Abstract
Twist1 is a basic helix-loop-helix-containing transcription factor that is expressed in the dental mesenchyme during the early stages of tooth development. To better delineate its roles in tooth development, we generated Twist1 conditional knockout embryos (Twist2Cre/+;Twist1fl/fl) by breeding Twist1 floxed mice (Twist1fl/fl) with Twist2-Cre recombinase knockin mice (Twist2Cre/+). The Twist2Cre/+;Twist1fl/fl embryos formed smaller tooth germs and abnormal cusps during early tooth morphogenesis. Molecular and histological analyses showed that the developing molars of the Twist2Cre/+;Twist1fl/fl embryos had reduced cell proliferation and expression of fibroblast growth factors 3, 4, 9, and 10 and FGF receptors 1 and 2 in the dental epithelium and mesenchyme. In addition, 3-week-old renal capsular transplants of embryonic day 18.5 Twist2Cre/+;Twist1fl/fl molars showed malformed crowns and cusps with defective crown dentin and enamel. Immunohistochemical analyses revealed that the implanted mutant molars had defects in odontoblast differentiation and delayed ameloblast differentiation. Furthermore, in vitro ChIP assays demonstrated that Twist1 was able to bind to a specific region of the Fgf10 promoter. In conclusion, our findings suggest that Twist1 plays crucial roles in regulating tooth development and that it may exert its functions through the FGF signaling pathway.
Keywords: cell differentiation, dentin, tooth, tooth development, transplantation, Twist1, Twist2, enamel formation, odontoblast differentiation, tooth morphogenesis
Introduction
Tooth morphogenesis is an advancing process through which the dental epithelium thickens, buds, grows, and folds to form a bell-shaped enamel organ (1). This process involves a series of sequential and reciprocal interactions between the dental epithelium and the underlying mesenchyme. This series is mediated by a number of growth factors, cell membrane receptors, and intracellular transcription factors (2–5).
Human and mouse genetic studies have demonstrated that the FGF signaling pathway is essential for tooth development (6). The epithelially derived Fgf4, Fgf8, and Fgf9 bind to FGF receptor 2c (Fgfr2c) and regulate mesenchymal cell proliferation, whereas mesenchymally derived Fgf3 and Fgf10 interact with Fgfr2b and stimulate epithelial cell proliferation (7–9). This reciprocal interaction is responsible for the formation of critical masses of dental epithelial and mesenchymal tissues that are shaped, sized, and positioned for the terminal differentiation of odontoblasts and ameloblasts. Previous studies have shown that Fgf3 and Fgf10 are involved in determining the size of teeth (8, 10–12). Further studies have revealed that a decrease in Fgf3 dosage in mice and humans led to a phenotype resembling the progressive reappearance of the dental morphogenesis of primitive rodent and primate fossils (13). These findings demonstrate the importance of fine-tuning FGF signaling in both early tooth morphogenesis and late tooth cusp formation.
Twist1 is a basic helix-loop-helix-containing transcription factor and is co-expressed with Fgf3 and Fgf10 as well as Fgfr2c in the neural crest-derived dental mesenchyme throughout tooth development (7, 14–16). This overlapping expression pattern suggests that Twist1 may be a transcription factor regulating FGF signaling within and from the dental mesenchyme, thereby coupling the epithelial and mesenchymal FGF pathways. However, this hypothesis has not been tested in vivo in animal models because of the embryonic lethality of Twist1-null embryos (17).
Twist2, also called Dermo-1, shares a high degree of sequence identity with Twist 1, particularly in the basic helix-loop-helix region and the C-terminal region (18). Although Twist2 is expressed temporally after Twist1, they show an extensive overlapping expression pattern, especially when they are both expressed in the craniofacial mesenchyme during embryonic development (19). Twist2-heterozygous mice display no apparent developmental defects (20). The Twist2-null mouse phenotype depends on the genetic background. In an inbred 129/Sv genetic background, Twist2-null mice die perinatally, but, in the 129/C57 mixed genetic background, they develop only a mild focal, facial, dermal, dysplasia-like phenotype (21). The role of Twist2 in tooth development is unknown.
During the histodifferentiation of crown formation, the terminal differentiation of ameloblasts and odontoblasts also involves a series of reciprocal interactions between the inner enamel epithelium and the cells of the outer layer of the dental papilla. Presumably, a defect in odontoblast differentiation may adversely affect ameloblast differentiation. Our previous studies have demonstrated that Twist1 promotes the differentiation of human dental pulp stem cells into odontoblast-like cells in vitro (22). However, it remains to be determined whether Twist1 plays a role in regulating the differentiation of odontoblasts in vivo or whether it indirectly affects ameloblast differentiation.
To overcome the embryonic lethality of Twist1-null embryos, Twist1-floxed mice were generated and used widely to study the roles of Twist1 in the development of various organs (23–25). In this study, we generated Twist1 conditional knockout mouse embryos by breeding Twist1-floxed mice with Twist2-Cre recombinase knockin mice and examined their phenotypic and molecular changes in the developing tooth germs. In addition, we determined the ability of the Twist1-deficient tooth germs to form crown dentin and enamel using the kidney capsule implantation approach.
Experimental Procedures
Experimental Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at Texas A&M University Baylor College of Dentistry. Twist1 floxed mice (Twist1fl/fl), Twist2-Cre knockin mice (Twist2Cre/+), Sox2-Cre transgenic mice, and ROSA26 conditional reporter mice (R26RLacZ/LacZ) have been described previously (23, 26–28). Twist1fl/fl mice were mated with Sox2-Cre mice to generate Twist1-heterozygous mice (Sox2-Cre;Twist1fl/+). Twist1fl/fl mice were bred with Twist2Cre/+ mice to generate compound Twist1 and Twist2 heterozygous mice (Twist2Cre/+;Twist1fl/+), which were crossed again with the Twist1fl/fl mice to obtain embryos with the conditional deletion of the Twist1 gene in tissues where Twist2 is expressed (Twist2Cre/+;Twist1fl/fl). Twist1fl/+ and Twist1fl/fl embryos were used as controls. R26RLacZ/LacZ mice were bred with Twist2Cre/+ mice to generate Twist2Cre/+;R26RLacZ/+ embryos.
X-Gal Staining
X-gal staining was performed to detect β-gal activity in the tooth germs of the Twist2Cre/+;R26RLacZ/+ embryos, as described previously (29). Briefly, embryos at embryonic days (E)4 12.5–18.5 were fixed in 4% paraformaldehyde for 1 h, soaked in 30% sucrose overnight, and then embedded in optimum cutting temperature Compound (Tissue-Tek). Samples were cut with a cryostat at 10 μm, and sections were stained overnight at 37 °C in 1% X-gal solution (Invitrogen).
Whole-mount Bone and Cartilage Staining
Whole-mount E18.5 control and Twist2Cre/+;Twist1fl/fl embryos were stained with Alizarin red and Alcian blue to stain the bone and cartilage, respectively, as described previously (26). The stained specimens were washed, stored, and photographed in aqueous 95% ethanol 40:60 glycerol solution.
Histology and BrdU Cell Proliferation Analysis
The embryos were harvested at the desired developmental stages and fixed in 4% paraformaldehyde overnight at 4 °C. For histological testing, the embryos were then dehydrated through a series of graded ethanol and embedded in paraffin wax. Serial sections of 6 μm were cut and stained with H&E.
To detect cell proliferation in the tooth germs, we injected the BrdU labeling reagent (Roche, 10 μl/g body weight) intraperitoneally once a day at gestational day 15.5. Thirty minutes after injection, the embryos were dissected, fixed overnight in 4% paraformaldehyde at 4 °C, embedded in paraffin, and sectioned at 6 μm in the coronal plane. Cell proliferation was assessed using the Invitrogen BrdU staining kit according to the instructions of the manufacturer. For color reaction, 3,3′diaminobenzidine (Vector Laboratories, DAKO) was used as a substrate.
Real-time RT-PCR (Quantitative RT-PCR)
Total RNA was extracted from the mandibular first molars of E14.5 Twist2Cre/+;Twist1fl/fl and control embryos using an RNeasy® mini kit (Qiagen, Germantown, MD) according to the instructions of the manufacturer (Invitrogen) and reverse-transcribed into cDNA with a reverse transcription kit (Qiagen). Quantitative real-time PCR was performed using a Go Tag® quantitative PCR master mix system (Promega, Madison, WI). Mouse 18S rRNA primers (catalog no. PPM57735E-200, Qiagen) were used for normalization. The primers for Fgfr1 and Fgfr2 have been reported elsewhere (26). The primers for Fgf3 were 5′-TAAGGTACCTTCCTGCCTAGCTCCAAGAG-3′ and 5′-CCCAGGATGCACACACTCAG-3′. The primers for Fgf10 were 5′-TCAAAGCCATCAACAGCAAC-3′ and 5′-GGAGGAAGTGAGCAGAGGTG-3′. All experiments were performed in triplicate on three animals. The data were analyzed using the 2−ΔΔCT method as described previously (26).
In Situ Hybridization and Immunohistochemistry
In situ hybridization on paraformaldehyde-fixed, paraffin-embedded sections was performed as described previously (30). Digoxigenin-labeled, single-stranded RNA probes of Shh, Fgf3, Fgf10, Fgfr1, Fgf4, and Fgf9 were used in this study. A 640-bp fragment of mouse Shh cDNA, a 1.2-kb fragment of mouse Fgfr1 cDNA, a 0.62-kb fragment of mouse Fgf4 cDNA, and a 0.41-kb fragment of mouse Fgf9 cDNA were used to generate antisense riboprobes. The Fgf3 and Fgf10 RNA probes were generated as described previously (31, 32). Deparaffinized sections were immunostained with polyclonal anti-digoxigenin antibodies, conjugated to alkaline phosphatase (Roche), and developed with a NBT/BCIP (nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate) substrate system. At least three individual samples were analyzed for each probe.
For immunohistochemistry testing, paraffin-embedded sections were treated with polyclonal rabbit anti-Twist1 (Abcam), polyclonal rabbit anti-ameloblastin (Santa Cruz Biotechnology) and the polyclonal rabbit antibody against dentin sialoprotein (DSP) as the primary antibodies. The signals were visualized with 3,3′diaminobenzidine (Vector Laboratories, DAKO) or with Alexa Fluor 555-coupled secondary antibody (Invitrogen) for immunofluorescence.
Kidney Capsule Grafting of Tooth Germs
The Twist2Cre/+;Twist1fl/fl mice were embryonically lethal. Therefore, it was impossible to determine the roles of Twist1 in postnatal tooth development. To overcome this limitation, kidney capsule grafting of tooth germs was performed. Briefly, the tooth germs of E18.5 mandibular first molars were dissected from Twist2Cre/+;Twist1fl/fl and control embryos and subsequently transplanted under the kidney capsule of anesthetized C57BL/6J mice as described by Otsu et al. (33). Three weeks after transplantation, the mice were sacrificed, and the kidneys with implanted tooth germs were removed carefully for further analysis.
Plain X-ray and Micro-computed Tomography (μCT) Analysis
For plain x-ray radiography, the tooth germs implanted under the kidney capsule for 3 weeks were analyzed with a Faxitron MX-20 specimen radiography system (Faxitron X-ray Corp., Buffalo Grove, IL). Then the control and mutant molars were scanned in a μCT system (μCT35, Scanco Medical, Basserdorf, Switzerland). The scans were performed at 55 kV, 145 μA, and 300 integration time and a resolution of 3 μm.
Cell Culture and ChIP Assay
Odontoblast-like MDPC-23 cells were cultured in a 6-well plate with α-modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen). The ChIP assay was performed according to the instructions of the manufacturer using the MAGnifyTM chromatin immunoprecipitation system (Invitrogen). Briefly, the cells were treated with 1% formaldehyde for 15 min to generate protein-DNA cross-links between molecules in close proximity within the chromatin complex. The odontoblast-like cells (MDPC-23) were then lysed for 5 min using lysis buffer, and chromatin was released from the nuclei and sheared by sonication to reduce the average DNA fragment size to 100∼300 bp. Then the cross-linked Twist1 protein was immunoprecipitated using a specific ChIP-qualified anti-Twist1 antibody conjugated to Dynabeads® protein A/G. The formaldehyde cross-linking was reversed by heat treatment at 55 °C for 15 min, and the DNA associated with that Twist1 protein was purified by DNA purification magnetic beads (Invitrogen). The purified DNA was analyzed by PCR with the following primers: forward, 5′-CGCCAAGAGAGAGGCTATGT-3′; reverse, 5′-AGCTTCGTTTCCTCCTCTCC-3′. The primers amplified a product of 169 bp of the Fgf10 promoter, spanning −1101∼1269 nt. As a positive control for PCR, DNA prepared from samples prior to immunoprecipitation was used as input DNA.
Data Quantification and Statistical Analysis
All experiments were repeated at least three times, and at least four samples of each phenotype were collected for each experiment. The data analysis was conducted with Student's t test for two-group comparison and with one-way analysis of variance for multiple-group comparison. If significant differences were found with one-way analysis of variance, the Bonferroni method was used to determine which groups were significantly different from others. The quantified results are presented as mean ± S.E. p < 0.05 was considered statistically significant.
Results
Delayed Development of the Third Molars in Twist2Cre/+;Twist1fl/+ Mice
We first generated Twist1 and Twist2 single heterozygous embryos and analyzed the morphology of the developing tooth germs at E13.5, E15.5, E16.5, and E18.5. Twist1 heterozygous embryos were generated by breeding Twist1fl/fl mice with Sox2-Cre mice. H&E staining showed that deleting one allele of the Twist1 or Twist2 gene had no apparent effect on early tooth morphogenesis (Fig. 1, A–D”). Then we generated compound Twist2Cre/+;Twist1fl/+ mice by breeding Twist1fl/fl mice with Twist2Cre/+ mice so that one allele of Twist1 gene was specifically deleted in the tissues, including the dental mesenchyme, where the Twist2 is expressed. To ensure that Twist2-Cre was active in the dental mesenchyme, we generated Twist2Cre/+;R26RLacZ/+ embryos and examined whether the Twist2-Cre could activate a β-gal expression in the dental mesenchyme of the ROSA26 embryos. X-gal staining showed that β-gal-positive cells were specifically restricted to the neural crest-derived ectomesenchyme but not in the dental epithelium in the molars from the bud to bell stages (E12.5-E18.5) (Fig. 2, A–F). Although individual Twist1 and Twist2 heterozygous mice showed no apparent defects in tooth development, the compound Twist2Cre/+;Twist1fl/+ mice exhibited delayed development of the third molars. Histological analysis showed that the third molars had fully developed to late bell stage in the control mice (Fig. 1E), whereas they were missing in the double-heterozygous mice by 1 week (Fig. 1F). However, x-ray radiography demonstrated that the third molars were also observed in the double-heterozygous mice by 3. weeks (Fig. 1H), although they appeared to be smaller than those in the control mice (Fig. 1G). These observations suggest that Twist1 and 2 might have a redundant function and that the superimposed haploinsufficiency of these two molecules is sufficient to cause defects in tooth development.
FIGURE 1.

Individual Twist1 and Twist2 heterozygous mice showed no apparent defects in tooth development. A–D”, hematoxylin and eosin-stained coronal sections through the developing first molar tooth germs of control (A–D), Sox2-Cre;Twist1fl/+ (A'–D'), and Twist2Cre/+mutant (A”–D”) mouse embryos from E13.5 to E18.5 are shown. EO, enamel organ; DF, dental follicle; DP, dental papilla; DE, dental epithelium; DM, dental mesenchyme. E and F, delayed development of the third molars (M3) in Twist2Cre/+;Twist1fl/+ mice. H&E staining showed that the third molar had developed to late bell stage in the control mice (E), whereas they were missing in the Twist2Cre/+;Twist1fl/+ mice (F) at the age of 1 week. G and H, X-radiography demonstrated that the third molars were also observed in the Twist2Cre/+;Twist1fl/+ mice at the age of 3 weeks (H), although they appeared to be smaller than those in the control mice (G). Scale bars = 100 μm (A–D”) and 200 μm (E and F).
FIGURE 2.
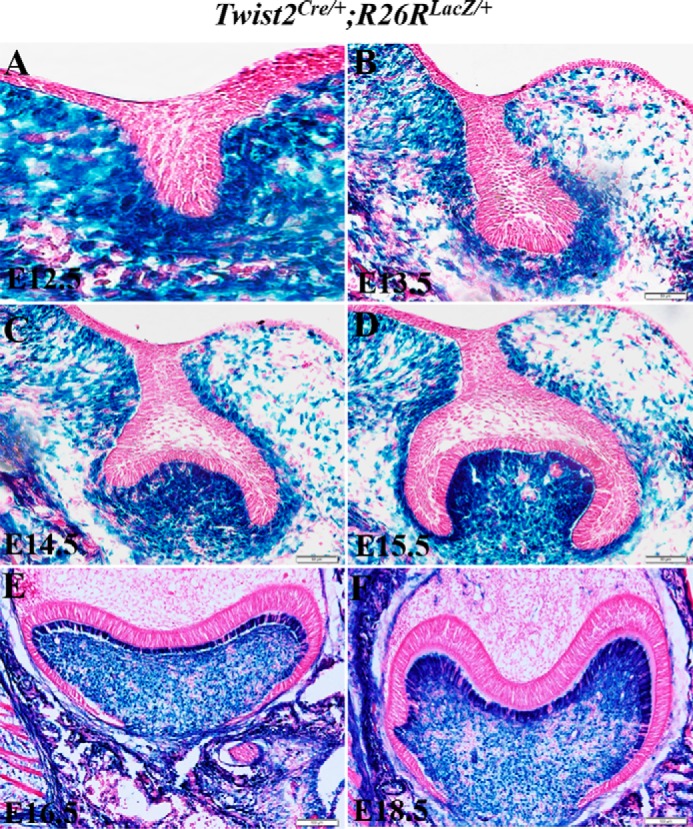
β-Gal expression in the developing molars of Twist2Cre/+;R26RLacZ/+ embryos. A–F, X-gal stained coronal sections of the mandibular first molar of E12.5-E18.5 Twist2Cre/+;R26RLacZ/+ embryos. β-Gal-positive cells were restricted in the dental mesenchyme but not in the dental epithelium at all stages examined. Scale bars = 50 μm.
Delayed Tooth Development and Reduced Cell Proliferation in Developing Molars of Twist2Cre/+;Twist1fl/fl Embryos
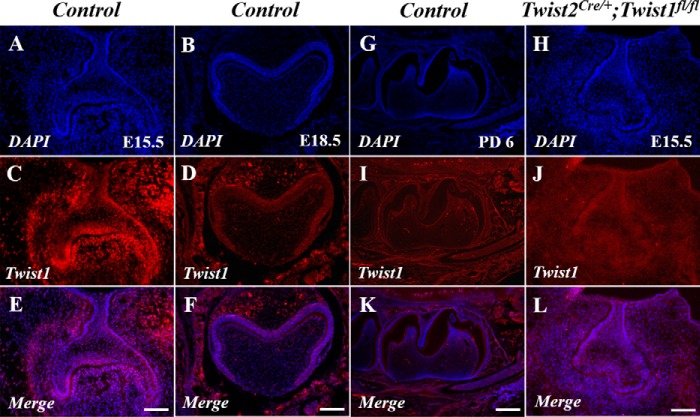
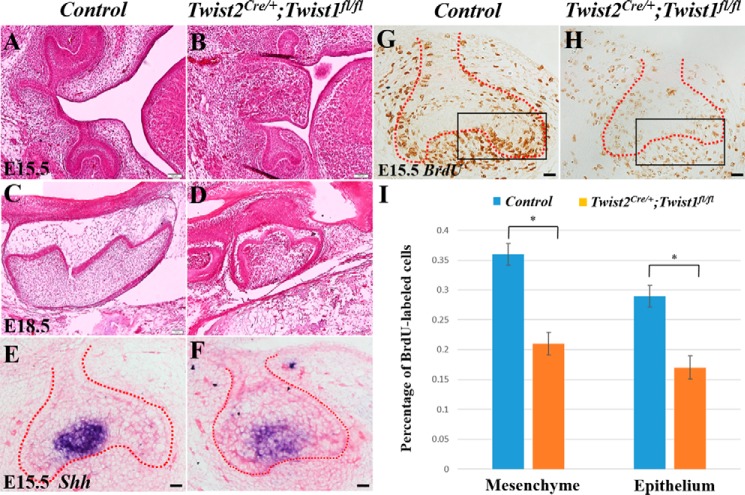
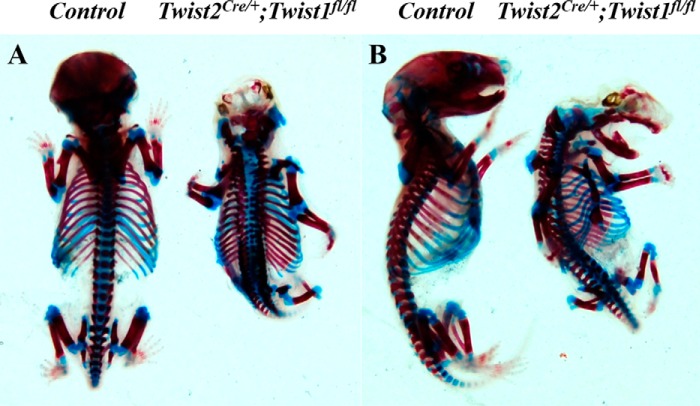
Next we generated Twist2Cre/+;Twist1fl/fl embryos by breeding Twist2Cre/+;Twist1fl/+ mice with Twist1fl/fl mice. Immunofluorescent staining showed that Twist1 protein signals were observed in the dental mesenchyme at E15.5 (Fig. 3, A, C, and E) but were barely detectable in the dental mesenchyme at E18.5 and postnatal day 6 (Fig. 3, B, D, F, G, I, and K). As expected, no Twist1 protein signals were detected in the dental mesenchyme of the Twist2Cre/+;Twist1fl/fl embryos at E15.5 (Fig. 3, H, J, and L). We found that all Twist2Cre/+;Twist1fl/fl embryos died before birth because of severe abdominal hernias, brain defects, and cleft palates. The histological analyses demonstrated that the Twist2Cre/+;Twist1fl/fl embryos had smaller tooth germs with abnormal cusps at E15.5 and E18.5 (Fig. 4, A–D). At E15.5, the control molars had developed to late cap stage (Fig. 4A), whereas the Twist2Cre/+;Twist1fl/fl molars were much smaller and were still at the early cap stage (Fig. 4B). At E18.5, the Twist2Cre/+;Twist1fl/fl molars (Fig. 4D) were smaller and had abnormal cusps compared with the control molars (Fig. 4C). Sonic hedgehog (Shh) is a marker for primary enamel knots (34–36). In situ hybridization showed that Shh transcription was reduced in the molars of Twist2Cre/+;Twist1fl/fl embryos compared with the control embryos (Fig. 4, E and F). Consistent with the smaller size of the tooth germs, the Twist2Cre/+;Twist1fl/fl embryos showed a significantly reduced cell proliferation rate in both the dental epithelium and mesenchyme in the first mandibular molars compared with the control embryos (Fig. 4, G, H, and I). These results suggest that Twist1 regulates cell proliferation during early tooth development. Alizarin red/Alcian blue staining was performed to assess the overall skeletal structures of E18.5 Twist2Cre/+;Twist1fl/fl embryos. The mutant embryos showed severe skeletal developmental defects, lack of skull bone, poorly developed frontonasal bones, and lack of fibula bones compared with the control embryos (Fig. 5, A and B).
FIGURE 3.
Deletion of Twist1 in the dental mesenchyme by Twist2-Cre. Immunofluorescent staining of the control and Twist2Cre/+;Twist1fl/fl molars with an antibody against Twist1. Strong Twist1 immunofluorescent signals (red) were observed in the dental mesenchyme in the control molars at E15.5 (A, C, and E). However, no signals were detected in the Twist2Cre/+;Twist1fl/fl molars (H, J, and L). The nuclei were counterstained with DAPI (blue). Twist1 was not expressed in the control molars at E18.5 (B, D, and F) and postnatal day 6 (G, I, and K). Scale bars = 50 μm.
FIGURE 4.
Delayed tooth development and reduced cell proliferation rate in Twist2Cre/+;Twist1fl/fl embryos. Shown are coronal sections (A, B, E, F, G, and H) and sagittal sections (C and D) of the control and Twist2Cre/+;Twist1fl/fl molars. Also shown are representative H&E staining images of molars from the control (A and C) and Twist2Cre/+;Twist1fl/fl embryos (B and D). At E15.5, the control molars had developed to late cap stage (A), whereas the Twist2Cre/+;Twist1fl/fl molars were much smaller and were still at the early cap stage (B). At E18.5, the Twist2Cre/+;Twist1fl/fl molars (D) were smaller and had abnormal cusps compared with the control molars (C). In situ hybridization showed reduced Shh transcripts in the primary enamel knot in the Twist2Cre/+;Twist1fl/fl molars (F) compared with the control molars (E). BrdU labeling was performed to determine cell proliferation in the control (G) and Twist2Cre/+;Twist1fl/fl molars (H) at E15.5. Proliferating cells incorporating BrdU are stained brown. The proliferation rate in the dental epithelium and mesenchyme was on the basis of the ratio of BrdU-incorporated epithelial and mesenchymal cells to the total dental epithelial and mesenchymal cells. The boxes indicate the area where the labeled cells and total cell number were counted and compared. The cell proliferation rate was reduced significantly in both the dental epithelium and the underlying mesenchyme in the Twist2Cre/+;Twist1fl/fl molars compared with the control molars (G–I). Error bars indicate mean ± S.E. *, p < 0.05. Scale bars = 100 μm (A–D) and 50 μm (E–H).
FIGURE 5.
Twist2Cre/+;Twist1fl/fl mutant mice developed severe craniofacial and skeletal defects. A and B, whole-mount embryos of the control and Twist2Cre/+;Twist1fl/fl at E18.5 were stained with Alcian blue (blue) and Alizarin red (red) to visualize the cartilage and bone, respectively.
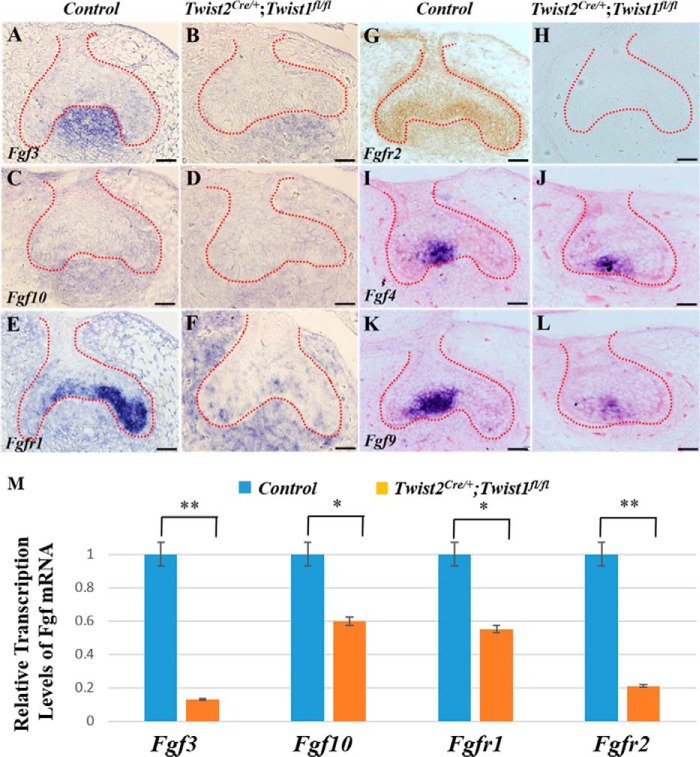
Reduced Fgf and Fgfr Expression in Developing Molars of Twist2Cre/+;Twist1fl/fl Embryos
FGF signaling plays a crucial role in regulating cell proliferation during tooth development (6). Therefore, we examined whether the expression of FGF ligands and receptors was affected by the deletion of Twist1 in the developing tooth germs in the Twist2Cre/+;Twist1fl/fl embryos at E15.5 (Fig. 6, A–L). In situ hybridization showed that the Fgf3 and Fgf10 transcripts were observed in the dental mesenchyme, whereas the Fgfr1, Fgf4, and Fgf9 transcripts were primarily found in the dental epithelium. The levels of Fgf3, Fgf10, Fgfr1, Fgf4, and Fgf9 transcripts were reduced dramatically reduced in the Twist2Cre/+;Twist1fl/fl embryos (Fig. 6, B, D, F, J, and L) compared with the controls (Fig. 6, A, C, E, I, and K). Immunohistochemistry showed that the Fgfr2 protein was located in both the dental epithelium and mesenchyme of the control embryos (Fig. 6G) but was barely detectable in the Twist2Cre/+;Twist1fl/fl embryos (Fig. 6H). Quantitative real-time PCR further confirmed that the Fgf3, Fgf10, Fgfr1, and Fgfr2 transcripts decreased markedly in the developing tooth germs in the Twist2Cre/+;Twist1fl/fl embryos compared with the control embryos at E14.5 (Fig. 6M). These data suggest that FGF signaling might be compromised in the Twist2Cre/+;Twist1fl/fl embryos.
FIGURE 6.
Reduced expression of Fgf and Fgfr in Twist2Cre/+;Twist1fl/fl mice. In situ hybridization was performed to determine the expression of Fgf3, Fgf10, Fgfr1, Fgf4, and Fgf9 in the developing control and Twist2Cre/+;Twist1fl/fl molars at E15.5 (A–L, signal in blue). Fgf3 and Fgf10 transcripts were observed in the dental mesenchyme, and Fgfr1, Fgf4, and Fgf9 transcripts were primarily found in the dental epithelium. The expression of Fgf3, Fgf10, Fgfr1, Fgf4, and Fgf9 was reduced dramatically in Twist2Cre/+;Twist1fl/fl molars compared with the control molars. Immunohistochemical staining showed that the Fgfr2 protein (signal in brown) was localized in both the dental epithelium and mesenchyme in the control molars (G) but was barely detectable in the Twist2Cre/+;Twist1fl/fl molars at E15.5 (H). The dashed lines in A–L indicate the boundary between the dental epithelium and mesenchyme. Quantitative PCR analysis also showed that the Fgf3, Fgf10, Fgfr1, and Fgfr2 transcripts were reduced remarkably in the Twist2Cre/+;Twist1fl/fl molars compared with the control molars at E14.5 (M). Error bars indicate mean ± S.E. *, p < 0.05; **, p < 0.01. Scale bars = 50 μm.
Twist1 is Essential to Odontoblast Differentiation
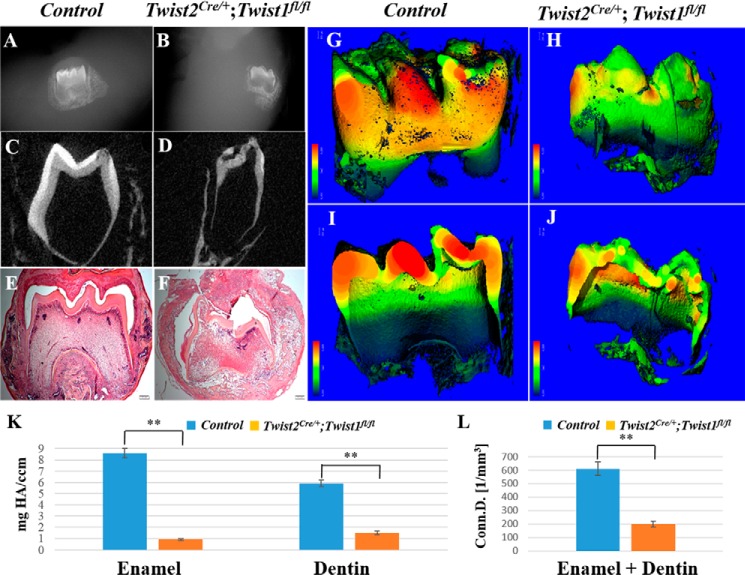
Because the Twist2Cre/+;Twist1fl/fl embryos died before birth, we employed the in vivo kidney capsule grafting approach to determine the postnatal roles of Twist1 in odontoblast differentiation. In vivo kidney capsule grafting has been used widely to study tooth development because the tooth germs continue to develop normally under the kidney capsule (33). We implanted tooth germs of the E18.5 Twist2Cre/+;Twist1fl/fl embryos under the kidney capsule of adult C57BL/6 female mice for 3 weeks and then examined the implanted tooth germs. Plain x-ray, μCT, and histological analyses showed that the implanted control molars had a well developed crown and roots (Fig. 7, A, C, E, G, and I), with well formed enamel and dentin. However, the Twist2Cre/+;Twist1fl/fl molars displayed a malformed crown and roots, abnormal cusps, and poorly formed enamel and dentin (Fig. 7, B, D, F, H, and J). The μCT quantification analysis showed that the density of both the dentin and enamel was decreased dramatically in the Twist2Cre/+;Twist1fl/fl molars compared with the control molars (Fig. 7, K and L). These observations demonstrate that Twist1 is essential for normal crown and root development.
FIGURE 7.
Defects in crown dentin and enamel formation. Plain x-ray radiography and μCT analysis showed that the E18.5 control molars had well developed crown dentin, enamel, and roots (A, C, G, and I), whereas the E18.5 Twist2Cre/+;Twist1fl/fl molars displayed severe defects in crown dentin and enamel as well as abnormal root development 3 weeks after kidney capsule transplantation (B, D, H, and J). The μCT quantification analysis showed that the density of both the dentin and enamel was decreased dramatically in the Twist2Cre/+;Twist1fl/fl molars compared with the control molars (K and L). Histologic analysis further confirmed the abnormal crown formed in the E18.5 Twist2Cre/+;Twist1fl/fl molars (F) compared with the E18.5 control molars (E) 3 weeks after kidney capsule transplantation. Error bars indicate mean ± S.E. **, p < 0.01. Scale bars = 200 μm.
Next we investigated whether the dentin defects in the Twist2Cre/+;Twist1fl/fl molars were associated with defects in odontoblast differentiation. Dentin sialophosphoprotein is a terminal differentiation marker for odontoblasts. We analyzed its expression in the odontoblasts in the implanted Twist2Cre/+;Twist1fl/fl molars at both transcript and protein levels. The in situ hybridization analysis showed that the Dspp transcripts were strongly expressed in the odontoblasts in the transplanted control molars (Fig. 8A) but reduced markedly in the Twist2Cre/+;Twist1fl/fl molars (Fig. 8B). Immunohistochemical staining with polyclonal anti-DSP antibody further verified that DSP, the amino-terminal fragment of dentin sialophosphoprotein, was barely detectable in the Twist2Cre/+;Twist1fl/fl molars (Fig. 8, D and F) compared with the control molars (Fig. 8, C and E). These findings suggest that Twist1 is required for normal odontoblast differentiation.
FIGURE 8.
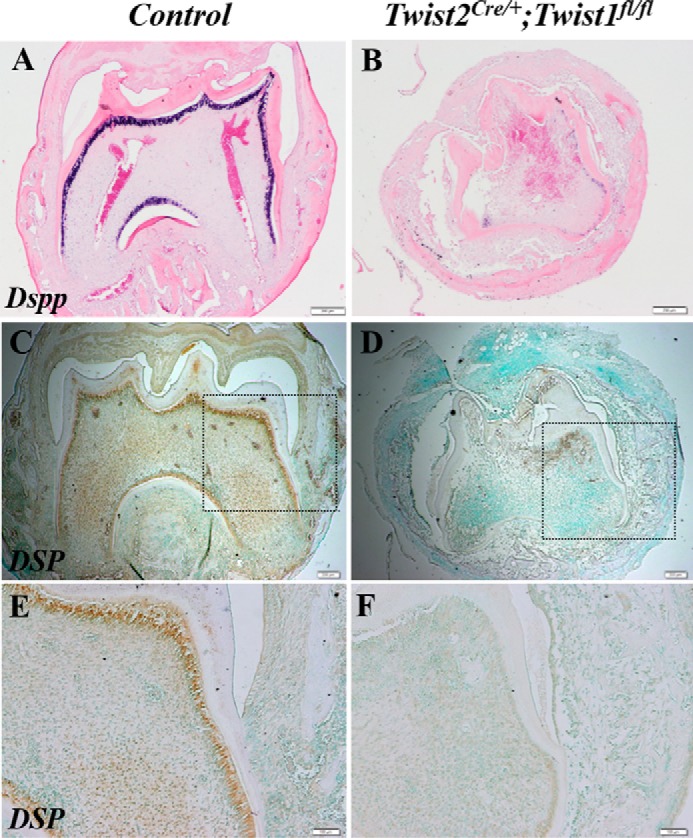
Reduced expression of Dspp in odontoblasts in Twist2Cre/+;Twist1fl/fl molars. In situ hybridization analysis showed that Dspp transcripts (signal in blue) were reduced markedly in the odontoblasts in the E18.5 Twist2Cre/+;Twist1fl/fl molars (B) compared with the E18.5 control molars (A) 3 weeks after kidney capsule transplantation. Immunohistochemical staining confirmed the marked reduction of DSP protein (signal in brown) in the Twist2Cre/+;Twist1fl/fl molars (C and D). E and F, high-magnification views of the boxed areas in C and D. Scale bars = 200 μm (A–D) and 100 μm (E and F).
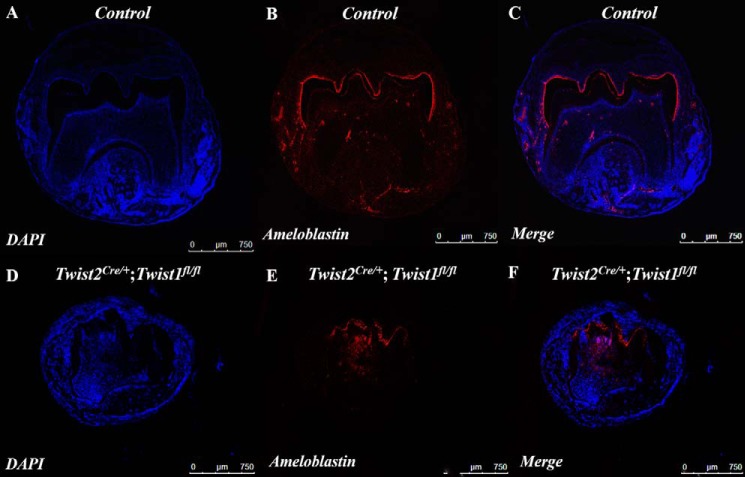
We then determined whether there were any defects in ameloblast differentiation in the Twist2Cre/+;Twist1fl/fl molars. Ameloblastin is a terminal differentiation marker for ameloblasts. We analyzed its expression in ameloblasts in the implanted Twist2Cre/+;Twist1fl/fl molars at the protein level. Immunofluorescent staining showed that the ameloblastin-positive cells were distributed over the entire tooth crown in the control molars (Fig. 9, A--C), whereas they were restricted to the cuspal region in the Twist2Cre/+;Twist1fl/fl molars (Fig. 9, D–F). In addition, the signal intensity of the ameloblastin protein appeared to be weaker in the Twist2Cre/+;Twist1fl/fl molars than in the control molars. These findings suggest that the ameloblast differentiation in the Twist2Cre/+;Twist1fl/fl molars was delayed and compromised.
FIGURE 9.
Ameloblastin expression in ameloblasts in Twist2Cre/+;Twist1fl/fl molars. Immunofluorescence staining of the E18.5 control and the E18.5 Twist2Cre/+;Twist1fl/fl molars 3 weeks after kidney capsule transplantation with an antibody against ameloblastin. Stronger ameloblastin immunofluorescent signals (red) were strictly observed in the ameloblasts of the cuspal regions in the control molars (A–C). However, a much weaker intensity of the signals was detected in the Twist2Cre/+;Twist1fl/fl molars (D--F). Nuclei were counterstained with DAPI (blue). Scale bars = 750 μm.
Binding of Twist1 to the Fgf10 Promoter
Fgf10 is expressed in the dental mesenchyme, and its expression was decreased in the dental mesenchyme of the Twist2Cre/+;Twist1fl/fl embryos. We demonstrated consistently that Twist1 regulates the 5.9-kb Fgf10 promoter activity in vitro (37). Here we further examined by ChIP assay whether Twist1 was able to bind to the Fgf10 promoter. We found that Twist1 bound to a region of Fgf10 promoter between −1101∼−1269 bp relative to the transcription start site (Fig. 10). This finding suggests that Twist1 might directly bind to the Fgf10 promoter and up-regulate the Fgf10 expression in the dental mesenchyme during tooth development.
FIGURE 10.
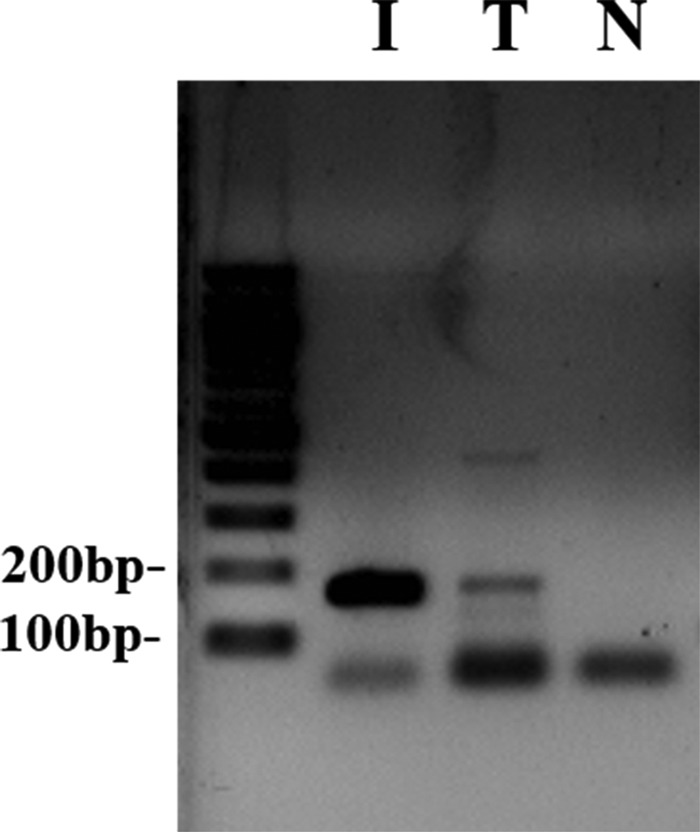
Binding of Twist1 to the Fgf10 promoter. A ChIP assay was conducted with a Twist1-specific antibody and nuclear extracts from MDPC23 cells. The PCR analysis identified a specific PCR product of 169 bp corresponding to the region −1101∼1269 nt of the Fgf10 promoter in the input added with Anti-Twist1 antibody (T) but not in the input with normal IgG as a negative control (N). I, the nuclear extracts were used as a PCR-positive control.
Discussion
In this study, we analyzed the phenotypic and molecular changes of the developing teeth from mouse embryos with the Twist1 alleles conditionally deleted by Twist2-Cre recombinase. Our findings demonstrate that Twist1 is essential for both early tooth morphogenesis and late odontoblast differentiation. Our data also imply that Twist1 may be the key transcription factor that mediates FGF signaling in the dental mesenchyme during tooth development.
Twist1 is essential for early tooth morphogenesis. The conventional deletion of the Twist1 gene in mice results in embryonic lethality at E11.5 (17), thereby preventing us from studying the functions of Twist1 in tooth development. In this study, we generated Twist2Cre/+;Twist1fl/fl mice by breeding Twist1fl/fl mice with Twist2Cre/+ mice. We found that the Twist2Cre/+;Twist1fl/fl embryos formed smaller tooth germs and abnormal cusps.
We further demonstrated that Twist1 regulates early tooth morphogenesis through up-regulating FGF signaling. Twist2Cre/+;Twist1fl/fl mutant embryos developed smaller tooth germs and abnormal cusps, accompanied by reduced cell proliferation in both the dental epithelium and mesenchyme, compared with the control embryos. It has been well documented that FGF signaling plays essential roles in determining the size and morphology of teeth (8, 10–13). Therefore, we examined the expressions of two mesenchymal FGF ligands, Fgf3 and Fgf10, two epithelial FGF ligands, Fgf4 and Fgf9, and two FGF receptors, Fgfr1 and Fgfr2, in the developing tooth germs of Twist2Cre/+;Twist1fl/fl mutant embryos. We found that all expression of Fgf3, Fgf10, Fgf4, Fgf9, Fgfr1, and Fgfr2 was decreased in the developing molars of Twist2Cre/+;Twist1fl/fl mutant embryos at E14.5 and E15.5. Furthermore, our previous and current in vitro studies suggest that Twist1 was able to bind to the Fgf10 promoter and regulate Fgf10 promoter activity (37). Guenou et al. (38) have shown that Twist1 could bind to the Fgfr2 promoter and regulate Fgfr2 expression. Collectively, these studies support the notion that Twist1 is the key transcription factor in the dental mesenchyme that couples the FGF signaling pathways in the dental epithelium and dental mesenchyme.
Twist1 is essential for odontoblast differentiation. Using the kidney capsule grafting approach, we were able to prolong the growth of the E18.5 Twist2Cre/+;Twist1fl/fl molar tooth germs to determine the effects of Twist1 deficiency on the terminal differentiation of odontoblasts and dentin deposition. We found that the implanted Twist2Cre/+;Twist1fl/fl molars had poorly formed crown dentin compared with the control molars. The defects in crown dentin formation were apparently due to a defect in odontoblast differentiation, as shown by the dramatically reduced Dspp expression. This finding is consistent with our previous in vitro data showing that Twist1 promotes the odontoblast-like differentiation of human dental pulp stem cells (22). However, we and others have demonstrated that Twist1 is barely detectable in the dental mesenchyme at E18.5 and onwards, when the terminal differentiation of odontoblasts starts (14, 15). These observations suggest that Twist1 may be required for preparing the dental mesenchymal cells for their terminal differentiation into odontoblasts.
In addition, the implanted Twist2Cre/+;Twist1fl/fl molars had poorly formed enamel, crown, and roots. We found that low levels of ameloblastin-expressing ameloblasts were restricted to the cuspal regions of the Twist2Cre/+;Twist1fl/fl molars, suggesting a delayed and compromised ameloblast differentiation. During crown formation, the interactions between the dental epithelium and underlying mesenchyme lead to the formation of a bell-shaped tooth germ in which the inner enamel epithelial cells and outer layer of dental papilla cells are positioned properly next to each other and get ready for terminal differentiation and enamel/dentin formation (39). The initial deposition of enamel and dentin occurs at the future cuspal/incisal regions of the bell-shaped tooth germ, which also involves a series of reciprocal interactions that occur between the inner enamel epithelial cells and subjacent outer layer of dental papilla cells. Therefore, the defective ameloblast differentiation might be secondary to the odontoblast differentiation defects. Alternatively, the defects in ameloblast differentiation might also be due to the reduced FGF signaling in the dental epithelium. Because root formation begins after crown formation is completed, it is likely that the defects in root formation may be due to the abnormal root odontoblast differentiation as well as the abnormal crown formation in the implanted Twist2Cre/+;Twist1fl/fl molars.
Twist2 might play a similar role as Twist1 in tooth development. Twist1 and Twist2 share a high degree of amino acid sequence identity, particularly in the basic-helix-loop-helix domain and in the C-terminal domain (18). In addition, they exhibit significant overlapping expression patterns during embryonic development (19). We have demonstrated previously that both Twist1 and Twist2 stimulate 4.9-kb Fgfr2 promoter activity and play a redundant function in regulating skeletal development through modulating FGF signaling (26). In this study, we have shown that, although there were no apparent defects in tooth development in either the Twist1 or Twist2 single heterozygous embryos, Twist1 and Twist2 compound heterozygous (Twist2Cre/+;Twist1fl/+) mice showed delayed development of the third molars. These findings suggest that the loss of one allele of the Twist2 gene (i.e. Twist2Cre/+) may contribute to the tooth phenotype of the Twist2Cre/+;Twist1fl/fl embryos. However, the conditional deletion of the Twist1 gene alone in the neural crest-derived cells mediated either by Wnt1-Cre or by Hand2-Cre also results in the formation of small first lower molars (40, 41). Therefore, whether Twist2 plays a definitive role similar to Twist1 in tooth development remains to be investigated further.
In summary, we have demonstrated that Twist1 plays essential roles during tooth development. It is required for early tooth morphogenesis, odontoblast differentiation, and crown formation.
Author Contributions
T. M. contributed to the conception and design of this work, data acquisition, analysis, and interpretation and drafted and critically revised the manuscript. Y. H., S. W., and H. Z. contributed to data acquisition. P. C. D., X. W., C. Q., and B. S. provided technical assistance and contributed to data analysis and preparation of the figures. Y. L. and R. N. D. contributed to the conception, design, and data analysis and drafted and critically revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgment
We thank Jeanne Santa Cruz for assistance with the editing of this article.
This work was supported by NIDCR/National Institutes of Health Grants DE13368 (to R. D. S.) and DE021773 (to Y. L.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- E
- embryonic day
- DSP
- dentin sialoprotein
- μCT
- micro-computed tomography.
References
- 1.Thesleff I., and Nieminen P. (1996) Tooth morphogenesis and cell differentiation. Curr. Opin. Cell Biol. 8, 844–850 [DOI] [PubMed] [Google Scholar]
- 2.Thesleff I. (2003) Epithelial-mesenchymal signalling regulating tooth morphogenesis. J. Cell Sci. 116, 1647–1648 [DOI] [PubMed] [Google Scholar]
- 3.Thesleff I., and Hurmerinta K. (1981) Tissue interactions in tooth development. Differentiation 18, 75–88 [DOI] [PubMed] [Google Scholar]
- 4.Thesleff I., and Mikkola M. (2002) The role of growth factors in tooth development. Int. Rev. Cytol. 217, 93–135 [DOI] [PubMed] [Google Scholar]
- 5.Tummers M., and Thesleff I. (2009) The importance of signal pathway modulation in all aspects of tooth development. J. Exp. Zoo. Part B Mol. Dev. Evol. 312, 309–319 [DOI] [PubMed] [Google Scholar]
- 6.Li C. Y., Prochazka J., Goodwin A. F., and Klein O. D. (2014) Fibroblast growth factor signaling in mammalian tooth development. Odontology 102, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kettunen P., Laurikkala J., Itäranta P., Vainio S., Itoh N., and Thesleff I. (2000) Associations of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis. Dev. Dyn. 219, 322–332 [DOI] [PubMed] [Google Scholar]
- 8.Ohuchi H., Hori Y., Yamasaki M., Harada H., Sekine K., Kato S., and Itoh N. (2000) FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem. Biophys. Res. Commun. 277, 643–649 [DOI] [PubMed] [Google Scholar]
- 9.Ornitz D. M., Xu J., Colvin J. S., McEwen D. G., MacArthur C. A., Coulier F., Gao G., and Goldfarb M. (1996) Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292–15297 [DOI] [PubMed] [Google Scholar]
- 10.Milunsky J. M., Zhao G., Maher T. A., Colby R., and Everman D. B. (2006) LADD syndrome is caused by FGF10 mutations. Clin. Genet. 69, 349–354 [DOI] [PubMed] [Google Scholar]
- 11.Tekin M., Himi B. O., Fitoz S., Ozda H., Cengiz F. B., Sirmaci A., Aslan I., Inceolu B., Yüksel-Konuk E. B., Yilmaz S. T., Yasun O., and Akar N. (2007) Homozygous mutations in fibroblast growth factor 3 are associated with a new form of syndromic deafness characterized by inner ear agenesis, microtia, and microdontia. Am. J. Hum. Genet. 80, 338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X. P., Suomalainen M., Felszeghy S., Zelarayan L. C., Alonso M. T., Plikus M. V., Maas R. L., Chuong C. M., Schimmang T., and Thesleff I. (2007) An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 5, e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charles C., Lazzari V., Tafforeau P., Schimmang T., Tekin M., Klein O., and Viriot L. (2009) Modulation of Fgf3 dosage in mouse and men mirrors evolution of mammalian dentition. Proc. Natl. Acad. Sci. U.S.A. 106, 22364–22368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice R., Thesleff I., and Rice D. P. (2005) Regulation of Twist, Snail, and Id1 is conserved between the developing murine palate and tooth. Dev. Dyn. 234, 28–35 [DOI] [PubMed] [Google Scholar]
- 15.Bourgeois P., Bolcato-Bellemin A. L., Danse J. M., Bloch-Zupan A., Yoshiba K., Stoetzel C., and Perrin-Schmitt F. (1998) The variable expressivity and incomplete penetrance of the twist-null heterozygous mouse phenotype resemble those of human Saethre-Chotzen syndrome. Hum. Mol. Genet. 7, 945–957 [DOI] [PubMed] [Google Scholar]
- 16.Connerney J., Andreeva V., Leshem Y., Mercado M. A., Dowell K., Yang X., Lindner V., Friesel R. E., and Spicer D. B. (2008) Twist1 homodimers enhance FGF responsiveness of the cranial sutures and promote suture closure. Dev. Biol. 318, 323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z. F., and Behringer R. R. (1995) twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 9, 686–699 [DOI] [PubMed] [Google Scholar]
- 18.Franco H. L., Casasnovas J., Rodríguez-Medina J. R., and Cadilla C. L. (2011) Redundant or separate entities? Roles of Twist1 and Twist2 as molecular switches during gene transcription. Nucleic Acids Res. 39, 1177–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L., Cserjesi P., and Olson E. N. (1995) Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Dev. Biol. 172, 280–292 [DOI] [PubMed] [Google Scholar]
- 20.Šošić D., Richardson J. A., Yu K., Ornitz D. M., and Olson E. N. (2003) Twist regulates cytokine gene expression through a negative feedback loop that represses NF-κB activity. Cell 112, 169–180 [DOI] [PubMed] [Google Scholar]
- 21.Tukel T., Šošić D., Al-Gazali L. I., Erazo M., Casasnovas J., Franco H. L., Richardson J. A., Olson E. N., Cadilla C. L., and Desnick R. J. (2010) Homozygous nonsense mutations in TWIST2 cause Setleis syndrome. Am. J. Hum. Genet. 87, 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y., Lu Y., Maciejewska I., Galler K. M., Cavender A., and D'Souza R. N. (2011) TWIST1 promotes the odontoblast-like differentiation of dental stem cells. Adv. Dent. Res. 23, 280–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y. T., Akinwunmi P. O., Deng J. M., Tam O. H., and Behringer R. R. (2007) Generation of a Twist1 conditional null allele in the mouse. Genesis 45, 588–592 [DOI] [PubMed] [Google Scholar]
- 24.Goodnough L. H., Chang A. T., Treloar C., Yang J., Scacheri P. C., and Atit R. P. (2012) Twist1 mediates repression of chondrogenesis by β-catenin to promote cranial bone progenitor specification. Development 139, 4428–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y., Xu Y., Liao L., Zhou N., Theissen S. M., Liao X. H., Nguyen H., Ludwig T., Qin L., Martinez J. D., Jiang J., and Xu J. (2013) Inducible knockout of Twist1 in young and adult mice prolongs hair growth cycle and has mild effects on general health, supporting Twist1 as a preferential cancer target. Am. J. Pathol. 183, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y., Meng T., Wang S., Zhang H., Mues G., Qin C., Feng J. Q., D'Souza R. N., and Lu Y. (2014) Twist1- and Twist2-haploinsufficiency results in reduced bone formation. PLoS ONE 9, e99331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi S., Lewis P., Pevny L., and McMahon A. P. (2002) Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech. Dev. 119, S97-S101 [DOI] [PubMed] [Google Scholar]
- 28.Soriano P. (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- 29.Chai Y., Jiang X., Ito Y., Bringas P. Jr., Han J., Rowitch D. H., Soriano P., McMahon A. P., and Sucov H. M. (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671–1679 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Zhao X., Hu Y., St Amand T., Zhang M., Ramamurthy R., Qiu M., and Chen Y. (1999) Msx1 is required for the induction of Patched by Sonic hedgehog in the mammalian tooth germ. Dev. Dyn. 215, 45–53 [DOI] [PubMed] [Google Scholar]
- 31.Liu C., Gu S., Sun C., Ye W., Song Z., Zhang Y., and Chen Y. (2013) FGF signaling sustains the odontogenic fate of dental mesenchyme by suppressing beta-catenin signaling. Development 140, 4375–4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Z., Liu C., Iwata J., Gu S., Suzuki A., Sun C., He W., Shu R., Li L., Chai Y., and Chen Y. (2013) Mice with Tak1 deficiency in neural crest lineage exhibit cleft palate associated with abnormal tongue development. J. Biol. Chem. 288, 10440–10450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otsu K., Fujiwara N., and Harada H. (2012) Organ cultures and kidney-capsule grafting of tooth germs. Methods Mol. Biol. 887, 59–67 [DOI] [PubMed] [Google Scholar]
- 34.Chuong C. M., Patel N., Lin J., Jung H. S., and Widelitz R. B. (2000) Sonic hedgehog signaling pathway in vertebrate epithelial appendage morphogenesis: perspectives in development and evolution. Cell Mol. Life Sci. 57, 1672–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thesleff I., Keränen S., and Jernvall J. (2001) Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv. Dent. Res. 15, 14–18 [DOI] [PubMed] [Google Scholar]
- 36.Thesleff I., and Jernvall J. (1997) The enamel knot: a putative signaling center regulating tooth development. Cold Spring Harbor Symp. Quant. Biol. 62, 257–267 [PubMed] [Google Scholar]
- 37.Lu Y., Li Y., Cavender A. C., Wang S., Mansukhani A., and D'Souza R. N. (2012) Molecular studies on the roles of Runx2 and Twist1 in regulating FGF signaling. Dev. Dyn. 241, 1708–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guenou H., Kaabeche K., Mée S. L., and Marie P. J. (2005) A role for fibroblast growth factor receptor-2 in the altered osteoblast phenotype induced by Twist haploinsufficiency in the Saethre-Chotzen syndrome. Hum. Mol. Genet. 14, 1429–1439 [DOI] [PubMed] [Google Scholar]
- 39.Simmer J. P., Papagerakis P., Smith C. E., Fisher D. C., Rountrey A. N., Zheng L., and Hu J. C. (2010) Regulation of dental enamel shape and hardness. J. Dent. Res. 89, 1024–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bildsoe H., Loebel D. A., Jones V. J., Chen Y. T., Behringer R. R., and Tam P. P. (2009) Requirement for Twist1 in frontonasal and skull vault development in the mouse embryo. Dev. Biol. 331, 176–188 [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y., Blackwell E. L., McKnight M. T., Knutsen G. R., Vu W. T., and Ruest L. B. (2012) Specific inactivation of Twist1 in the mandibular arch neural crest cells affects the development of the ramus and reveals interactions with hand2. Dev. Dyn. 241, 924–940 [DOI] [PMC free article] [PubMed] [Google Scholar]