Background: The functions of integrin α5 on cell proliferation and the underlying mechanisms remain unclear.
Results: Loss of N-glycosylation on α5 increased the phosphorylation and internalization of EGFR and abolished its inhibitory effects on cell proliferation.
Conclusion: Integrin α5 regulates EGFR-mediated signaling through N-glycosylation.
Significance: N-Glycosylation plays important roles in the cross-talk between integrins and growth factor receptors.
Keywords: cell growth, epidermal growth factor receptor (EGFR), glycobiology, glycosylation, integrin, lipid raft, signaling
Abstract
Integrin α5β1-mediated cell adhesion regulates a multitude of cellular responses, including cell proliferation, survival, and cross-talk between different cellular signaling pathways. Integrin α5β1 is known to convey permissive signals enabling anchorage-dependent receptor tyrosine kinase signaling. However, the effects of integrin α5β1 on cell proliferation are controversial, and the molecular mechanisms involved in the regulation between integrin α5β1 and receptor tyrosine kinase remain largely unclear. Here we show that integrin α5 functions as a negative regulator of epidermal growth factor receptor (EGFR) signaling through its N-glycosylation. Expression of WT integrin α5 suppresses the EGFR phosphorylation and internalization upon EGF stimulation. However, expression of the N-glycosylation mutant integrin α5, S3–5, which contains fewer N-glycans, reversed the suppression of the EGFR-mediated signaling and cell proliferation. In a mechanistic manner, WT but not S3–5 integrin α5 forms a complex with EGFR and glycolipids in the low density lipid rafts, and the complex formation is disrupted upon EGF stimulation, suggesting that the N-glycosylation of integrin α5 suppresses the EGFR activation through promotion of the integrin α5-glycolipids-EGFR complex formation. Furthermore, consistent restoration of those N-glycans on the Calf-1,2 domain of integrin α5 reinstated the inhibitory effects as well as the complex formation with EGFR. Taken together, these data are the first to demonstrate that EGFR activation can be regulated by the N-glycosylation of integrin α5, which is a novel molecular paradigm for the cross-talk between integrins and growth factor receptors.
Introduction
Epidermal growth factor receptor (EGFR),2 a member of the ErbB receptor tyrosine kinase family, converts extracellular cues into intracellular effectors to trigger appropriate cellular responses (1–3), which play a key role in normal epithelial developmental biology and in tumor metastasis (4). A dysregulation of EGFR signaling, including receptor overexpression and/or activation, is a common feature in tumorigenesis (5). Due to this aberrant activity in the pathology of cancer, EGFR has emerged as an attractive candidate for anticancer therapy (6), which prompted us to examine the underlying molecular mechanisms for EGFR activation.
EGFR activation forms a complex signaling network with several regulators, including related cytoplasmic proteins, microRNAs, tyrosine kinase inhibitors, and other coupled receptors (7–9). However, studies that address these direct or indirect regulations of EGFR have focused mainly on the inner membrane, particularly the cytoplasmic kinase domain of EGFR. The underlying mechanisms of the outer membrane remain unknown.
Current insight into this regulation derives largely from studies around EGFR-related microdomains, so-called lipid rafts (10–13), which are thought to act as platforms for EGFR signaling (14, 15). These molecules are localized in the microdomains of the cell membrane and are usually rich in cholesterol, glycosphingolipids, and glycoproteins (15–17). Several studies have associated glycosphingolipids, including gangliosides GM1, GM3, and GD3, with the regulation of EGFR signaling (18–20). In addition to glycosphingolipids, some glycoproteins that are located in the EGFR-related lipid rafts play important roles in the regulation of EGFR signaling (12, 13, 21–23). These limited results highlight the possibility that glycosylation might act as a “linker” in lipid rafts for the regulation of EGFR. However, little is known about how glycosylation controls regulation. Therefore, elucidation of the underlying mechanisms involved in the glycosylation-mediated regulation of EGFR is very important for a complete view of the biological functions of EGFR.
Integrins are important members of the EGFR lipid raft-related glycoproteins, as mentioned above, and are major carriers of N-glycans, which are thought to play crucial roles in many biological functions. In response to cell adhesion, integrins not only directly initiate certain cytoplasmic signals for cell spreading but also indirectly modulate the transmission of EGFR signaling, which is referred to as cross-talk (24–30). The integrins mediate cooperation with EGFR mainly through α-cytoplasmic domains (31), which are also restricted to the inner membrane. Until recently, integrin α5β1, a major fibronectin receptor, was believed to be a well characterized integrin, with N-glycosylation functions in cell adhesion (32, 33). However, the function of N-glycosylation on cell proliferation and cellular signaling remains unclear. In fact, there have been several controversial reports about the regulation of EGFR-mediated signaling by integrin α5 (34, 35).
To resolve these issues, we have examined the relationship between integrin α5 and EGFR and found that the expression of integrin α5 negatively regulates EGFR-mediated cellular signaling and cell proliferation. We used N-glycosylation mutants of integrin α5 to clarify the roles of the N-glycosylation of α5 in EGFR-mediated signaling and cross-talk with EGFR and found that the regulation was strictly controlled via the N-glycans of integrin α5, particularly the N-glycans on the Calf-1,2 domain. Our results clearly demonstrate the importance of the N-glycans of integrin in the regulation of EGFR-mediated cellular signaling and provide new insights into the cross-talk between growth factor receptors and integrins.
Experimental Procedures
Antibodies and Reagents
The experiments were performed using the following antibodies: mAbs against integrin α5 (catalog no. 610634), ERK1 (catalog no. 610031), and caveolin-1 (catalog no. 610407) obtained from BD Biosciences; mAb against human α5β1 (MAB1999) from Millipore; the supernatant of the hybridoma of hamster integrin β1 subunit (7E2) from the Developmental Studies Hybridoma Bank, University of Iowa; mouse polyclonal antibody to EGFR (sc-120) from Santa Cruz Biotechnology, Inc.; rabbit mAbs to EGFR (catalog no. 4267), phospho-EGFR (catalog no. 3777), phospho-ERK1/2 (catalog no. 4370), AKT (catalog no. 9272), and phospho-AKT (catalog no. 4060) from Cell Signaling Technology; and mAb against α-tubulin from Sigma. Alexa Fluor® 647 goat anti-mouse IgG was obtained from Invitrogen. The peroxidase-conjugated goat against mouse and rabbit IgG antibodies were obtained from Promega and Cell Signaling Technology, respectively. The methyl-β-cyclodextrin, biotin-conjugated cholera toxin B subunit, primaquine, MesNa, iodoacetamide, and fibronectin (FN) were from Sigma; EGF (AF-100) was from PeproTech; EGF-Alexa Fluor® 555 was from Invitrogen; the control mouse IgG1 was from TONBO biosciences; sulfo-NHS-SS-biotin was from Thermo Scientific; and TO-PRO-3 was from Molecular Probes. The agarose-conjugated anti-GFP antibody (RQ2) and the Streptavidin-conjugated agarose were obtained from Medical & Biological Laboratories Co. Ltd. (Nagoya, Japan) and Millipore, respectively.
Cell Lines and Cell Culture
The 293T and HeLa cell lines were provided by the RIKEN cell bank (Japan). The phoenix and MDA-MB-231 cell lines were purchased from ATCC. The integrin α5 subunit-deficient CHO-K1 cell line (CHO-B2) and the U-251MG cell line were gifts from Dr. Rudolf Juliano (School of Medicine, University of North Carolina, Chapel Hill, NC) (36) and Prof. Jun Nakayama (Shinshu University Graduate School of Medicine, Japan), respectively. The stable cell lines used in this study were established as mentioned below. All cell lines were maintained at 37 °C in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (FBS), under a humidified atmosphere containing 5% CO2, except for the virus production.
Generation of CRISPR/Cas9-based Integrin α5-knock-out (KO) Cells
The CRISPR/Cas9-based integrin α5-KO cells were established as described previously (37). Briefly, the sgRNA-specifying oligonucleotide sequences spanning human integrin α5 exon 3 (5′-CACCGGGGCAACAGTTCGAGCCCA-3′ and 5′-AAACTGGGCTCGAACTGTTGCCCC-3′) were chosen from the human KO library sgRNAs (38). After annealing, the double-stranded guide oligonucleotides were cloned into the pSpCas9(BB)-2A-GFP (Addgene plasmid ID: 48138) vector. The expression vector was transfected into the indicated cell lines by electroporation according to the manufacturer's instructions. The GFP-positive cells were sorted by FACSAria II (BD Bioscience) after culture for 3 days. The integrin α5-positive but GFP-negative cells were then sorted three times during the following 2-week culture. The α5-KO cells were confirmed by flow cytometry and Western blot analyses as described below.
EGFR and Integrin α5 Expression Vectors
The cDNA of human EGFR (a generous gift from Dr. Motoko Takahashi, Sapporo Medical University, Japan) was inserted into a cloning entry vector (pENTR1A, Invitrogen) according to the manufacturer's protocol for the In-Fusion kit (Takara Bio) using the following primers: 5′-ATCCGGTACCGAATTCACCATGCGACCCTCCGG-3′ and 5′-TCTAGATATCTCGAGTGCTCCAATAAATTC-3′. The vectors of GFP and GFP-tagged integrin α5 with altered N-glycosylation sites (WT, S3–5, and Δ10–14) were previously established in our laboratory (33). The S3–5,10–14 mutation vector was also constructed according to the in-fusion kit using the following primers: 5′-TACATTATCAGAGCAAGAGCCG-3′, 5′-ATCCAACTCCAGGCCCTTTGGG-3′, 5′-CAAAGGGCCTGGAGTTGGAT-3′, and 5′-GCTCTTGCTCTGATAATGTAGG-3′. The resultant cDNAs were sequenced to confirm the presence of the desired mutations. We used the GatewayTM cloning system kit (Invitrogen) to acquire all of the expression vectors. Briefly, the LR clonase enzyme (Invitrogen) was used to transfer the cDNAs of EGFR and integrin α5 from the entry vectors into pBABE-puro-Rfa (32) and CSII-CMV-Rfa (kindly provided by Dr. H. Miyoshi (RIKEN, Tokyo, Japan)), respectively.
Virus Production and Infection
The virus production and infection were performed as described previously (32, 39). In brief, the pBABE-puro-Rfa-based retrovirus vectors and pLP/VSVG (Invitrogen) were transfected into Phoenix cells with Lipofectamine 2000 (Invitrogen). The CSII-CMV-Rfa-based lentivirus vectors were cotransfected with pCAG-HIVgp and pCMV-VSV-G-RSV-Rev into 293T cells. After transfection for 48 h, the retrovirus and lentivirus supernatants were collected. The CHO-B2 cells were infected with the resultant viral supernatant containing 10 μg/ml Polybrene (Sigma) at 32 °C overnight and then selected in the presence of 2.0 mg/ml puromycin (Nacalai Tesque, Kyoto, Japan) for 7 days. The antibiotic-resistant positive colonies (CHO-B2/EGFR) were picked up as a control. In the case of integrin α5-KO cells expressing GFP, WT, or mutant integrin α5, the GFP-positive cells were sorted three times using FACSAria II after lentivirus infection for 72 h. The stable cell lines were used in subsequent studies.
Western Blot (WB) and Immunoprecipitation
For WB, the indicated cells were washed with ice-cold PBS and then lysed in the cell lysate (20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Triton X-100) with protease and phosphatase inhibitors (Nacalai Tesque, Kyoto, Japan) for 30 min. After centrifugation at 1,000 × g for 10 min, the supernatant was collected, and protein concentrations were determined using a BCA protein assay kit (Pierce). The protein lysates were resolved by non-reducing SDS-PAGE for integrin α5 and β1 or reducing SDS-PAGE for other proteins. After electrophoresis, the proteins were transferred to a PVDF membrane (Millipore) and detected with the indicated primary and secondary antibodies using an Immobilon Western Chemiluminescent HRP Substrate (Millipore), according to the manufacturer's instructions. For immunoprecipitates, cells were lysed with detergent-free TBS buffer (20 mm Tris-HCl, pH 7.4, 150 mm NaCl) by being passed through a 21-gauge needle as described previously (18). Briefly, cells were resuspended in the TBS with protease and phosphatase inhibitors and lysed by being passed through a 21-gauge needle 30 times. After centrifugation at 1,000 × g for 10 min, the supernatant was collected. The remaining pellet was again syringed 30 times. After centrifugation at 1,000 × g for 10 min, the second postnuclear supernatant was combined with the first, and protein concentrations were determined using a BCA protein assay kit. Equivalent amounts (600 μg) of the supernatants were immunoprecipitated with anti-GFP-agarose, anti-EGFR antibody, or cholera toxin B subunit-biotin and Streptavidin-conjugated agarose for 1 h at 4 °C with rotation, and then the immunoprecipitates were washed twice with lysis buffer and subjected to 6% SDS-PAGE.
Cell Growth and Colony Formation Analysis
The growth of the indicated cells was estimated by determination of cell growth curves or colony formation assays. To assay the cell growth curves, the cells (3 × 104) were seeded in 6-cm dishes overnight and then serum-starved for either 24 or 48 h (for MDA-MB-231 cells). After starvation, the cells were supplied with DMEM containing 10% FBS with or without EGF (0.1 ng/ml), control IgG (10 μg/ml), or anti-EGFR-blocking Ab (10 μg/ml). The photographs of the same areas on the cultured dishes were taken at the indicated times (0, 24, 48, and 72 h), and the cell numbers were counted. Cell numbers were normalized to those at 0 h and statistically analyzed.
To assay the colony formation, the control, GFP, WT, and S3–5 group cells (0.6 × 103) were seeded in the 6-cm dishes. Cells were incubated for 14 days to allow colony formation, cells were stained with 0.25% crystal violet for 15 min, and images were taken. Quantification of the colonies was obtained by measuring the OD595 after digesting the colonies in each dish with 1 ml of 10% acetic acid. The OD595 values from GFP, WT, and S3–5 were normalized to that of the control cells.
Cell Surface Biotinylation
Cell surface biotinylation was performed as described previously with minor modifications (33). Briefly, cells were gently washed twice with PBS and then incubated with ice-cold PBS containing 0.2 mg/ml sulfo-NHS-SS-biotin for 1 h at 4 °C. After incubation, cells were washed three times with ice-cold PBS, and the cells were harvested and lysed in the lysis buffer. The biotinylated proteins were precipitated with Streptavidin-conjugated agarose and then detected by WB, as described above.
Flow Cytometry Analysis of Cells
Flow cytometric analysis was performed as described previously (32). Briefly, the indicated semiconfluent cells were detached from the 10-cm culture dishes using trypsin containing 1 mm EDTA and were subsequently stained with either the mouse IgG or primary mouse anti-α5β1 or anti-EGFR antibody for 1 h on ice, followed by incubation with Alexa Fluor® 647 goat anti-mouse IgG for 1 h. During the incubation, the cells were mixed gently every 10 min by flicking. After incubation, cells were washed three times with PBS and then analyzed using a FACSCalibur flow cytometer and Cell Quest Pro software (BD Biosciences).
Isolation of Detergent-free Lipid Raft Fractions
Preparation of detergent-free lipid raft fractions was preformed as described previously, with minor modifications (18). Briefly, 150-mm dishes of the indicated CHO-B2 cells were washed twice with PBS and then lysed twice with a total of 1 ml (each time 0.5 ml) of detergent-free base buffer (20 mm Tris·HCl, 250 mm sucrose, pH 7.8, containing 1 mm CaCl2 and 1 mm MgCl2) with protease and phosphatase inhibitors by being passed through a 21-gauge needle as described above. After centrifugation at 1,000 × g for 10 min, the postnuclear supernatant (∼1 ml) was mixed with the same volume of the base buffer containing 50% (v/v) OptiPrep and then added to the bottom of a 5-ml ultracentrifuge tube. Subsequently, each 0.6 ml of 20, 15, 10, 5, and 0% of OptiPrep in the base buffer was sequentially overlaid to the ultracentrifuge tube. The gradient was centrifuged at 5.2 × 104 rpm for 18 h at 4 °C, using an ultracentrifuge (Hitachi himac CS100GX). A total of 12 fractions (0.4 ml for each) were carefully collected from top to bottom of the gradient and analyzed by WB, as described above.
Cell-spreading Assay
The cell-spreading assay was performed as described previously with minor modifications (33). Briefly, 6-well plates were coated with FN (10 μg/ml) in PBS overnight at 4 °C and then blocked with 1% bovine serum albumin (BSA) in DMEM for 1 h at 37 °C. The indicated CHO-B2 cells were detached and suspended in serum-free DMEM with 0.1% BSA at 3 × 104 cells/ml. After replating on the FN-coated dishes for 20 min, non-adherent cells were removed by washing with PBS, and the attached cells were fixed with 4% paraformaldehyde in PBS, and representative photographs were then taken by phase-contrast microscopy.
Immunofluorescence
To assay the EGF-Alexa 555-based EGFR endocytosis, the indicated CHO-B2 cells were grown on coverslips (MatTek Corp., Ashland, MA) and starved for 24 h, followed by stimulation with a serum-free medium containing 50 ng/ml Alexa Fluor® 555-conjugated EGF (EGF-555) and 0.3 mm primaquine, a recycling inhibitor, for the indicated times (0, 2.5, 5, 10, and 15 min). Cells were washed and fixed in 4% paraformaldehyde for 20 min at room temperature, followed by two rinses with PBS. A nonspecific blocking solution was applied (PBS, 0.1% Triton X-100, 10% BSA) at room temperature for 1 h followed by incubation with TO-PRO-3 for 1 h in the dark. Cells were washed three times with PBS and were then immediately mounted using a fluorescent mounting medium (Dako). The confocal images were acquired using a ×60/1.35 numerical aperture oil immersion objective lens (FV1000; Olympus). The numbers of internalized EGF-555 puncta per cell in random fields were quantified.
Biotinylation-based EGFR Internalization Assay
The indicated CHO-B2 cells grown on 15-cm dishes were serum-starved for 5 h prior to the assay and washed in ice-cold PBS, and surface proteins were biotinylated with 0.2 mg/ml sulfo-NHS-SS-biotin in cold PBS for 1 h, followed by washing in TBS and placement on ice. For internalization, cells were then incubated in prewarmed DMEM containing 0.1 ng/ml EGF and 0.3 mm primaquine at 37 °C for the indicated duration (0, 2.5, 5, 10, and 15 min), whereas the control groups (without MesNa) remained on ice. Surface biotin was then stripped from the cells with a 10-min incubation in 50 mm MesNa in TBS (pH 8.6) twice, followed by washing and quenching of the MesNa with 20 mm iodoacetamide in TBS for 10 min. The control group cells were not subjected to surface reduction (no MesNa) in order to obtain total surface-labeled EGFR. After quenching, the cells were lysed, precipitated with Streptavidin-conjugated agarose, and subjected to WB. The percentage of internalized EGFR was calculated from the signal intensity of MesNa-resistant (internalized) EGFR at each time point relative to the control groups (no MesNa), which was the total EGFR on the cell surface in the three independent experiments.
Xenograft Assay
The left flank of NOD/SCID mice (5-week-old female mice; Charles River Laboratories, Japan) were injected subcutaneously with the indicated CHO-B2 cells (1 × 106). After 21 days of growth, tumors were dissected, and their volumes and weights were noted. Tumor volumes were calculated using the formula, V = (L × W2) × 0.5 (where V is volume, L is length, and W is width). All animal procedures were carried out according to experimental protocols approved by the Tohoku Pharmaceutical University Research Ethics Board.
Statistical Analysis
Results are presented as the mean ± S.E. Statistical analyses were performed using Student's t test using GraphPad Prism version 5. Statistical significance was defined as p < 0.05 (not significant (n.s), p > 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
Results
Inhibition of Cell Proliferation and Tumor Formation by the Expression of Integrin α5 via N-Glycosylation
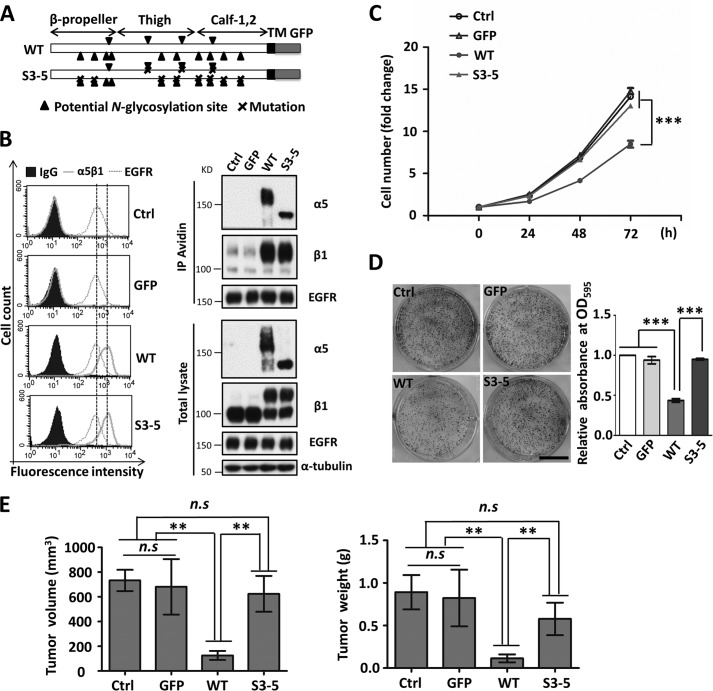
Given the evidence that integrin α5 cooperates with EGFR to drive cell cycle progression (40), we established a simple cell model to clarify the underlying molecular mechanism for the cooperation between integrin α5 and EGFR. First, human EGFR cDNA was transfected into the CHO-B2 cell line that lacks the α5 subunit, in order to establish a stable EGFR-overexpressed CHO-B2 cell line (CHO-B2/EGFR). The CHO-B2/EGFR cells then were respectively reconstituted with either a GFP-tagged wild-type (WT) or a N-glycosylation mutant α5 subunit, S3–5, which contained only three of the 14 N-glycosylation sites (Fig. 1A). The three sites on the β-propeller domain of integrin α5 are essential for α5β1 heterodimerization, cell surface expression, and cell adhesion in CHO-B2 cells (33). As shown in Fig. 1B, when analyzed by flow cytometry analysis and biotinylation, these cells exhibited almost the same expression levels of EGFR and α5β1 on the cell surface and in the whole-cell lysates, suggesting that the level of α5 subunits had no effect on the expression of EGFR. Unexpectedly, the expression of WT, but not S3–5 α5 subunits, significantly suppressed cell proliferation, compared with those found in control cells with or without overexpression of a GFP tag only (Fig. 1C). The colony formation consistently included colony numbers (Fig. 1D) and sizes (data not shown) that were clearly decreased in the WT, compared with those in the S3–5 and control cells.
FIGURE 1.
Effects of WT and S3–5 mutant α5 subunit expression on cell growth in CHO cells. A, schematic diagram of potential N-glycosylation sites on the WT and S3–5 integrin α5 subunit. Putative N-glycosylation sites (N84Q, N182Q, N297Q, N307Q, N316Q, N524Q, N530Q, N593Q, N609Q, N675Q, N712Q, N724Q, N773Q, and N868Q) are indicated by triangles, and point mutations are indicated by crosses. B, the control, GFP, WT, and S3–5 cells exhibited same expression levels of EGFR and integrin α5β1. The stable cell lines were established as described under “Experimental Procedures” section. The expression levels of integrin α5β1 and EGFR on the cell surfaces and in total cell lysates were analyzed by flow cytometry analysis (left) or biotinylation (top right) and WB with the indicated antibodies (bottom right), respectively. The IgG and α-tubulin were used as controls. C, the WT but not S3–5 transfectants exhibited a decrease in cell growth. After starvation for 24 h, cells were supplied with DMEM containing 10% FBS, and then cell numbers were counted and statistically analyzed at the indicated times (n = 3 individual experiments). D, colony formation ability was inhibited in WT but not S3–5 cells. Images of cell colonies are shown on the left. Colonies were stained with crystal violet after 14 days of seeding on plastic dishes. The OD595 values of GFP, WT, and S3–5 groups were normalized to that of the control group (n = 3 individual experiments). E, WT but not S3–5 cells showed a decrease in the tumorigenicity in vivo. The indicated cells (1 × 106) were injected subcutaneously into the left flank. Tumors were dissected, and their volumes (left) and weights (right) were noted after 21 days (n = 4). All values are means ± S.E. (error bars), Student's t test; n.s, not significant (p > 0.05); **, p < 0.01; ***, p < 0.001. Scale bar, 1 cm (D).
These in vitro observations encouraged us to investigate the effects of α5 expression on tumorigenicity in vivo. As shown in Fig. 1E, the control, GFP, and S3–5 cells permitted vigorous tumor formation. Tumor formation was significantly suppressed, however, in the WT cells, as reflected by both the tumor volume and weight. Taken together, these results suggested that the remaining N-glycosylation sites, with the noted exception of sites 3–5 of integrin α5, play an important role in the inhibition of cell growth and tumorigenicity.
EGFR-mediated Cellular Signaling Is Suppressed by the Expression of Wild-type Integrin α5
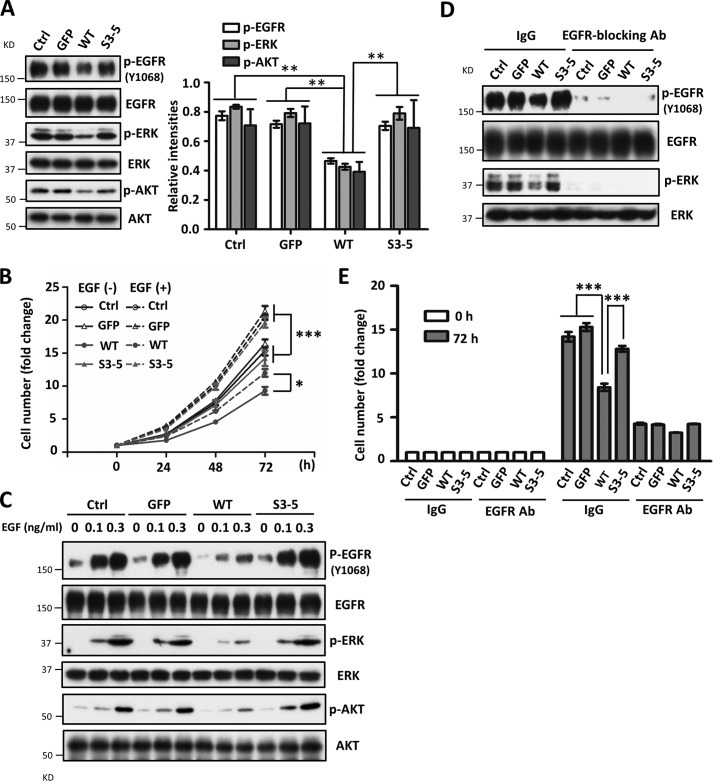
Considering the overexpression of EGFR in these cells, we wondered whether EGFR-mediated cellular signaling was modulated by α5. Surprisingly, under normal culture conditions, the phosphorylation levels of EGFR and its downstream molecules ERK and AKT were significantly decreased in WT cells compared with those in other cells (Fig. 2A). Furthermore, the responses for exogenous EGF-induced cell proliferation (Fig. 2B) and cellular signaling (Fig. 2C) were also significantly suppressed in WT cells but neither in S3–5 cells nor in the other control cells.
FIGURE 2.
Expression of WT but not S3–5 integrin α5 inhibits EGFR-mediated cellular signaling. A, EGFR-related cellular signaling was inhibited in the WT cells. Cell lysates from the indicated cells were subjected to WBs with the indicated antibodies (left). The relative ratios (phospho-EGFR, phospho-ERK, and phospho-AKT versus EGFR, ERK, and AKT, respectively) are shown on the right (n = 3 individual experiments). B, comparison of cell growth among WT, S3–5, and control cells upon EGF stimulation. After starvation, cells were supplied with complete medium with or without EGF (0.1 ng/ml). Cell numbers were counted at the indicated times and statistically analyzed (n = 3 individual experiments). C, expression of WT α5 decreased responses for EGF. After starvation for 24 h, cells were treated with EGF at the indicated concentrations for 5 min. WB analysis was performed with the indicated antibodies. D and E, effects of the treatment with anti-EGFR-blocking antibody on EGFR-related cellular signaling (D) and cell growth (E) under normal culture conditions (without EGF stimulation). Cells were cultured with complete medium containing 10 μg/ml IgG (control) or anti-EGFR-blocking Ab for 72 h, the resultant cell extracts were subjected to WB with the indicated antibodies (Ab) (D), and the cell numbers were counted and statistically analyzed (n = 3 individual experiments) (E), respectively. All values are the means ± S.E. (error bars), Student's t test; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Integrin α5 also is known to cooperate with other growth factor receptors, such as c-Met or VEGFR (41); thus, we next investigated the contributions of EGFR to cell growth. Both the phosphorylation levels of EGFR and ERK were completely blocked in the presence of anti-EGFR blocking antibody (Fig. 2D); meanwhile, the abilities and differences among cells for cell proliferation were largely cancelled (Fig. 2E). Together, these results supported our hypothesis that the N-glycosylation-mediated inhibitory effects of α5 on cell growth happen primarily thorough EGFR signaling.
Inhibitory Effects of Integrin α5 on Cell Growth and EGFR Cellular Signaling Are Also Observed in Several Human Cancer Cell Lines
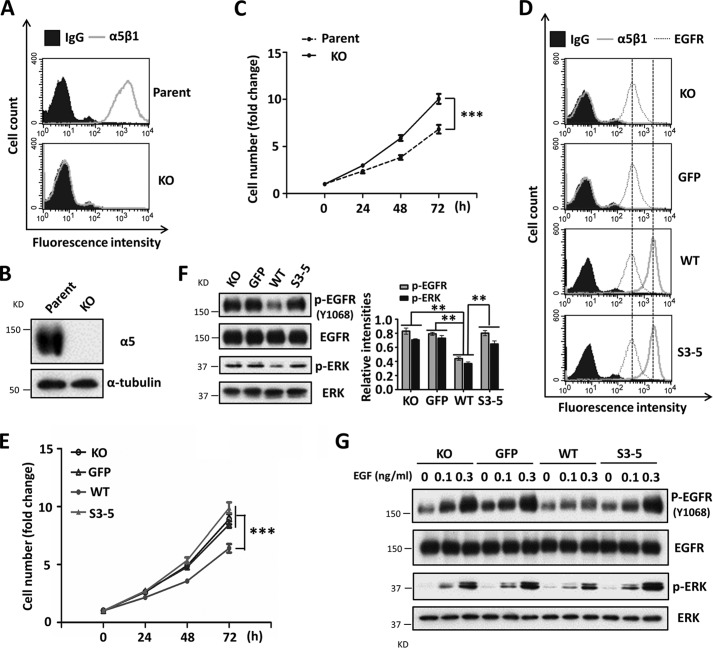
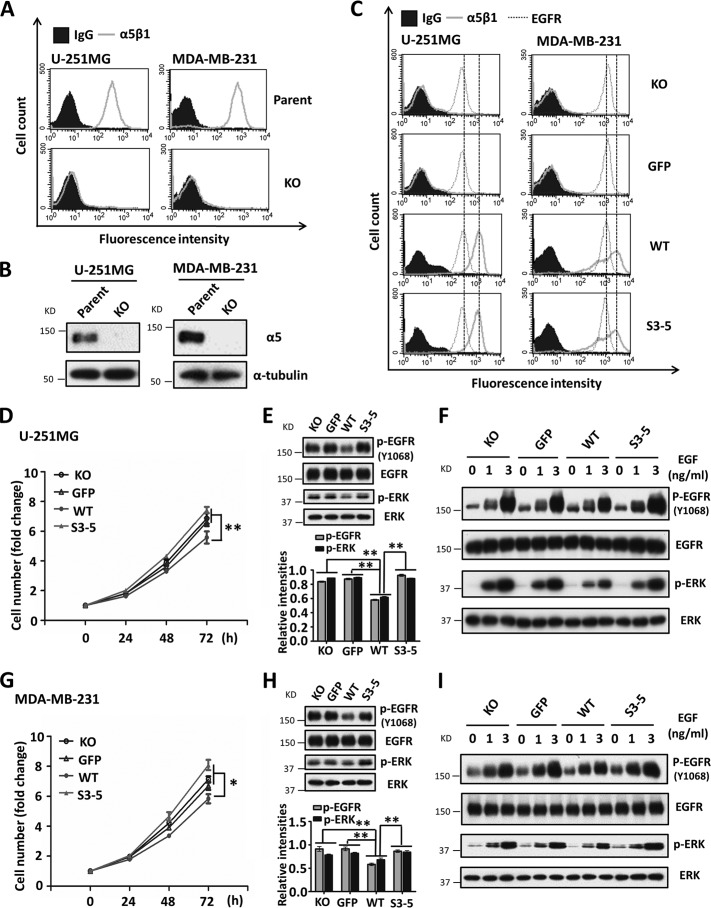
As described above, α5 negatively regulated EGFR-mediated signaling via N-glycosylation in CHO-B2/EGFR cells. Therefore, we selected several human cancer cell lines, such as HeLa, U-251MG, and MDA-MB-231 cells, which express relatively high levels of endogenous EGFR, in order to assess whether the phenomenon is common to other mammalian cells. To eliminate influences from endogenous α5, that gene was deleted by using a CRISPR/Cas9 knock-out system. The knock-out efficiencies were assessed by flow cytometry analysis and Western blot in HeLa, U-251MG, and MDA-MB-231 cells (Figs. 3 (A and B) and 4 (A and B)). As shown in Fig. 3C, the KO HeLa cells exhibited an increased proliferation ability compared with the parent cells, indicating that α5 also acts as a proliferation suppressor in HeLa cells. Furthermore, the α5-KO cells were reconstituted with a GFP tag, WT or S3–5, and each cell line showed similar expression levels of EGFR (Figs. 3D and 4C). Consistent with the data of CHO-B2 cells, the expression of WT cells had an inhibitory effect on cell proliferation (Figs. 3E and 4 (D and G)) and phospho-EGFR as well as subsequent downstream phospho-ERK (Figs. 3F and 4 (E and H)), which differed from the expression of S3–5 integrin α5 cells. Moreover, the responses to EGF were also attenuated in WT, compared with that in other cells (Fig. 3G). Almost the same tendencies were observed in the U-251MG and MDA-MB-231 cells (Fig. 4, F and I). Together, these results further suggest that integrin α5 is a negative regulator for EGFR-mediated signaling through N-glycosylation. To clarify the underlying molecular mechanism, we further employed CHO-B2/EGFR cells in subsequent experiments.
FIGURE 3.
Expression of integrin α5 inhibits cell growth and EGFR cellular signaling in HeLa cells. A and B, the efficiency of the knock-out of the α5 gene using the CRISP/Cas9 system was assessed by flow cytometry analysis (A) and WB (B). The integrin α5-KO HeLa cells were established as described under “Experimental Procedures.” C, the α5 KO-HeLa cells exhibited an increased cell growth ability. An analysis of cell growth was performed as described in the legend to Fig. 1C (n = 3 individual experiments). D, expression levels of integrin α5β1 and EGFR on the cell surface were assessed by flow cytometry analysis among revived cells with WT and S3–5 mutant of α5 in the α5 KO-HeLa cells. E, comparison of cell growth among those transfectants. An analysis of the KO, GFP, WT, and S3–5 HeLa cell growth was performed as described above (n = 3 individual experiments). F, the phosphorylation levels of EGFR and ERK were significantly suppressed in the WT cells, compared with the other cells. Left, WB pattern; right, quantitative analysis (n = 3 individual experiments). G, the WT cells also exhibited attenuated EGFR responses upon EGF stimulation. The responses to the indicated concentrations of EGF were performed by WB as described in Fig. 2C. All values are means ± S.E. (error bars), Student's t test; **, p < 0.01; ***, p < 0.001.
FIGURE 4.
Expression of integrin α5 also inhibits cell growth and EGFR cellular signaling in U-251MG and MDA-MB-231 cells. The relevant integrin α5-KO cells were established as described under “Experimental Procedures.” The established α5-KO U-251MG and MDA-MB-231 cells were assessed by flow cytometry analysis (A) and WB (B). C, the expression levels of integrin α5β1 and EGFR in the relevant integrin α5-rescued U-251MG and MDA-MB-231 cells were assessed by flow cytometry analysis. IgG and α-tubulin were used as controls. D–I, these rescued cells with WT, but not S3–5, α5 also exhibited growth retardations, decrease in EGFR signaling, and attenuated EGF responses. These phenomena were similar to those observed in HeLa cells, as shown in Fig. 3. All values are the means ± S.E. (error bars), Student's t test; *, p < 0.05; **, p < 0.01 (n = 3 individual experiments).
Expression of Integrin α5 Delays EGFR Internalization upon EGF Stimulation
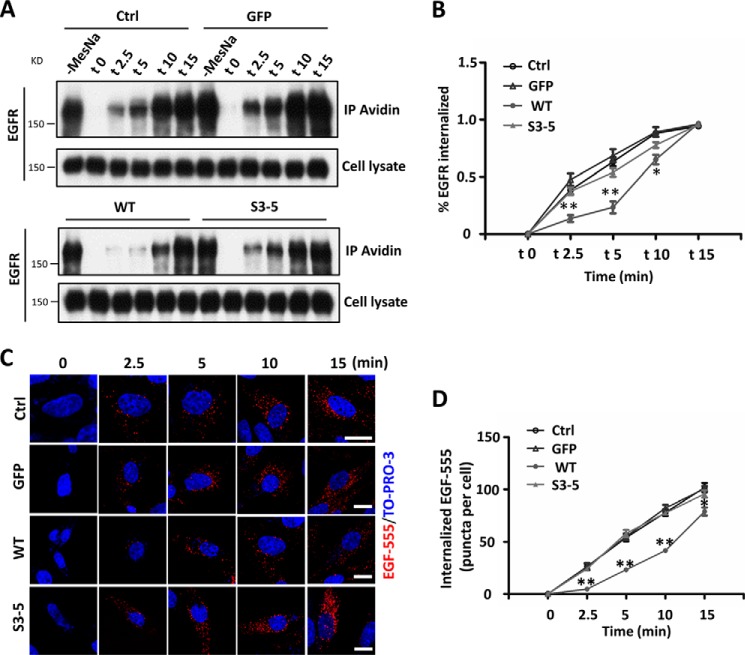
Upon EGF binding, EGFR is involved in a series of trafficking events, including internalization, degradation, and recycling, which ultimately regulate its signal amplification and propagation (42). Also, integrins are known to regulate the trafficking of some membrane proteins (43). Therefore, we conducted a biotinylation-based internalization assay (Fig. 5A) and an EGF-555-based EGFR endocytosis assay (Fig. 5B) in order to determine if the kinetics of EGFR internalization are affected by α5. To increase sensitivity for detection, primaquine, a receptor recycling inhibitor, was added to the culture medium to block the recycling of internalized EGFR to the cell membrane. For the EGF-555-based EGFR endocytosis assay, the cells were stimulated by EGF-555 at 10, 30, and 50 ng/ml concentrations, which are approximately equal to 1, 3, and 5 ng/ml EGF, respectively, because the molecular mass of EGF-555 (∼63 kDa) is about 10 times higher than that of EGF (6 kDa). We found that the treatment at 50 ng/ml provided clear signals (Fig. 5B), and this concentration was also used in other cells (18). A lower concentration, such as 10 or 30 ng/ml, showed no, or only marginal, endocytosis signals (data not shown). Of particular interest, the EGFR internalization after EGF treatment during the first 10-min duration was greatly retarded in the WT cells, compared with that in both the S3–5 and control cells, although the levels of the internalized EGFR were similar at the 15-min chase point. These results clearly indicated that EGFR internalization was delayed by α5 via N-glycosylation, which might explain why the EGFR signaling was inhibited in the WT cells.
FIGURE 5.
Integrin α5 decreases EGFR internalization upon EGF stimulation in CHO-B2 cells. A, the internalization of EGFR was inhibited in WT cells, compared with the other cells. A biochemical internalization assay of EGFR was performed, as described under “Experimental Procedures.” The internalized EGFRs at indicated times were immunoprecipitated by avidin-agarose and then subjected to WB for detection of EGFR. The cell lysates were used as controls to show similar expression levels of EGFR among these cells. B, the rates of internalized EGFR were statistically analyzed (right; n = 3 individual experiments). C, EGFR endocytosis was inhibited in the WT but not S3–5 cells upon EGF stimulation. Shown is a representative image for EGFR endocytosis after 50 ng/ml EGF-555 treatment at the indicated times. The image was merged with EGF-555 and TO-PRO-3 staining. D, the numbers of internalized EGF-555 puncta/cell in random fields were quantified (n = 9, from triplicate experiments). All values are means ± S.E. (error bars), Student's t test; *, p < 0.05; **, p < 0.01; ***, p < 0.001. Scale bar, 10 μm (C).
Integrin α5 Interacts with EGFR and Plays a Critical Role in EGFR Localization in Lipid Rafts
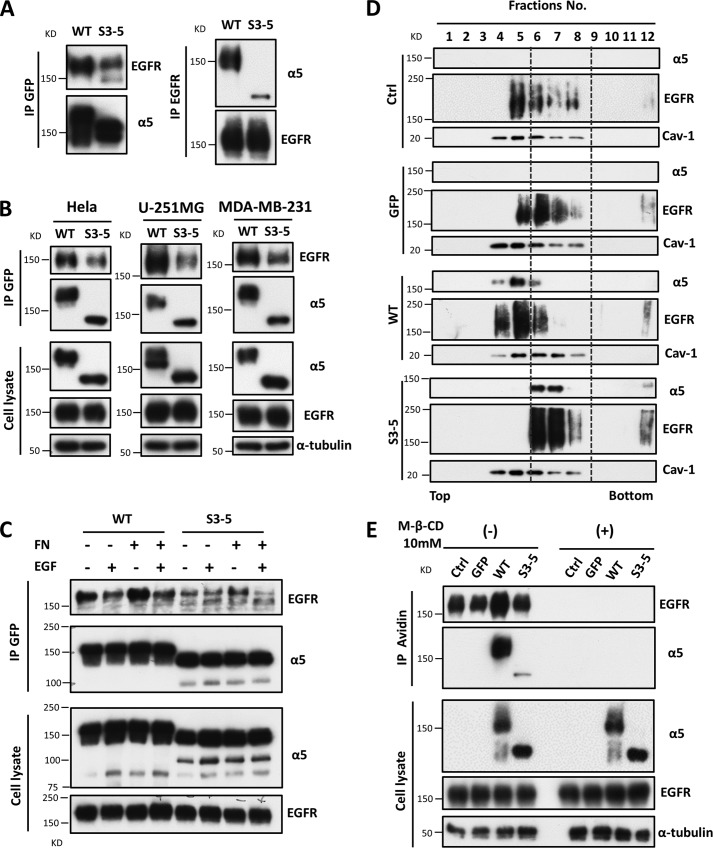
As described above, the N-glycosylation of integrin α5 regulated EGFR internalization and activation. Therefore, we wondered how N-glycosylation participates in regulation and whether integrin α5 is associated with EGFR through the N-glycosylation of α5. Reciprocal immunoprecipitates with anti-GFP-agarose and anti-EGFR antibody showed that integrin α5 indeed interacted with EGFR (Fig. 6A), which was consistent with previous reports (44). This interaction was significantly decreased in the S3–5 cells by comparison with WT (Fig. 6A). The decreased interactions between S3–5 integrin and EGFR were also observed in HeLa, U-251MG, and MDA-MB-231 cells (Fig. 6B). These results suggest that the N-glycosylation of integrin α5 is required for the interaction between integrin α5 and EGFR. Next, we wondered whether this interaction is also modulated by EGF stimulation. As shown in Fig. 6C, the interaction was decreased following EGF stimulation both in WT and S3–5 cells. Additionally, the interaction was enhanced when those cells were attached to FN-coated dishes, but it was decreased upon EGF stimulation. These results strongly suggested that the interaction of EGFR and integrin α5 restricts EGFR activation and decreases EGFR responsiveness upon EGF treatment.
FIGURE 6.
Integrin α5 associates with EGFR and regulates EGFR localization in lipid rafts. A, the association of integrin α5 with EGFR was decreased in S3–5 cells compared with the WT cells. The indicated CHO-B2 cell extracts were immunoprecipitated (IP) with anti-GFP agarose (left panels) or anti-EGFR antibody (right panels), and then subjected to WB, reciprocally followed by anti-EGFR and integrin α5 antibodies for detection. B, the interaction between integrin α5 and EGFR was also decreased in the HeLa, U-251MG, and MDA-MB-231 S3–5 mutant cells. The indicated cell extracts were immunoprecipitated as described in A. Crude cell extracts were also subjected to WB as “input” using the indicated antibodies (bottom panels). C, the interaction between integrin α5 and EGFR was decreased upon EGF stimulation. The indicated CHO-B2 cells were cultured on dishes coated with or without 10 μg/ml FN for 24 h and then stimulated with or without EGF at 0.1 ng/ml for 5 min. The resultant cell lysates (as an input; bottom panels) were directly blotted with anti-EGFR and integrin α5 antibodies or immunoprecipitated with anti-GFP agarose (top panels) and then blotted with anti-EGFR antibody. D, comparison of localization of EGFR and integrin α5 in the lower density lipid rafts in the indicated CHO-B2. Distributions of integrin α5 and EGFR from the indicated cell lysates in the lipid raft fractions were prepared as described under “Experimental Procedures.” Fraction 1 is the top fraction of the gradient, and fraction 12 is the bottom fraction. The localization shifts of the integrin α5 and EGFR are highlighted with gray dotted lines. Caveolin-1 (Cav-1) was used to act as a marker for lipid rafts. E, the complex of EGFR and integrin α5 is mediated by GM1. The indicated CHO-B2 cells were pretreated with or without methyl-β-cyclodextrin (M-β-CD) (10 mm) for 1 h. After the treatment, cell lysates were incubated with biotinylated cholera toxin B subunit (CTB) for another 1 h and then immunoprecipitated with avidin-agarose beads. The immunoprecipitates were subjected to WB detected with anti-EGFR and integrin α5 antibodies (top panels). The whole cell lysates were also subjected to WB with the indicated antibodies (bottom panel; as an input).
Given the evidence that EGFR localizes in the lipid rafts, which results in its signal transduction (10, 18, 45), we speculated that the N-glycans on integrin α5 might affect the distribution of EGFR in the lipid rafts on a cell membrane. When using the OptiPrep density gradient ultracentrifugation method for lipid raft fractions, the EGFR and integrin α5 from each of the cell lines were basically distributed in the fractions ranging from 4 to 8, in which caveolin-1, a marker for lipid raft, could be detected (Fig. 6D). Interestingly, the distribution of EGFR and α5 was shifted from the low-density fractions (fractions 4 and 5) in the WT cells to higher density fractions (fractions 6–8) in the S3–5 and control cells, indicating that the N-glycans on integrin α5 may be a switch that influences EGFR and integrin α5 localization on the cell membrane.
The glycosylation of integrin α5 could affect its translocation into or out of a glycosphingolipid-enriched microdomain (46); thus, we tested its association with ganglioside GM1 and EGFR in these cells. Co-immunoprecipitation using biotinylated cholera toxin subunit B, which specifically binds ganglioside GM1, showed that the association of EGFR and α5 was much higher in the WT cells than it was in either the S3–5 or the control cells (Fig. 6E). These interactions among GM1, EGFR, and α5 were completely disrupted in the presence of methyl-β-cyclodextrin, a cholesterol depletion reagent. Taken together, these results clearly showed that the N-glycosylation of α5 plays a crucial role in its localization and complex formation with other receptors on the cell membrane.
N-Glycosylation on the Calf-1,2 Domain of Integrin α5 Is Important for Its Inhibitory Functions
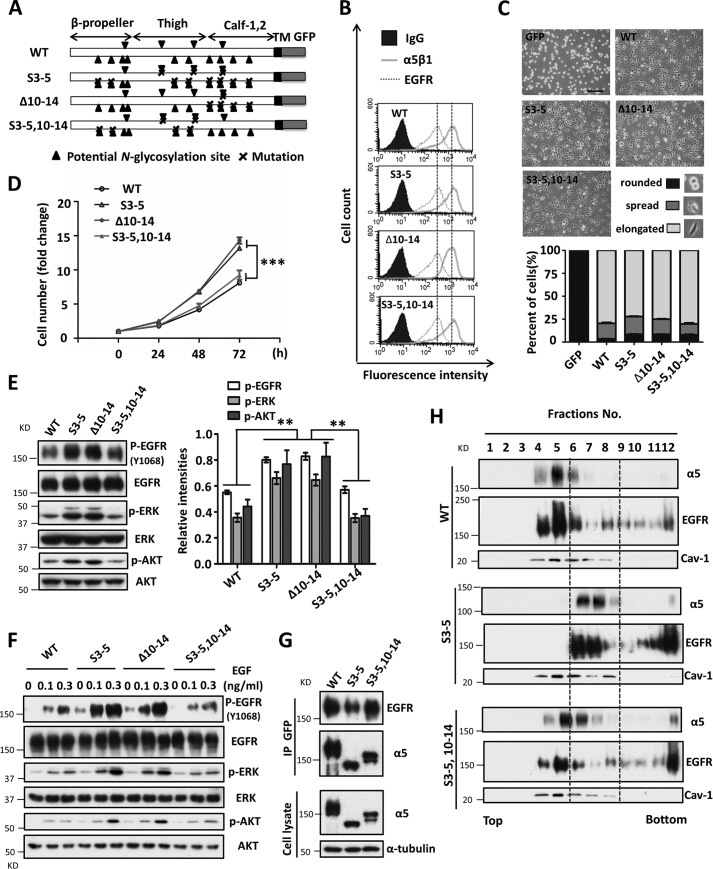
The data provided above led us to seek the N-glycosylation site(s) of α5 that was essential for its growth inhibitory effect. To address this, we focused on the N-glycosylation of the Calf domain (sites 10–14) (Fig. 7A), which happens in the vicinity of the cell membrane. Therefore, we restored N-glycosylation on the Calf domain in the S3–5 mutant cells (S3–5,10–14). Both of the N-glycosylation-rescued cell lines (Δ10–14 and S3–5,10–14) exhibited similar expression levels of EGFR and α5β1 on the cell surface (Fig. 7B) and abilities for cell spreading on FN (Fig. 7C), compared with those in the S3–5 cells. Restoration of N-glycosylation on the Calf domain in the S3–5 mutant, S3–5,10–14, decreased cell proliferation, which was similar to that in WT cells (Fig. 7D). Furthermore, deletion of the N-glycosylation sites on the Calf domain (Δ10–14) of α5 abolished its inhibitory effects on cell growth (Fig. 7D). These results indicated that N-glycosylation on sites 10–14 of α5 plays a crucial role in its inhibitory functions. Consistently, the levels of phospho-EGFR, phospho-ERK, and phospho-AKT in S3–5,10–14 cells were similar to those in the WT but different from those in S3–5 or Δ10–14 cells (Fig. 7E). The attenuated response to EGF in WT cells was also observed in S3–5,10–14 but not Δ10–14 cells (Fig. 7F). The decreased association with EGFR (Fig. 7G) and the aberrant localization of EGFR and α5 in lipid rafts (Fig. 7H) in S3–5 cells could be reversed in the S3–5,10–14 cells, which was similar to the situation in the WT cells. Taken together, these results clearly showed that the N-glycosylation on the Calf-1,2 domain of integrin α5 plays a crucial role in the regulation of EGFR-mediated cell signaling, which demonstrates a novel regulator for EGFR inhibition.
FIGURE 7.
N-Glycosylation on the Calf-1,2 domain of integrin α5 mediates its growth-inhibitory function. A, schematic diagram of potential N-glycosylation mutational integrin α5 subunit (WT, S3–5, Δ10–14, and S3–5,10–14). B, the integrin α5 mutant cells express equally α5β1 and EGFR levels on cell surface, compared with the WT cells. The stable rescued CHO-B2 cell lines (10–14 and S3–5,10–14) were also established as described under “Experimental Procedures.” The expression levels of both α5β1 and EGFR on cell surface were analyzed by flow cytometry analysis. The IgG was used as a control. C, the α5 mutant cells exhibited comparable abilities for cell spreading as WT ones. Cells were detached and then replanted on the FN-coated dishes. After incubation for 20 min, cells were fixed, and the images were taken. The percentages of the rounded, spread, and elongated cells were statistically analyzed (bottom panel, n = 9, from triplicate experiments). D, the S3–5,10–14, but not 10–14 cells exhibit a similar inhibitory ability for cell growth, compared with the WT cells. The cell growth abilities of WT, S3–5,10–14, and S3–5, 10–14 were analyzed as described in the legend to Fig. 1C (n = 3 individual experiments). E, the EGFR-related cellular signaling was revived in the S3–5,10–14 cells. Cell lysates from the indicated cells were subjected to WB with indicated antibodies (left). The relative ratios were statistically analyzed (right, n = 3 individual experiments). F, the response to EGF was decreased in S3–5,10–14 cells. Cells were serum-starved for 24 h, followed by a treatment with the indicated concentrations of EGF for 5 min, and then the resultant cell extracts were subjected to WB analysis with indicated antibodies. G, the interaction between integrin α5 and EGFR was also rescued in the S3–5,10–14 cells. The indicated cell extracts were immunoprecipitated with anti-GFP agarose, followed by anti-EGFR and α5 antibodies for WB (top panels). The whole cell extracts were also subjected to WB using the indicated antibodies (bottom panels; as an input). H, the localizations of EGFR and integrin α5 in the lower density lipid raft were also revived in the S3–5,10–14 cells. Distributions of integrin α5 and EGFR in lipid raft fractions were detected, as described in the legend to Fig. 6D. The localization shifts of integrin α5 and EGFR are highlighted with gray dotted lines. The caveolin-1 (Cav-1) was used to act as a positive control for lipid rafts. All values are means ± S.E. (error bars), Student's t test; **, p < 0.01; ***, p < 0.001. Scale bar, 120 μm.
Discussion
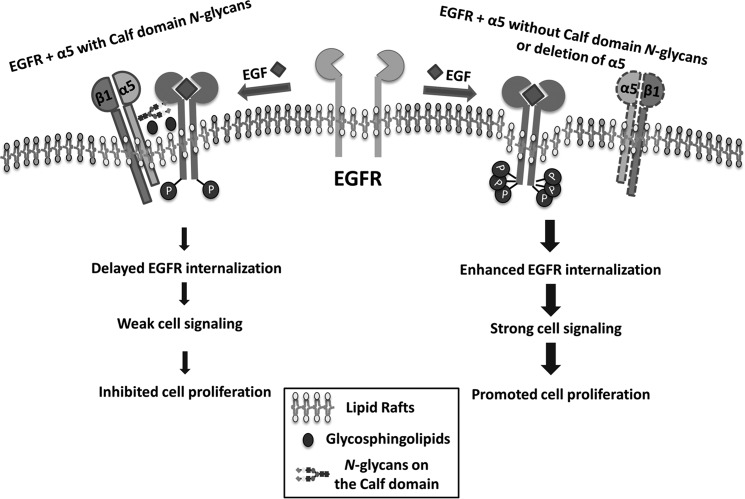
We describe here how cell growth could be down-regulated by integrin α5 and clearly demonstrate how the N-glycosylation of α5 is a key factor for α5 regulation of EGFR signaling. Among 14 potential N-glycosylation sites of α5, sites on the Calf domain played a crucial role in the inhibitory effect on EGFR-mediated signaling, through regulation of the complex formation between EGFR and α5, localization in lipid rafts, and internalization (Fig. 8). Thus, the present study outlines the novel underlying mechanism responsible for the inhibition of EGFR and also provides new insight into the role of N-glycosylation in the regulation of cellular signaling to maintain cell properties via a cross-talk manner among glycoproteins on the cell surface.
FIGURE 8.
Proposed molecular mechanism for the regulation of EGFR cellular signaling by the N-glycosylations of integrin α5. In the integrin α5 with N-glycosylation on its Calf domain cells (left), integrin α5β1 forms a complex with EGFR as well as glycosphingolipids in the lipid raft, which may restrict the EGFR internalization and the related signaling upon EGF stimulation, resulting in an inhibition of cell proliferation. However, in the integrin α5-deficient or mutant (deletion of N-glycosylation on its Calf domain) cells (right), the majority of EGFR is located in lipid rafts without integrin α5, leading to a rapid activation and internalization of EGFR upon EGF stimulation. The thick arrow lines indicate a rapid EGF response and strong signal transduction, whereas the thinner arrow lines indicate that these events are inhibited.
Integrin α5 is thought to play crucial roles in many biological functions, but there are several controversial reports concerning the functions of integrin α5 in cancer. Low expression levels of α5 have been linked to the growth of tumors in gastric, colorectal, colon, and breast cancers (47), whereas the overexpression of integrin α5 inhibited cell growth in several cell lines, such as 4T1, HT29, and CHO (48–52). Furthermore, integrin α5 has been used to down-regulate the HER2 pathway in Caco-2 and HT-29 cells (34). However, these findings are contradicted by other studies showing that integrin α5 functions as an oncogene and was associated with several instances of tumorigenesis (53, 54) as well as promoting EGFR activation in some cancer cells (35, 44, 55). The reasons for the contradictions in the functioning of α5 in cancers remain unclear. Of course, it could be speculated that α5 might differentially affect cellular signaling in different cell types or may exhibit intrinsic limitations in different cell models. However, based on our observation, integrin α5 inhibits cell proliferation through a negative regulation of EGFR via N-glycosylation and shows tumor suppressor-like activity. Coincidently, similar to integrin α5, integrin α1 also negatively regulated EGFR (26). We believe that the different effects of α5 on cell growth can be ascribed to distinct N-glycosylations, which may be altered in different cells and tissues and cancers. Further investigation is obviously needed to support this hypothesis.
The N-glycosylation of integrin α5 plays crucial roles in several biological functions, including cell adhesion and cell migration. However, little is known about the functioning of individual N-glycosylation. We previously underscored the importance of N-glycans on N-glycosylation potential sites 3–5 of α5 in its assembly of β1 (33). Although the interaction-mediated cross-talk between α5 and EGFR was described previously (41, 44), the precise underlying mechanisms of how α5 regulates EGFR signaling and the molecular basis for this interaction remain unclear. In the present study, we find that the N-glycosylation on the Calf domain is essential for integrin α5-EGFR complex formation and is a key regulator for EGFR-mediated signaling. Most studies have focused on the cytoplasmic domain of integrins for the regulation of cell functions. For example, Caswell et al. (56) reported that the Rab-coupling protein could mediate the complex formation between integrin α5 and EGFR through their cytoplasmic domains to regulate cell migration; Mattila et al. (26) described how integrin α1 negatively regulates EGFR-mediated cellular signaling through the activation of a protein tyrosine phosphatase in the cytosol. These theories are plausible because any output (downstream) must happen inside a cell. However, these explanations are insufficient to clarify the molecular mechanisms in detail. In the present study, we highlight an input (upstream), post-translational modification of the extracellular domains of α5 and show its importance in complex formation and cellular signaling. Similar to integrin α5, we previously showed how the N-glycosylation on integrin β4 regulates its association with EGFR and EGFR signaling (12). Recently, Paszek et al. (22) demonstrated that a bulky glycocalyx in ECM might facilitate integrin clustering and then promote a tumor phenotype by increasing integrin adhesion and signaling. Those results further support the notion that glycosylation plays an important role in protein-protein interaction (57).
It is worth noting that the N-glycans on the Calf domain of α5 represent a key switch for the regulation of EGFR-mediated signaling. Previous study of the crystal structure of the ectodomain of integrin α5β1 showed that the N-glycans on α5, particularly those surrounding the RGD-binding pocket, play an important role in its binding to FN (58). Thus, based on molecular modeling, we speculate that the N-glycans of a Calf domain may not participate in the cell adhesion process, but, instead, a location near the cell membrane suggests that they may be involved in the association with other molecules on the cell surface. In fact, restoration of all N-glycosylation sites on the Calf domain (sites 10–14) in an S3–5 mutant completely rescued the complex formation and the capacity for inhibitory effects of EGFR-mediated signaling, which were observed in wild-type α5-expressing cells. In consideration of the fact that the specific conformation of integrin α5β1 is essential for its mediated functions (59–61), future studies will require an extensive analysis of the structures of these N-glycosylation mutants as well as that of specific N-glycans on individual sites.
It is well known that an appropriate lipid raft formation is required for normal EGFR signaling transduction (62, 63). We demonstrate here that N-glycosylation on integrin α5 serves as a regulator for its association with EGFR and GM1, one of the gangliosides in lipid rafts, which regulates the EGF response. In fact, other gangliosides, such as GM3 and GD3, can also interact with the N-glycans on EGFR and regulate EGFR signaling, indicating the importance of the carbohydrate-to-carbohydrate interaction in cellular signaling (12, 18, 20, 62, 63). These results lead us to speculate that the N-glycans on the Calf domain may be the most suitable for those interactions among the α5, EGFR, and gangliosides at a distance. Of course, more evidence is needed to prove this hypothesis. Although the underlying mechanism for the complex formation that down-regulates EGFR internalization upon EGF stimulation remains unclear, the observation could partly explain why the cell growth was inhibited in the wild-type α5 cells because enhancement of EGFR internalization and recycling plays an important role in signal transduction for persistent proliferation and tumor carcinogenesis in cells (42, 64).
Given the existence of the mutations of several integrins and EGFR during pathological processes (65–67), particularly the N-glycosylation mutation of integrin α3 (68), and the apparent irregular expression of integrin α5 in multiple tumors (47, 53, 54), it is tempting to speculate that the N-glycosylation of integrin α5 may undergo mutations during tumorigenesis. Integrin α5 modified by different N-glycosylations exhibited different effects on cell proliferation and tumorigenesis, suggesting a possibility that the remodeling of N-glycosylation on this integrin may serve as a novel approach to tumor treatment. Furthermore, the present study clearly demonstrates how specific N-glycosylation on integrin α5 functions as a negative regulator for EGFR, which may provide a new perspective on the cross-talk between growth factor receptors and integrins.
Author Contributions
Q. H. performed all of the experiments with the help of T. I., T. F., S. H., and S. I. Q. H. and T. I. constructed the virus expression and the α5-knockout vectors. Q. H., T. I., and S. I. performed the cell-sorting experiments. Q. H., T. F., and S. H. contributed the generation of xenograft tumors. J. G. designed the experiment. Q. H. and J. G. analyzed the data, prepared the figures, and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Acknowledgments
We thank Dr. Motoko Takahashi (Sapporo Medical University), Prof. Rudolf Juliano (School of Medicine, University of North Carolina, Chapel Hill, NC), and Prof. Jun Nakayama (Shinshu University Graduate School of Medicine) for kindly providing the cDNA of human EGFR, α5 subunit-deficient CHO-K1 cell line (CHO-B2), and U-251MG cell line, respectively.
This work was supported in part by Grants-in-Aid for Scientific Research 15H04354 (to J. G.) and 24570169 (to T. I.) and for Challenging Exploratory Research 15K14408 (to J. G.) from the Japan Society for the Promotion of Science and by a Strategic Research Foundation Grant-aided Project for Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The authors declare that they have no conflicts of interest with the contents of this article.
- EGFR
- epidermal growth factor receptor
- FN
- fibronectin
- WB
- Western blot
- EGF-555
- Alexa Fluor® 555-conjugated EGF
- GM1
- Galβ1,3GalNAcβ1,4(NeuAcα2,3)-Galβ1,4Glc-ceramide
- GM3
- NeuAcα2,3Galβ1,4Glc-ceramide
- GD3
- Neu5Acα2,8Neu5Acα2,3Galβ1,4Glc-ceramide.
References
- 1.Bogdan S., and Klämbt C. (2001) Epidermal growth factor receptor signaling. Curr. Biol. 11, R292–R295 [DOI] [PubMed] [Google Scholar]
- 2.Citri A., and Yarden Y. (2006) EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 7, 505–516 [DOI] [PubMed] [Google Scholar]
- 3.Hynes N. E., and Lane H. A. (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5, 341–354 [DOI] [PubMed] [Google Scholar]
- 4.Izumi Y., Xu L., di Tomaso E., Fukumura D., and Jain R. K. (2002) Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 416, 279–280 [DOI] [PubMed] [Google Scholar]
- 5.Salomon D. S., Brandt R., Ciardiello F., and Normanno N. (1995) Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 19, 183–232 [DOI] [PubMed] [Google Scholar]
- 6.Grandis J. R., and Sok J. C. (2004) Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol. Ther. 102, 37–46 [DOI] [PubMed] [Google Scholar]
- 7.Hampton K. K., and Craven R. J. (2014) Pathways driving the endocytosis of mutant and wild-type EGFR in cancer. Oncoscience 1, 504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mlcochova J., Faltejskova P., Nemecek R., Svoboda M., and Slaby O. (2013) MicroRNAs targeting EGFR signalling pathway in colorectal cancer. J. Cancer Res. Clin. Oncol. 139, 1615–1624 [DOI] [PubMed] [Google Scholar]
- 9.Sebastian S., Settleman J., Reshkin S. J., Azzariti A., Bellizzi A., and Paradiso A. (2006) The complexity of targeting EGFR signalling in cancer: from expression to turnover. Biochim. Biophys. Acta 1766, 120–139 [DOI] [PubMed] [Google Scholar]
- 10.Coskun Ü., Grzybek M., Drechsel D., and Simons K. (2011) Regulation of human EGF receptor by lipids. Proc. Natl. Acad. Sci. U.S.A. 108, 9044–9048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guéguinou M., Gambade A., Félix R., Chantôme A., Fourbon Y., Bougnoux P., Weber G., Potier-Cartereau M., and Vandier C. (2015) Lipid rafts, KCa/ClCa/Ca channel complexes and EGFR signaling: novel targets to reduce tumor development by lipids? Biochim. Biophys. Acta 1848, 2603–2620 [DOI] [PubMed] [Google Scholar]
- 12.Kariya Y., and Gu J. (2011) N-Glycosylation of β4 integrin controls the adhesion and motility of keratinocytes. PLoS One 6, e27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lajoie P., Partridge E. A., Guay G., Goetz J. G., Pawling J., Lagana A., Joshi B., Dennis J. W., and Nabi I. R. (2007) Plasma membrane domain organization regulates EGFR signaling in tumor cells. J. Cell Biol. 179, 341–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown D. A., and London E. (2000) Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275, 17221–17224 [DOI] [PubMed] [Google Scholar]
- 15.Simons K., and Toomre D. (2000) Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 [DOI] [PubMed] [Google Scholar]
- 16.Lingwood D., and Simons K. (2010) Lipid rafts as a membrane-organizing principle. Science 327, 46–50 [DOI] [PubMed] [Google Scholar]
- 17.Munro S. (2003) Lipid rafts: elusive or illusive? Cell 115, 377–388 [DOI] [PubMed] [Google Scholar]
- 18.Wang J., and Yu R. K. (2013) Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal ability of mouse neural stem cells in vitro. Proc. Natl. Acad. Sci. U.S.A. 110, 19137–19142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X. Q., Sun P., and Paller A. S. (2005) Gangliosides inhibit urokinase-type plasminogen activator (uPA)-dependent squamous carcinoma cell migration by preventing uPA receptor/αβ integrin/epidermal growth factor receptor interactions. J. Invest. Dermatol. 124, 839–848 [DOI] [PubMed] [Google Scholar]
- 20.Yoon S. J., Nakayama K., Hikita T., Handa K., and Hakomori S. I. (2006) Epidermal growth factor receptor tyrosine kinase is modulated by GM3 interaction with N-linked GlcNAc termini of the receptor. Proc. Natl. Acad. Sci. U.S.A. 103, 18987–18991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Midgley A. C., Rogers M., Hallett M. B., Clayton A., Bowen T., Phillips A. O., and Steadman R. (2013) Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J. Biol. Chem. 288, 14824–14838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paszek M. J., DuFort C. C., Rossier O., Bainer R., Mouw J. K., Godula K., Hudak J. E., Lakins J. N., Wijekoon A. C., Cassereau L., Rubashkin M. G., Magbanua M. J., Thorn K. S., Davidson M. W., Rugo H. S., Park J. W., Hammer D. A., Giannone G., Bertozzi C. R., and Weaver V. M. (2014) The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X. Q., Sun P., and Paller A. S. (2003) Ganglioside GM3 blocks the activation of epidermal growth factor receptor induced by integrin at specific tyrosine sites. J. Biol. Chem. 278, 48770–48778 [DOI] [PubMed] [Google Scholar]
- 24.Yamada K. M., and Even-Ram S. (2002) Integrin regulation of growth factor receptors. Nat. Cell Biol. 4, E75–E76 [DOI] [PubMed] [Google Scholar]
- 25.Larsen M., Artym V. V., Green J. A., and Yamada K. M. (2006) The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 18, 463–471 [DOI] [PubMed] [Google Scholar]
- 26.Mattila E., Pellinen T., Nevo J., Vuoriluoto K., Arjonen A., and Ivaska J. (2005) Negative regulation of EGFR signalling through integrin-α1β1-mediated activation of protein tyrosine phosphatase TCPTP. Nat. Cell Biol. 7, 78–85 [DOI] [PubMed] [Google Scholar]
- 27.Miranti C. K., and Brugge J. S. (2002) Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4, E83–E90 [DOI] [PubMed] [Google Scholar]
- 28.Schwartz M. A., and Ginsberg M. H. (2002) Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4, E65–E68 [DOI] [PubMed] [Google Scholar]
- 29.Seguin L., Kato S., Franovic A., Camargo M. F., Lesperance J., Elliott K. C., Yebra M., Mielgo A., Lowy A. M., Husain H., Cascone T., Diao L., Wang J., Wistuba I. I., Heymach J. V., Lippman S. M., Desgrosellier J. S., Anand S., Weis S. M., and Cheresh D. A. (2014) An integrin β3-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol. 16, 457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streuli C. H., and Akhtar N. (2009) Signal co-operation between integrins and other receptor systems. Biochem. J. 418, 491–506 [DOI] [PubMed] [Google Scholar]
- 31.Liu S., Calderwood D. A., and Ginsberg M. H. (2000) Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 113, 3563–3571 [DOI] [PubMed] [Google Scholar]
- 32.Isaji T., Sato Y., Fukuda T., and Gu J. (2009) N-Glycosylation of the I-like domain of β1 integrin is essential for beta1 integrin expression and biological function: identification of the minimal N-glycosylation requirement for α5β1. J. Biol. Chem. 284, 12207–12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isaji T., Sato Y., Zhao Y., Miyoshi E., Wada Y., Taniguchi N., and Gu J. (2006) N-Glycosylation of the β-propeller domain of the integrin α5 subunit is essential for α5β1 heterodimerization, expression on the cell surface, and its biological function. J. Biol. Chem. 281, 33258–33267 [DOI] [PubMed] [Google Scholar]
- 34.Kuwada S. K., Kuang J., and Li X. (2005) Integrin α5/β1 expression mediates HER-2 down-regulation in colon cancer cells. J. Biol. Chem. 280, 19027–19035 [DOI] [PubMed] [Google Scholar]
- 35.Kuwada S. K., and Li X. (2000) Integrin α5/β1 mediates fibronectin-dependent epithelial cell proliferation through epidermal growth factor receptor activation. Mol. Biol. Cell 11, 2485–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreiner C. L., Bauer J. S., Danilov Y. N., Hussein S., Sczekan M. M., and Juliano R. L. (1989) Isolation and characterization of Chinese hamster ovary cell variants deficient in the expression of fibronectin receptor. J. Cell Biol. 109, 3157–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., and Zhang F. (2013) Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shalem O., Sanjana N. E., Hartenian E., Shi X., Scott D. A., Mikkelsen T. S., Heckl D., Ebert B. L., Root D. E., Doench J. G., and Zhang F. (2014) Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isaji T., Im S., Gu W., Wang Y., Hang Q., Lu J., Fukuda T., Hashii N., Takakura D., Kawasaki N., Miyoshi H., and Gu J. (2014) An oncogenic protein Golgi phosphoprotein 3 up-regulates cell migration via sialylation. J. Biol. Chem. 289, 20694–20705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mettouchi A., Klein S., Guo W., Lopez-Lago M., Lemichez E., Westwick J. K., and Giancotti F. G. (2001) Integrin-specific activation of Rac controls progression through the G1 phase of the cell cycle. Mol. Cell 8, 115–127 [DOI] [PubMed] [Google Scholar]
- 41.Alam N., Goel H. L., Zarif M. J., Butterfield J. E., Perkins H. M., Sansoucy B. G., Sawyer T. K., and Languino L. R. (2007) The integrin-growth factor receptor duet. J. Cell. Physiol. 213, 649–653 [DOI] [PubMed] [Google Scholar]
- 42.Gao M., Patel R., Ahmad I., Fleming J., Edwards J., McCracken S., Sahadevan K., Seywright M., Norman J., Sansom O., and Leung H. Y. (2012) SPRY2 loss enhances ErbB trafficking and PI3K/AKT signalling to drive human and mouse prostate carcinogenesis. EMBO Mol. Med. 4, 776–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.del Pozo M. A., Alderson N. B., Kiosses W. B., Chiang H. H., Anderson R. G., and Schwartz M. A. (2004) Integrins regulate Rac targeting by internalization of membrane domains. Science 303, 839–842 [DOI] [PubMed] [Google Scholar]
- 44.Morozevich G. E., Kozlova N. I., Ushakova N. A., Preobrazhenskaya M. E., and Berman A. E. (2012) Integrin α5β1 simultaneously controls EGFR-dependent proliferation and Akt-dependent pro-survival signaling in epidermoid carcinoma cells. Aging 4, 368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambert S., Ameels H., Gniadecki R., Hérin M., and Poumay Y. (2008) Internalization of EGF receptor following lipid rafts disruption in keratinocytes is delayed and dependent on p38 MAPK activation. J. Cell. Physiol. 217, 834–845 [DOI] [PubMed] [Google Scholar]
- 46.Kazui A., Ono M., Handa K., and Hakomori S. (2000) Glycosylation affects translocation of integrin, Src, and caveolin into or out of GEM. Biochem. Biophys. Res. Commun. 273, 159–163 [DOI] [PubMed] [Google Scholar]
- 47.Samandari E., Visarius T., Zingg J. M., and Azzi A. (2006) The effect of γ-tocopherol on proliferation, integrin expression, adhesion, and migration of human glioma cells. Biochem. Biophys. Res. Commun. 342, 1329–1333 [DOI] [PubMed] [Google Scholar]
- 48.Giancotti F. G., and Ruoslahti E. (1990) Elevated levels of the α5β1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell 60, 849–859 [DOI] [PubMed] [Google Scholar]
- 49.Varner J. A., Emerson D. A., and Juliano R. L. (1995) Integrin α5β1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol. Biol. Cell 6, 725–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schirner M., Herzberg F., Schmidt R., Streit M., Schöning M., Hummel M., Kaufmann C., Thiel E., and Kreuser E. D. (1998) Integrin α5β1: a potent inhibitor of experimental lung metastasis. Clin. Exp. Metastasis 16, 427–435 [DOI] [PubMed] [Google Scholar]
- 51.Zhou G. F., Ye F., Cao L. H., and Zha X. L. (2000) Over expression of integrin α5β1 in human hepatocellular carcinoma cell line suppresses cell proliferation in vitro and tumorigenicity in nude mice. Mol. Cell Biochem. 207, 49–55 [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Shenouda S., Baranwal S., Rathinam R., Jain P., Bao L., Hazari S., Dash S., and Alahari S. K. (2011) Integrin subunits α5 and α6 regulate cell cycle by modulating the chk1 and Rb/E2F pathways to affect breast cancer metastasis. Mol. Cancer 10, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adachi M., Taki T., Higashiyama M., Kohno N., Inufusa H., and Miyake M. (2000) Significance of integrin α5 gene expression as a prognostic factor in node-negative non-small cell lung cancer. Clin. Cancer Res. 6, 96–101 [PubMed] [Google Scholar]
- 54.Qian F., Zhang Z. C., Wu X. F., Li Y. P., and Xu Q. (2005) Interaction between integrin α5 and fibronectin is required for metastasis of B16F10 melanoma cells. Biochem. Biophys. Res. Commun. 333, 1269–1275 [DOI] [PubMed] [Google Scholar]
- 55.Liu D., Aguirre Ghiso J., Estrada Y., and Ossowski L. (2002) EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell 1, 445–457 [DOI] [PubMed] [Google Scholar]
- 56.Caswell P. T., Chan M., Lindsay A. J., McCaffrey M. W., Boettiger D., and Norman J. C. (2008) Rab-coupling protein coordinates recycling of α5β1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J. Cell Biol. 183, 143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ono M., Handa K., Withers D. A., and Hakomori S. (2000) Glycosylation effect on membrane domain (GEM) involved in cell adhesion and motility: a preliminary note on functional α3, α5-CD82 glycosylation complex in ldlD 14 cells. Biochem. Biophys. Res. Commun. 279, 744–750 [DOI] [PubMed] [Google Scholar]
- 58.Nagae M., Re S., Mihara E., Nogi T., Sugita Y., and Takagi J. (2012) Crystal structure of α5β1 integrin ectodomain: atomic details of the fibronectin receptor. J. Cell Biol. 197, 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mould A. P., and Humphries M. J. (2004) Regulation of integrin function through conformational complexity: not simply a knee-jerk reaction? Curr. Opin. Cell Biol. 16, 544–551 [DOI] [PubMed] [Google Scholar]
- 60.Clark K., Pankov R., Travis M. A., Askari J. A., Mould A. P., Craig S. E., Newham P., Yamada K. M., and Humphries M. J. (2005) A specific α5β1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation. J. Cell Sci. 118, 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell I. D., and Humphries M. J. (2011) Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 10.1101/cshperspect.a004994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miljan E. A., Meuillet E. J., Mania-Farnell B., George D., Yamamoto H., Simon H. G., and Bremer E. G. (2002) Interaction of the extracellular domain of the epidermal growth factor receptor with gangliosides. J. Biol. Chem. 277, 10108–10113 [DOI] [PubMed] [Google Scholar]
- 63.Handa K., and Hakomori S. I. (2012) Carbohydrate to carbohydrate interaction in development process and cancer progression. Glycoconj. J. 29, 627–637 [DOI] [PubMed] [Google Scholar]
- 64.Tomas A., Futter C. E., and Eden E. R. (2014) EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 24, 26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camidge D. R., Pao W., and Sequist L. V. (2014) Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat. Rev. Clin. Oncol. 11, 473–481 [DOI] [PubMed] [Google Scholar]
- 66.Kawahara E., Saito A., Kobayashi J., Maenaka S., Minamoto T., Imai M. A., and Oda Y. (2005) Adhesiveness of β5 integrin variant lacking FNK767–769 is similar to that of the prototype containing FNKFNK764–769. Cell Biol. Int. 29, 521–528 [DOI] [PubMed] [Google Scholar]
- 67.Yoo N. J., Soung Y. H., Lee S. H., Jeong E. G., and Lee S. H. (2007) Mutational analysis of proapoptotic integrin β3 cytoplasmic domain in common human cancers. Tumori 93, 281–283 [DOI] [PubMed] [Google Scholar]
- 68.Nicolaou N., Margadant C., Kevelam S. H., Lilien M. R., Oosterveld M. J., Kreft M., van Eerde A. M., Pfundt R., Terhal P. A., van der Zwaag B., Nikkels P. G., Sachs N., Goldschmeding R., Knoers N. V., Renkema K. Y., and Sonnenberg A. (2012) Gain of glycosylation in integrin α3 causes lung disease and nephrotic syndrome. J. Clin. Invest. 122, 4375–4387 [DOI] [PMC free article] [PubMed] [Google Scholar]