Background: The mechanism underlying sperm PLCζ interaction with its target membrane is unresolved.
Results: EF-hand mutations introduced into PLCζ reduce in vivo Ca2+ oscillation inducing activity and in vitro interaction with PIP2.
Conclusion: EF-hand domain is essential for targeting PLCζ to PIP2-containing membranes.
Significance: We propose a novel mechanism by which sperm PLCζ is anchored to its physiological membrane substrate.
Keywords: calcium intracellular release; fertilization; phosphatidylinositol signaling; phospholipase C; sperm; EF-hand domain; PIP2; inositol 1,4,5-trisphosphate
Abstract
Sperm-specific phospholipase C-ζ (PLCζ) is widely considered to be the physiological stimulus that triggers intracellular Ca2+ oscillations and egg activation during mammalian fertilization. Although PLCζ is structurally similar to PLCδ1, it lacks a pleckstrin homology domain, and it remains unclear how PLCζ targets its phosphatidylinositol 4,5-bisphosphate (PIP2) membrane substrate. Recently, the PLCδ1 EF-hand domain was shown to bind to anionic phospholipids through a number of cationic residues, suggesting a potential mechanism for how PLCs might interact with their target membranes. Those critical cationic EF-hand residues in PLCδ1 are notably conserved in PLCζ. We investigated the potential role of these conserved cationic residues in PLCζ by generating a series of mutants that sequentially neutralized three positively charged residues (Lys-49, Lys-53, and Arg-57) within the mouse PLCζ EF-hand domain. Microinjection of the PLCζ EF-hand mutants into mouse eggs enabled their Ca2+ oscillation inducing activities to be compared with wild-type PLCζ. Furthermore, the mutant proteins were purified, and the in vitro PIP2 hydrolysis and binding properties were monitored. Our analysis suggests that PLCζ binds significantly to PIP2, but not to phosphatidic acid or phosphatidylserine, and that sequential reduction of the net positive charge within the first EF-hand domain of PLCζ significantly alters in vivo Ca2+ oscillation inducing activity and in vitro interaction with PIP2 without affecting its Ca2+ sensitivity. Our findings are consistent with theoretical predictions provided by a mathematical model that links oocyte Ca2+ frequency and the binding ability of different PLCζ mutants to PIP2. Moreover, a PLCζ mutant with mutations in the cationic residues within the first EF-hand domain and the XY linker region dramatically reduces the binding of PLCζ to PIP2, leading to complete abolishment of its Ca2+ oscillation inducing activity.
Introduction
During fertilization, the spermatozoon initiates activation of egg development by triggering an acute rise in cytosolic free Ca2+ concentration (1). In mammals, this manifests as a series of distinctive cytosolic Ca2+ oscillations, beginning soon after sperm-egg fusion and persisting for several hours (2). The weight of evidence now suggests that Ca2+ oscillations appear to be caused by a sperm-specific protein, phospholipase C-ζ (PLCζ),3 which is introduced into the egg upon sperm-egg fusion and leads to cycles of inositol 1,4,5-trisphosphate (IP3) production following PIP2 hydrolysis, thus activating IP3 receptor-mediated Ca2+ release from intracellular stores in the egg (3–12). The closest PLC homologue of sperm PLCζ is PLCδ1 (47% similarity, 33% identity), which is only able to cause Ca2+ oscillations in mouse eggs at non-physiological concentrations, because it has a >50-fold lower potency (2, 3, 12). The superior fertilization potency of the sperm PLCζ over somatic PLCs has not yet been fully explained.
PLCζ is the smallest PLC with the simplest domain organization among all the mammalian isoforms. PLCζ consists of four tandem EF-hand domains, the characteristic X and Y catalytic domains in the center of the molecule, and a C-terminal C2 domain. All these domains are common to the other PLC isoforms (β, γ, δ, ϵ, and η), but they appear to individually have an essential role in the unique mode of regulation of this distinctive PLC isozyme (2). A notable structural difference between PLCζ and the other somatic PLC isoforms is that PLCζ lacks a pleckstrin homology (PH) domain at the N terminus (2, 3, 13). The membrane binding of somatic PLCs appears to be mediated by the PH domain, a well defined structural module of ∼120-amino acid residues identified in numerous proteins (14). The PH domain of PLCδ1 is essential for interaction with its phospholipid substrate PIP2 in the plasma membrane (15). The absence of a PH domain from PLCζ sequence raises questions about how PLCζ can bind to membranes.
We have previously proposed that the PLCζ XY-linker, a segment between the X and Y catalytic domains that is notably different from the corresponding XY-linker region of somatic PLCs, is involved in the targeting of PLCζ to its membrane-bound substrate PIP2 (16, 17). The XY-linker region of PLCζ is extended in length and consists of more basic residues relative to its PLCδ1 counterpart. The affinity of the XY-linker for PIP2 appears to involve a polybasic charged region that is found in a number of other membrane-associated proteins (16, 18). These positively charged amino acids in the XY-linker appear to assist the anchoring of PLCζ to membranes by enhancing the local PIP2 concentration adjacent to the XY catalytic domain via electrostatic interactions with the negatively charged PIP2 (16, 17). However, the XY-linker might not be the only domain that mediates the binding of PLCζ to PIP2-containing membranes. We have demonstrated that the absence of the XY-linker from PLCζ significantly diminishes, but does not completely abolish, the in vivo Ca2+ oscillation inducing activity (19). This suggests that other domain(s) may also be involved in anchoring PLCζ to its target membrane.
A recent study reported that the N-terminal lobe of the EF-hand domain of PLCδ1 binds anionic phospholipids, and this binding is due to interactions with cationic and hydrophobic residues in the first EF-hand sequence of PLCδ1 (20). The authors propose a general mechanism that may apply to other PLC isoforms by suggesting that EF-hand domain interactions with anionic phospholipids in the target membrane provides a tether that facilitates proper substrate access and binding in the active site (20). Importantly, the cationic residues in the first EF-hand domain of PLCδ1 that contribute to anionic lipid vesicle binding are all conserved in PLCζ.
The aim of this study is to investigate the potential importance of a conserved cluster of cationic residues at the N-terminal lobe of the EF-hand domain of PLCζ in association with anionic lipids and its substrate PIP2. A series of full-length mouse PLCζ mutants were prepared that sequentially neutralized two positively charged lysine and one arginine residues within the first EF-hand domain. The Ca2+ oscillation-inducing properties of these mutants were experimentally tested relative to wild-type PLCζ by microinjection of cRNA into unfertilized mouse eggs. The various PLCζ mutants' enzymatic properties were analyzed using an in vitro PIP2 hydrolysis assay. A protein-lipid overlay and a liposome binding/enzyme assay were employed to assess the binding properties of wild-type PLCζ to phosphatidylserine (PS), phosphatidic acid (PA), and PIP2. Furthermore, the binding properties of mutant EF-hand PLCζ proteins to PIP2 were examined. Our results suggest that PLCζ possesses significant affinity only for PIP2 but not for PA or PS. We also find that sequential reduction of the net positive charge within the first EF-hand domain significantly reduces both in vivo Ca2+ oscillation inducing activity and the in vitro interaction of PLCζ with PIP2. Moreover, we show that a PLCζ mutant where three cationic residues within the first EF-hand domain and three cationic residues within the XY-linker region of PLCζ were substituted by alanine is unable to trigger Ca2+ oscillations in mouse eggs. In vitro biochemical characterization suggests that this PLCζ mutant displays dramatically reduced binding to PIP2-containing liposomes compared with the wild-type PLCζ. Thus, we propose a novel mechanism for the sperm PLCζ interaction with PIP2-containing membranes mediated by electrostatic interactions between the anionic PIP2 with both the first EF-hand domain and the XY-linker region of PLCζ, which are rich in cationic residues.
Experimental Procedures
Plasmid Construction
A pCR3-mouse PLCζ-luciferase (PLCζ-luc) construct (21) was subjected to site-directed mutagenesis (QuikChange II, Stratagene) to sequentially generate the three single, one double, and one triple substitutions at Lys-49, Lys-53, and Arg-57, thus producing the PLCζK49A, PLCζK53A, PLCζR57A, PLCζK49A,R57A, and PLCζK49A,K53A,R57A mutants.
pCR3-PLCζK49A,K53A,R57A,K374A,K375A,K377A-luc construct was generated by a three-step cloning strategy. PLCζEFK49A,K53A,R57A (1–149 amino acids) was amplified from the PLCζK49A,K53A,R57A-luc plasmid by PCR with primers to incorporate a 5-KpnI site and a 3-EcoRI site and then cloned into the pCR3 vector. PLCζΔEFK374A,K375A,K377A (150–647 amino acids) was then amplified from the PLCζK374A,K375A,K377A-luc plasmid (17) with primers to incorporate a 5-EcoRI site and a 3-NotI site in which the stop codon had been removed and cloned into the pCR3-PLCζEFK49A,K53A,R57A plasmid. Finally, luciferase was amplified from pGL2 with primers incorporating NotI sites, and the product was cloned into the NotI site of the pCR3-PLCζK49A,K53A,R57A,K374A,K375A,K377A plasmid.
All the above PLCζ mutants were amplified from their corresponding pCR3 plasmid with the appropriate primers to incorporate a 5-SalI site and a 3-NotI site, and the products were cloned into the pETMM60 vector to enable bacterial protein expression. The primers used for the amplifications were as follows: 5′-GAACGTCGACATGGAAAGCCAACTTCATGAGCTCGC-3′ (forward) and 5′-GGAAGCGGCCGCTCACTCTCTGAAGTACCAAAC-3′ (reverse). Successful mutagenesis and cloning of the above expression vector constructs were confirmed by dideoxynucleotide sequencing (Applied Biosystems Big-Dye Version 3.1 chemistry and model 3730 automated capillary DNA sequencer by DNA Sequencing & ServicesTM).
cRNA Synthesis
Following linearization of wild-type and mutated PLCζ plasmids, cRNA was synthesized using the mMessage Machine T7 kit (Ambion) and then was polyadenylated using the poly(A) tailing kit (Ambion), as per the manufacturer's instructions.
Preparation and Handling of Gametes
Female mice were super-ovulated and mature MII eggs were collected from excised oviducts 13.5–14.5 h after injection of human chorionic gonadotrophin and maintained in droplets of M2 media (Sigma) under mineral oil at 37 °C. Experimental recordings of Ca2+ release or luciferase expression were carried out with mouse eggs in Hepes-buffered media (H-KSOM), as described previously (22). All compounds were from Sigma unless stated otherwise. All procedures using animals were performed in accordance with the United Kingdom Home Office Animals Procedures Act and were approved by the Cardiff University Animals Ethics Committee.
Microinjection and Measurement of Intracellular Ca2+ and Luciferase Expression
Mouse eggs were washed in M2 and microinjected with cRNA diluted in injection buffer (120 mm KCl, 20 mm Hepes, pH 7.4). The volume injected was estimated from the diameter of cytoplasmic displacement caused by the bolus injection. All injections were 3–5% of the oocyte volume. Eggs were microinjected with the appropriate cRNA in the injection buffer, mixed with an equal volume of 1 mm Oregon Green 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-dextran (Life Technologies, Inc.). Eggs were then maintained in H-KSOM containing 100 μm luciferin and imaged on a Nikon TE2000 microscope equipped with a cooled intensified CCD camera (Photek Ltd., UK). The luminescence (luciferase expression) and fluorescence (for Ca2+ measurements) from eggs were collected by switching back and forth between the two modes on a 10-s cycle (23, 24). These two signals were then displayed as two separate signals over the same time period for each egg. The fluorescent light used to measure Ca2+ is shown in relative units. Luminescence was recorded as photon counts/s and plotted as a running average over 5 min. All live imaging experiments on eggs were made during a 1-month period.
Protein Expression and Purification
For NusA-His6-fusion protein expression, Escherichia coli (BL21-CodonPlus(DE3)-RILP; Stratagene) cells were transformed with the appropriate pETMM60 plasmid and cultured at 37 °C until the A600 reached 0.6, and protein expression was induced for 18 h at 16 °C with 0.1 mm isopropyl 1-thio-β-d-galactopyranoside (ForMedium). Cells were harvested (6000 × g for 10 min), resuspended in PBS containing a protease inhibitor mixture (EDTA-free; Roche Applied Science), and sonicated four times for 15 s on ice. Soluble NusA-His6-tagged fusion protein was purified on nickel-nitrilotriacetic acid resin following standard procedures (Qiagen) and eluted with 250 mm imidazole. Eluted proteins were dialyzed overnight (10,000 molecular weight cutoff; Pierce) at 4 °C against 4 liters of PBS and concentrated with centrifugal concentrators (Sartorius; 10,000 molecular weight cutoff).
Assay of PLC Activity
PIP2 hydrolytic activity of recombinant PLCζ proteins was assayed as described previously (17, 21). The final concentration of PIP2 in the reaction mixture was 220 μm, containing 0.05 μCi of [3H]PIP2. The assay conditions were optimized for linearity, requiring a 1-min incubation of 200 pmol of PLCζ protein sample at 25 °C. In assays to determine dependence on PIP2 concentration, 0.05 μCi of [3H]PIP2 was mixed with cold PIP2 to give the appropriate final concentration. In assays examining Ca2+ sensitivity, Ca2+ buffers were prepared by EGTA/CaCl2 admixture, as described previously (17, 21).
Protein Lipid Overlay Assay
PIP array membranes (Echelon Biosciences) were blocked for 2 h with binding buffer (TBS-T: 20 mm Tris, 137 mm NaCl, 0.1% Tween 20, pH 7.4) containing 3% bovine serum albumin (lipid-free) and incubated with 25 pmol of each NusA-PLCζ fusion protein for 1 h at room temperature. After washing three times in TBS-T, NusA-PLCζ fusion protein interaction with the inositol phosphate lipids was detected by first incubating the PIP array membranes with penta-His monoclonal antibody (Qiagen, 1:5000 dilution in 5 ml of binding buffer) overnight at 4 °C, followed by three 15-min washes. This was followed by incubation with horseradish peroxidase-conjugated anti-mouse antibody in the same binding buffer for 1 h at room temperature, followed by three 15-min washes with TBS-T. Detection of horseradish peroxidase-coupled secondary antibody was achieved using enhanced chemiluminescence detection (ECL; Amersham Biosciences).
Liposome Preparation and Binding Assay
Unilamellar liposomes were prepared as described previously (17, 25) by the extrusion method using a laboratory extruder (LiposoFast-Pneumatic, Avestin Inc., Ottawa, Ontario, Canada) with lipids purchased from Avanti Polar Lipids Inc. (Alabaster, AL). In a typical experiment for preparing a 2-ml dispersion of liposomes, 0.038 mmol (19 × 10−3 m) of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (PtdCho), 0.019 mmol (9.5 × 10−3 m) of cholesterol (CHOL; molar ratio of PtdCho/CHOL, 2:1), 0.0095 mmol (4.8 × 10−3 m) of 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (PtdEtn; molar ratio of PtdCho/PtdEtn, 4:1), and 5% 1,2-diacyl-sn-glycero-3-phospho-l-serine (PS) or 1–5% 1,2-diacyl-sn-glycero-3-phosphate sodium salt (PA) or 1% of 1,2-diacyl-sn-glycero-3-phospho-(1-d-myo-inositol 4,5-bisphosphate) sodium salt (PIP2) were dissolved in a chloroform/methanol solution (2:1 v/v) for the formation of lipid films. The film was hydrated with 2 ml of PBS, and the resultant suspension was extruded through two stacked polycarbonate filters of 100-nm pore size. Twenty five cycles of extrusion were applied at 50 °C. Dynamic light scattering was employed to determine the size of the liposomes, which used a light scattering apparatus (AXIOS-150/EX, Triton Hellas, Thessaloniki, Greece) with a 30-milliwatt laser source and an Avalanche photodiode detector set at a 90° angle. Dynamic light scattering measurements of the extruded lipid preparation showed a narrow monomodal size distribution with average liposome diameter of 100 nm and a polydispersity index of 0.20–0.25. For protein binding studies, liposomes (100 μg) were incubated with 1 μg of recombinant protein for 30 min at room temperature and centrifuged for 5 h at 4 °C. The supernatant and pellet were then analyzed either by SDS-PAGE and Coomassie Blue staining or by the [3H]PIP2 hydrolysis assay described above.
SDS-PAGE and Western Blotting
Recombinant proteins were separated by SDS-PAGE as described previously (25, 26). Separated proteins were transferred onto polyvinylidene difluoride membranes (Immobilon-P; Millipore) using a semi-dry transfer system (Trans-Blot S.D.; Bio-Rad) in transfer buffer (48 mm Tris, 39 mm glycine, 0.0375% SDS, 20% v/v methanol) at 20 V for 1 h. Membranes were incubated overnight at 4 °C in Tris-buffered saline, 0.1% Tween 20 (TBS-T) containing 5% nonfat milk powder, and probed with a penta-His mouse monoclonal antibody (Novagen, 1:100,000 dilution). Detection of horseradish peroxidase-coupled secondary antibody was achieved using enhanced chemiluminescence detection (ECL; Amersham Biosciences).
Mathematical Modeling of Oocyte Ca2+ Dynamics
Theoretical predictions of the oscillatory Ca2+ activity associated with the various PLCζ constructs were provided by a mathematical model of oocyte IP3/Ca2+ dynamics. The mathematical model, which has previously been presented in detail (25), employs three inter-dependent variables, namely free cytosolic Ca2+, Ca2+ sequestered in the endoplasmic reticulum, and intracellular concentrations of IP3. To account for the specific binding activity of each PLCζ variant, the effective activity of a PLCζ concentration is defined as: VePLC = VPLC·b, where VPLC is the nominal PLCζ concentration, and b is the binding activity estimated experimentally for each construct. Coefficient b assumes a value between 0 and 1, whereas VPLC can assume values beyond the physiological range when the protein is overexpressed. The mathematical model was coded and numerically integrated on both C++ and a MATLAB platform (MathWorks).
Results
Effect of EF-hand Mutations on PLCζ-mediated Ca2+ Oscillations in Mouse Eggs
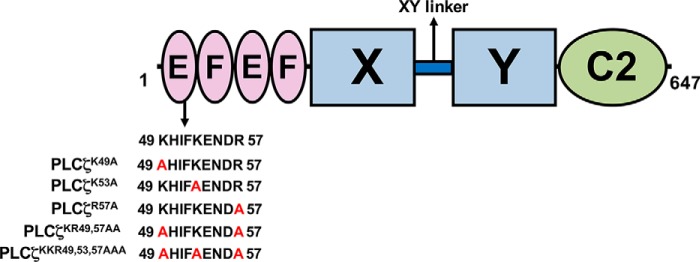
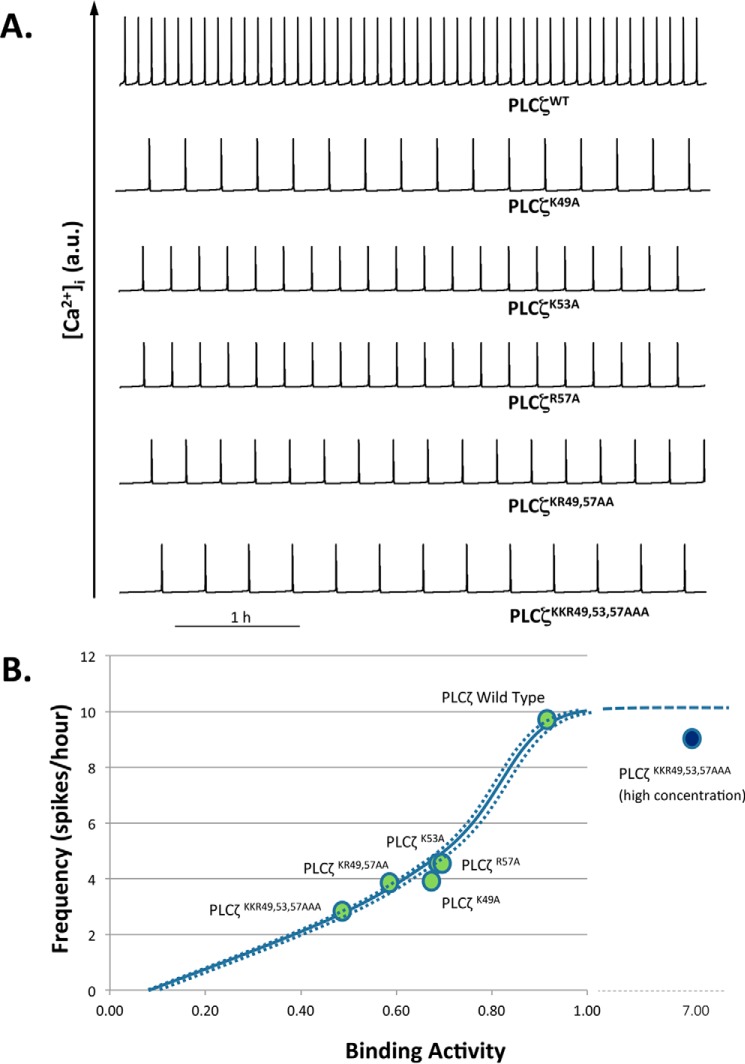
To investigate the potential importance of a cluster of cationic residues within the first EF-hand unit of the first pair of PLCζ EF-hand domains (Fig. 1), we performed site-directed mutagenesis to produce a panel of cumulative mutations within this positively charged region of the full-length mouse PLCζ. Thus, the residues Lys-49, Lys-53, and Arg-57 were sequentially substituted by the neutral amino acid, alanine, to create three single (PLCζK49A, PLCζK53A, and PLCζR57A) mutants, as well as one double (PLCζK53A,K57A) and one triple (PLCζK49A,K53A,R57A) PLCζ mutant. To test the Ca2+ oscillation inducing activity of PLCζK49A, PLCζK53A, PLCζR57A, PLCζK49A,R57A, and PLCζK49A,K53A,R57A mutants and to verify that these constructs were faithfully expressed as proteins in cRNA-microinjected mouse eggs, we generated C-terminal luciferase-tagged versions of these constructs to enable quantitation of relative protein expression by luminescence detection of the expressed PLCζ-luciferase fusion protein, as described previously (17, 21). Prominent Ca2+ oscillations were observed in PLCζWT-luciferase cRNA-injected mouse eggs (9.7 spikes in the 1st h of oscillations) following successful protein expression to a level indicated by a luminescence reading of 0.47 counts/s (Fig. 2 and Table 1), in accord with previous reports (17, 21). Microinjection of cRNA encoding the three single PLCζ mutants (PLCζK49A, PLCζK53A, and PLCζR57A) also triggered Ca2+ oscillations (Fig. 2), but these exhibited a lower frequency relative to PLCζWT (3.6, 4.4, and 4.3 spikes in the 1st h, respectively), although the proteins were expressed at comparable expression levels (Table 1). Similarly, egg microinjection with cRNA encoding either the double PLCζK49A,R57A or the triple PLCζK49A,K53A,R57A mutant resulted in a significant reduction in the frequency of Ca2+ oscillations compared with PLCζWT, causing 3.7 and 2.8 spikes/1 h, respectively, again when protein was expressed at comparable levels (Fig. 2 and Table 1). These data indicate that the substitution of even one Lys or Arg residue for a neutral Ala within the positively charged cluster of the PLCζ EF-hand domain can significantly alter their Ca2+ oscillation inducing activity in mouse eggs by reducing the frequency of Ca2+ spikes.
FIGURE 1.
Generation of PLCζ EF-hand mutants. Schematic representation of the domain structure of mouse PLCζ identifying the location of the successive Lys or Arg residue substitutions to Ala, between residues 49 and 57 within the first EF-hand domain that are prepared by site-directed mutagenesis for this study.
FIGURE 2.
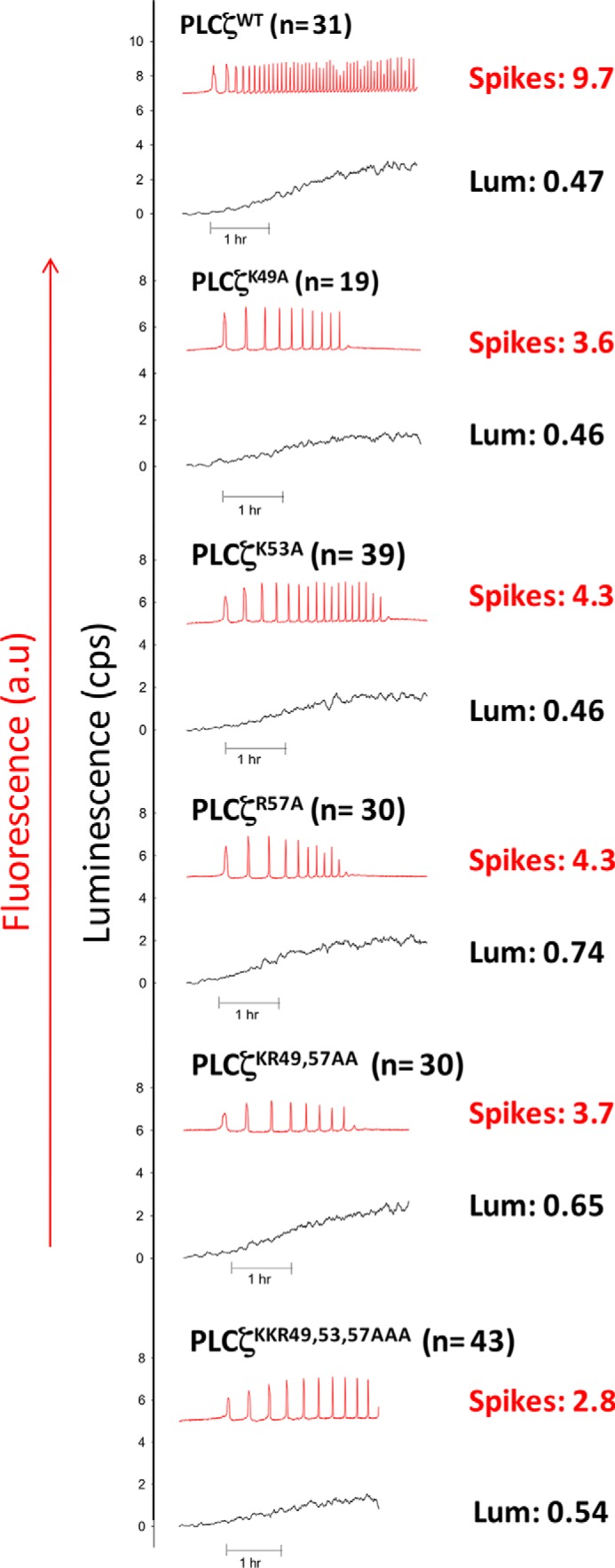
Expression of wild-type and mutant PLCζ constructs (PLCζK49A, PLCζK53A, PLCζR57A, PLCζK53A,K57A, and PLCζK49A,K53A,R57A) in unfertilized mouse eggs. Fluorescence and luminescence (Lum) recordings reported the Ca2+ changes (red traces; Ca2+) and luciferase expression ((black traces; luminescence, in counts/s), respectively, in unfertilized mouse eggs following microinjection of cRNA encoding luciferase-tagged PLCζ constructs. Mean number of Ca2+ oscillations in the 1st h of oscillating (spikes) and mean luminescence (cps) in the 1st h of oscillating (Lum) are shown. a.u., arbitrary units.
TABLE 1.
Properties of PLCζ-luciferase EF-hand mutants expressed in unfertilized mouse eggs
Ca2+ oscillation inducing activity (number of Ca2+ spikes in the 1st h of oscillations) and luciferase luminescence levels (counts/s of luminescence in 1st h of oscillating) are summarized for mouse eggs microinjected with each of the PLCζ-luciferase mutants as follows: PLCζK49A, PLCζK53A, PLCζR57A, PLCζK53A,R57A, PLCζK49A,K53A,R57A, PLCζDMM, and wild type PLCζ-luciferase (see Figs. 2, 3, and 8B). The data are expressed to two significant figures, with means ± S.E. Ratios of total luminescence (counts) per spike in the 1st h of oscillating are also shown expressed to two significant figures with means ± S.E. This is not shown where the number of spikes is zero or where the expression of PLCζ is sufficiently high that the relationship between number of spikes and expression is no longer linear. The results of Mann-Whitney tests for significant differences between the number of spikes for each of the wild-type and mutant PLCζ-luciferase constructs are indicated with p values. The results of this same test for a significant difference between the PLCζ mutant ratios (counts/spike) and the wild-type PLCζ-luciferase ratio are also denoted in this way. NA, not applicable.
| PLCζ cRNA | No. of eggs | Mean no. of oscillations in 1st h of spiking | Mean expression in 1st h of spiking | No. of spikes significantly different from wild type? | Mean total counts/spike in 1st h of oscillating (counts/spike) | Counts/spike significantly different from wild type? |
|---|---|---|---|---|---|---|
| cps | ||||||
| PLCζWT | 31 | 9.7 ± 0.63 | 0.47 ± 0.038 | NA | 14.61 ± 2.05 | NA |
| PLCζK49A | 19 | 3.6 ± 0.16 | 0.48 ± 0.024 | Yes (p = <0.001) | 25.92 ± 1.17 | Yes (p = <0.001) |
| PLCζK53A | 39 | 4.4 ± 0.13 | 0.46 ± 0.014 | Yes (p = <0.001) | 20.78 ± 0.85 | Yes (p = <0.001) |
| PLCζR57A | 30 | 4.3 ± 0.13 | 0.74 ± 0.032 | Yes (p = <0.001) | 38.21 ± 2.1 | Yes (p = <0.001) |
| PLCζK49A,R57A | 30 | 3.7 ± 0.14 | 0.65 ± 0.030 | Yes (p = <0.001) | 34.96 ± 1.94 | Yes (p = <0.001) |
| PLCζK49A,K53A,R57A | 43 | 2.8 ± 0.074 | 0.54 ± 0.031 | Yes (p = <0.001) | 37.35 ± 2.24 | Yes (p = <0.001) |
| PLCζK49A,K53A,R57A | 11 | 8.6 ± 1.7 | 7.65 ± 0.92 | No (p = 0.15) | NA | NA |
| PLCζDMM | 25 | 0 ± 0 | 0.53 ± 0.046 | Yes (p = <0.001) | NA | NA |
| PLCζDMM | 20 | 0 ± 0 | 14.43 ± 0.80 | Yes (p = <0.001) | NA | NA |
Overexpression of PLCζK49A,K53A,R57A in Mouse Eggs Rescues Its Defective Ca2+ Oscillation-inducing Phenotype
Judging by the number of Ca2+ spikes observed within the 1st h of oscillations per unit of recombinant fusion protein expression (cps), PLCζWT can be seen to be about ∼3.5 times more effective at causing Ca2+ oscillations than the PLCζK49A,K53A,R57A triple mutant. To investigate whether we could rescue the low frequency of Ca2+ oscillations induced by PLCζK49A,K53A,R57A, we overexpressed this PLCζ mutant in mouse eggs. As shown in Fig. 3 and Table 1, the overexpression of PLCζK49A,K53A,R57A (7.65 cps) indeed led to 8.6 spikes in the 1st h of oscillations, comparable with that for PLCζWT, suggesting that loading the egg with large amounts of this PLCζ mutant can rescue its defective Ca2+ oscillation-inducing phenotype.
FIGURE 3.
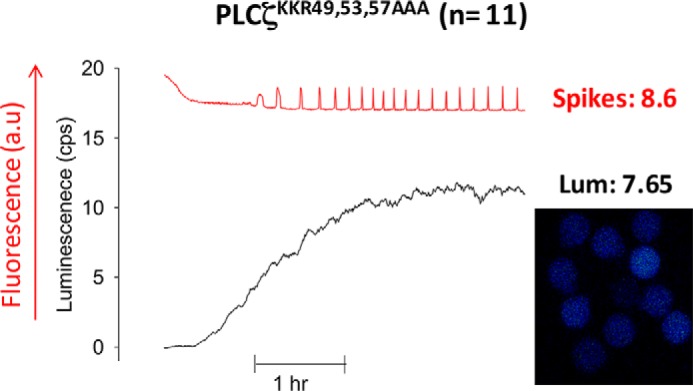
Overexpression of PLCζK49A,K53A,R57A in unfertilized mouse eggs. The left panel shows representative fluorescence (a.u., arbitrary units) and luminescence (cps) recordings reporting the Ca2+ concentration changes (red traces; Ca2+) and luciferase expression (black traces; Lum), respectively, in a mouse egg following microinjection of PLCζK49A,K53A,R57A-luciferase cRNA. The right panel shows an integrated image of luciferase luminescence from eggs microinjected with the corresponding PLCζK49A,K53A,R57A-luciferase cRNA for the 1st h of recording. The mean luminescence in the 1st h of oscillating (Lum) and mean number of Ca2+ spikes in the 1st h of oscillating (spikes) are shown. a.u., arbitrary units.
Expression and Enzymatic Characterization of PLCζ EF-hand Mutants
Each of the PLCζK49A, PLCζK53A, PLCζR57A, PLCζK49A,R57A, and PLCζK49A,K53A,R57A mutants was subcloned into the pETMM60 vector and purified as NusA-His6 fusion proteins by affinity chromatography. We have recently demonstrated that NusA is an effective fusion protein partner for PLCζ, significantly increasing soluble expression of PLCζ protein in E. coli, as well as enhancing the enzymatic stability of the purified protein over time (11). Following expression of NusA-PLCζ fusion proteins in E. coli and purification by nickel-nitrilotriacetic acid affinity chromatography, samples of each protein were analyzed by SDS-PAGE followed by Coomassie Brilliant Blue staining and immunoblotting using an anti-NusA monoclonal antibody. Fig. 4A shows that the major protein band following affinity isolation, with mobility corresponding to the predicted molecular mass of ∼134 kDa for each construct, was present for all fusion proteins analyzed (left panel), and these major bands were also recognized in the corresponding anti-NusA immunoblot (right panel), confirming the appropriate expression of all PLCζ mutants. Some intermediate molecular mass bands detected by the anti-NusA antibody are the probable result of some degradation occurring through the various protein expression and purification procedures. Similarity of protein expression profile, including degradation products, for the various PLCζ constructs being examined suggests that experimental comparison of relative enzymatic data may be appropriate. Hence, the specific PIP2 hydrolytic enzyme activity for PLCζWT and each recombinant mutant protein was determined by the standard micellar [3H]PIP2 hydrolysis assay. The histogram of Fig. 4B and Table 2 summarize the enzyme specific activity values obtained for each recombinant protein. The enzymatic activities of all recombinant proteins was very similar, suggesting that mutating the basic residues of the first pair of EF-hands to a neutral residue has no effect on the ability of PLCζ to hydrolyze PIP2 in vitro. Moreover, to investigate the impact of the EF-hand mutations on Ca2+ sensitivity of PLCζ enzyme activity, we assessed the ability of these PLCζ recombinant proteins to hydrolyze [3H]PIP2 at different Ca2+ concentrations ranging from 0.1 nm to 0.1 mm. These experiments indicated that there was no significant difference in the Ca2+ sensitivity of PIP2 hydrolysis for the wild type, and the five EF-hand mutants (Fig. 4C) with a very similar EC50 value (67–85 nm) displayed by all recombinant PLCζ proteins (Table 2). To compare the enzyme kinetics of wild-type and mutant PLCζs, the Michaelis-Menten constant, Km, was calculated for each construct (Table 2). The Km values obtained were similar for human PLCζWT (84 μm), PLCζK49A (121 μm), and PLCζR57A (115 μm), whereas the Km value for PLCζK53A (169 μm) and PLCζK49A,R57A (219 μm) mutants was ∼2- and ∼2.6-fold higher compared with that of PLCζWT. Interestingly, the Km value for PLCζK49A,K53A,R57A (432 μm) was ∼5.1-fold higher compared with PLCζWT (84 μm), suggesting that replacement of these three positively charged residues within the first EF-hand domain affects the in vitro affinity of PLCζ for PIP2 without affecting the Ca2+ sensitivity of this enzyme.
FIGURE 4.
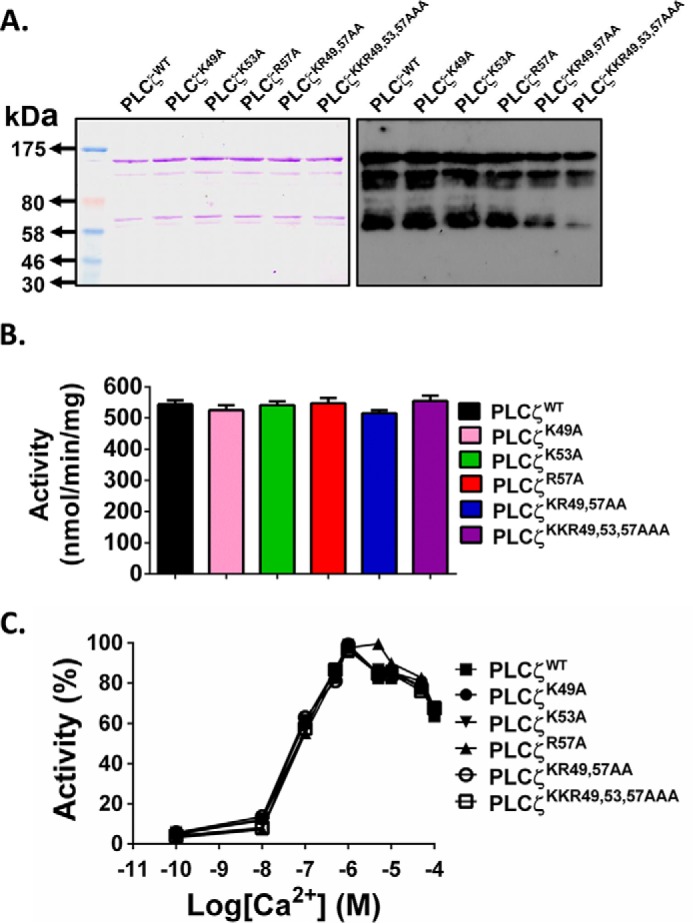
Expression and enzymatic characterization of recombinant NusA-His6-PLCζ EF-hand mutants. A, expression of recombinant NusA-His6-PLCζWT and the various mutant PLCζ proteins. Affinity-purified PLCζ proteins (1 μg) were analyzed by SDS-PAGE followed by either Coomassie Brilliant Blue staining (left panel) or immunoblot analysis using the anti-NusA monoclonal antibody at 1:100,000 dilution (right panel). B, enzyme activity of the various PLCζ mutants. PIP2 hydrolysis enzyme activities of the purified NusA-His6-PLCζ fusion proteins were determined with the standard [3H]PIP2 cleavage assay, n = 4 ± S.E., using two different preparations of each recombinant protein. An unpaired Student's t test showed no significant statistical difference between the enzymatic activities of PLCζ and PLCζ EF-hand mutants. In all cases, p > 0.1. C, effect of varying [Ca2+] on the normalized activity of NusA-His6-tagged wild-type and mutant PLCζ fusion proteins. For these assays, n = 4 ± S.E., using two different preparations of each recombinant protein.
TABLE 2.
In vitro enzymatic properties of NusA-His6-PLCζ EF-hand mutants
Summary of specific enzyme activity and Km and EC50 values of Ca2+ dependence for PIP2 hydrolysis determined by non-linear regression analysis (GraphPad Prism 5) for the NusA-His6 fusion proteins (see Figs. 4 and 9) is shown.
| PLC protein | PIP2 hydrolysis enzyme activity | Ca2+ dependence EC50 | Km |
|---|---|---|---|
| nmol/min/mg | nm | μm | |
| PLCζWT | 544 ± 23 | 72 | 84 |
| PLCζK49A | 525 ± 28 | 68 | 121 |
| PLCζK53A | 541 ± 22 | 75 | 169 |
| PLCζR57A | 547 ± 30 | 85 | 115 |
| PLCζK49A,R57A | 515 ± 17 | 67 | 219 |
| PLCζK49A,K53A,R57A | 555 ± 30 | 79 | 432 |
| PLCζDMM | 434 ± 28 | 108 | 4975 |
Binding of PLCζ to PS, PA, and PIP2
To examine the ability of PLCζ to bind the membrane lipids, PS, PA, and PIP2, we employed three different approaches. First, we used a protein-lipid overlay assay to assess the binding of PLCζ to membrane-spotted arrays of inositol phospholipids containing PS, PA, or PIP2. As shown in Fig. 5A, no binding to PS or PA was evident, although PLCζ was able to bind to membrane arrays containing PIP2. This result is consistent with our liposome binding assays (Fig. 5B). For these binding assays, we made unilamellar liposomes composed of phosphatidylcholine/CHOL/phosphatidylethanolamine (4:2:1) with incorporation of either 5% PS, 1 or 5% PA, and 1% PIP2. To diminish any nonspecific protein binding to highly charged lipids, the liposome binding assays were performed in the presence of a near-physiological concentration of MgCl2 (0.5 mm). PLCζ displayed robust binding only to liposomes containing 1% PIP2, whereas the protein was only detected in the supernatant of liposomes containing 5% PS and 1 or 5% PA (Fig. 5B). Finally, we incubated 1 μg of PLCζ recombinant protein with the liposomes composed of the different phospholipids, and after centrifugation, the supernatants were separated and assayed for their ability to hydrolyze PIP2 in vitro, using the standard [3H]PIP2 hydrolysis assay. As shown in Fig. 5C, only the supernatant obtained after the interaction of recombinant PLCζ protein with the liposomes containing 1% PIP2 showed a dramatic ∼94% reduction in its PIP2 hydrolytic activity. All these data suggest that the PLCζ binds specifically to PIP2, not generically to any anionic phospholipid.
FIGURE 5.
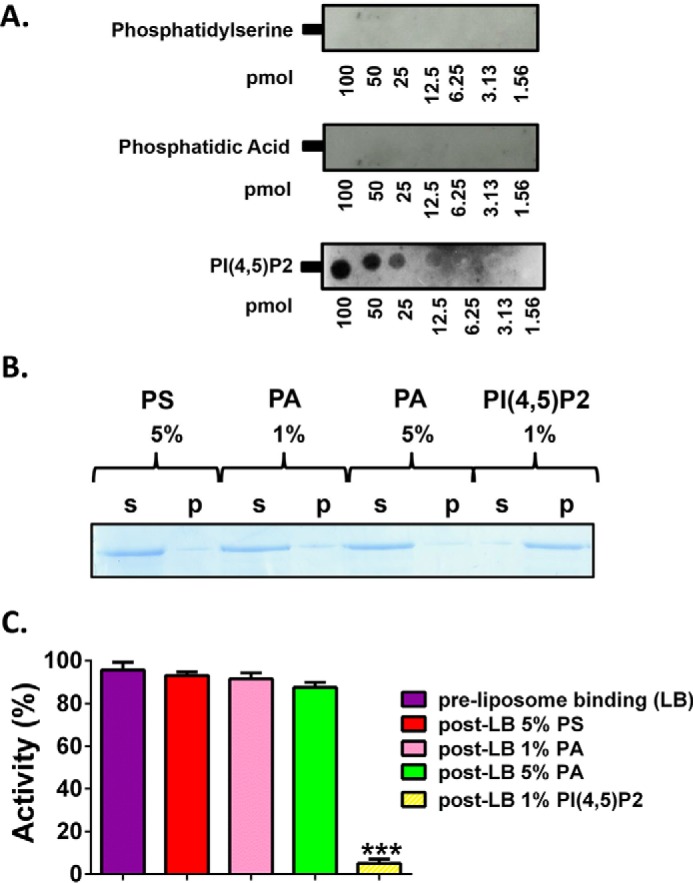
In vitro binding of wild-type PLCζ to PS, PA and PIP2. A, PLCζ protein-lipid overlay assays. Recombinant protein binding to spotted phospholipids on the PIP arrays was detected using the monoclonal penta-His antibody. B, liposome “pulldown” assay of PLCζ. Unilamellar liposomes containing either PS (5%), or PA (1 or 5%), or PIP2 (1%) were incubated with PLCζ recombinant protein. Following liposome centrifugation, both the supernatant (s) and liposome pellet (p) were subjected either to SDS-PAGE and Coomassie Brilliant Blue staining. C, supernatants were assayed for their ability to hydrolyze PIP2 in vitro, using the standard [3H]PIP2 hydrolysis assay, n = 4 ± S.E., using two different preparations of recombinant protein. Significant statistical differences (asterisks) were calculated by an unpaired Student's t test; ***, p < 0.0005 (GraphPad, Prism 5).
Binding of PLCζ EF-hand Mutants to PIP2-containing Liposomes
To investigate the effect of cumulative EF-hand mutations on the PIP2-binding properties of wild-type PLCζ, we employed the liposome/activity binding assay as described above (see Fig. 5C). Thus, 1 μg of recombinant protein corresponding to PLCζWT and the five EF-hand mutants were each incubated with liposomes containing 1% PIP2. After centrifugation, the supernatants were separated, and the PIP2 hydrolytic activity was assayed using the standard [3H]PIP2 hydrolysis assay. Based on the percentage of the PIP2 hydrolytic activity pre- and post-liposome binding, we estimated the relative binding of each PLCζ protein to the PIP2-containing liposomes. As shown in Fig. 6, although 94% of PLCζWT bound to the liposomes, the three single EF-hand mutants (PLCζK49A, PLCζK53A, and PLCζR57A) showed ∼71–75% liposome binding. The effect of the double and the triple mutation was even more notable, as PLCζK53A,K57A displayed ∼59% and PLCζK49A,K53A,R57A ∼49% relative liposome binding. These data indicate that sequential neutralization of the basic residues within the EF-hand region substantially reduces the PIP2-binding ability of PLCζ.
FIGURE 6.
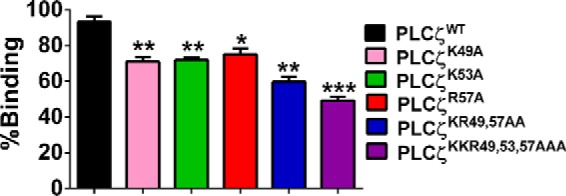
Binding of PLCζ mutants to PIP2-containing liposomes. Normalized binding of PLCζWT, PLCζK49A, PLCζK53A, PLCζR57A, PLCζK53A,K57A, and PLCζK49A,K53A,R57A to unilamellar liposomes containing 1% PIP2 is shown. Following centrifugation, the supernatants were assayed for their ability to hydrolyze PIP2 in vitro, using the standard [3H]PIP2 hydrolysis assay (n = 4 ± S.E., using two different preparations of recombinant protein). Based on the percentage of the PIP2 hydrolytic activity pre- and post- liposome binding, the relative binding of each PLCζ protein to the PIP2-containing liposomes was determined. Significant statistical differences (asterisks) were calculated by an unpaired Student's t test; *, p < 0.05; **, p < 0.005; and ***, p < 0.0005, (GraphPad, Prism 5).
Modeling of Ca2+ Oscillations Induced by PLCζ EF-hand Mutants
The Ca2+ oscillatory activity associated with each of the PLCζ mutants constructed was simulated by using the parameters calculated in Fig. 6 and Tables 1 and 2. The most marked differentiation between constructs is the binding activity of each protein (Fig. 6), which is in agreement with a progressive destabilization of the EF-hand binding regime. By contrast, the Ca2+ dependence of IP3 production (plotted in Fig. 4 and quantified in Table 2 as Ca2+-dependent EC50 value) is very similar for each of the PLCζ constructs. Ca2+ oscillations simulated with this set of parametric values (Fig. 7, top panel) closely match those observed experimentally for each construct (Fig. 2) in terms of frequency. The theoretical relationship between Ca2+ oscillatory frequency and binding activity was produced by the mathematical model for EC50 = 65, 75, and 85 nm (Fig. 7, bottom panel,, lines left to right). The experimentally computed operating points of PLCζ wild-type and its various constructs (Fig. 7, bottom panel, circles) are located very close to the theoretical curves, confirming that the variability in Ca2+ oscillatory frequency can be accounted for almost exclusively by the gradual reduction in binding activity. When PLCζK49A,K53A,R57A was highly overexpressed, the oscillatory activity was largely restored, as indicated by the operating point of this scenario (Fig. 7, bottom panel, solid circle at the right of the panel). The fact that the circle lies below the theoretical frequency curve (Fig. 7, bottom panel, dashed line) may be due to the sub-optimal binding of the protein to PIP2 at non-physiologically elevated concentrations.
FIGURE 7.
Simulated time series of Ca2+ oscillations within an egg for wild-type and the various PLCζ mutants. Top panel, physiological parameters were taken from experimental measurements summarized in Fig. 6 and Tables 1 and 2. The theoretical relationship between PIP2 binding activity of the PLCζ constructs and the Ca2+ oscillatory frequency is plotted in the bottom panel as a solid line for EC50 = 75 nm. The solid curve is framed by two dotted lines corresponding to EC50 = 65 and 85 nm (left and right panels, respectively) to account for the small variability in EC50 estimated for the various constructs (indicated by circles). The curve is plotted against a normalized range of 0 to 1 to account for the binding activity estimated as a percentile in Fig. 6. Oscillatory activity associated with overexpressed PLCζK49A,K53A,R57A is indicated by the solid circle (top right). This point lies below the theoretical binding activity versus frequency curve (dashed line).
Ca2+ Oscillation Inducing Activity of PLCζ Double Motif Mutant Expressed in Mouse Eggs
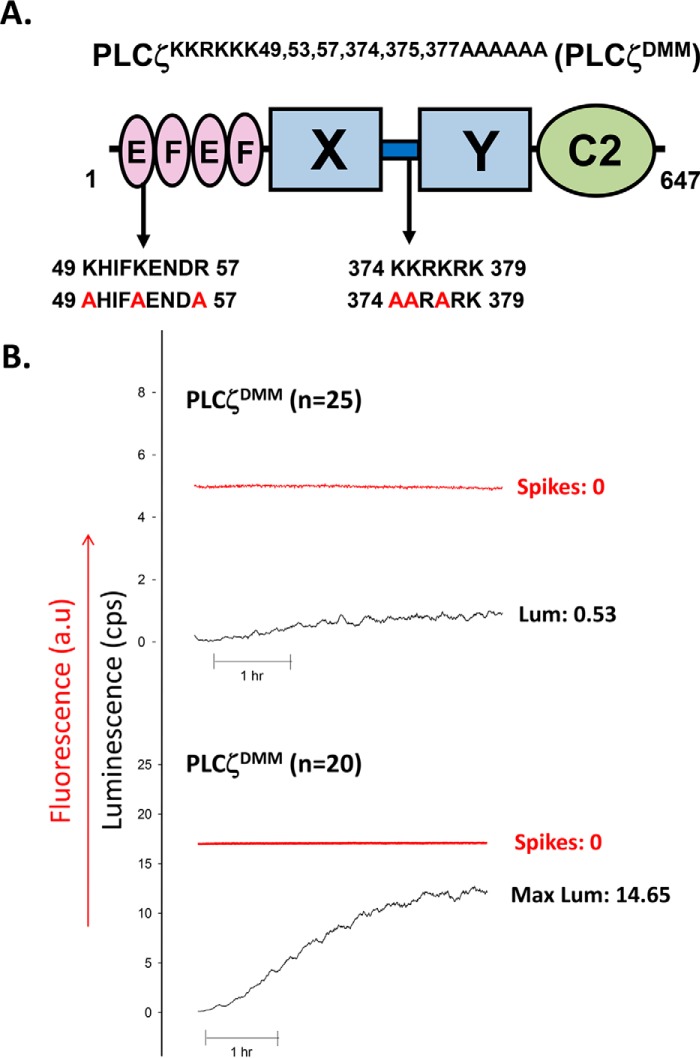
To investigate whether there is synergy between the cationic residues of the first EF-hand domain and the XY-linker region of PLCζ and whether these residues are necessary and sufficient to anchor this sperm protein to its PIP2-containing membranes, we generated a PLCζ mutant, in which charge-neutralization mutations were introduced within these two PLCζ motifs. Thus, the residues Lys-49, Lys-53, and Arg-57 within the first EF-hand domain and the residues Lys-374, Lys-375, and Lys-377 within the XY-linker of PLCζ were substituted by the neutral Ala residue giving rise to a PLCζ double motif mutant (PLCζK49A,K53A,R57A,K374A,K375A,K377A; PLCζDMM) containing six neutralization mutations (Fig. 8A). Interestingly, microinjection of cRNA encoding a luciferase-tagged version of PLCζDMM failed to cause any Ca2+ release, even after relatively high levels of protein expression in unfertilized mouse eggs (Fig. 8B and Table 1).
FIGURE 8.
Generation and expression of PLCζK49A,K53A,R57A,K374A,K375A,K377A (PLCζDMM) in unfertilized mouse eggs. A, schematic representation of mouse PLCζ domain structure identifying the location of the successive Lys or Arg residue substitutions to Ala, between residues 49 and 57 within the first EF-hand domain, as well as the location of the successive Lys substitutions to Ala between residues 374 and 379 in the XY-linker region. B, traces showing the changes in fluorescence (a.u., arbitrary units) and luminescence (cps) denoting alterations in Ca2+ concentrations (red trace) and luciferase expression (black trace), respectively, following the microinjection of high and low concentrations of PLCζDMM-luciferase cRNA into unfertilized mouse eggs. The mean values for the number of Ca2+ oscillations (spikes) and luminescence (Lum) during the 1st h of oscillating are shown.
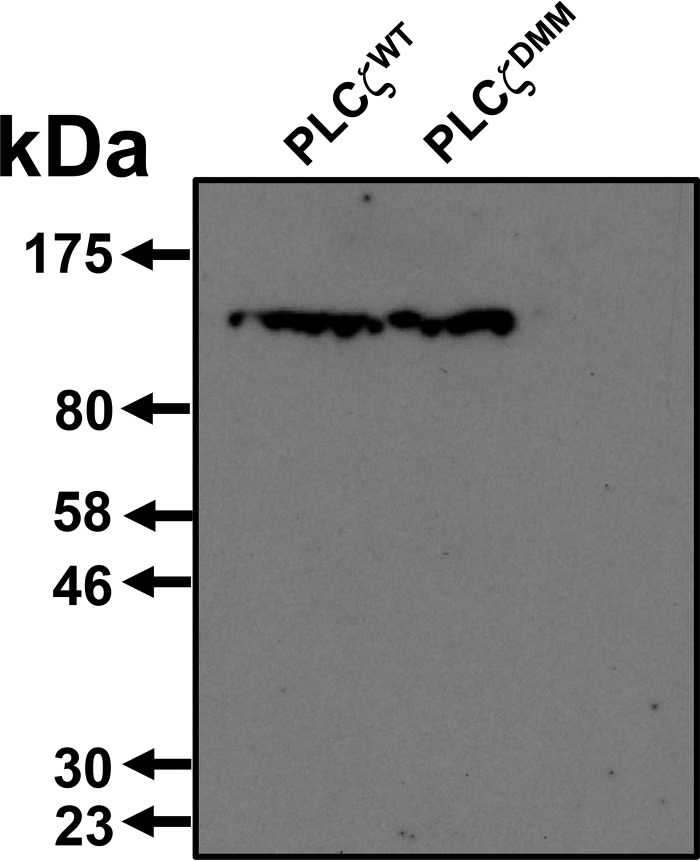
To investigate whether the luciferase-tagged PLCζWT and PLCζDMM fusion constructs were expressed as structurally intact proteins in mouse eggs, we performed immunoblot analysis of two groups of mouse eggs microinjected with 0.5 μg/μl cRNA encoding either PLCζWT-LUC or the PLCζDMM-LUC mutant. Expression was followed for ∼3 h and then the two groups of eggs were analyzed by SDS-PAGE and immunoblot detection using an anti-luciferase antibody. A single protein band was observed with mobility corresponding to the predicted molecular mass (∼129 kDa) for both PLCζWT-LUC and PLCζDMM-LUC fusion proteins (Fig. 9), suggesting that each of the two cRNAs was faithfully expressed as full-length PLC-luciferase proteins and at similar expression levels in the cRNA-injected mouse eggs.
FIGURE 9.
Confirmation of expression of PLCζWT- and PLCζDMM-LUC fusion proteins in mouse eggs. Two sets of mouse eggs (50 eggs each) were microinjected with 0.5 μg/μl cRNA corresponding to either PLCζWT or PLCζDMM. Expression was allowed for ∼3 h, and then the two sets of eggs were analyzed by SDS-PAGE and Western blotting using an anti-firefly luciferase antibody (1:10,000; Pierce).
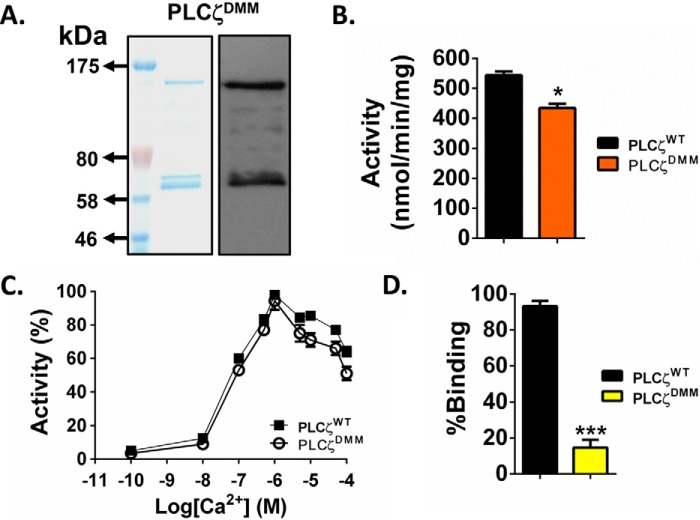
Expression, Enzymatic Characterization, and in Vitro Binding of PLCζ Double Motif Mutant to PIP2-containing Liposomes
PLCζDMM was then subcloned into the pETMM60 vector and bacterially expressed and purified as a NusA-His6-tagged fusion protein. Fig. 10A shows NusA-His6-PLCζDMM recombinant protein analyzed by SDS-PAGE (left panel) and immunoblot detection with the anti-NusA monoclonal antibody (right panel). The corresponding protein with the appropriate molecular mass (∼134 kDa) was observed as the top band in both Coomassie Brilliant Blue staining and on the immunoblot (Fig. 10A). Some low molecular weight bands were also detected by the anti-NusA antibody, and these are probably the result of protein degradation occurring through the bacterial expression and purification processes. Enzymatic analysis using the [3H]PIP2 hydrolysis assay showed that PLCζDMM retained ∼80% of the enzymatic activity of PLCζWT (434 ± 28 versus 544 ± 23 nmol/min/mg) (Fig. 10B) and that there was no significant difference in the Ca2+ sensitivity of PIP2 hydrolysis for PLCζWT and PLCζDMM, with a very similar EC50 value (72 versus 108 nm) (Fig. 10C and Table 2). However, the Km value for PLCζDMM (4975 μm) was ∼59-fold higher compared with PLCζWT (84 μm). More interestingly, when we performed the liposome/activity binding assay for PLCζDMM, we found that this mutant displayed only ∼15% relative liposome binding compared with PLCζWT (Fig. 10D). These data indicate that neutralization of the positively charged residues within the first EF-hand and the XY-linker region dramatically reduces the binding of PLCζ to PIP2, leading to complete loss of its in vivo Ca2+ oscillation inducing activity.
FIGURE 10.
Expression, enzymatic characterization and in vitro binding of NusA-His6-PLCζDMM to PIP2-containing liposomes. A, expression of recombinant PLCζDMM protein. Affinity-purified NusA-His6-tagged PLCζDMM protein (1 μg) was analyzed by SDS-PAGE followed by either Coomassie Brilliant Blue staining (left panel) or immunoblot analysis using the anti-NusA monoclonal antibody at 1:100,000 dilution (right panel). B, enzyme activity of PLCζDMM. PIP2 hydrolysis enzyme activities of the purified recombinant proteins were determined with the standard [3H]PIP2 cleavage assay, n = 4 ± S.E., using two different preparations of each recombinant protein. Significant statistical differences (asterisks) were calculated by an unpaired Student's t test; *, p < 0.05 (GraphPad, Prism 5). C, effect of varying [Ca2+] on the normalized activity of NusA-His6-tagged PLCζDMM fusion protein. For these assays n = 4 ± S.E., using two different preparations of each recombinant protein. D, normalized binding of PLCζDMM to unilamellar liposomes containing 1% PIP2 (n = 4 ± S.E., using two different preparations of recombinant protein). Significant statistical differences (asterisks) were calculated by an unpaired Student's t test; ***, p < 0.0005, (GraphPad, Prism 5).
Discussion
A significant body of scientific and clinical evidence suggests that the sperm-specific PLCζ protein is the physiological molecule that, following sperm-egg fusion, stimulates cytoplasmic Ca2+ oscillations, egg activation, and early embryonic development to effect mammalian fertilization (3, 5, 7, 8, 11, 21, 27). The most compelling observation is that solely introducing PLCζ mimics all of the signaling processes initiated by the sperm, triggering the same pattern of Ca2+ release as seen at normal fertilization and leading to the successful development of a blastocyst embryo. Thus, the current model of egg activation at fertilization is that the PLCζ of a fertilizing spermatozoon is introduced into the egg cytoplasm where it catalyzes PIP2 hydrolysis, stimulating the IP3 signaling pathway, and leading to Ca2+ oscillations (5, 13).
The sperm PLCζ is the smallest, with the most elementary domain organization, of all the mammalian PLC isoforms (3). Hence, the intrinsic ability of sperm PLCζ to cause robust Ca2+ oscillations in eggs is significant because all the other PI-specific PLCs are unable trigger Ca2+ oscillations in eggs at physiological protein expression levels. It therefore appears most plausible that PLCζ employs a novel mechanism to potently induce Ca2+ release in eggs and each of its individual domains appears to play an important role in the distinct molecular and biochemical characteristics, as well as in the unique regulatory mechanism of this sperm-derived PLC isozyme (2, 12). PLCζ shares the greatest homology with PLCδ1, but one major structural difference that distinguishes PLCζ from PLCδ1 is the lack of an N-terminal PH domain (2, 13). This is mechanistically interesting because the PH domain of PLCδ1 in particular is known to specifically bind PIP2 in the plasma membrane (15, 28). In contrast, we have recently shown that PLCζ does not localize to the plasma membrane-bound PIP2, but instead it targets distinct vesicular structures inside the egg cortex (29). Interestingly, the chimeric addition of a PH domain at the N terminus of the PLCζ sequence does not alter the ability of PLCζ to trigger Ca2+ oscillations in mouse eggs, and the PH-PLCζ chimera is unable to target PLCζ to the plasma membrane PIP2 (25). The precise mechanism employed by PLCζ to enable interaction with the PIP2-containing vesicular membranes inside the egg cytosol is not understood.
Although the precise identity of the intracellular PIP2-containing vesicles is currently unknown, we have proposed that PLCζ associates with vesicular PIP2 via electrostatic interactions mediated by the positively charged XY-linker region, assisting in anchoring PLCζ to membranes, while enhancing local concentrations of the negatively charged PIP2 (16, 17). In PLCζ, the XY-linker region is more extended compared with that of PLCδ1, and the proximal part to the Y catalytic domain contains a distinctive cluster of basic amino acid residues not found in the homologous region of any of the other somatic PLC isoforms (3). It is also notable that the XY-linker of somatic PLCs confers potent inhibition of their enzymatic activity (30, 31). In contrast, the XY-linker of PLCζ does not confer enzymatic auto-inhibition but conversely appears to be required for maximal enzymatic activity (19). We have recently shown that deletion of PLCζ XY-linker significantly diminishes its in vivo Ca2+ oscillation inducing activity but does not completely abolish it (19). This suggests that the XY-linker is essential for the association of PLCζ with PIP2-containing vesicular membranes, but it is not the sole region of PLCζ responsible for this association.
Another candidate region that might be involved in the sequestration of PLCζ to membranes containing its substrate PIP2 is the C2 domain. The current data indicate that the C2 domain of PLCζ may interact, albeit with low affinity, with membrane phospholipids (17, 32). Indeed, such interactions were observed in vitro with phosphatidylinositol 3-phosphate and phosphatidylinositol 5-phosphate. It is possible that the association of the C2 domain with phosphatidylinositol 3-phosphate may play a role in PLCζ localization, or even perhaps regulation of enzymatic activity, as the presence of phosphatidylinositol 3-phosphate reduced PIP2 hydrolysis by PLCζ in vitro (32).
A recent study demonstrated that the N-terminal lobe of the EF-hand domain of PLCδ1 binds to anionic phospholipid-containing vesicles, suggesting that the EF-hand domain aids substrate binding in the active site when the protein is membrane-anchored (20). The binding of the PLCδ1 EF-hand domain to anionic phospholipid is mediated by a number of cationic residues within the first EF-hand motif of PLCδ1. Interestingly, the positively charged residues that have been shown to contribute to the binding of PLCδ1 (Arg-182, Lys-183, and Arg-186) by vesicles containing anionic lipids are specifically conserved in PLCζ. We have shown that PLCζ EF-hand domains play an important role in the high Ca2+ sensitivity relative to the other PLC isoforms, especially in comparison with PLCδ1 (21). PLCζ appears to be 100-fold more sensitive to Ca2+ than PLCδ1, which would enable the enzyme to be active at the resting nanomolar Ca2+ levels within the egg cytosol (21). Deletion of one or both pairs of EF-hand domains of PLCζ completely abolishes its Ca2+ oscillation inducing activity in mouse eggs (21). Our current data suggest that this might be the result of both altered Ca2+ sensitivity and loss of ability to associate with PIP2-containing membranes, as these PLCζ EF-hand deletion constructs were unable to trigger Ca2+ release even when overexpressed in mouse eggs (21). Our mutagenesis analysis indicates that the substitution of even one Lys or Arg residue to Ala within the positively charged cluster of the PLCζ EF-hand domain diminishes the Ca2+ oscillation inducing activity of PLCζ (Fig. 2) without affecting its ability to hydrolyze PIP2 in vitro or the Ca2+ sensitivity of its enzymatic activity (Fig. 4). Interestingly, the Km value for the triple mutant PLCζK49A,K53A,R57A (432 μm) was ∼5.1-fold higher compared with PLCζWT (84 μm), suggesting that replacement of these three positively charged residues within the first EF-hand domain has an effect on the in vitro binding ability of PLCζ to PIP2 (Table 2). Moreover, we used a variety of approaches and demonstrated that PLCζ binds only to PIP2-containing liposomes, and sequential neutralization of these basic residues within the first EF-hand region of PLCζ can significantly diminish the PIP2-binding ability of PLCζ (Figs. 5 and 6). As shown in our proposed mechanism in Fig. 11, which is supported by our studies on the PLCζK49A,K53A,R57A,K374A,K375A,K377A mutant (PLCζDMM), it is plausible that PLCζ is attracted to the anionic PIP2-containing component of the intracellular vesicular membranes through electrostatic interactions with both the first EF-hand domain and the XY-linker regions, which are rich in basic residues.
FIGURE 11.
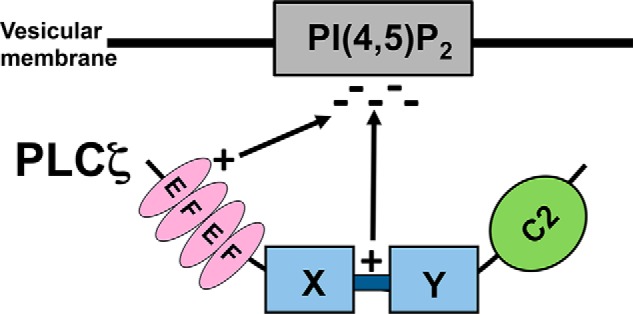
Schematic illustration of the proposed mechanism that PLCζ utilizes to target intracellular vesicular PIP2-containing membranes. Association of PLCζ with the negatively charged PIP2 involves electrostatic interactions with the positively charged first EF-hand domain and the XY-linker region. The catalytic XY domain subsequently proceeds with the enzymatic cleavage of PIP2.
Our study provides an important advance in understanding the complex regulatory mechanism of PLCζ and suggests that the N-terminal lobe of the EF-hand domain of PLCζ has an essential role in the interaction of this enzyme with its target membrane, which together with the XY-linker may combine to provide a tether that facilitates proper PIP2 substrate access and binding in the PLCζ active site.
Author Contributions
M. N., K. S., and F. A. L. designed the study; J. R. S. conducted the oocyte experiments; D. P. generated the simulation data; M. N., L. B., B. L. C., P. S., A. S., and M. C. performed the molecular cloning, protein expression, purification, and characterization experiments; Z. S. prepared the liposomes, and all authors contributed to manuscript preparation. M. N. compiled the figures and together with F. A. L. prepared the final draft.
This work was supported by EU-FP7 Marie-Curie Intra-European Fellowship 628634 (to M. N.) and an Institute for Molecular and Experimental Medicine Research Scholarship (to J. R. S.). F. A. L. and K. S. hold patents on PLCζ with Cardiff University.
- PLCζ
- phospholipase C-ζ
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- IP3
- inositol 1,4,5-trisphosphate
- PH
- pleckstrin homology
- PtdCho
- 1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- CHOL
- cholesterol
- PtdEtn
- 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine
- PS
- phosphatidylserine
- PA
- phosphatidic acid.
References
- 1.Stricker S. A. (1999) Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 211, 157–176 [DOI] [PubMed] [Google Scholar]
- 2.Nomikos M., Kashir J., Swann K., and Lai F. A. (2013) Sperm PLCζ: from structure to Ca2+ oscillations, egg activation and therapeutic potential. FEBS Lett. 587, 3609–3616 [DOI] [PubMed] [Google Scholar]
- 3.Saunders C. M., Larman M. G., Parrington J., Cox L. J., Royse J., Blayney L. M., Swann K., and Lai F. A. (2002) PLCζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 129, 3533–3544 [DOI] [PubMed] [Google Scholar]
- 4.Cox L. J., Larman M. G., Saunders C. M., Hashimoto K., Swann K., and Lai F. A. (2002) Sperm phospholipase Cζ from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction 124, 611–623 [DOI] [PubMed] [Google Scholar]
- 5.Kouchi Z., Fukami K., Shikano T., Oda S., Nakamura Y., Takenawa T., and Miyazaki S. (2004) Recombinant phospholipase Cζ has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J. Biol. Chem. 279, 10408–10412 [DOI] [PubMed] [Google Scholar]
- 6.Knott J. G., Kurokawa M., Fissore R. A., Schultz R. M., and Williams C. J. (2005) Transgenic RNA interference reveals role for mouse sperm phospholipase Cζ in triggering Ca2+ oscillations during fertilization. Biol. Reprod. 72, 992–996 [DOI] [PubMed] [Google Scholar]
- 7.Yoon S. Y., Jellerette T., Salicioni A. M., Lee H. C., Yoo M. S., Coward K., Parrington J., Grow D., Cibelli J. B., Visconti P. E., Mager J., and Fissore R. A. (2008) Human sperm devoid of PLCζ1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J. Clin. Invest. 118, 3671–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heytens E., Parrington J., Coward K., Young C., Lambrecht S., Yoon S. Y., Fissore R. A., Hamer R., Deane C. M., Ruas M., Grasa P., Soleimani R., Cuvelier C. A., Gerris J., Dhont M., et al. (2009) Reduced amounts and abnormal forms of phospholipase C ζ (PLCζ) in spermatozoa from infertile men. Hum. Reprod. 24, 2417–2428 [DOI] [PubMed] [Google Scholar]
- 9.Kashir J., Konstantinidis M., Jones C., Lemmon B., Lee H. C., Hamer R., Heindryckx B., Deane C. M., De Sutter P., Fissore R. A., Parrington J., Wells D., and Coward K. (2012) A maternally inherited autosomal point mutation in human phospholipase C-ζ (PLCζ) leads to male infertility. Hum. Reprod. 27, 222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nomikos M., Elgmati K., Theodoridou M., Calver B. L., Cumbes B., Nounesis G., Swann K., and Lai F. A. (2011) Male infertility-linked point mutation disrupts the Ca2+ oscillation-inducing and PIP2 hydrolysis activity of sperm PLCζ. Biochem. J. 434, 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomikos M., Yu Y., Elgmati K., Theodoridou M., Campbell K., Vassilakopoulou V., Zikos C., Livaniou E., Amso N., Nounesis G., Swann K., and Lai F. A. (2013) Phospholipase Cζ rescues failed oocyte activation in a prototype of male factor infertility. Fertil. Steril. 99, 76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nomikos M. (2015) Novel signalling mechanism and clinical applications of sperm-specific PLCζ. Biochem. Soc. Trans. 43, 371–376 [DOI] [PubMed] [Google Scholar]
- 13.Nomikos M., Swann K., and Lai F. A. (2012) Starting a new life: sperm PLC-ζ mobilizes the Ca2+ signal that induces egg activation and embryo development: an essential phospholipase C with implications for male infertility. Bioessays 34, 126–134 [DOI] [PubMed] [Google Scholar]
- 14.Dowler S., Currie R. A., Campbell D. G., Deak M., Kular G., Downes C. P., and Alessi D. R. (2000) Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 351, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lomasney J. W., Cheng H. F., Wang L. P., Kuan Y., Liu S., Fesik S. W., and King K. (1996) Phosphatidylinositol 4,5-bisphosphate binding to the pleckstrin homology domain of phospholipase C-δ1 enhances enzyme activity. J. Biol. Chem. 271, 25316–25326 [DOI] [PubMed] [Google Scholar]
- 16.Nomikos M., Mulgrew-Nesbitt A., Pallavi P., Mihalyne G., Zaitseva I., Swann K., Lai F. A., Murray D., and McLaughlin S. (2007) Binding of phosphoinositide-specific phospholipase C-ζ (PLC-ζ) to phospholipid membranes: potential role of an unstructured cluster of basic residues. J. Biol. Chem. 282, 16644–16653 [DOI] [PubMed] [Google Scholar]
- 17.Nomikos M., Elgmati K., Theodoridou M., Calver B. L., Nounesis G., Swann K., and Lai F. A. (2011) Phospholipase Cζ binding to PtdIns(4,5)P2 requires the XY-linker region. J. Cell Sci. 124, 2582–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin S., and Murray D. (2005) Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605–611 [DOI] [PubMed] [Google Scholar]
- 19.Nomikos M., Elgmati K., Theodoridou M., Georgilis A., Gonzalez-Garcia J. R., Nounesis G., Swann K., and Lai F. A. (2011) Novel regulation of PLCζ activity via its XY-linker. Biochem. J. 438, 427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai J., Guo S., Lomasney J. W., and Roberts M. F. (2013) Ca2+-independent binding of anionic phospholipids by phospholipase C δ1 EF-hand domain. J. Biol. Chem. 288, 37277–37288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nomikos M., Blayney L. M., Larman M. G., Campbell K., Rossbach A., Saunders C. M., Swann K., and Lai F. A. (2005) Role of phospholipase C-ζ domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J. Biol. Chem. 280, 31011–31018 [DOI] [PubMed] [Google Scholar]
- 22.Swann K. (2013) Measuring Ca2+ oscillations in mammalian eggs. Methods Mol. Biol. 957, 231–248 [DOI] [PubMed] [Google Scholar]
- 23.Campbell K., and Swann K. (2006) Ca2+ oscillations stimulate an ATP increase during fertilization of mouse eggs. Dev. Biol. 298, 225–233 [DOI] [PubMed] [Google Scholar]
- 24.Swann K., Campbell K., Yu Y., Saunders C., and Lai F. A. (2009) Use of luciferase chimaera to monitor PLCζ expression in mouse eggs. Methods Mol. Biol. 518, 17–29 [DOI] [PubMed] [Google Scholar]
- 25.Theodoridou M., Nomikos M., Parthimos D., Gonzalez-Garcia J. R., Elgmati K., Calver B. L., Sideratou Z., Nounesis G., Swann K., and Lai F. A. (2013) Chimeras of sperm PLCζ reveal disparate protein domain functions in the generation of intracellular Ca2+ oscillations in mammalian eggs at fertilization. Mol. Hum. Reprod. 19, 852–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomikos M., Sanders J. R., Theodoridou M., Kashir J., Matthews E., Nounesis G., Lai F. A., and Swann K. (2014) Sperm-specific post-acrosomal WW-domain binding protein (PAWP) does not cause Ca2+ release in mouse oocytes. Mol. Hum. Reprod. 20, 938–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanusi R., Yu Y., Nomikos M., Lai F. A., and Swann K. (2015) Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Cζ. Mol. Hum. Reprod. DOI 10.1093/molehr/gav042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebecchi M. J., and Pentyala S. N. (2000) Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 80, 1291–1335 [DOI] [PubMed] [Google Scholar]
- 29.Yu Y., Nomikos M., Theodoridou M., Nounesis G., Lai F. A., and Swann K. (2012) PLCζ causes Ca2+ oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P(2). Mol. Biol. Cell 23, 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicks S. N., Jezyk M. R., Gershburg S., Seifert J. P., Harden T. K., and Sondek J. (2008) General and versatile autoinhibition of PLC isozymes. Mol. Cell 31, 383–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gresset A., Hicks S. N., Harden T. K., and Sondek J. (2010) Mechanism of phosphorylation-induced activation of phospholipase C-γ isozymes. J. Biol. Chem. 285, 35836–35847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kouchi Z., Shikano T., Nakamura Y., Shirakawa H., Fukami K., and Miyazaki S. (2005) The role of EF-hand domains and C2 domain in regulation of enzymatic activity of phospholipase Cζ. J. Biol. Chem. 280, 21015–21021 [DOI] [PubMed] [Google Scholar]