Background: B7-H6, the ligand for NKp30, is expressed on tumor cells.
Results: Tumor therapeutics promote B7-H6 expression in tumor cells and enhance tumor sensitivity to NK cell cytolysis.
Conclusion: Tumor therapeutics work as stress inducers to enhance tumor sensitivity to NK cell cytolysis.
Significance: These results suggest that B7-H6 could be a potential target for tumor therapy in the future.
Keywords: immunotherapy, natural killer cells (NK cells), stress, tumor immunology, tumor therapy
Abstract
Immune cells are believed to participate in initiating anti-tumor effects during regular tumor therapy such as chemotherapy, radiation, hyperthermia, and cytokine injection. One of the mechanisms underlying this process is the expression of so-called stress-inducible immunostimulating ligands. Although the activating receptor NKG2D has been proven to play roles in tumor therapy through targeting its ligands, the role of NKp30, another key activating receptor, is seldom addressed. In this study, we found that the NKp30 ligand B7-H6 was widely expressed in tumor cells and closely correlated to their susceptibility to NK cell lysis. Further studies showed that treatment of tumor cells with almost all standard tumor therapeutics, including chemotherapy (cisplatin, 5-fluorouracil), radiation therapy, non-lethal heat shock, and cytokine therapy (TNF-α), could up-regulate the expression of B7-H6 in tumor cells and enhance tumor sensitivity to NK cell cytolysis. B7-H6 shRNA treatment effectively dampened sensitization of tumor cells to NK-mediated lysis. Our study not only reveals the possibility that tumor therapeutics work as stress inducers to enhance tumor sensitivity to NK cell cytolysis but also suggests that B7-H6 could be a potential target for tumor therapy in the future.
Introduction
Increasing evidence supports the notion that complete remission of tumors relies on eliciting endogenous anti-tumor immunity (1, 2). Appropriate chemotherapy, radiation therapy, hyperthermia therapy, and immunotherapy have been proven to break the immunosuppressive state at the tumor location and activate anti-tumor immune effects. Tumor therapy elicits endogenous anti-tumor immune responses via various mechanisms including promoting the activation of natural killer cells (NK)3 cells and tumor-specific CTLs (3–5). However, although NK cells have been proven vital in initiating the anti-tumor response and are extensively applied in tumor treatments (6–10), the mechanisms by which NK cells are activated in tumor treatments remain largely unexplored.
The balance of activating receptors and inhibitory receptors manipulates the activation of NK cells. Activating receptors mainly include C-type lectin like receptors NKG2D, natural cytotoxicity receptors including NKp30, NKp44, and NKp46, as well as activating members of the Ly49 family (6). Up-regulation of NKG2D ligands on tumor cells mediated by tumor therapy-induced cell stress has been extensively studied. For example, the DNA damage response induced by irradiation and chemotherapeutic reagents is reported to up-regulate the expression of NKG2D ligands on tumor cells (11). Non-lethal heat shock, mimicking hyperthermia therapy, increases the expressions of Mult-1 (12), MICA, and MICB on tumor cells (13). Among the cytokines used for immunotherapy, TNF-α has been shown to induce the expression of MICA in human primary tissue cells and tumor cells (14, 15).
NKp30 is another important activating receptor expressed on NK cells (16, 17), and increasing the expression of NKp30 ligands on tumor cells may represent another approach to tumor therapy using NK cells. B7-H6, the only membrane-binding ligand among the three known NKp30 ligands, is widely expressed on tumor cells but not healthy tissue cells (18–20), implying that B7-H6 may be a useful target for tumor therapy. However, regulation of B7-H6 expression on tumor cells by tumor therapy-induced cell stress remains elusive.
In this study, we investigated the influence of tumor therapy on B7-H6 expression on tumor cells and found that chemotherapy, radiation therapy, hyperthermia therapy, and immunotherapy could increase B7-H6 expression on tumor cells. When subjected to NK cell-mediated cytolysis, target cells exposed to tumor therapy were more sensitive to NK cell lysis when compared with untreated cells. Moreover, lentivirus-delivered B7-H6-specific shRNA treatment reduced the sensitivity of tumor cells to NK cell lysis, implying that increased B7-H6 expression contributed to tumor sensitization to NK cell cytolysis. Thus, our findings showed that tumor therapeutics work as stress inducers to enhance tumor sensitivity to NK cell cytolysis, suggesting that increasing the expression of B7-H6 on tumor cells may be a promising approach for tumor therapy.
Materials and Methods
Cells, Radiation, and Heat Shock
NK-92 cells were purchased from the ATCC (American Type Culture Collection) and maintained in MEM-α containing 12.5% FBS, 12.5% horse serum, 100 IU/ml IL-2 (Changsheng, Changchun, China), 0.2 mm inositol, 0.02 mm folic acid, and 2-ME. NKG cells were generated in our laboratory (21) and maintained in MEM-α containing 10% FBS, 10% horse serum, 100 IU/ml IL-2. HEK293 (human embryonic kidney epithelial cell), HepG2 (hepatocellular carcinoma), SK-LU-1 (lung adenocarcinoma), and HeLa (cervical carcinoma) cells were maintained in DMEM containing 10% FBS. U937 (acute myeloid leukemia), H7402 (hepatocarcinoma), H460 (lung carcinoma) and Ho8910 (ovarian carcinoma) cells were maintained in RPMI 1640 medium containing 10% FBS. K562 (chronic myeloid leukemia) and HL60 (acute myeloid leukemia) were maintained in IMDM containing 10% FBS. A549 (lung carcinoma) and Du145 (prostate carcinoma) cells were maintained in F-12 medium containing 10% FBS. Huh-7 (hepatocarcinoma) cells were maintained in DMEM containing 15% FBS. All cells were passaged every 2–3 days. For irradiation, HEK293 cells were diluted to a concentration of 1 × 106/ml and irradiated with 137Cs at a dose rate of 100 cGy/min. For non-lethal heat-shock treatment, cells were diluted to a concentration of 1 × 106/ml and exposed to 42 °C for 30 min.
Reagents
Anti-B7-H6 polyclonal serum was generated by immunizing rabbits with recombinant B7-H6 (25–260 AA)-6His. B7-H6 antibody was purified with Protein A-Sepharose (GE) and then with B7-H6–6His-coupled CNBr-activated Sepharose (GE). 5-Fluorouracil (5-FU) was purchased from Sigma. Cisplatin injection was obtained from Nanjing Pharmaceutical Factory Co., Ltd. TNF-α was purchased from PeproTech. FITC-goat anti-mouse IgG were purchased from Santa Cruz Biotechnology and 647- goat anti-rabbit IgG were purchased from Invitrogen. The blocking antibody anti-NKp30 (final concentration 1 ng/ml), anti-NKG2D (final concentration 2 ng/ml) and control mouse IgG (final concentration 3 ng/ml) were purchased from BioLegend.
Quantitative RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. RNA was reverse transcribed using M-MLV reverse transcriptase (Invitrogen), and the resulting cDNA was used for quantitative PCR. SYBR Premix Ex TaqII (Takara) was for quantitative PCR. The cycling parameters were 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The following primers were used: B7-H6-F, 5′-TTTTCCATTCATTGGTGGCCTA-3′; B7-H6-R, 5′-TGCCCGAGTGCAAAAGAATATG-3′; α-actin-F, 5′-CTGGAACGGTGAAGGTGACA-3′; and α-actin-R, 5′-AAGGGACTTCCTGT AACAATGCA-3′.
Western Blotting
Cells were washed twice with ice-cold PBS and then lysed in RIPA Lysis Buffer (Beyotime) supplemented with 0.25 mg/ml PMSF (Sigma), 1× protease inhibitor (Roche). An equal volume of 2× sample loading buffer (62.5 mm Tris-HCl, 25% glycerol, 2% SDS, 0.02% bromphenol blue, 5% β-mercaptoethanol, pH 6.8) was added, and cleared extracts were frozen at −80 °C. The protein concentration was determined using the Bradford method (Bio-Rad). Equal amounts of protein were denatured, electrophoresed, and blotted. For chemiluminescence, HRP-conjugated secondary antibodies (Boster) were applied, and ECL substrate (Bio-Rad) was used for detection.
Construction of Expression Vectors
B7-H6 expression vector was constructed by inserting B7-H6 coding sequence into pcDNA 3.0 plasmid. For myc-B7-H6 expression vector, human CD8a signal peptide sequence (ATGGCCTTACCAGTGACCGCCTTGCTCCTGCCGCTGGCCTTGCTGCTCCACGCCGCCAGG) and three tandem myc-tag sequence (GAACAA AAACTC ATCCTA GAAGAG GATCTG) fused at N-terminal with B7-H6 coding sequence excluding signal peptide sequence were inserted into pcDNA 3.0 plasmid. Both expression vectors were validated by DNA sequencing.
Lentiviral Construction and Transduction of Cells
shB7-H6-1 segments (top, 5′- CGCGTGCCCTGCTCTCCTAACAGTTTTCAGAGAAACTGTTAGGAGAGCAGGGTTTTAT-3′; bottom, 5′- CGATAAAACCCTGCTCTCCTAACAGTTTCTCTGAAAACTGTTAGGAGAGCAGGGCA-3′), shB7-H6-2 segments (top, 5′-CGCGT GGTTCTACCCAGAGGCTATTCAAGAGATAGCCTCTGGGTAGAACCTTTTAT-3′; bottom, 5′-CGATAAAAGGTTCTACCCAGAGGCTATCTCTTGAATAGCCTCTGGGTAGAACCA-3′) and shScr were inserted into the pLVTHM vector with the ClaI and MluI restriction sites. Lentiviral supernatants were generated by co-transfecting 293T cells with pLVTHM, psPAX2, and pMD2G plasmids using Lipofectamine 2000 reagent (Invitrogen). Culture supernatants collected 36 h after transfection were added directly to cells plated in 6-well culture plates, and Polybrene (Sigma) was added at a final concentration of 5 ìg/ml for 24 h. Transduced cells were characterized by GFP expression.
Cytotoxicity Assay
NK-92 cells were used as effector cells and co-cultured with target cells at specific ratios for 4 h, and cytotoxicity was determined using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega). The data shown represent at least three independent experiments.
Statistical Analysis
Results were analyzed using two-tailed Student's t-tests. The data were expressed as the means ± S.E. Values of p < 0.05 were considered statistically significant. Significance was denoted as *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Results
B7-H6 Is Widely Expressed in Tumor Cells
To investigate the expression of B7-H6 in tumor cells, anti-B7-H6 polyclonal antibody was generated in our laboratory because of the lack of appropriate commercial antibody. The stained bands could only be detected in B7-H6 transfection group but not in untreated group and control vector group in CHO-K1 and HEK293 cells (Fig. 1, A and B), suggesting that the anti-B7-H6 antibody was specific and suitable for Western blotting analysis of B7-H6 expression in cells, which was further confirmed by myc-B7-H6 transfection and staining in HEK293 cell (Fig. 1B). Furthermore, the B7-H6 antibody was also suitable for flow cytometry, which was determined by comparing the expressions of B7-H6 and myc-B7-H6 on cell surface detected by anti-B7-H6 antibody and anti-myc antibody, respectively (Fig. 1, C and D). Thus, the anti-B7-H6 polyclonal antibody generated in our laboratory was specific and suitable for B7-H6 expression analysis by Western blotting and flow cytometry.
FIGURE 1.
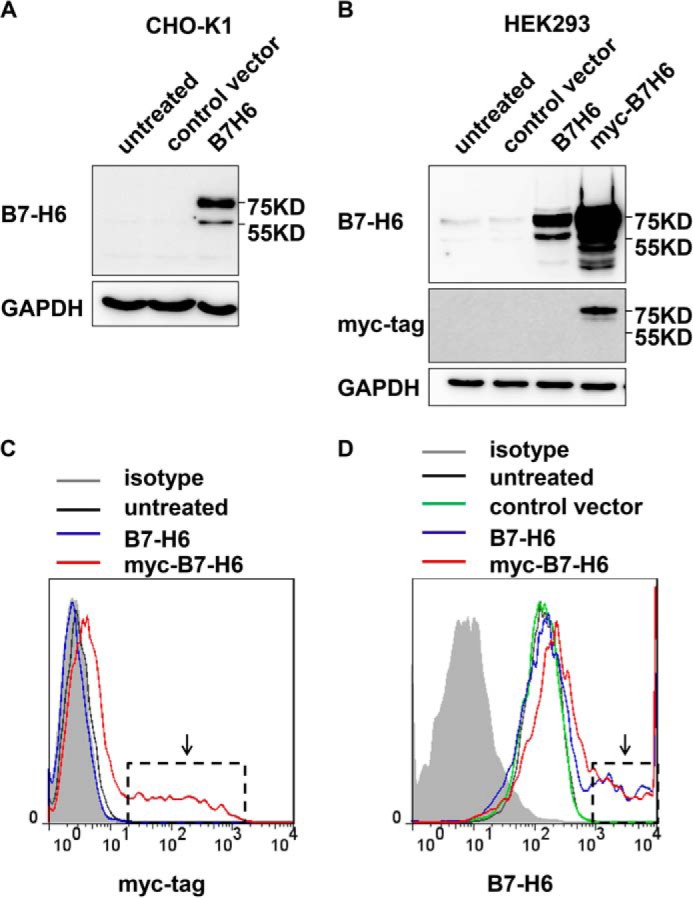
Specificity of anti-B7-H6 antibody. A, CHO-K1 cells were transfected with pcDNA3-B7-H6 and then were harvested 24 h later. B7-H6 expression was analyzed by Western blotting. B, HEK293 cells were transfected with pcDNA3-B7-H6 or pcDNA3-myc-B7-H6 and then were harvested 24 h later. B7-H6 and myc-tag expressions were analyzed by Western blotting. C and D, HEK293 cells were transfected with pcDNA3-B7-H6 or pcDNA3-myc-B7-H6 and then were harvested 24 h later. myc-tag (C) and B7-H6 (D) expressions were analyzed by flow cytometry.
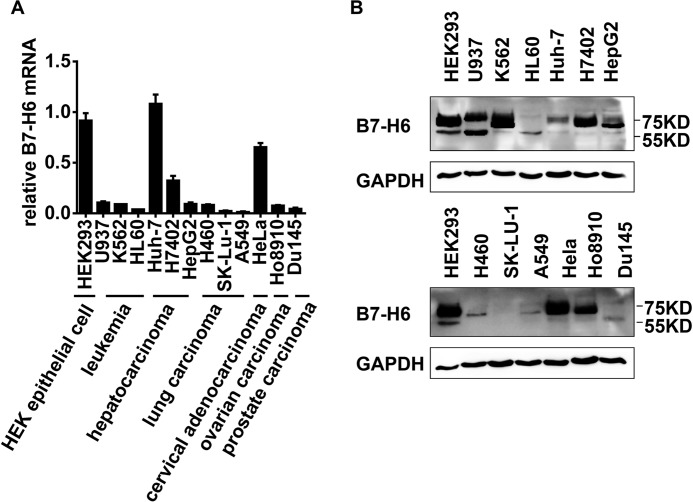
Next, to evaluate whether B7-H6 could be a potential target for tumor therapy to activate NK cells, the expression of B7-H6 was examined by real-time PCR and Western blotting in 12 tumor cell lines derived from leukemia, hepatocarcinoma, lung carcinoma, cervical carcinoma, ovarian carcinoma, and prostate carcinoma. B7-H6 expression was detected in all tumor cell lines, although the level of expression varied among the cell lines (Fig. 2, A and B). Moreover, more than one band could be detected using the B7-H6 antibody in some tumor cell lines (Fig. 2B), suggesting that there were differential post-translational modifications of B7-H6, which is consistent with a previous study (22). Thus, these data suggested that B7-H6 was extensively expressed in tumor cells.
FIGURE 2.
B7-H6 is widely expressed in tumor cell lines. A, B7-H6 expression in human tumor cell lines was analyzed by real-time PCR. B, B7-H6 expression in human tumor cells was analyzed by Western blotting.
B7-H6 Expressed in Tumor Cells Correlates with Their Susceptibility to NK Cell Lysis
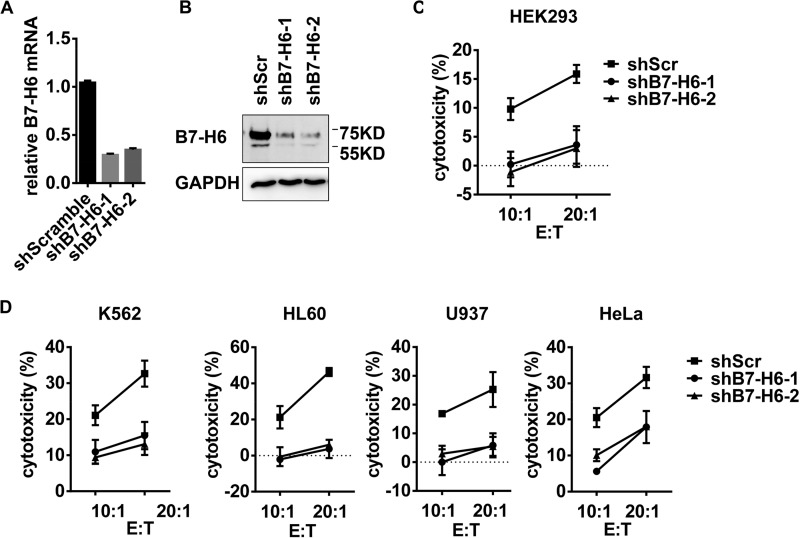
To evaluate the impact of B7-H6 expression on tumor sensitivity to NK cell-mediated lysis, B7-H6 expression in tumor cell lines were silenced with shRNA. In order to avoid the off-target effects of shRNA, we used two shB7-H6 sequences, shB7-H6-1, and shB7-H6-2, to treat tumor cells. shRNA treatment effectively down-regulated the B7-H6 expression at mRNA and protein levels (Fig. 3, A and B) and reduced the killing activity of NK-92 cells to target cells HEK293 (Fig. 3C), suggesting that the shRNA was effective. Furthermore, knockdown of B7-H6 expression increased the resistance of tumor cell lines, including leukemia (K562, HL60, and U937) and cervical adenocarcinoma (HeLa), to lysis by NK-92 cells (Fig. 3D). Thus, these data suggest that the level of B7-H6 expression in tumor cells correlates with their susceptibility to NK cell lysis.
FIGURE 3.
B7-H6 expression in tumor cells correlates to their susceptibility to NK killing. A and B, HEK293 cells were transduced with lentivirus delivering scramble shRNA (shScr) or shRNA targeting B7-H6 (shB7-H6-1, shB7-H6-2), and B7-H6 expression was then analyzed by real-time PCR (A) and Western blotting (B). C, lysis of HEK293 cells transduced with shScr, shB7-H6-1 or shB7-H6-2 by NK-92 cells was determined in a 4 h cytotoxicity assay. D, lysis of K562, HL60, U937, or HeLa cells transduced with shScr, shB7-H6-1, or shB7-H6-2 by NK-92 cells was determined in a 4 h cytotoxicity assay.
Chemotherapy Up-regulates B7-H6 Expression in Tumor Cells and Enhances Tumor Sensitivity to NK Cell Cytolysis
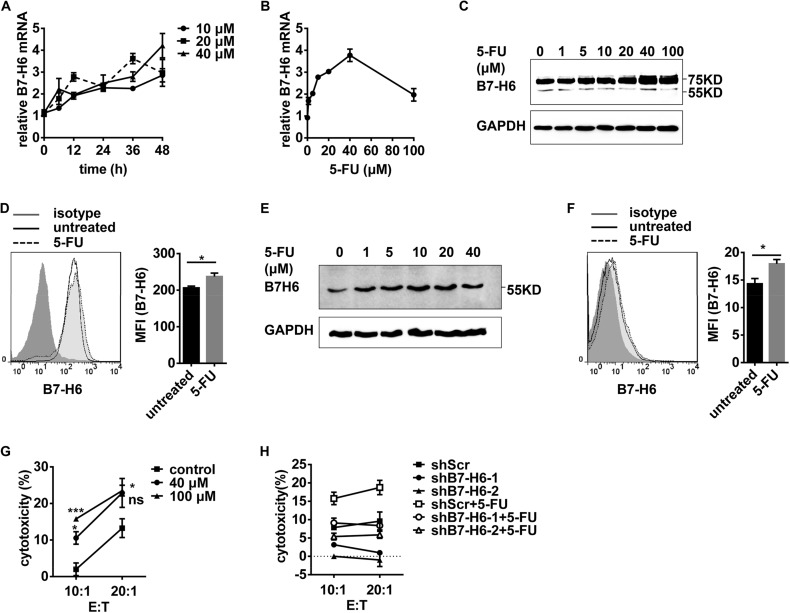
Chemotherapy, including treatment with cisplatin or 5-FU, has been proven effective in certain cancer treatments. To explore whether chemotherapy could increase the sensitivity of tumor cells to NK cell lysis by promoting B7-H6 expression, HEK293 cells were treated with chemotherapy reagents. And B7-H6 expression was then analyzed by real-time PCR, Western blotting and Flow cytometry. Treatment with cisplatin (Fig. 4, A–D) or 5-FU (Fig. 5, A–D) increased the expression of B7-H6 at mRNA and protein levels as well as on the cell surface. Similarly, leukemia HL60 cells with low B7-H6 expression up-regulated B7-H6 expressions at both protein level and cell surface after cisplatin or 5-FU treatment (Figs. 4, E–F and 5, E–F). Cisplatin or 5-FU treatment caused the tumor cells to become more sensitive to NK-92-mediated killing (Figs. 4G and 5G). Silencing B7-H6 expression in target cells significantly decreased NK cell-mediated cytolysis (Figs. 4H and 5H). Thus, these data suggest that chemotherapy could promote the expression of B7-H6 in tumor cells and thereby increase the sensitivity of tumor cells to NK cell lysis.
FIGURE 4.
Cisplatin treatment increases B7-H6 expression and sensitizes target cells to NK cell cytolysis. A, HEK293 cells were stimulated with 10 μm, 20 μm or 40 μm cisplatin for the indicated times. B7-H6 expression was analyzed by real-time PCR. B, HEK293 cells were stimulated with various doses of cisplatin for 24 h. B7-H6 expression was analyzed by real-time PCR. C, HEK293 cells were treated with various doses of cisplatin for 48 h. B7-H6 expression was analyzed by Western blotting. D, HEK293 cells were treated with 40 μm cisplatin for 48 h. Cell surface B7-H6 expression was analyzed by flow cytometry. E, HL60 cells were stimulated with various doses of cisplatin for 48 h. B7-H6 expression was analyzed by Western blotting. F, HL60 cells were treated with 40 μm cisplatin for 48 h. B7-H6 expression on cell surface was analyzed by flow cytometry. G, HEK293 cells were stimulated with 20 μm or 40 μm cisplatin for 48 h and then subjected to NK-92-mediated lysis. H, HEK293/shScr, HEK293/shB7-H6-1, and HEK293/shB7-H6-2 were stimulated with 40 μm cisplatin for 48 h or not and then subjected to NK-92-mediated lysis.
FIGURE 5.
5-FU treatment increases B7-H6 expression and sensitizes target cells to NK cell cytolysis. A, HEK293 cells were stimulated with 10, 20, or 40 μm 5-FU for the indicated times. B7-H6 expression was analyzed by real-time PCR. B, HEK293 cells were stimulated with various doses of 5-FU for 48 h. B7-H6 expression was analyzed by real-time PCR. C, HEK293 cells were treated with various doses of 5-FU for 48 h. B7-H6 expression was analyzed by Western blotting. D, HEK293 cells were treated with 40 μm 5-FU for the indicated times. Cell surface B7-H6 expression was analyzed by flow cytometry. E, HL60 cells were stimulated with various doses of 5-FU for 48 h. B7-H6 expression was analyzed by Western blotting. F, HL60 cells were treated with 40 μm 5-FU for 48 h. B7-H6 expression on cell surface was analyzed by Flow cytometry. G, HEK293 cells were stimulated with 40 or 100 μm 5-FU for 48 h and then subjected to NK-92-mediated lysis. H, HEK293/shScr, HEK293/shB7-H6-1, and HEK293/shB7-H6-2 were stimulated with 100 μm 5-FU for 48 h or not and then subjected to NK-92-mediated lysis.
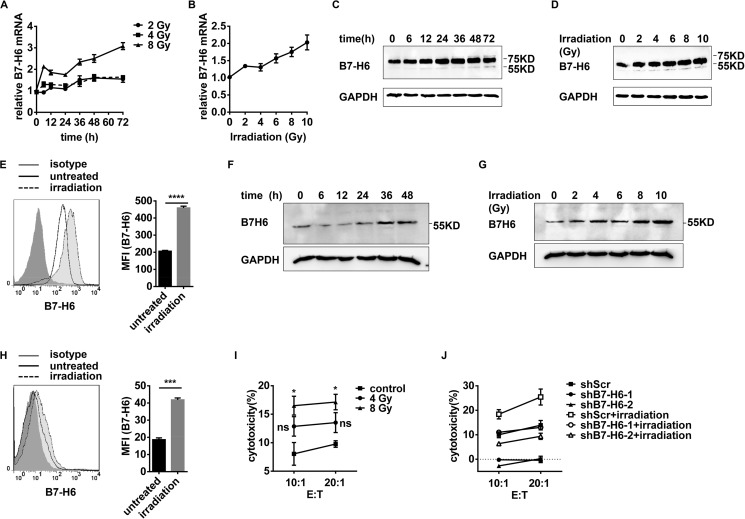
Irradiation Increases B7-H6 Expression and Makes Target Cells More Sensitive to NK Cell Cytolysis
Radiation therapy has been reported to contribute to eliciting the anti-tumor immune response (23). HEK293 cells were treated with γ-irradiation, and then B7-H6 expression was analyzed by real-time PCR, Western blotting, and flow cytometry. Irradiation increased the B7-H6 expression at mRNA and protein levels in a time- and dose-dependent manner (Fig. 6, A–D). The expression of B7-H6 on cell surface was also increased upon irradiation (Fig. 6E). Similar results were obtained in HL60 cells upon irradiation (Fig. 6, F–H). Importantly, the irradiation-treated tumor cells became more sensitive to NK cell lysis when co-cultured with the NK-92 cell line (Fig. 6I). Treating with B7-H6 shRNA before irradiation significantly decreased the sensitivity of the HEK293 cell to NK cell lysis (Fig. 6J). These data thus suggest that irradiation could increase the expression of B7-H6 in tumor cells and that B7-H6 was involved in making tumor cells more sensitive to lysis by NK cells.
FIGURE 6.
Irradiation increases B7-H6 expression and makes target cells more sensitive to NK cell cytolysis. A, HEK293 cells were treated with 2 Gy, 4 Gy or 8 Gy irradiation and then cultured for the indicated times. B7-H6 expression was analyzed by real-time PCR. B, HEK293 cells were treated with various doses of irradiation and then cultured for 24 h. B7-H6 expression was analyzed by real-time PCR. C, HEK293 cells were treated with 8 Gy irradiation and then cultured for the indicated times. B7-H6 expression was analyzed by Western blotting. D, HEK293 cells were exposed to various doses of irradiation and then cultured for 24 h. B7-H6 expression was analyzed by Western blotting. E, HEK293 cells were treated with 8 Gy irradiation and then cultured for 24 h. B7-H6 expression was analyzed by flow cytometry. F, HL60 cells were treated with 8 Gy irradiation and then cultured for the indicated times. B7-H6 expression was analyzed by Western blotting. G, HL60 cells were treated with various doses of irradiation and then cultured for 48 h. B7-H6 expression was analyzed by Western blotting. H, HL60 cells were exposed to 8 Gy irradiation and then cultured for 48 h. B7-H6 expression on cell surface analyzed by flow cytometry. I, HEK293 cells were treated with 0, 4, or 8 Gy irradiation, cultured for 24 h and subjected to NK-92-mediated lysis. J, HEK293/shScr, HEK293/shB7-H6-1 and HEK293/shB7-H6-2 were treated with 8 Gy irradiation or not, then cultured for 24 h and subjected to NK-92-mediated lysis.
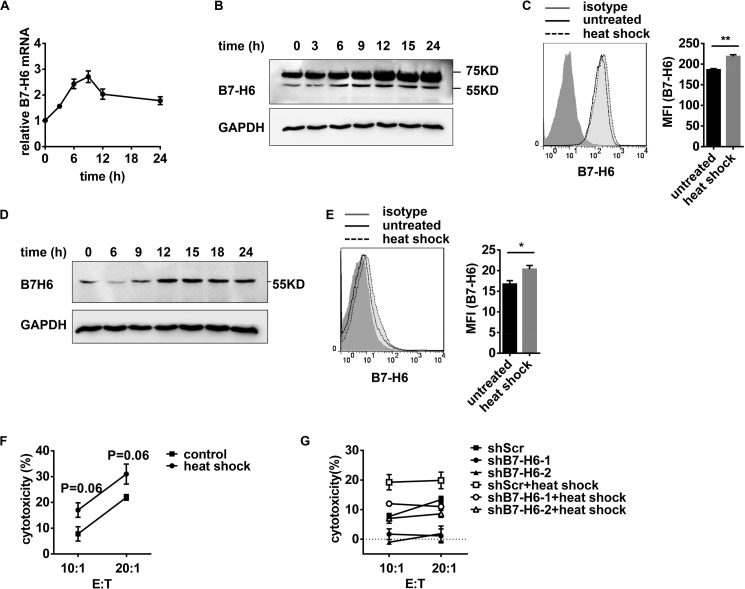
Heat Shock Increases B7-H6 Expression and Makes Target Cells More Sensitive to NK Cell Cytolysis
To evaluate whether hyperthermia could affect tumor sensitivity to NK cells in a B7-H6-dependent manner, HEK293 cells were exposed to non-lethal heat shock by incubation at 42 °C for 30 min. Heat shock treatment caused a transient up-regulation of B7-H6 mRNA (Fig. 7A) and protein (Fig. 7B), peaking at 9 h and 12 h post treatment, respectively, and declined quickly thereafter. Further flow cytometry analysis displayed that B7-H6 expression on cell surface increased upon heat shock treatment (Fig. 7C). Heat shock treatment also promoted B7-H6 expression in HL60 cells (Fig. 7, D and E). When subjected to NK-92-mediated lysis, HEK293 cells exposed to heat shock treatment became more sensitive to NK cell lysis (Fig. 7F). Treatment with B7-H6 shRNA before heat-shock treatment significantly decreased the sensitivity of the HEK293 cells to NK cell lysis (Fig. 7G). These data thus suggest that hyperthermia may enhance the sensitivity of tumor cells to NK cell lysis by up-regulating B7-H6 expression.
FIGURE 7.
Non-lethal heat shock increases B7-H6 expression and makes target cells more sensitive to NK cell cytolysis. A and B, HEK293 cells were exposed to 42 °C for 30 min and then cultured at 37 °C for the indicated times. B7-H6 expression was analyzed by real-time PCR (A), Western blotting (B). HEK293 cells were exposed to 42 °C for 30 min and then cultured at 37 °C for 12 h and B7-H6 expression was analyzed by flow cytometry (C). D, HL60 cells were exposed to 42 °C for 30 min and then cultured at 37 °C for the indicated times. B7-H6 expression was analyzed by Western blotting. E, HL60 cells were exposed to 42 °C for 30 min and then cultured at 37 °C for 12 h. B7-H6 expression on cell surface was analyzed by flow cytometry. F, HEK293 cells were incubated at 42 °C for 30 min, cultured at 37 °C for 11 h, and then subjected to NK-92-mediated lysis. G, HEK293/shScr, HEK293/shB7-H6-1, and HEK293/shB7-H6-2 were exposed to 42 °C for 30 min or not, then cultured at 37 °C for 11 h and subjected to NK-92-mediated lysis.
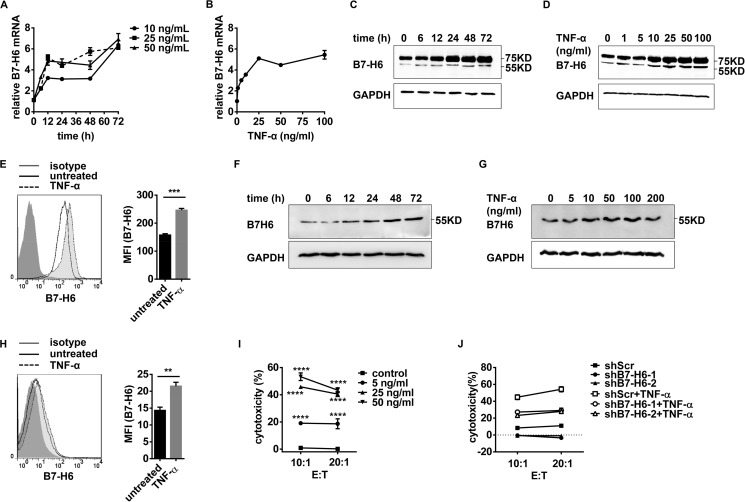
Cytokine Treatment Increases B7-H6 Expression and Sensitizes Target Cells to NK Cell Cytolysis
Localized administration of TNF-α has been demonstrated to be effective in controlling tumor growth (24). To explore whether TNF-α treatment would directly affect B7-H6 expression in tumor cells, HEK293 cells were stimulated with TNF-α, and B7-H6 expression was then analyzed by real-time PCR, Western blotting and Flow cytometry. TNF-α treatment increased the expression of B7-H6 at mRNA and protein levels as well as on cell surface (Fig. 8, A–E). Similar results were also acquired in HL60 cells after TNF-α treatment (Fig. 8, F–H). Moreover, TNF-α treatment increased tumor cell sensitivity to NK cell lysis (Fig. 8I). B7-H6 shRNA treatment significantly decreased the sensitivity of the HEK293 cells to NK cell lysis (Fig. 8J). Thus, these data suggest that TNF-α treatment could enhance the sensitivity of tumor cells to NK cell lysis by up-regulating B7-H6 expression.
FIGURE 8.
TNF-α treatment increases B7-H6 expression and sensitizes target cells to NK cell cytolysis. A, HEK293 cells were stimulated with 10 ng/ml, 25 ng/ml or 50 ng/ml TNF-α for the indicated times. B7-H6 expression was analyzed by real-time PCR. B, HEK293 cells were stimulated with various doses of TNF-α for 72 h. B7-H6 expression was analyzed by real-time PCR. C, HEK293 cells were treated with 50 ng/ml TNF-α for the indicated times. B7-H6 expression was analyzed by Western blotting. D, HEK293 cells were treated with various doses of TNF-α for 72 h. B7-H6 expression was analyzed by Western blotting. E, HEK293 cells were treated with 50 ng/ml TNF-α for 48 h. B7-H6 expression was analyzed by flow cytometry. F, HL60 cells were stimulated with 50 ng/ml TNF-α for the indicated times. B7-H6 expression was analyzed by Western blotting. G, HL60 cells were stimulated with various doses of TNF-α for 48 h. B7-H6 expression was analyzed by Western blotting. H, HL60 cells were treated with 50 ng/ml TNF-α for 48 h. B7-H6 expression on cell surface was analyzed by flow cytometry. I, HEK293 cells were stimulated with 5 ng/ml, 25 ng/ml, or 50 ng/ml TNF-α for 72 h and then subjected to NK-92-mediated lysis. J, HEK293/shScr, HEK293/shB7-H6-1, and HEK293/shB7-H6-2 were stimulated with 50 ng/ml TNF-α for 72 h or not and then subjected to NK-92-mediated lysis.
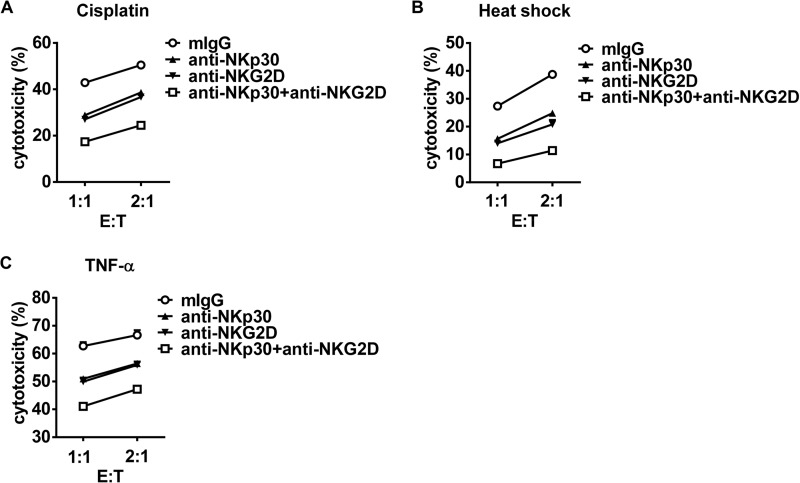
Enhancing Tumor Sensitivity to NK Cell Cytolysis by Tumor Therapeutics Depends on the Combined Action of NKG2D and NKp30 Signals
NKG2D ligands have been reported to be up-regulated upon tumor therapy and contribute to tumor sensitization to NK cell cytolysis. To validate our proposal that increased B7-H6 expression induced by tumor therapeutics also participate in tumor sensitivity to NK cell cytolysis, anti-NKp30, and anti-NKG2D blocking antibodies were used during cytolysis. Blocking NKp30 or NKG2D function alone dampened NK cell-mediated cytolysis to tumor cells, while simultaneously blocking both receptors lead to the least cytolysis (Fig. 9, A–C). Thus, these data suggest that enhancing tumor sensitivity to NK cell cytolysis upon tumor therapy depended on not only increase of NKG2D ligands but also increase of NKp30 ligand B7-H6 on tumor cells.
FIGURE 9.
Anti-NKp30 and anti-NKG2D blocking antibodies treatment dampens NK cell-mediated cytolysis to tumor cells. A, HEK293 cells were stimulated with 40 μm cisplatin for 48 h and then subjected to NKG-mediated cytolysis in the presence of anti-NKp30 mAb and/or anti-NKG2D mAb. B, HEK293 cells were incubated at 42 °C for 30 min, then cultured at 37 °C for 11 h, and finally subjected to NKG-mediated lysis in the presence of anti-NKp30 mAb and/or anti-NKG2D mAb. C, HEK293 cells were stimulated with 50 ng/ml TNF-α for 48 h and then subjected to NKG-mediated lysis in the presence of anti-NKp30 mAb and/or anti-NKG2D mAb.
Discussion
In many cases, an intact immune system is essential for complete remission during tumor therapy (1, 2). Deficiency of immune-related molecules or cells dampens the efficacy of conventional tumor therapy (23, 25). Studies have shown that in successful tumor treatments, including radiation therapy (3, 23, 26, 27), hyperthermia therapy (4) and chemotherapy (5, 28–30), endogenous anti-tumor immunity is elicited and is demonstrably effective. Recently, the success of PD-1 and PD-L1 antibodies in combating tumors has provided support for the notion that endogenous anti-tumor immunity participates in tumor elimination (31, 32).
Tumor-specific CTLs, as the effector cells with the strongest anti-tumor effects, have been extensively investigated. Studies have revealed that CD8+ T cells are indispensable for tumor elimination in certain murine tumor models (3, 33). Tumor-specific CTLs induced by appropriate tumor therapy mediate the anti-tumor effects not only locally but also at distal sites (3–5, 23). However, although CTLs play a critical role in the elimination of tumor cells, the functions of other immune cells, such as innate immune cell NK cells (9), cannot be neglected in this process.
NK cells have been shown to participate in tumor immunosurveillance in humans and mice (6). Low-dose radiation administered to NK cells directly enhances their cytotoxic ability without changing their phenotype (34, 35). Tumor therapy also promotes NK activation via DAMPs released from destroyed cells (26). NK cells mediate tumor cytolysis directly via perforin and granzymes or indirectly by inducing tumor apoptosis via death receptors (FasL and TRAIL). IFN-α secreted by activated NK cells promotes Th1 cell polarization and induces tumor-specific CTLs (16, 17).
NK cell activation is regulated by activating receptors and inhibitory receptors (36). The NK cell activating receptor NKG2D has been proved indispensable in several murine tumor models (37, 38). Regulation of NKG2D ligand expression on tumor cells has been extensively studied. γ-Radiation, cisplatin therapy, and 5-FU therapy up-regulate the expression of the NKG2D ligands MICA and ULBP-1,2,3 on tumor cells in humans (13). NKp30 is another important activating receptor of NK cells, but compared with NKG2D, study of NKp30 has lagged behind. Nonetheless, in vitro studies have demonstrated that NKp30 is involved in tumor elimination (39).
NKp30 ligands include MCMV protein pp65, nuclear protein BAT3, and B7-H6 (18–20). However, B7-H6 is the only transmembrane ligand of NKp30. Most importantly, B7-H6 is selectively expressed on tumor cells under steady-state conditions (40), implying that the interaction between NKp30 and B7-H6 may be involved in promoting the activation of NK cells and mediating the NK cell lysis of tumor cells. It has been reported that B7-H6 is down-regulated by HDAC inhibitor (41). In this study, we found that B7-H6 was widely expressed in tumor cells, and B7-H6 expression levels in tumor cells correlated to their susceptibility to NK cell lysis. Further studies showed that almost all tumor therapies could work as stress inducers to up-regulate the expression of B7-H6 in tumor cells.
Although we have found that tumor therapies can up-regulate the expression of B7-H6 in tumor cells, the underlying mechanisms that regulate the expression of B7-H6 remain unknown. Based on previous studies, we speculate that treatment with irradiation, cisplatin or 5-FU induces the DNA damage response and treatment with TNF-α activates the NK-κB signaling pathway, thereby potentially increasing the expression of B7-H6 in tumor cells. Further studies about the mechanisms underlying B7-H6 expression are needed.
Author Contributions
G. C., J. W., Z. T., and R. S. conceived and designed the experiments. G. C. performed the experiments. G. C., J. W., Z. T., and R. S. analyzed the data. X. Z. and H. W. contributed reagents/materials/analysis tools. G. C., J. W., Z. T., and R. S. wrote the manuscript. All authors analyzed the results and approved the final version of the manuscript.
This work was supported by the Ministry of Science & Technology of China (2013CB530506, 2012ZX10002014, and 2012AA020901) and Natural Science Foundation of China (#91029303). The authors declare that they have no conflicts of interest with the contents of this article.
- NK
- natural killer cells
- 5-FU
- 5-fluorouracil
- TNF
- tumor necrosis factor.
References
- 1.Zitvogel L., Apetoh L., Ghiringhelli F., André F., Tesniere A., and Kroemer G. (2008) The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 118, 1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zitvogel L., Kepp O., and Kroemer G. (2011) Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat. Rev. Clin. Oncol. 8, 151–160 [DOI] [PubMed] [Google Scholar]
- 3.Takeshima T., Chamoto K., Wakita D., Ohkuri T., Togashi Y., Shirato H., Kitamura H., and Nishimura T. (2010) Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 70, 2697–2706 [DOI] [PubMed] [Google Scholar]
- 4.Toraya-Brown S., Sheen M. R., Zhang P., Chen L., Baird J. R., Demidenko E., Turk M. J., Hoopes P. J., Conejo-Garcia J. R., and Fiering S. (2014) Local hyperthermia treatment of tumors induces CD8+ T cell-mediated resistance against distal and secondary tumors. Nanomedicine 10, 1273–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarnowski G. S., Faanes R. B., Ralph P., and Williams N. (1978) Suppression and restoration of cytotoxic T-cell activity during chemotherapy of a mouse T-cell lymphoma and a macrophage tumor. Cancer Res. 38, 4540–4545 [PubMed] [Google Scholar]
- 6.Vivier E., Raulet D. H., Moretta A., Caligiuri M. A., Zitvogel L., Lanier L. L., Yokoyama W. M., and Ugolini S. (2011) Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moretta L., Pietra G., Montaldo E., Vacca P., Pende D., Falco M., Del Zotto G., Locatelli F., Moretta A., and Mingari M. C. (2014) Human Nk cells: from surface receptors to the therapy of leukemias and solid tumors. Front. Immunol. 5, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng M., Chen Y., Xiao W., Sun R., and Tian Z. (2013) NK cell-based immunotherapy for malignant diseases. Cell Mol. Immunol. 10, 230–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Childs R. W., and Carlsten M. (2015) Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat. Rev. Drug. Discov. 14, 487–498 [DOI] [PubMed] [Google Scholar]
- 10.Luevano M., Madrigal A., and Saudemont A. (2012) Generation of natural killer cells from hematopoietic stem cells in vitro for immunotherapy. Cell Mol. Immunol. 9, 310–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasser S., Orsulic S., Brown E. J., and Raulet D. H. (2005) The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436, 1186–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nice T. J., Coscoy L., and Raulet D. H. (2009) Posttranslational regulation of the NKG2D ligand Mult1 in response to cell stress. J. Exp. Med. 206, 287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkataraman G. M., Suciu D., Groh V., Boss J. M., and Spies T. (2007) Promoter region architecture and transcriptional regulation of the genes for the MHC Class I-related chain A and B ligands of NKG2D. J. Immunol. 178, 961–969 [DOI] [PubMed] [Google Scholar]
- 14.Jinushi M., Takehara T., Kanto T., Tatsumi T., Groh V., Spies T., Miyagi T., Suzuki T., Sasaki Y., and Hayashi N. (2003) Critical role of MHC Class I-related chain A and B expression on IFN-α-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J. Immunol. 170, 1249–1256 [DOI] [PubMed] [Google Scholar]
- 15.Lin D., Lavender H., Soilleux E. J., and O'Callaghan C. A. (2012) NF-κB regulates MICA gene transcription in endothelial cell through a genetically inhibitable control site. J. Biol. Chem. 287, 4299–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vitale M., Della Chiesa M., Carlomagno S., Pende D., Aricò M., Moretta L., and Moretta A. (2005) NK-dependent DC maturation is mediated by TNFα and IFNγ released upon engagement of the NKp30 triggering receptor. Blood 106, 566–571 [DOI] [PubMed] [Google Scholar]
- 17.Morandi B., Mortara L., Chiossone L., Accolla R. S., Mingari M. C., Moretta L., Moretta A., and Ferlazzo G. (2012) Dendritic cell editing by activated natural killer cells results in a more protective cancer-specific immune response. PLoS ONE. 7, e39170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnon T. I., Achdout H., Levi O., Markel G., Saleh N., Katz G., Gazit R., Gonen-Gross T., Hanna J., Nahari E., Porgador A., Honigman A., Plachter B., Mevorach D., Wolf D. G., and Mandelboim O. (2005) Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat. Immunol. 6, 515–523 [DOI] [PubMed] [Google Scholar]
- 19.Pogge von Strandmann E., Simhadri V. R., von Tresckow B., Sasse S., Reiners K. S., Hansen H. P., Rothe A., Böll B., Simhadri V. L., Borchmann P., McKinnon P. J., Hallek M., and Engert A. (2007) Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 27, 965–974 [DOI] [PubMed] [Google Scholar]
- 20.Brandt C. S., Baratin M., Yi E. C., Kennedy J., Gao Z., Fox B., Haldeman B., Ostrander C. D., Kaifu T., Chabannon C., Moretta A., West R., Xu W., Vivier E., and Levin S. D. (2009) The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 206, 1495–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng M., Ma J., Chen Y., Zhang J., Zhao W., Zhang J., Wei H., Ling B., Sun R., and Tian Z. (2011) Establishment, characterization, and successful adaptive therapy against human tumors of NKG cell, a new human NK cell line. Cell Transplantation 20, 1731–1746 [DOI] [PubMed] [Google Scholar]
- 22.Schlecker E., Fiegler N., Arnold A., Altevogt P., Rose-John S., Moldenhauer G., Sucker A., Paschen A., von Strandmann E. P., Textor S., and Cerwenka A. (2014) Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res. 74, 3429–3440 [DOI] [PubMed] [Google Scholar]
- 23.Lee Y., Auh S. L., Wang Y., Burnette B., Wang Y., Meng Y., Beckett M., Sharma R., Chin R., Tu T., Weichselbaum R. R., and Fu Y.-X. (2009) Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manusama E. R., Stavast J., Durante N. M., Marquet R. L., and Eggermont A. M. (1996) Isolated limb perfusion with TNFα and melphalan in a rat osteosarcoma model: a new anti-tumour approach. Eur. J. Surg. Oncol. 22, 152–157 [DOI] [PubMed] [Google Scholar]
- 25.Apetoh L., Ghiringhelli F., Tesniere A., Obeid M., Ortiz C., Criollo A., Mignot G., Maiuri M. C., Ullrich E., Saulnier P., Yang H., Amigorena S., Ryffel B., Barrat F. J., Saftig P., Levi F., Lidereau R., Nogues C., Mira J.-P., Chompret A., Joulin V., Clavel-Chapelon F., Bourhis J., André F., Delaloge S., Tursz T., Kroemer G., and Zitvogel L. (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 [DOI] [PubMed] [Google Scholar]
- 26.Frey B., Rubner Y., Kulzer L., Werthmöller N., Weiss E.-M., Fietkau R., and Gaipl U. S. (2014) Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol. Immunother. 63, 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park B., Yee C., and Lee K.-M. (2014) The effect of radiation on the immune response to cancers. Int. J. Mol. Sci. 15, 927–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zitvogel L., Apetoh L., Ghiringhelli F., and Kroemer G. (2008) Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 8, 59–73 [DOI] [PubMed] [Google Scholar]
- 29.Ramakrishnan R., Assudani D., Nagaraj S., Hunter T., Cho H.-I., Antonia S., Altiok S., Celis E., and Gabrilovich D. I. (2010) Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J. Clin. Invest. 120, 1111–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghiringhelli F., and Apetoh L. (2014) The interplay between the immune system and chemotherapy: emerging methods for optimizing therapy. Expert Review of Clinical Immunology. 10, 19–30 [DOI] [PubMed] [Google Scholar]
- 31.Brahmer J. R., Tykodi S. S., Chow L. Q. M., Hwu W.-J., Topalian S. L., Hwu P., Drake C. G., Camacho L. H., Kauh J., Odunsi K., Pitot H. C., Hamid O., Bhatia S., Martins R., Eaton K., Chen S., Salay T. M., Alaparthy S., Grosso J. F., Korman A. J., Parker S. M., Agrawal S., Goldberg S. M., Pardoll D. M., Gupta A., and Wigginton J. M. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topalian S. L., Hodi F. S., Brahmer J. R., Gettinger S. N., Smith D. C., McDermott D. F., Powderly J. D., Carvajal R. D., Sosman J. A., Atkins M. B., Leming P. D., Spigel D. R., Antonia S. J., Horn L., Drake C. G., Pardoll D. M., Chen L., Sharfman W. H., Anders R. A., Taube J. M., McMiller T. L., Xu H., Korman A. J., Jure-Kunkel M., Agrawal S., McDonald D., Kollia G. D., Gupta A., Wigginton J. M., and Sznol M. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsui S., Ahlers J. D., Vortmeyer A. O., Terabe M., Tsukui T., Carbone D. P., Liotta L. A., and Berzofsky J. A. (1999) A model for CD8+ CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J. Immunol. 163, 184–193 [PubMed] [Google Scholar]
- 34.Halpern J. N., Cohen E., Mekori T., Beny A. N., Kuten A., Rosenblatt E. A., and Robinson E. (1991) The effect of in vitro irradiation on human NK cell activity. A preliminary report. J. Med. 22, 243–254 [PubMed] [Google Scholar]
- 35.Sonn C. H., Choi J. R., Kim T.-J., Yu Y.-B., Kim K., Shin S. C., Park G.-H., Shirakawa T., Kim H. S., and Lee K.-M. (2012) Augmentation of natural cytotoxicity by chronic low-dose ionizing radiation in murine natural killer cells primed by IL-2. J. Radiat. Res. 53, 823–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bryceson Y. T., March M. E., Ljunggren H.-G., and Long E. O. (2006) Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 214, 73–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smyth M. J., Swann J., Cretney E., Zerafa N., Yokoyama W. M., and Hayakawa Y. (2005) NKG2D function protects the host from tumor initiation. J. Exp. Med. 202, 583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guerra N., Tan Y. X., Joncker N. T., Choy A., Gallardo F., Xiong N., Knoblaugh S., Cado D., Greenberg N. R., and Raulet D. H. (2008) NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28, 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pende D., Parolini S., Pessino A., Sivori S., Augugliaro R., Morelli L., Marcenaro E., Accame L., Malaspina A., Biassoni R., Bottino C., Moretta L., and Moretta A. (1999) Identification and molecular characterization of Nkp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J. Exp. Med. 190, 1505–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matta J., Baratin M., Chiche L., Forel J.-M., Cognet C., Thomas G., Farnarier C., Piperoglou C., Papazian L., Chaussabel D., Ugolini S., Vély F., and Vivier E. (2013) Induction of B7-H6, a ligand for the natural killer cell–activating receptor NKp30, in inflammatory conditions. Blood 122, 394–404 [DOI] [PubMed] [Google Scholar]
- 41.Fiegler N., Textor S., Arnold A., Rölle A., Oehme I., Breuhahn K., Moldenhauer G., Witzens-Harig M., and Cerwenka A. (2013) Down-regulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood 122, 684–693 [DOI] [PubMed] [Google Scholar]