Abstract
Enteropathogenic Escherichia coli (EPEC), including diffusely adhering atypical E. coli, strains use a type III secretion system to deliver effector proteins into the membrane and cytoplasm of infected cells. The E. coli secreted proteins A, B, and D (EspA, EspB, and EspD) are required for the formation of the characteristic attaching and effacing (A/E) lesions. EspB and EspD are thought to form a translocation pore in the host cell membrane through which effector proteins are injected into the host cytosol. Besides its function in pore formation, EspB has been found in the cytosol and implicated to function as an effector protein. We screened for putative host cell proteins interacting with EspB of atypical EPEC strains and identified α1-antitrypsin (AAT) as a binding partner for EspB. AAT binds to EspB in pull-down and overlay experiments and also to EspD in overlay experiments. In agreement with the role of EspB and EspD in pore formation, EPEC-mediated hemolysis of red blood cells is strongly reduced by AAT in a concentration-dependent manner, indicating that AAT interferes with type III secretion by inhibiting the formation of the translocation pore. This is further supported by a decreased actin polymerization after infection of HeLa or CaCo-2 cells with EPEC in the presence of physiologically relevant concentrations of AAT. In this study, we identify AAT as a new binding partner for EspB and EspD, suggesting a previously unappreciated role for AAT in host cell defense against EPEC infections and potentially also against other bacterial pathogens.
Locally adhering typical enteropathogenic E. coli (LA-EPEC) and diffusely-adhering atypical EPEC (DA-EPEC) are implicated as a major cause of infantile diarrhea in children in developing countries (25, 26). Typical EPEC strains possess the EAF plasmid, coding for bundle-forming pili, which confers the local-adherence phenotype on host cells. In contrast, the EAF plasmid is missing in atypical EPEC strains, resulting in diffuse adherence of the bacteria (26). In typical as well as in atypical EPEC strains, pathogenesis and the characteristic attaching and effacing (A/E) phenotype is associated with a 35-kb pathogenicity island termed the locus of enterocyte effacement (LEE) (3, 22). The LEE encodes a type III secretion system designed to deliver effector proteins across the bacterial and host cell membranes into the cytosol of the host cell, a recurring theme in the pathogenesis of many gram-negative bacteria (11). The importance of the LEE pathogenicity island for virulence is further documented by the fact that LEE homologues were identified in enterohemorrhagic E. coli (EHEC), rabbit diarrheagenic E. coli (RDEC), and the mouse pathogen Citrobacter rodentium, where they are also responsible for the induction of A/E lesions on epithelial cells (7, 29, 40). The A/E phenotype is characterized by intimate attachment of the bacteria to the host cell, effacement of brush border microvilli, and formation of pedestals on which the bacteria reside (9). Intimate attachment is mediated by binding of the bacterial adhesin intimin to Tir, which, after translocation into the target cell membrane, serves as a receptor. Both proteins are encoded on the LEE pathogenicity island. Binding of intimin to Tir results in a rearrangement of the host cytoskeleton and subsequent pedestal formation (16). The type III secreted proteins EspA, EspB, and EspD are required for translocation of the effector proteins into the host cytosol (9). EspA forms a filamentous structure on the surface of the bacteria, connecting it to the host cell and thereby forming a conduit through which effector proteins are translocated (20, 30). EspB and EspD insert into the membrane of infected cells, where they are thought to form a translocation pore. Pore formation correlates with the induction of hemolysis of red blood cells (RBC) (12, 32, 37, 38). Additional type III-secreted proteins include EspF, EspG, EspH, and Map. EspF disrupts intestinal barrier function and induces apoptosis (5, 23). Until now, there has been no experimental evidence for a function of EspG during infection; however, homology to VirA of Shigella flexneri suggests a role in intestinal colonization (8). EspH localizes to membranes of infected cells and interferes with the formation of filopodia and pedestals (36). Map (Orf19) localizes to mitochondria and disrupts their membrane potential (18). In addition, Map induces the formation of Cdc42-dependent filopodia in host cells and is involved in EPEC invasion (14, 17). Although EspB inserts into the host membrane and, once there, plays a structural role in effector protein translocation, it can also be detected in the cytoplasm of infected cells, implying an additional function as a type III-secreted effector protein (35). Accordingly, overproduction of EspB in HeLa cells leads to a change in cellular morphology and reduction in the number of stress fibers (34). Moreover, espB mutant strains of EPEC, RDEC, and C. rodentium are reduced for virulence in humans (EPEC) and animal models (RDEC and C. rodentium) (1, 27, 33). Further support for a role of EspB as an effector protein comes from studies with EHEC, in which α-catenin was identified as a binding protein for EspB (21). To expand the current understanding of EspB function during host cell infection, we aimed at identifying putative host cell proteins interacting with EspB of atypical EPEC. In this study, we show that EspB binds to α1-antitrypsin (AAT). This interaction interferes with hemolysis of RBC and actin accumulation in infected host cells.
AAT is an acute-phase protein and the most abundant circulating serine protease inhibitor. The concentration of acute-phase proteins in serum increases in response to inflammation or injury, providing a nonspecific protection of the host against microorganisms. AAT concentrations in the serum vary between 1.5 and 3.5 mg/ml but may increase fourfold during inflammation (31). Although AAT is synthesized primarily in the liver, it can also be found in extrahepatic tissues and intestinal enterocytes (24). AAT is a physiological inhibitor of various proteases, but its primary role is to protect the lower respiratory tract from proteolytic destruction by neutrophil elastase (6). In this paper, we describe a novel role for AAT in host defense that might have implications for the virulence of pathogens besides EPEC.
MATERIALS AND METHODS
Bacterial strains, tissue culture cell lines, and culture conditions.
The atypical DA-EPEC strain 3431 was described previously (13). The typical EPEC strain E2348/69 was obtained from J. B. Kaper (Baltimore, Md.). The EPEC E2348/69 ΔespB and ΔespD mutant strains UMD864 and UMD870, respectively, were provided by M. Donnenberg (Baltimore, Md.). All strains were routinely grown in Standard-I medium. For the induction of type III secretion, EPEC strains were statically grown in Dulbecco's minimal essential medium (DMEM) under a 10% CO2 atmosphere. HeLa cells (ATCC CCL 2) were routinely grown at 37°C, under a 10% CO2 atmosphere, in DMEM supplemented with 10% (vol/vol) fetal calf serum, 1 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. CaCo-2 cells (subclone BBe2; American Type Culture Collection) were routinely grown at 37°C, under a 5% CO2 atmosphere in DMEM supplemented with 10% (vol/vol) fetal calf serum, 4 mM glutamine, 10 μg of human transferrin per ml, 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
Western blot analysis of type III secreted proteins and overlay experiments.
Bacteria were grown without agitation (static cultures) in DMEM for 3 h at 37°C in the presence of 10% CO2. Bacterial supernatants were precipitated by the addition of 10% (final concentration) trichloroacetic acid, subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (12.5% polyacrylamide), and transferred to nitrocellulose membranes for immunoblot analysis. The blots were blocked for 1 h at room temperature in 5% (wt/vol) skim milk-Tris-buffered saline (TBS), incubated for 1 h at room temperature with primary antibody diluted in 0.5% skim milk-TBS, washed with TBS, and incubated for 1 h at room temperature with secondary antibody diluted in 0.5% skim milk-TBS. Primary antibodies were polyclonal rabbit antiserum against AAT (Biomeda) at a 1:1,000 dilution and polyclonal rabbit antiserum against EspB at a 1:10,000 dilution. As a secondary antibody, we used alkaline phosphatase-conjugated goat anti-rabbit antibody (Jackson ImmunoResearch, West Grove, Pa.) at a 1:7,500 dilution. For overlay experiments, the blots were blocked as described above and incubated for 2 h at room temperature with 1 mg of AAT per ml in 0.5% skim milk-TBS. The membranes were washed with TBS and subsequently incubated with AAT antiserum as described above.
Quantification of hemolysis of SRBC induced by bacteria and bacterial supernatants.
The hemolysis assays were performed essentially as described previously (12, 13). Briefly, sheep RBC (SRBC) were mixed 1:1 with bacteria that were grown for 3 h in DMEM without agitation or with an equal volume of bacterial supernatant and incubated for 1 h at 37°C in the presence of 10% CO2; various concentrations of AAT (Sigma, St. Louis, Mo.) (1 to 200 μM) were added to the bacterial culture or the supernatant immediately before incubation with SRBC. The SRBC were pelleted, and lysis was monitored by photometrically measuring the release of hemoglobin into the supernatant at 450 nm.
Plasmid construction and protein purification.
The espB gene was amplified with oligonucleotides THisB+ (5′-GGAATTCCATATGAATACTATTGATAATAAT-3′) and THisB− (5′-CCGCTCGAGCCCGGCTAAGCGACCCGATTG-3′) (NdeI and XhoI restriction sites are underlined) and with chromosomal DNA from atypical EPEC strain 3431 as the template. The amplified fragment was digested with NdeI and XhoI and ligated into pET24b (Novagen, Schwalbach, Germany), a vector encoding a His6 epitope tag, resulting in plasmid pEspB3431His. Expression results in the production of a C-terminally His-tagged EspB protein. For the production of His-tagged EspB, overnight cultures of E. coli BL21(DE3) harboring plasmid pEspB3431His were diluted 1:200 in 150 ml of Standard-I medium containing 100 μg of ampicillin per ml and grown to an optical density at 600 nm of 0.6. After addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to induce EspB expression, the cultures were further incubated for an additional 3 h at 37°C. Subsequently, bacteria were harvested by centrifugation. His-tagged proteins were purified by Ni-nitrilotriacetate (NTA)-agarose affinity purification under nondenaturing conditions as recommended by the supplier (Qiagen, Hilden, Germany). Protein concentrations were evaluated by Coomassie brilliant blue staining of SDS-polyacrylamide gels in comparison with standard proteins.
Pull-down experiments.
Lysates of E. coli BL21(DE3) (pEspB3431His) grown under conditions to allow the expression of EspB with a C-terminal His tag were incubated with Ni-NTA agarose for at least 1 h at 4°C to allow binding of EspB-His to the matrix. The mixture was subsequently washed twice with washing buffer (50 mM Tris [pH 8.0], 500 mM NaCl, 20 mM imidazole, 10% glycerol, 0.1% Triton X-100) and once with Dulbecco's phosphate-buffered saline (D-PBS) to remove nonspecifically bound proteins. HeLa or Caco-2 cell monolayers were washed with D-PBS and lysed with lysis buffer (50 mM Tris [pH7.6], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 30% [vol/vol] glycerol, 1% [vol/vol] Triton X-100) containing complete EDTA-free protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany). The cell lysates were diluted 1:10 in D-PBS and preincubated with Ni-NTA-agarose for 30 min at 4°C. After removal of the matrix by centrifugation, the cellular lysates were added to the matrix suspension containing EspB-His and incubated for at least 2 h at 4°C. The suspension was loaded onto a column, and after it was washed twice with D-PBS, proteins were eluted with elution buffer (50 mM Tris [pH 8.0], 500 mM NaCl, 250 mM imidazole, 10% glycerol, 0.1% Triton X-100) in four 500-μl fractions and further investigated by SDS-PAGE and immunoblot analysis.
Immunofluorescence analysis.
HeLa cells were seeded at a density of 105 cells per well in 24-well tissue culture plates on round coverslips and grown in DMEM to 60 to 80% confluency. The cells were washed twice with D-PBS (containing MgCl2 and CaCl2) before being infected with 108 bacteria per well (grown statically in Standard-I medium for 15 h at 37°C) in 500 μl of low-serum DMEM (2% [vol/vol] fetal calf serum, 2 mM glutamine) containing 1% (wt/vol) methyl-α-d-mannoside (to block adhesion mediated by type I fimbriae) and incubated for 3 h at 37°C in a 10% CO2 atmosphere. Infected HeLa cells were washed with D-PBS to remove nonadherent bacteria and fixed for 15 min in 4% paraformaldehyde plus D-PBS. The fixed cells were washed three times with D-PBS, quenched in 1 M NH4Cl plus D-PBS for 10 min, and permeabilized with 0.1% Triton X-100 in D-PBS plus 4% paraformaldehyde for 4 min. The cells were blocked with 3% (wt/vol) bovine serum albumin in D-PBS for 1 h. For fluorescent-actin staining (FAS) assays, phalloidin-Texas Red was used at a 1:100 dilution. For AAT detection, we used polyclonal rabbit antiserum against AAT at a 1:400 dilution and Cy2-conjugated goat anti-rabbit antiserum as the secondary antibody. All antibodies were diluted in 0.3% bovine serum albumin.
RESULTS
Identification of AAT as a binding partner of EspB.
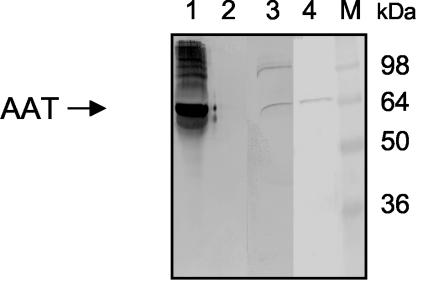
To identify host cell proteins that bind to EspB of the atypical EPEC strain 3431, we incubated recombinant EspB harboring a carboxy-terminal His6 tag (rEspB) with HeLa cell lysates and analyzed the lysates for cellular proteins that copurify with rEspB after Ni-NTA-agarose affinity purification of rEspB. SDS-PAGE analysis of eluted fractions revealed a prominent band corresponding to a polypeptide with an apparent molecular mass of approximately 60 kDa that was absent when cell lysates were incubated with Ni-NTA-agarose alone (data not shown). Determination of the N-terminal amino acid sequence of this protein by Edman degradation revealed the sequence X-Val-Leu-Gln-Gly-His-Ala-Val-Gln-Glu. A database search identified this protein clearly as AAT. Pull-down experiments were performed using rEspB and Caco-2 cell lysates, and the precipitates obtained were analyzed by SDS-PAGE and Western blot analysis with AAT-specific antibodies for detection. AAT can be detected in elution fractions of rEspB incubated with HeLa and CaCo-2 cell lysates but not in control fractions (Fig. 1). To confirm the binding of AAT to rEspB, we incubated purified AAT with rEspB and Ni-NTA-agarose. AAT was precipitated with Ni-NTA-bound rEspB but not with Ni-NTA-agarose alone, indicating a direct interaction between rEspB and AAT (Fig. 1). These results show that rEspB interacts directly with AAT derived from HeLa and Caco-2 cells.
FIG. 1.
Binding of AAT to rEspB in a pull-down assay. rEspB was incubated with purified AAT, HeLa, or Caco-2 cell lysate. Pull-down experiments were performed as described in Materials and Methods. AAT was detected using anti-AAT antiserum. rEspB precipitates purified AAT (lane 1) and also AAT from HeLa (lane 3) and Caco-2 (lane 4) cell lysate. However, AAT was not detected when HeLa cell lysate was incubated with Ni-NTA-agarose matrix alone (lane 2). Molecular masses of standard proteins are indicated in kilodaltons (lane M).
Purified AAT binds to EspB from EPEC culture supernatants.
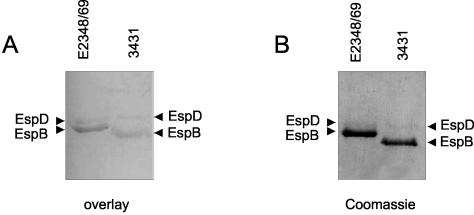
To determine if AAT also binds to type III-secreted EspB, we employed an overlay assay to investigate the binding of purified AAT to supernatants of the atypical EPEC strain 3431 grown under conditions that induce the type III secretion system. Esp-containing supernatants were separated by SDS-PAGE and blotted onto a nitrocellulose membrane, which was subsequently blocked and incubated with purified AAT. After the membrane was washed, bound AAT was detected on the membrane by using anti-AAT antibody. We identified two bands that correspond in size to the EspB and EspD proteins, respectively, of the atypical EPEC strain 3431 (Fig. 2). The identity of these proteins was confirmed in Western blot analysis with EspB- and EspD-specific antibodies (data not shown). Furthermore, AAT is able to bind to type III-secreted EspB and EspD from typical EPEC strain E2348/69 in an overlay assay, whereas the corresponding band is specifically missing in the respective mutant strains UMD864 (ΔespB) and UMD870 (ΔespD) (Fig. 2 and data not shown). These results indicate that EspB is able to bind AAT in solution, as assayed by the pull-down analysis, and also immobilized on a membrane, as demonstrated by the overlay assay. Furthermore, the results of the overlay experiments also show that not only EspB but also EspD from typical and atypical EPEC strains is able to bind to AAT.
FIG. 2.
Binding of AAT to EspB and EspD in an overlay assay. (A) Supernatant proteins of EPEC strains 3431 and E2348/69 grown under conditions inducing the secretion of Esp's were separated by SDS-PAGE and blotted to nitrocellulose, which was subsequently incubated with AAT. Binding of AAT to EspB and EspD was detected with anti-AAT antiserum. (B) Additionally, equivalent amounts of protein were separated by SDS-PAGE and stained with Coomassie brilliant blue to determine the relative amounts of proteins in the supernatants.
AAT inhibits hemolysis mediated by EPEC.
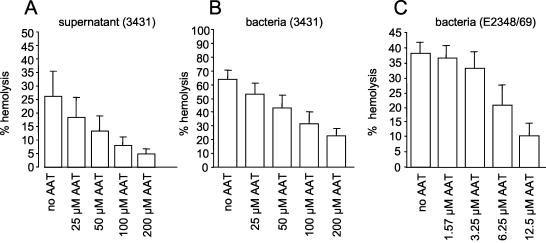
Classical LA-EPEC as well as atypical EPEC strains are able to induce hemolysis of RBC (12, 38). The hemolytic activity of atypical EPEC 3431 is contact independent and involves the type III-secreted proteins EspB and EspD (12). We speculated that the interaction of AAT with EspB might influence the hemolytic activity of atypical EPEC supernatants. Therefore, we performed hemolysis assays employing SRBC with atypical EPEC 3431 grown under conditions that induce the secretion of Esp proteins in the presence of different concentrations of AAT. AAT inhibited the hemolytic activity of atypical EPEC in a concentration-dependent manner (Fig. 3B). While 25 μM AAT reduced hemolysis by about 10%, only about one-third of the initial activity remained in the presence of 200 μM AAT. Contact-independent hemolytic activity was measured by incubating SRBC with supernatants of bacteria grown under conditions inducing Esp secretion in the presence or absence of AAT (Fig. 3A). Although hemolysis caused by supernatant alone is generally less pronounced than hemolysis mediated by whole bacterial cells (12), the effect of AAT in this assay is even more striking: 200 μM AAT leads to an approximately fivefold reduction of hemolytic activity, indicating that AAT inhibits Esp-mediated hemolysis, presumably by interacting with free EspB and/or EspD. To analyze the effect of AAT on lysis of SRBC independent of secreted EspB and EspD, we performed hemolysis assays using the typical EPEC strain E2348/69, which is able to lyse RBC in only a contact-dependent matter. As demonstrated in Fig. 3C, very small amounts of AAT were needed to inhibit hemolysis, indicating that the activity of AAT is not restricted to binding of free EspB and EspD from bacterial supernatants. Importantly, the AAT concentrations used in the experiments had no influence on growth of the bacteria (data not shown) and are of physiological relevance. A 50% inhibition of hemolysis is achieved with AAT concentrations ranging from 0.3 mg/ml (contact-dependent hemolysis by EPEC strain E2348/69) to 5.2 mg/ml (contact-dependent hemolysis by DA-EPEC strain 3431) and with a concentration of 2.6 mg/ml for contact-independent hemolysis by supernatants from DA-EPEC strain 3431.
FIG. 3.
AAT inhibits EPEC-mediated lysis of SRBC. Atypical EPEC strain 3431, typical EPEC strain E2348/69, or supernatant derived from atypical EPEC strain 3431 grown under conditions inducing the secretion of Esp proteins was incubated with SRBC in the presence or absence of different concentrations of AAT. The release of hemoglobin was monitored by measuring the optical density at 450 nm.
AAT inhibits atypical EPEC-induced actin polymerization.
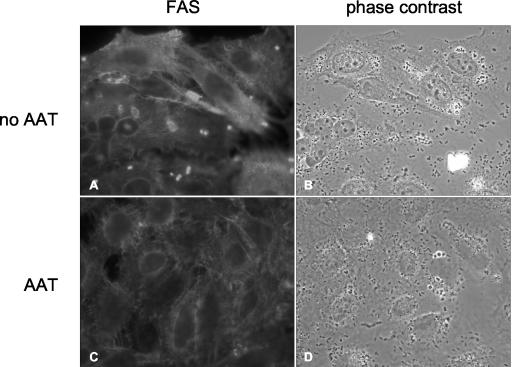
EspA, EspB, and EspD are necessary for the induction in host cells of pedestal formation characteristic of EPEC and atypical EPEC (9). Since AAT binds EspB and inhibits EPEC-induced hemolysis, we were interested in whether AAT also affects actin polymerization in host cells after infection with atypical EPEC. Therefore, HeLa and Caco-2 cells were infected with atypical EPEC strain 3431 in the presence or absence of 2.6 mg of AAT per ml. The actin polymerization induced by the infection was analyzed in a FAS test (19). As shown for HeLa cells (Fig. 4), the number of FAS-positive cells was greatly reduced in the presence of AAT. We measured the reduction in the number of FAS-positive cells by counting a subset of cells infected with atypical EPEC in the presence or absence of AAT. AAT inhibited actin polymerization mediated by atypical EPEC by more than 60%. To exclude the possibility that this effect is due to an influence of AAT on adherence of atypical EPEC to HeLa cells, we determined the number of adherent bacteria per cell in the presence or absence of AAT. No effect of AAT on adherence of the atypical EPEC strain 3431 to HeLa cells could be detected (data not shown). To analyze if AAT has a similar effect on actin polymerization after infection with the typical LA-EPEC strain E2348/69, HeLa cells were infected in the presence or absence of 2.6 mg of AAT per ml. As expected, the presence of AAT led to a 40% decrease in the number of FAS-positive cells compared to infection in the absence of AAT (data not shown). These results indicate that AAT is able to inhibit EPEC-induced actin polymerization in host cells, presumably by binding to EspB and/or EspD.
FIG. 4.
AAT inhibits atypical EPEC-mediated actin polymerization. HeLa cells were infected with the atypical EPEC strain 3431 in the presence or absence of AAT as described in Materials and Methods. Immunofluorescent staining revealed reduced accumulation of actin in HeLa cells beneath adherent bacteria in the presence but not in the absence of AAT.
AAT is not recruited to the site of atypical EPEC adherence.
AAT is a secreted protease inhibitor that is abundant in human serum but can also be found intracellularly in enterocytes (24). In cells infected with typical LA-EPEC, EspB can be found in the cytosol as well as in the membrane (37, 39). EspB of the classical EPEC strain E2348/69 seems to be localized immediately beneath attached bacteria (39). To determine if AAT can be detected at the site of bacterial attachment in cells infected with atypical EPEC strain 3431, HeLa and Caco-2 cells were mock infected or infected for 3 h, fixed, stained with AAT-specific antiserum, and analyzed by immunofluorescence microscopy. It was found that AAT was localized throughout the cells that were either mock infected or infected with atypical EPEC strain 3431, and no difference in the distribution of AAT as a result of infection could be observed. In summary, we conclude that EspB or another protein derived from atypical EPEC apparently does not recruit AAT to the site of bacterial adherence.
DISCUSSION
The type III-secreted EspB protein has multiple functions for the virulence of LEE-positive pathogenic E. coli strains. First, together with EspD, it inserts into host cell membranes and is thought to constitute part of a pore through which effector proteins are translocated into the host cell (12, 37). Second, it can be detected in the cytoplasm of infected host cells, where it might function as a cytoskeletal toxin (34, 35, 39). Third, it was shown that EspB recruits α-catenin to the site of EHEC infection in host cells, where it is essential for A/E lesion formation (21). For these reasons, it is not surprising that EspB represents a critical determinant for the pathogenesis of EPEC infections (1,33).
In this study, we have identified AAT as a novel binding partner for the type III-secreted EspB protein. Recombinant as well as type III-secreted EspB proteins from culture supernatants of atypical EPEC strains bound to AAT in pull-down and overlay assays. Further analysis revealed that AAT binding to EspB and presumably also to EspD interferes with the function of these proteins, since AAT reduced the ability of EPEC bacteria and supernatants to lyse RBC. Furthermore, when HeLa and Caco-2 cells were infected with atypical or typical EPEC in the presence of AAT, this interaction resulted in a reduction in the number of FAS-positive cells compared to cells infected in the absence of AAT. These results point to a previously unidentified role of AAT in host defense against infection and expand our knowledge about the role of EspB in EPEC infection.
AAT is one of the most abundant circulating protease inhibitors in humans and plays a role as an acute phase protein. It is synthesized primarily in the liver but is also produced in extrahepatic tissues and cells in the intestinal epithelium of the small bowel (24). The physiological role of AAT is the maintenance of the protease/antiprotease balance. The primary site of activity of AAT is in the lower respiratory tract, where it prevents damage from proteolytic destruction by inhibiting neutrophil elastase (6, 15). As a result of the relatively common genetic disorder leading to AAT deficiency, the risk of developing pulmonary emphysema is enhanced (4). Interaction of AAT with a bacterial virulence factor has not been described so far. One would expect that AAT as a serpin would preferentially bind to serine proteases; however, a protease motif could not be identified in EspB. Therefore, AAT has to interact with other domains of EspB and potentially also with other bacterial proteins that remain to be identified in future studies. Recently, the binding domain for α-catenin was identified in the N-terminal region of EspB by sequential deletion analysis (21). Similar experiments directed at the identification of EspB domains involved in AAT binding are under way in our laboratory.
Decreased hemolysis and reduced actin accumulation, as indicated by a FAS assay in the presence of AAT after atypical EPEC infection, indicate that AAT interferes with the stability or function, or both, of EspB and EspD. This interaction presumably inhibits the integration of EspB and EspD into the host cell membrane and the formation of the type III translocation pore. It has been described that AAT is able to inhibit human immunodeficiency virus type 1 (HIV-1), presumably by blocking viral entry into the cell, a process that is mediated by the viral gp120 and gp41 envelope glycoproteins (31). However, it has been speculated that AAT disrupts the interaction of gp120 with cell surface proteases involved in the fusion process. In a different study, the AAT variant α1-PDX is able to inhibit membrane fusion of HIV-1 by inhibiting furin-dependent cleavage of gp160 to gp120 and gp41, a processing step that is necessary for HIV-1 infectivity (2). In contrast to the effect of AAT on HIV-1 infection, the reduced infectivity of atypical EPEC in the presence of AAT presumably does not involve an inhibition of host proteases. Instead, AAT seems to inhibit EspB/EspD-dependent pore formation by directly interacting with the bacterial proteins, thereby inhibiting their membrane fusogenic potential.
Our results indicate that EspB interacts primarily with secreted AAT. While atypical and typical EPEC strains showed reduced hemolytic activity and a reduced potential to polymerize actin in host cells in the presence of AAT, we did not observe a recruitment of AAT to the site of atypical EPEC infection or a colocalization of EspB with AAT in infected cells (data not shown). However, a putative intracellular association of AAT with EspB cannot be excluded and should be investigated in more detail in future studies.
Besides AAT, other protease inhibitors have been identified to play a role in host defense against infection by bacteria, fungi, and viruses. Like antimicrobial peptides, these proteins have a microbicidal activity that is distinct from their protease inhibitory activity (10). Again, AAT seems to act in a distinctly different fashion on atypical EPEC, since bacteria grow in the presence of AAT without any growth impairment or other obvious defect (data not shown). Recently, lactoferrin was identified as a protein of the innate immune system that has proteolytic activity against the EPEC proteins EspA, EspB, and EspD, with the strongest effect on EspB (28). Although the mechanism by which lactoferrin impairs EPEC virulence functions appears to be distinct from the mechanism of the effect of AAT on EspB described in our study, it emphasizes the concerted function of multiple host defense factors to counteract the effects of type III-secreted proteins on the host. In addition, it can be speculated that AAT might act not only on proteins secreted by atypical EPEC but also on virulence-associated proteins of other pathogens. Future studies will be aimed at identifying the mechanism underlying the binding of AAT to EspB and EspD.
Acknowledgments
This study has been supported by grants of the Deutsche Forschungsgemeinschaft (DFG) (DFG SFB 293/B5) and the Federal Ministry for Science and Technology (BMFT) (PTJ-BIO/03U213B VBIII PG3) and an Innovative Medical Research grant (IMF) (HE 120201) of the Medical School of the University of Münster.
Editor: J. B. Bliska
REFERENCES
- 1.Abe, A., U. Heczko, R. G. Hegele, and B. B. Finlay. 1998. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J. Exp. Med. 188:1907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, E. D., L. Thomas, J. S. Hayflick, and G. Thomas. 1993. Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed α1-antitrypsin variant. J. Biol. Chem. 268:24887-24891. [PubMed] [Google Scholar]
- 3.Beinke, C., S. Laarmann, C. Wachter, H. Karch, L. Greune, and M. A. Schmidt. 1998. Diffusely adhering Escherichia coli strains induce attaching and effacing phenotypes and secrete homologs of Esp proteins. Infect. Immun. 66:528-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brantly, M. L., L. D. Paul, B. H. Miller, R. T. Falk, M. Wu, and R. G. Crystal. 1988. Clinical features and history of the destructive lung disease associated with α1-antitrypsin deficiency of adults with pulmonary symptoms. Am. Rev. Respir. Dis. 138:327-336. [DOI] [PubMed] [Google Scholar]
- 5.Crane, J. K., B. P. McNamara, and M. S. Donnenberg. 2001. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell. Microbiol. 3:197-211. [DOI] [PubMed] [Google Scholar]
- 6.Crystal, R. G. 1990. Alpha-1-antitrypsin deficiency, emphysema, and liver disease. Genetic basis and strategies for therapy. J. Clin. Investig 85:1343-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng, W. Y. Li, B. A. Vallance, and B. B. Finlay. 2001. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 69:6323-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott, S. J., E. O. Krejany, J. L. Mellies, R. M. Robins-Browne, C. Sasakawa, and J. B. Kaper. 2001. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect. Immun. 69:4027-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 10.Hiemstra, P. S. 2002. Novel roles of protease inhibitors in infection and inflammation. Biochem. Soc. Trans. 30:116-120. [DOI] [PubMed] [Google Scholar]
- 11.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ide, T., S. Laarmann, L. Greune, H. Schillers, H. Oberleithner, and M. A. Schmidt. 2001. Characterization of translocation pores inserted into plasma membranes by type III-secreted Esp proteins of enteropathogenic Escherichia coli. Cell. Microbiol. 3:669-679. [DOI] [PubMed] [Google Scholar]
- 13.Ide, T., S. Michgehl, S. Knappstein, G. Heusipp, and M. A. Schmidt. 2003. Differential modulation by Ca2+ of type III secretion of diffusely adhering enteropathogenic Escherichia coli. Infect. Immun. 71:1725-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jepson, M. A., S. Pellegrin, L. Peto, D. N. Banbury, A. D. Leard, H. Mellor, and B. Kenny. 2003. Synergistic roles for the Map and Tir effector molecules in mediating uptake of enteropathogenic Escherichia coli (EPEC) into non-phagocytic cells. Cell. Microbiol. 5:773-783. [DOI] [PubMed] [Google Scholar]
- 15.Kalsheker, N., S. Morley, and K. Morgan. 2002. Gene regulation of the serine proteinase inhibitors α1 antitrypsin and α1-antichymotrypsin. Biochem. Soc. Trans. 30:93-98. [DOI] [PubMed] [Google Scholar]
- 16.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 17.Kenny, B., S. Ellis, A. D. Leard, J. Warawa, H. Mellor, and M. A. Jepson. 2002. Co-ordinate regulation of distinct host cell signalling pathways by multifunctional enteropathogenic Escherichia coli effector molecules. Mol. Microbiol. 44:1095-1107. [DOI] [PubMed] [Google Scholar]
- 18.Kenny, B., and M. Jepson. 2000. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell. Microbiol. 2:579-590. [DOI] [PubMed] [Google Scholar]
- 19.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodama, T., Y. Akeda, G. Kono, A. Takahashi, K. Imura, T. Iida, and T. Honda. 2002. The EspB protein of enterohaemorrhagic Escherichia coli interacts directly with alpha-catenin. Cell. Microbiol. 4:213-222. [DOI] [PubMed] [Google Scholar]
- 22.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23:399-407. [DOI] [PubMed] [Google Scholar]
- 23.McNamara, B. P., A. Koutsouris, C. B. O'Connell, J. P. Nougayrede, M. S. Donnenberg, and G. Hecht. 2001. Translocated EspF protein fromenteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Investig. 107:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molmenti, E. P., D. H. Perlmutter, and D. C. Rubin. 1993. Cell-specific expression of α1-antitrypsin in human intestinal epithelium. J. Clin. Investig. 92:2022-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nataro, J. P., M. M. Baldini, J. B. Kaper, R. E. Black, N. Bravo, and M. M. Levine. 1985. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J. Infect. Dis. 152:560-565. [DOI] [PubMed] [Google Scholar]
- 26.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman, J. V., B. A. Zabel, S. S. Jha, and D. B. Schauer. 1999. Citrobacter rodentium espB is necessary for signal transduction and for infection of laboratory mice. Infect. Immun. 67:6019-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochoa, T. J., M. Noguera-Obenza, F. Ebel, C. A. Guzman, H. F. Gomez, and T. G. Cleary. 2003. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect. Immun. 71:5149-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perna, N. T., G. F. Mayhew, G. Posfai, S. Elliott, M. S. Donnenberg, J. B. Kaper, and F. R. Blattner. 1998. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 66:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekiya, K., M. Ohishi, T. Ogino, K. Tamano, C. Sasakawa, and A. Abe. 2001. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. USA 98:11638-11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapiro, L., G. B. Pott, and A. H. Ralston. 2001. Alpha-1-antitrypsin inhibits human immunodeficiency virus type 1. FASEB J. 15:115-122. [DOI] [PubMed] [Google Scholar]
- 32.Shaw, R. K., S. Daniell, F. Ebel, G. Frankel, and S. Knutton. 2001. EspA filament-mediated protein translocation into red blood cells. Cell. Microbiol. 3:213-222. [DOI] [PubMed] [Google Scholar]
- 33.Tacket, C. O., M. B. Sztein, G. Losonsky, A. Abe, B. B. Finlay, B. P. McNamara, G. T. Fantry, S. P. James, J. P. Nataro, M. M. Levine, and M. S. Donnenberg. 2000. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect. Immun. 68:3689-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor, K. A., P. W. Luther, and M. S. Donnenberg. 1999. Expression of the EspB protein of enteropathogenic Escherichia coli within HeLa cells affects stress fibers and cellular morphology. Infect. Immun. 67:120-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor, K. A., C. B. O'Connell, P. W. Luther, and M. S. Donnenberg. 1998. The EspB protein of enteropathogenic Escherichia coli is targeted to the cytoplasm of infected HeLa cells. Infect. Immun. 66:5501-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu, X., I. Nisan, C. Yona, E. Hanski, and I. Rosenshine. 2003. EspH, a new cytoskeleton-modulating effector of enterohaemorrhagic and enteropathogenic Escherichia coli. Mol. Microbiol. 47:595-606. [DOI] [PubMed] [Google Scholar]
- 37.Wachter, C., C. Beinke, M. Mattes, and M. A. Schmidt. 1999. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 31:1695-1707. [DOI] [PubMed] [Google Scholar]
- 38.Warawa, J., B. B. Finlay, and B. Kenny. 1999. Type III secretion-dependent hemolytic activity of enteropathogenic Escherichia coli. Infect. Immun. 67:5538-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolff, C., I. Nisan, E. Hanski, G. Frankel, and I. Rosenshine. 1998. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 28:143-155. [DOI] [PubMed] [Google Scholar]
- 40.Zhu, C., T. S. Agin, S. J. Elliott, L. A. Johnson, T. E. Thate, J. B. Kaper, and E. C. Boedeker. 2001. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infect. Immun. 69:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]