlying model was controlled for the effects of age and scanner field-strength. Z-statistic images were thresholded using a z-value of 2.3 and a p-value of 0.001 (Worsley, 2001). For additional information, see SM 1.5.
3. Results
3.1. Schizophrenia clusters
The clusters of individuals with schizophrenia (Fig. 1 and SM Fig. 1) derived from k-means analyses had the following neuropsychological profiles: Cluster 1 showed diminished verbal fluency with signs of impaired processing speed. Cluster 2 was characterized by diminished verbal episodic memory with poor fine motor control and signal detection. The third cluster exhibited impaired face episodic memory and slowed processing speed, but above average verbal fluency for individuals with schizophrenia. Cluster 4 was characterized by a deficit in general intellectual function. For an overview of the original neuropsychological test scores of patients and healthy controls, refer to SM Table 4 and the description in Sponheim et al. (2010). The demographics and illness-related parameters of the schizophrenia clusters and healthy controls are displayed in Tables 2 and 3. The four schizophrenia clusters differed in relatively few variables: length of illness (cluster 4 < cluster 2), positive symptoms (clusters 3 and 4 > cluster 1), WRAT3 (clusters 1 and 3 > cluster 4) and years of education (clusters 1 and 3 > cluster 4). Cluster 1 thus appears to have fewer positive symptoms, shorter illness duration, and more education. Cluster 2 tends to be of a longer length of illness. Cluster 3 consists of individuals who have more education, and what appears to be a later onset of disorder and a tendency toward more depressive symptoms. Cluster 4 consists of patients who have a shorter duration of illness, less education, and lower estimated premorbid cognitive function. For an overview of the association between neuropsychological components and the clinical variables, see SM Table 5.
Figure 1.
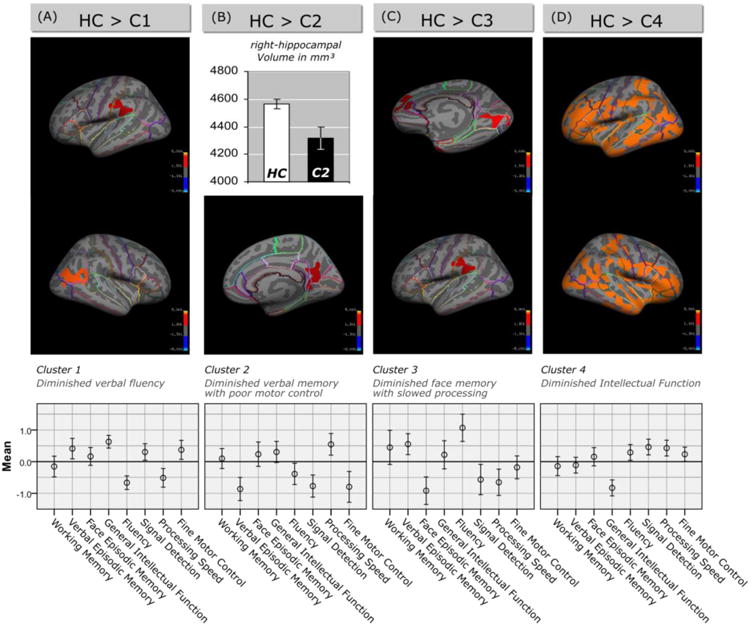
Top: Differences in brain structure between individual schizophrenia clusters and healthy controls (HC). In (A), (C) and (D) cortical statistical maps display regions of reduced cortical thickness in schizophrenia patients with distinct neuropsychological profiles compared to healthy controls. All statistical maps are shown on the inflated surface of the standard average subject, allowing visualization of data across the entire cortical surface without interference from cortical folding. CWP-values (corrected for multiple comparisons) are represented according to the color code and are all < 0.05. (B) The bar chart shows the difference in the right-hippocampal volume [mm³] between schizophrenia patients of cluster 2 (filled bar) and healthy controls (white bar). Bottom: Cognitive profile of the four schizophrenia clusters. The eight cognitive components (Working Memory, Verbal Episodic Memory, Face Episodic Memory, General Intellectual Function, Fluency, Signal Detection, Processing Speed, Fine Motor Control) were obtained by PCA of 18 neuropsychological scales. Baseline is the mean of the patients.
Table 2.
Demographics. Demographic characteristics for clusters of schizophrenia patients defined through cognitive variables, the entire schizophrenia sample, and healthy control participants. One-way ANOVAs indicated no significant differences between the four clusters of schizophrenia patients or between the clusters of schizophrenia patients and healthy controls in sex, age, handedness, parental SES (socioeconomic status), and scanner field strength during sMRI acquisition.
| Gender | Age | Handedness | WRAT3 Score | Education | Parental SES | sMRI Acquisition | fMRI Acquisition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Years | Total Score | Total Score | Years | Total Score | 1.5T / 3.0T | 1.5T / 3.0T | ||||||||
| N (fMRI) | N | % | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | N | N | |
| Cluster 1 | 38 (35) | 8 | 21 | 32.1 | 10.4 | 0.95 | 2.78 | 48.9 | 4.5 a | 14.0 | 2.3 c | 2.69 | 1.01 | 32 / 6 | 17 / 18 e |
| Cluster 2 | 26 (23) | 3 | 12 | 36.8 | 10.7 | 1.35 | 3.24 | 47.2 | 6.1 v | 13.3 | 1.3 x | 2.65 | 0.85 | 18 / 8 | 4 / 19 |
| Cluster 3 | 21 (20) | 6 | 29 | 37.6 | 13.2 | 1.38 | 3.07 | 49.2 | 5.6 b | 14.0 | 3.7 d | 2.76 | 1.14 | 18 / 3 | 8 / 12 |
| Cluster 4 | 44 (40) | 14 | 32 | 29.8 | 9.9 | 0.86 | 2.51 | 44.1 | 7.4 w,a,b | 12.2 | 2.5 y,c,d | 3.07 | 1.01 | 31 / 13 | 7 / 33 e,z |
| Total Schizophrenia Sample | 129 (118) | 31 | 24 | 33.2 | 11.1 | 1.07 | 2.82 | 47.0 | 6.4 | 13.3 | 2.6 | 2.83 | 1.01 | 99 / 30 | 36 / 82 |
| Healthy Control Sample | 165 (155) | 62 | 38 | 31.4 | 11.1 | 0.71 | 2.23 | 50.7 | 4.2 v,w | 15.2 | 2.1 x,y | 2.69 | 0.73 | 139 / 26 | 43 / 112 z |
Significant group differences confirmed by Sidak post hoc tests are denoted by same letters (a, b, c, w, x, y = p<0.005 and d, e, v, z = p<0.05). The distribution of subjects across clusters and sites for structural and functional imaging acquisition is shown in SM Table 3.
Table 3.
Clinical parameters of schizophrenia clusters. One-way ANOVA analysis indicated no significant differences between schizophrenia clusters in negative symptoms, disorganized symptoms, depression, extrapyramidal symptoms, akathisia, and antipsychotic medication.
| Length of Illness |
Positive Symptoms (SAPS) |
Negative Symptoms (SANS) |
Disorganized Symptoms (SAPS) |
Depression (CDS) |
Extrapyramidal Symptoms (SAS) |
Akathisia (BAS) |
Cumulative Medication |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | Total Score | Total Score | Total Score | Total Score | Total Score | Total Score | Cumulative dose years |
|||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Cluster 1 | 8.80 | 9.15 | 3.47 | 2.41 b,c | 7.89 | 3.41 | 1.71 | 1.83 | 3.71 | 3.35 | 0.97 | 1.78 | 0.53 | 0.80 | 32.38 | 55.61 |
| Cluster 2 | 15.19 | 10.85 a | 5.12 | 2.88 | 8.42 | 3.26 | 1.88 | 1.82 | 2.92 | 4.03 | 1.62 | 1.90 | 0.46 | 0.76 | 53.09 | 55.32 |
| Cluster 3 | 13.29 | 12.13 | 5.81 | 2.63 b | 7.90 | 3.05 | 1.14 | 1.68 | 5.95 | 6.45 | 0.95 | 1.24 | 0.57 | 0.81 | 36.85 | 63.25 |
| Cluster 4 | 8.14 | 8.92 a | 5.73 | 2.47 c | 7.43 | 4.60 | 1.77 | 1.95 | 3.09 | 4.33 | 1.09 | 1.63 | 0.73 | 0.82 | 48.57 | 160.35 |
| Total Schizophrenia Sample | 10.61 | 10.27 | 4.95 | 2.72 | 7.84 | 3.76 | 1.67 | 1.84 | 3.71 | 4.50 | 1.14 | 1.68 | 0.59 | 0.80 | 42.83 | 102.89 |
Significant group differences confirmed by Sidak post hoc tests are denoted by same letters (a = p<0.05; b = p<0.01; c = p<0.001).
3.2. Structural neural correlates of schizophrenia clusters
When we compared schizophrenia patients with distinct neuropsychological profiles to healthy controls, we found a significant decrease in cortical thickness in patients of cluster 1 in the supramarginal gyrus that includes portions of Wernicke's area (Brodmann Area 40, Fig. 1A). We observed no effects on cortical thickness in cluster 2. However, because cluster 2 was defined by marked verbal memory deficits, we investigated possible differences in hippocampal volumes. A regression analysis controlling for scanner field strength, sex, age and intra-cranial volume revealed differences between cluster 2 and healthy controls in right-hemispheric hippocampal volume [b = -0.172, t(184) = -2.81, p = 0.005] (Fig. 1B). Cluster 3 was characterized by cortical thinning in the lingual gyrus and face areas of the occipital lobe (Fig. 1C). Moreover, there were reductions of cortical thickness in the left superior frontal, rostral anterior cingulate and middle temporal gyrus (Fig. 1C). Patients of cluster 4 were characterized by widespread reductions of cortical thickness across both hemispheres (Fig. 1D). Furthermore, schizophrenia cluster 4 had significantly decreased cortical thickness in the right precentral area (Fig. 2) when compared with cluster 1, while the remaining between-cluster differences in cortical thickness were not statistically significant.
Figure 2.
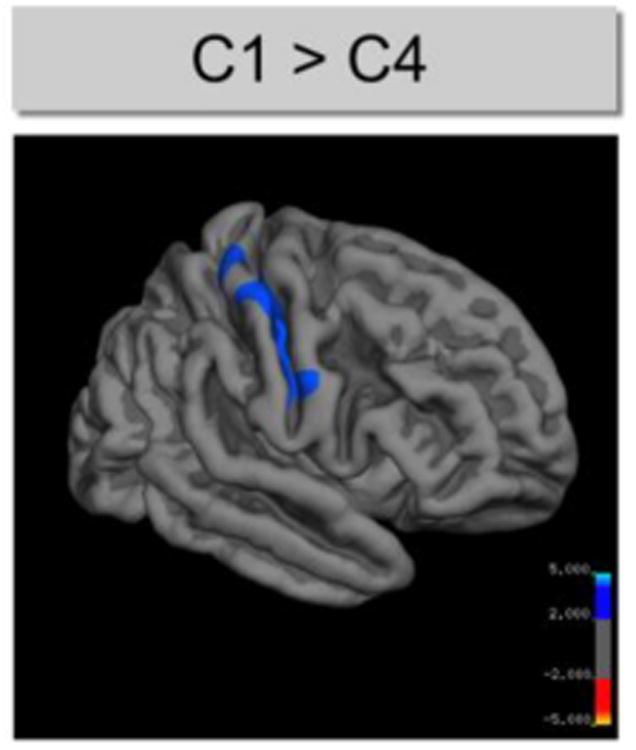
Structural differences between schizophrenia clusters. The cortical statistical map displays regions of reduced thickness (indicated by blue color) in the right hemisphere of schizophrenia patients of cluster 4 compared to cluster 1. CWP-values (corrected for multiple comparisons) are represented according to the color code and are all < 0.05.
3.3. Functional neural correlates of schizophrenia clusters
A comparison of working memory-elicited neural activity of the schizophrenia subgroups with healthy controls yielded significant findings for clusters 3 and 4 (Table 4). Patients of cluster 3 showed increased neural activity in the right planum temporale, while cluster 4 was characterized by an increased neural activity in the parietal operculum cortex, right planum temporale, and right precuneus cortex. Furthermore, the following between-cluster differences were found: Patients of cluster 4 showed increased activation in the left precentral gyrus extending to Broca's area and the insula when compared with cluster 1 as well as when compared with cluster 2 (Table 4 and SM Fig. 2).
Table 4.
Differences in working memory-elicited neural activity between the four schizophrenia clusters and healthy controls (HC) and between schizophrenia clusters. Coordinates of local maxima of the differences are given in MNI space.
| Local Maximum | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| MNI | |||||||
|
|
|||||||
| Contrast | Brain Area | Side | X | Y | Z | -log10(p) | z-value |
| C3 > HC | Planum Polare | R | 54 | 0 | -2 | 4.32 | 3.8 |
|
| |||||||
| C4 > HC | Parietal Operculum Cortex | L | -60 | -40 | 24 | 25.6 | 4.85 |
| Planum Polare/Insular Cortex | R | 38 | -14 | -10 | 16.5 | 5.21 | |
| Precuneus Cortex | R | 6 | -46 | 62 | 9.1 | 4.17 | |
|
| |||||||
| C1 < C4 | Precentral Gyrus | L | -58 | 4 | 20 | 4.86 | 4.08 |
|
| |||||||
| C2 < C4 | Precentral Gyrus | L | -60 | 2 | 20 | 3.38 | 4.37 |
4. Discussion
The present findings provide evidence that schizophrenia patients can be categorized into cognitive clusters that are meaningfully related to grey matter structures of the brain. We used an array of neuropsychological data and a cluster analysis technique to form schizophrenia clusters with distinct neuropsychological profiles. The cognitive clusters were generally consistent with neuropsychologically-defined clusters of individuals identified by other investigators and thus were considered as a possible means by which to characterize heterogeneity within the psychopathology of schizophrenia. Measures of brain structure and function provided external validation of the cognitive clusters.
An important question is whether the neuropsychological differences between the clusters were driven by variations in demographics or other external illness-related variables. Iatrogenic effects such as influences of medication may provoke new symptoms (e.g., extrapyramidal symptoms) or modify existing ones (e.g. anhedonia). However, our careful analyses showed no significant differences regarding most demographics or variables reflective of medical treatment. Most notably, there were no differences in antipsychotic medication, extrapyramidal or negative/ depressive symptoms. The lower level of education and reduced premorbid IQ in the cluster 4 may simply indicate a more severe prodrome that interfered with educational achievement. Such differences have been repeatedly described in previous reports using cognitive data and cluster analytic techniques (Heinrichs et al., 1997; Seaton et al., 1999). Cluster 4 is also characterized by a shorter duration of illness and increased positive symptoms, which could be indicators for active psychosis. In sum, findings of the present study provide evidence for schizophrenia being composed of subtypes of individuals with differing aspects of psychopathology (i.e., heterogeneity) that are not the result of demographic characteristics or iatrogenic influences of treatment such as antipsychotic medication. We will now briefly discuss the four different clusters in more detail:
Cluster 1, characterized by low verbal fluency and slowed processing speed, had diminished cortical thickness in the Wernicke's area and reduced working memory-elicited neural activity in the Broca's area, which are regions associated with forms of aphasia (Broca, 1861; Wernicke, 1874). Cortical thickness is assumed to reflect the arrangement and density of neural and glial cells, synaptic spines as well as passing axons (Parent and Carpenter, 1995; Garey, 2010). Postmortem studies in patients with schizophrenia showed reduced neural size and a decrease in interneural neuropil, dendritic trees, cortical afferents and synaptic spines (Harrison, 1999; Selemon and Goldman-Rakic, 1999; Garey, 2010), while no reduction in the number of neurons or signs of gliosis could be demonstrated (Selemon and Goldman-Rakic, 1999; Thune et al., 2001). Relationships between cortical grey matter in Brocas's/Wernicke's areas and verbal fluency have been shown in healthy controls as well as in patients with progressive aphasia (Sapolsky et al., 2010; Porter et al., 2011). Furthermore, structural and functional brain abnormalities in Broca's and Wernicke's area are widely replicated findings in studies of schizophrenia. For example, a quantitative post mortem study in schizophrenia patients showed reduced interhemispheric asymmetry of the Sylvian fissure (Falkai et al., 1992), and during verbal fluency tasks, Frith (1995) and Weiss (2006) found abnormal neural activity in the superior temporal cortex and Broca's area in schizophrenia patients, respectively. The absence of differences between cluster 1 patients and healthy controls in the fMRI domain in our study may be due the nature of the working memory task used here.
Cluster 2 was defined by diminished verbal memory and poor motor control. We found that patients of this cluster had reduced working memory-elicited neural activity in the left precentral gyrus extending to Broca's area and insula and a pronounced reduction of right-hemispheric hippocampal volume. The fMRI finding could be related to the impaired motor functioning in this subgroup, whereas the reduced hippocampus volume might be related to the episodic memory deficits. Larger hippocampal volumes have been associated with higher memory performance (Gur et al., 2000). Additionally, the worse signal detection in cluster 2 is reflective of a greater number of misidentifications of new items as old during episodic memory testing. In addition to memory related processes, the hippocampus has also been linked to the modulation of sensorimotor processes concerning control of motor responses to sensory stimuli (Giménez-Llort et al., 2002; Bast and Feldon, 2003; Zornoza et al., 2005). Both reductions of verbal memory functioning (Censits et al., 1997) and impaired fine motor control are prominent characteristics of schizophrenia patients (Sullivan et al., 1996), and the reduction of the hippocampal volume is one of the most consistent structural trait found in schizophrenia patients (Honea et al., 2005; Ehrlich et al., 2010) and in first-degree relatives of schizophrenia patients (Gur et al., 2000; O'Driscoll et al., 2001; Seidman et al., 2002).
Cluster 3 was characterized by poor face memory, slowed processing and poor signal detection. Our imaging analysis revealed that cluster 3 had cortical thinning in the lingual gyrus and adjacent areas (fusiform and occipital face areas) as well as in the superior frontal, rostral anterior cingulated and middle temporal gyrus. Interestingly, the temporal regions also showed aberrant activation pattern (increased neural responses) in this cluster during a working-memory task. The fusiform gyrus and occipital face areas are thought to be relevant for different forms of face perception (Mesulam, 1998; Rotshtein et al., 2007; Rhodes et al., 2009). Schizophrenia has long been associated with impaired face processing (Yoo et al., 2005; Anilkumar et al., 2008; Chen et al., 2008), and this neuropsychological deficit has even been observed in individuals at high risk for psychosis (Kim et al., 2010). Similarly, several previous studies have shown grey matter abnormalities in the lingual and fusiform gyrus as well as occipital face areas of schizophrenia patients (McDonald et al., 2000; Lee et al., 2002; Onitsuka et al., 2003; Schultz et al., 2010).
Cluster 4 was characterized by a deficit in general cognitive function as measured by tests with high loadings (>0.70) on general intellectual functioning (Sattler, 2001; Kaufman and Lichtenberger, 2005). Accordingly patients in this cluster were characterized by generalized cortical thinning. Cluster 4 patients were also found to have high positive symptoms, low education and low premorbid cognitive functioning. The widespread thinning may be consistent with a generalized cortical pathology that develops premorbidly, limits educational attainment and premorbid cognitive function, and eventually leads to higher expression of psychotic symptoms. Somewhat in line with that, Allen et al. (2000) reported that schizophrenia patients of a cluster with severely impaired cognitive functioning had greater bilateral sulcal widening on CT scans. Patients of cluster 4 also showed increased working-memory elicited neural activity in several working memory-related areas. This finding can be interpreted as a generalized tendency for the individuals with the greatest cortical atrophy to inefficiently activate neural structures during a relatively easy task. Manoach et al. (1999) was one of the first groups reporting prefrontal inefficiency in schizophrenia patients during working memory processing. A possible explanation for this common finding includes a deficit in automation, i.e. a subgroup of patients may fail to automate cognitive tasks, which in turn leads to decreased efficiency.
Despite a growing body of literature supporting the existence of cognitive subtypes of schizophrenia (Heinrichs and Awad, 1993; Insel et al., 2010) and promising neuroimaging findings from the present study, the issue of whether schizophrenia reflects a continuum of severity or a number of discrete subtypes remains to be settled. The partitioning of individuals with schizophrenia into separable subtypes perhaps reflecting distinct etiologic mechanisms and neurocognitive profiles holds potential for advancing our understanding of the genetics of schizophrenia (Jablensky, 2006). In the present study, we used a principal component analysis before applying the cluster analyses in order to reduce intercorrelation among the neuropsychological variables (Jolliffe, 2002; Sacco et al., 2012). Thus, it suggests that our clusters are based on separable cognitive performance dimensions. Our approach allowed us to map variance associated with separable cognitive impairments onto neural structures and function. Before definitive conclusions can be made, cross-validation and replication of both the neurocognitive clusters and their relation to brain phenotypes is necessary.
5. Limitations and conclusion
Our study is potentially limited by the fact that different acquisition sites (and MR scanners) contributed neuroimaging data. However, cross-site calibration of the acquisition sequences for each scanner (as well as the investigation of reliability, potential site and scanner differences) was carried out prior to this study (Jovicich et al., 2009, 2006) and the results were used to optimize the MRI scanning protocols. These cross-site calibration fMRI data revealed that activation indices varied more by individual than by scan site. Following a similar procedure as Walton et al. (2013) we included scanner field strength as a covariate into our analysis. Another potential limitation is related to a previous observation suggesting that neurocognitive clusters of schizophrenia patients may differ depending on the applied neuropsychological assessments (Goldstein et al., 1998). Further studies are needed to determine whether the identified subtypes remain stable over time and/or have different disease courses.
Taken together, data from the present study support the hypothesis that schizophrenia is a heterogeneous disorder and that variation across individuals does not simply reflect a range of impairment. Measures of cognitive function appear to provide a method for deriving clusters meaningfully related to brain structure and function that may be more stable than the symptom-based definitions of subtypes. Because the present study identified brain-based neural correlates of the cognitive clusters, the proposed groups of individuals with schizophrenia carry notable external validity. If replicated, the use of these clusters could facilitate research in molecular psychiatry and the identification of more specific therapeutic interventions.
Supplementary Material
Highlights.
Cluster analysis was applied to factorized data of neuropsychological performance
Four schizophrenia clusters based on distinct cognitive profiles were identified
Clusters are characterized by a specific pattern of structural brain changes
Acknowledgments
This work was supported by the National Institutes of Health (NIH/NCRR P41RR14075, 1RC1MH089257, and R01EB005846 (to VDC)), Department of Energy (DE-FG02-99ER62764), Mind Research Network, Morphometry BIRN (1U24, RR021382A),Function BIRN (U24RR021992-01, NIH.NCRR MO1 RR025758-01), the Deutsche Forschungsgemeinschaft (research fellowship to SE) and the NARSAD Young Investigator Award (to S.E.).
We are grateful to Claudia Schneider for helpful discussions and critical review of the manuscript. We thank the Center for Information Services and High Performance Computing (ZIH) at TU Dresden for generous allocations of computer time.
Footnotes
Conflict of interest: Veit Roessner has received lecture fees from Eli Lilly, Janssen-Cilag, Medice, Novartis, and was member of advisory boards of Eli Lilly, Novartis. All other authors declare no biomedical financial interests or other potential conflict of interests.
Contributors: Stefan Ehrlich designed the study, wrote the protocol and supervised the data analysis and the writing of the manuscript. Daniel Geisler conducted the statistical data analysis, managed the literature searches and wrote the first draft of the manuscript. Scott R. Sponheim and Melissa Naylor helped with the statistical analysis and assisted in manuscript preparation. S. Charles Schulz and Kelvin O. Lim assisted with the study design, interpretation of the results and revised the manuscript. Esther Walton helped with the literature searches, interpretation of the results and assisted in the preparation of the manuscript. Veit Roessner and Vince D. Calhoun helped with the data analysis, interpretation of the data and assisted in the preparation of the manuscript. Randy L. Gollub was responsible for the study design, supervision of subject assessments and data collection and manuscript revision. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophrenia Research. 1992;6:201–208. doi: 10.1016/0920-9964(92)90003-n. [DOI] [PubMed] [Google Scholar]
- Allen DN, Seaton BE, Goldstein G, Sanders RD, Gurklis JA, Jr, Peters JL, van Kammen DP. Neuroanatomic differences among cognitive and symptom subtypes of schizophrenia. Journal of Nervous and Mental Disease. 2000;188:381–384. doi: 10.1097/00005053-200006000-00010. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa City: 1984. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City: 1983. [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Archives of General Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Schultz S, Duzyurek S, Miller D. Diagnosis, methodology and subtypes of schizophrenia. Neuropsychobiology. 1997;35:61–63. doi: 10.1159/000119390. [DOI] [PubMed] [Google Scholar]
- Anilkumar APP, Kumari V, Mehrotra R, Aasen I, Mitterschiffthaler MT, Sharma T. An fMRI study of face encoding and recognition in first-episode schizophrenia. Acta Neuropsychiatrica. 2008;20:129–138. doi: 10.1111/j.1601-5215.2008.00280.x. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology (London, England: 1953) 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophrenia Research. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Arndt S, Andreasen NC, Flaum M, Miller D, Nopoulos P. A longitudinal study of symptom dimensions in schizophrenia. Prediction and patterns of change. Archives of General Psychiatry. 1995;52:352–360. doi: 10.1001/archpsyc.1995.03950170026004. [DOI] [PubMed] [Google Scholar]
- Barnes TR. A rating scale for drug-induced akathisia. British Journal of Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Bast T, Feldon J. Hippocampal modulation of sensorimotor processes. Progress in Neurobiology. 2003;70:319–345. doi: 10.1016/s0301-0082(03)00112-6. [DOI] [PubMed] [Google Scholar]
- Benton AL. The visual retention test as a constructional praxis task. Confinia Neurologica. 1962;22:141–155. doi: 10.1159/000104348. [DOI] [PubMed] [Google Scholar]
- Berrios GE. Positive and negative symptoms and Jackson. A conceptual history. Archives of General Psychiatry. 1985;42:95–97. doi: 10.1001/archpsyc.1985.01790240097011. [DOI] [PubMed] [Google Scholar]
- Bertisch H, Li D, Hoptman MJ, Delisi LE. Heritability estimates for cognitive factors and brain white matter integrity as markers of schizophrenia. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2010;153B:885–894. doi: 10.1002/ajmg.b.31054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5:125–142. [Google Scholar]
- Broca P. Perte de la parole: ramollissement chronique et destruction partielle du lobe anterieur gauche du cerveau. Bulletins de La Societe D'anthropologie. 1861;2:235–8. [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. The American Journal of Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. American Journal of Psychiatry. 1988;145:578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- Censits DM, Ragland JD, Gur RC, Gur RE. Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: a longitudinal study. Schizophrenia Research. 1997;24:289–298. doi: 10.1016/s0920-9964(96)00091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Norton D, Ongur D, Heckers S. Inefficient face detection in schizophrenia. Schizophrenia Bulletin. 2008;34:367–374. doi: 10.1093/schbul/sbm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ. The two-syndrome concept: origins and current status. Schizophrenia Bulletin. 1985;11:471. doi: 10.1093/schbul/11.3.471. [DOI] [PubMed] [Google Scholar]
- Dekker MCJ, Bonifati V, van Duijn CM. Parkinson's disease: piecing together a genetic jigsaw. Brain. 2003;126:1722–1733. doi: 10.1093/brain/awg172. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer J. Delis–Kaplan Executive Function System. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Brauns S, Yendiki A, Ho BC, Calhoun V, Schulz SC, Gollub RL, Sponheim SR. Associations of cortical thickness and cognition in patients with schizophrenia and healthy controls. Schizophrenia Bulletin. 2012a;38:1050–1062. doi: 10.1093/schbul/sbr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Morrow EM, Roffman JL, Wallace SR, Naylor M, Bockholt HJ, Lundquist A, Yendiki A, Ho BC, White T, Manoach DS, Clark VP, Calhoun VD, Gollub RL, Holt DJ. The COMT Val108/158Met polymorphism and medial temporal lobe volumetry in patients with schizophrenia and healthy adults. NeuroImage. 2010;53:992–1000. doi: 10.1016/j.neuroimage.2009.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Yendiki A, Greve DN, Manoach DS, Ho BC, White T, Schulz SC, Goff DC, Gollub RL, Holt DJ. Striatal function in relation to negative symptoms in schizophrenia. Psychological Medicine. 2012b;42(2):267–282. doi: 10.1017/S003329171100119X. [DOI] [PubMed] [Google Scholar]
- Falkai P, Bogerts B, Greve B, Pfeiffer U, Machus B, Fölsch-Reetz B, Majtenyi C, Ovary I. Loss of sylvian fissure asymmetry in schizophrenia: a quantitative post mortem study. Schizophrenia Research. 1992;7:23–32. doi: 10.1016/0920-9964(92)90070-L. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders (Research Version, Non-patient Edition) [Google Scholar]
- Frith CD, Friston KJ, Herold S, Silbersweig D, Fletcher P, Cahill C, Dolan RJ, Frackowiak RS, Liddle PF. Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. British Journal of Psychiatry. 1995;167:343–349. doi: 10.1192/bjp.167.3.343. [DOI] [PubMed] [Google Scholar]
- Garey L. When cortical development goes wrong: schizophrenia as a neurodevelopmental disease of microcircuits. Journal of Anatomy. 2010;217:324–333. doi: 10.1111/j.1469-7580.2010.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez-Llort L, Wang FH, Ogren SO, Ferré S. Local dopaminergic modulation of the motor activity induced by N-methyl-D-aspartate receptor stimulation in the ventral hippocampus. Neuropsychopharmacology. 2002;26:737–743. doi: 10.1016/S0893-133X(01)00411-0. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW. Sulcal thickness as a vulnerability indicator for schizophrenia. British Journal of Psychiatry. 2007;191:229–233. doi: 10.1192/bjp.bp.106.034595. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Greenstein D, Lenane M, Clasen L, Sharp W, Gochman P, Butler P, Evans A, Rapoport J. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Archives of General Psychiatry. 2007;64:772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. A case against subtyping in schizophrenia. Schizophrenia Research. 1995;17:147–152. doi: 10.1016/0920-9964(95)00060-Y. [DOI] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, Weinberger DR, Meyer-Lindenberg A. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Archives of General Psychiatry. 2009;66:467–477. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. The Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Allen DN, Seaton BE. A comparison of clustering solutions for cognitive heterogeneity in schizophrenia. Journal of the International Neuropsychological Society: JINS. 1998;4:353–362. [PubMed] [Google Scholar]
- Goldstein G, Shemansky WJ. Influences on cognitive heterogeneity in schizophrenia. Schizophrenia Research. 1995;18:59–69. doi: 10.1016/0920-9964(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Gollub RL, Shoemaker JM, King MD, White T, Ehrlich S, Sponheim SR, Clark VP, Turner JA, Mueller BA, Magnotta V, O'Leary D, Ho BC, Brauns S, Manoach DS, Seidman L, Bustillo JR, Lauriello J, Bockholt J, Lim KO, Rosen BR, Schulz SC, Calhoun VD, Andreasen NC. The MCIC Collection: a shared repository of multi-modal, multi-site brain image data from a clinical investigation of schizophrenia. Neuroinformatics. 2013;11(3):367–388. doi: 10.1007/s12021-013-9184-3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Gur RE, Calkins ME, Gur RC, Horan WP, Nuechterlein KH, Seidman LJ, Stone WS. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophrenia Bulletin. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC. Temporolimbic volume reductions in schizophrenia. Archives of General Psychiatry. 2000;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Hartberg CB, Lawyer G, Nyman H, Jönsson EG, Haukvik UK, Saetre P, Bjerkan PS, Andreassen OA, Hall H, Agartz I. Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Research: Neuroimaging. 2010;182:123–133. doi: 10.1016/j.pscychresns.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Wong MA. A k-means clustering algorithm. JSTOR: Applied Statistics. 1979;28:100–108. [Google Scholar]
- Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Archives of General Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW. The primacy of cognition in schizophrenia. American Psychologist. 2005;60:229–242. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Awad AG. Neurocognitive subtypes of chronic schizophrenia. Schizophrenia Research. 1993;9:49–58. doi: 10.1016/0920-9964(93)90009-8. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Ruttan L, Zakzanis KK, Case D. Parsing schizophrenia with neurocognitive tests: evidence of stability and validity. Brain and Cognition. 1997;35:207–224. doi: 10.1006/brcg.1997.0938. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hill SK, Ragland JD, Gur RC, Gur RE. Neuropsychological profiles delineate distinct profiles of schizophrenia, an interaction between memory and executive function, and uneven distribution of clinical subtypes. Journal of Clinical and Experimental Neuropsychology. 2002;24:765–780. doi: 10.1076/jcen.24.6.765.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M, DeLisi LE. Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. American Journal of Psychiatry. 1999;156:1336–1341. doi: 10.1176/ajp.156.9.1336. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two-factor Index of Social Position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. American Journal of Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jablensky A. Subtyping schizophrenia: implications for genetic research. Molecular Psychiatry. 2006;11:815–836. doi: 10.1038/sj.mp.4001857. [DOI] [PubMed] [Google Scholar]
- Jolliffe IT. Principal Component Analysis. Springer; 2002. [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, Maguire P, Rosas D, Makris N, Gollub R, Dale A, Dickerson BC, Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. NeuroImage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Glahn DC, van Erp TGM, Therman S, Huttunen M, Manninen M, Kaprio J, Cohen MS, Lönnqvist J, Cannon TD. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophrenia Research. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Lichtenberger EO. Assessing Adolescent and Adult Intelligence. John Wiley & Sons; 2005. [Google Scholar]
- Keefe RSE, Bilder RM, Harvey PD, Davis SM, Palmer BW, Gold JM, Meltzer HY, Green MF, Miller DD, Canive JM, Adler LW, Manschreck TC, Swartz M, Rosenheck R, Perkins DO, Walker TM, Stroup TS, McEvoy JP, Lieberman JA. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2006;31:2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- Kim HS, Shin NY, Choi JS, Jung MH, Jang JH, Kang DH, Kwon JS. Processing of facial configuration in individuals at ultra-high risk for schizophrenia. Schizophrenia Research. 2010;118:81–87. doi: 10.1016/j.schres.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SCR, van der Kouwe AJW, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lee CU, Shenton ME, Salisbury DF, Kasai K, Onitsuka T, Dickey CC, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Archives of General Psychiatry. 2002;59:775–781. doi: 10.1001/archpsyc.59.9.775. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biological Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Leonhard K. Classification of Endogenous Psychoses and Their Differentiated Etiology. Springer-Verlag Wien ; New York: 1999. 2nd, rev and enlarged ed ed. [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biological Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Marneros A, Deister A, Rohde A. Validity of the negative/positive dichotomy for schizophrenic disorders under long-term conditions. Schizophrenia Research. 1992;7:117–123. doi: 10.1016/0920-9964(92)90041-3. [DOI] [PubMed] [Google Scholar]
- McDonald B, Highley JR, Walker MA, Herron BM, Cooper SJ, Esiri MM, Crow TJ. Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: A postmortem study. American Journal of Psychiatry. 2000;157:40–47. doi: 10.1176/ajp.157.1.40. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, DeLuca H, Thompson PM. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cerebral Cortex (New York, NY: 1991) 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cerebral Cortex (New York, NY: 1991) 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia Research. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- O'Driscoll GA, Florencio PS, Gagnon D, Wolff ALV, Benkelfat C, Mikula L, Lal S, Evans AC. Amygdala–hippocampal volume and verbal memory in first-degree relatives of schizophrenic patients. Psychiatry Research: Neuroimaging. 2001;107:75–85. doi: 10.1016/S0925-4927(01)00095-6. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Shenton ME, Kasai K, Nestor PG, Toner SK, Kikinis R, Jolesz FA, McCarley RW. Fusiform gyrus volume reduction and facial recognition in chronic schizophrenia. Archives of General Psychiatry. 2003;60:349–355. doi: 10.1001/archpsyc.60.4.349. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex (New York, NY: 1991) 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Carpenter M. Human neuroanatomy. Williams & Wilkins; Baltimore, MD: 1995. [Google Scholar]
- Peralta V, Cuesta MJ, de Leon J. Positive and negative symptoms/syndromes in schizophrenia: reliability and validity of different diagnostic systems. Psychological Medicine. 1995;25:43–50. doi: 10.1017/s0033291700028075. [DOI] [PubMed] [Google Scholar]
- Porter JN, Collins PF, Muetzel RL, Lim KO, Luciana M. Associations between cortical thickness and verbal fluency in childhood, adolescence, and young adulthood. NeuroImage. 2011;55:1865–1877. doi: 10.1016/j.neuroimage.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, Manoach DS, Belger A, Diaz M, Wible CG, Ford JM, Mathalon DH, Gollub R, Lauriello J, O'Leary D, van Erp TG, Toga AW, Preda A, Lim KO. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophrenia Bulletin. 2009;35:19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MRC. The neurodevelopmental model of schizophrenia: update 2005. Molecular Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Rhodes G, Michie PT, Hughes ME, Byatt G. The fusiform face area and occipital face area show sensitivity to spatial relations in faces. European Journal of Neuroscience. 2009;30:721–733. doi: 10.1111/j.1460-9568.2009.06861.x. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Geng JJ, Driver J, Dolan RJ. Role of features and second-order spatial relations in face discrimination, face recognition, and individual face skills: behavioral and functional magnetic resonance imaging data. Journal of Cognitive Neuroscience. 2007;19:1435–1452. doi: 10.1162/jocn.2007.19.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Perceptual and Motor Skills. 1993;76:1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- Sacco R, Lenti C, Saccani M, Curatolo P, Manzi B, Bravaccio C, Persico AM. Cluster analysis of autistic patients based on principal pathogenetic components. Autism Research. 2012;5:137–147. doi: 10.1002/aur.1226. [DOI] [PubMed] [Google Scholar]
- Sapolsky D, Bakkour A, Negreira A, Nalipinski P, Weintraub S, Mesulam MM, Caplan D, Dickerson BC. Cortical neuroanatomic correlates of symptom severity in primary progressive aphasia. Neurology. 2010;75:358–366. doi: 10.1212/WNL.0b013e3181ea15e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler JM. Assessment of Children: Cognitive Applications. 4th. Jerome M; Sattler: 2001. [Google Scholar]
- Sautter FJ, McDermott BE, Cornwell J, Johnson J, Borges A, Wilson AF, Vasterling JJ, Foundas AL. A preliminary study of the neuropsychological heterogeneity of familial schizophrenia. Schizophrenia Research. 1995;18:1–7. doi: 10.1016/0920-9964(95)00015-1. [DOI] [PubMed] [Google Scholar]
- Schneider K. Clinical Psychopathology. Grune & Stratton; New York: 1959. [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, Nenadic I, Reichenbach JR, Sauer H, Schlösser RGM. Reduced cortical thickness in first episode schizophrenia. Schizophrenia Research. 2010;116:204–209. doi: 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Scott WK, Hauser ER, Schmechel DE, Welsh-Bohmer KA, Small GW, Roses AD, Saunders AM, Gilbert JR, Vance JM, Haines JL, Pericak-Vance MA. Ordered-subsets linkage analysis detects novel Alzheimer disease loci on chromosomes 2q34 and 15q22. American Journal of Human Genetics. 2003;73:1041–1051. doi: 10.1086/379083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton BE, Allen DN, Goldstein G, Kelley ME, van Kammen DP. Relations between cognitive and symptom profile heterogeneity in schizophrenia. Journal of Nervous and Mental Disease. 1999;187:414–419. doi: 10.1097/00005053-199907000-00004. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N, Toomey R, Kennedy D, Caviness VS, Tsuang MT. Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Archives of General Psychiatry. 2002;59:839–849. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Bearden CE, Christensen BK, Hawkins K, Heaton R, Keefe RSE, Heinssen R, Cornblatt BA. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Archives of General Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biological Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica. Supplementum. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Sommers AA. “Negative symptoms”: conceptual and methodological problems. Schizophrenia Bulletin. 1985;11:364–379. doi: 10.1093/schbul/11.3.364. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Jung RE, Seidman LJ, Mesholam-Gately RI, Manoach DS, O'Leary DS, Ho BC, Andreasen NC, Lauriello J, Schulz SC. Cognitive deficits in recent-onset and chronic schizophrenia. Journal of Psychiatric Research. 2010;44:421–428. doi: 10.1016/j.jpsychires.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. Memory-scanning: mental processes revealed by reaction-time experiments. American Scientist. 1969;57:421–457. [PubMed] [Google Scholar]
- Sullivan EV, Shear PK, Lim KO, Zipursky RB, Pfefferbaum A. Cognitive and motor impairments are related to gray matter volume deficits in schizophrenia. Biological Psychiatry. 1996;39:234–240. doi: 10.1016/0006-3223(95)00135-2. [DOI] [PubMed] [Google Scholar]
- Tandon R, Greden JF. Negative symptoms of schizophrenia: the need for conceptual clarity. Biological Psychiatry. 1991;30:321–325. doi: 10.1016/0006-3223(91)90287-v. [DOI] [PubMed] [Google Scholar]
- Thune JJ, Uylings HB, Pakkenberg B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. Journal of Psychiatric Research. 2001;35:15–21. doi: 10.1016/s0022-3956(00)00043-1. [DOI] [PubMed] [Google Scholar]
- Torrey EF. Studies of individuals with schizophrenia never treated with antipsychotic medications: a review. Schizophrenia Research. 2002;58:101–115. doi: 10.1016/s0920-9964(02)00381-x. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, Fischl B, Quinn BT, Makris N, Salat D, Reinvang I. Regional cortical thickness matters in recall after months more than minutes. NeuroImage. 2006;31:1343–1351. doi: 10.1016/j.neuroimage.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Walton E, Geisler D, Hass J, Liu J, Turner J, Yendiki A, Smolka MN, Ho BC, Manoach DS, Gollub RL, Roessner V, Calhoun VD, Ehrlich S. The impact of genome-wide supported schizophrenia risk variants in the neurogranin gene on brain structure and function. PloS One. 2013;8:e76815. doi: 10.1371/journal.pone.0076815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd. Psychological Corporation; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd. Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of General Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Hofer A, Golaszewski S, Siedentopf C, Felber S, Fleischhacker WW. Language lateralization in unmedicated patients during an acute episode of schizophrenia: a functional MRI study. Psychiatry Research: Neuroimaging. 2006;146:185–190. doi: 10.1016/j.pscychresns.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Der aphasische Symptomencomplex: eine psychologische Studie auf anatomischer Basis. Cohn & Weigert; 1874. [Google Scholar]
- White T, Magnotta VA, Bockholt HJ, Williams S, Wallace S, Ehrlich S, Mueller BA, Ho BC, Jung RE, Clark VP, Lauriello J, Bustillo JR, Schulz SC, Gollub RL, Andreasen NC, Calhoun VD, Lim KO. Global white matter abnormalities in schizophrenia: a multisite diffusion tensor imaging study. Schizophrenia Bulletin. 2011;37:222–232. doi: 10.1093/schbul/sbp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. Wide Range Achievement Test 3. Wide Range; Wilmington, DE: 1993. [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley K. Statistical analysis of activation images. Functional MRI: An Introduction to Methods. 2001:251–270. [Google Scholar]
- Yoo SS, Choi BG, Juh RH, Park JM, Pae CU, Kim JJ, Lee SJ, Lee C, Paik IH, Lee CU. Working memory processing of facial images in schizophrenia: fMRI investigation. The International Journal of Neuroscience. 2005;115:351–366. doi: 10.1080/00207450590520957. [DOI] [PubMed] [Google Scholar]
- Zornoza T, Cano-Cebrián MJ, Miquel M, Aragón C, Polache A, Granero L. Hippocampal dopamine receptors modulate the motor activation and the increase in dopamine levels in the rat nucleus accumbens evoked by chemical stimulation of the ventral hippocampus. Neuropsychopharmacology. 2005;30:843–852. doi: 10.1038/sj.npp.1300618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


