Background: The role of long non-coding RNAs (lncRNAs) in senescence is little known.
Results: Senescence-associated lncRNA (SALNR) expression is reduced during senescence. SALNR delays oncogene-induced senescence through suppressing NF90 nucleolus translocation.
Conclusion: lncRNA SALNR plays an important role in senescence.
Significance: We profiled lncRNAs in senescence and provided novel insights into the role of lncRNAs in senescence.
Keywords: aging; long noncoding RNA (long ncRNA, lncRNA); oncogene; Ras protein; senescence; NF90; SALNR
Abstract
Long non-coding RNAs (lncRNAs) have recently emerged as key players in many physiologic and pathologic processes. Although many lncRNAs have been identified, few lncRNAs have been characterized functionally in aging. In this study, we used human fibroblast cells to investigate genome-wide lncRNA expression during cellular senescence. We identified 968 down-regulated lncRNAs and 899 up-regulated lncRNAs in senescent cells compared with young cells. Among these lncRNAs, we characterized a senescence-associated lncRNA (SALNR), whose expression was reduced during cellular senescence and in premalignant colon adenomas. Overexpression of SALNR delayed cellular senescence in fibroblast cells. Furthermore, we found that SALNR interacts with NF90 (nuclear factor of activated T-cells, 90 kDa), an RNA-binding protein suppressing miRNA biogenesis. We demonstrated that NF90 is a SALNR downstream target, whose inhibition led to premature senescence and enhanced expressions of senescence-associated miRNAs. Moreover, our data showed that Ras-induced stress promotes NF90 nucleolus translocation and suppresses its ability to suppress senescence-associated miRNA biogenesis, which could be rescued by SALNR overexpression. These data suggest that lncRNA SALNR modulates cellular senescence at least partly through changing NF90 activity.
Introduction
Human fibroblasts have a limited ability to divide in culture, after which they remain viable for many weeks but fail to proliferate despite being given space, nutrients, or growth factors, which is known as senescence (1). Cellular senescence could be triggered through two closely related processes: telomere erosion (replicative senescence) and exposure to damaging conditions (premature senescence) (2). Senescent cells often have an enlarged morphology, elevated activity of lysosomal β-galactosidase, enhanced autophagy, and senescence-associated heterochromatic foci (SAHF).4 Senescent cells also display the characteristic senescence-associated secretory phenotype (2). Evidence has shown that cellular senescence not only acts as an important anti-cancer mechanism but also influences other disease, such as neurodegeneration, cardiovascular disease, and declining immune function (3).
A number of factors have been implicated in regulating senescence, including transcription factors of the p53 and Ets families (4), the post-transcriptional regulators AUF1 (AU-binding factor 1) (5) and HuR (human antigen R) (6), and small non-coding RNAs (7). A recent report (8) showed that long non-coding RNAs (lncRNAs) exhibit a special expression pattern during senescence and probably act as an important regulator of cellular senescence.
lncRNAs comprise a highly heterogeneous group of transcripts with a wide range of sizes, structures, and subcellular locations. lncRNAs can regulate gene expression by modulating the structure of chromatin remodeling factors and transcription factors and by forming DNA-RNA triple helices (9). lncRNAs also have scaffolding or decoy function affecting gene transcription (9). In addition, lncRNAs post-transcriptionally regulate gene expression via modulating precursor mRNA splicing, mRNA stability, or translation (10–12). The lncRNA-mediated gene expression is involved in cell proliferation, differentiation, chromosomal imprinting, and embryogenesis, whereas lncRNA function is associated with cancer, neurodegeneration, cardiovascular disease, and metabolic disorder (13–15).
Although a growing number of lncRNAs have been characterized in many physiologic and pathologic processes, their implications in senescence are largely unknown. In this study, we aimed to identify senescence-associated lncRNAs and intended to characterize their functions in premature senescence.
Experimental Procedures
Cell Cultures and Human Samples
Human embryonic lung diploid fibroblast 2BS cells (National Institute of Biological Products, Beijing, China) were isolated from female fetal lung fibroblast tissue, and they have been fully characterized previously (16). The cells were maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen). WI-38 normal human embryo lung fibroblasts (American Type Culture Collection, Manassas, VA) were cultured in the same manner as described for 2BS cells. Colon adenoma (n = 15) and adjacent normal tissue specimens (n = 15) were collected at Peking University First Hospital.
lncRNA and miRNA Expression Profiling
The Human Long Non-coding RNA Array and microarray chip have been described previously (17). The microarray analysis was performed by KangChen Bio-tech (Shanghai, China). We selected candidates with a minimum of 2-fold changes and p < 0.05. Differentially expressed lncRNAs and their chromosomal locations were mapped by Prism version 6.0 (GraphPad Software, La Jolla, CA). The expression profile of miRNAs was determined using the stem-loop RT-PCR-based TaqMan human microRNA array (Applied Biosystems, Foster City, CA) as described previously (18). Relative miRNA levels were determined by ΔΔCt using endogenous controls. The scatter plot is a visualization method used for assessing expression variations between pairs. The values of the x and y axes in the scatter plot are the averaged normalized signal values of the group (log2-scaled). The green lines are -fold change (the default -fold change given is 2.0). The lncRNAs or miRNAs above the top green line and below the bottom green line indicate >2-fold change between pairs.
Rapid Amplification of cDNA Ends (RACE) Mapping of SALNR
RACE was performed using a SMARTerTM RACE cDNA amplification kit (Clontech, Palo Alto, CA). The primers (5′Race_GSP1, 5′Race_GSP-1N, 3′Race_GSP1, and 3′Race_GSP-1N) used are shown in Table 1.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotides | Sequences |
|---|---|
| 5′Race_GSP1 | 5′-GGCCTGTAATCCCAGCACTTAGTCCG-3′ |
| 5′Race_GSP-1N | 5′-CGACAGTGCGAGACTCAAAAAAAGGGGA-3′ |
| 3′Race_GSP1 | 5′-CAAAGGGCTGGGATTACAGGTGTGAG-3′ |
| 3′Race_GSP-1N | 5′-CTTTGATTTCCAGTCTGTTGAAGGTG-3′ |
| SALNR_F | 5′-GTACAGGTGGGTTTTGGTTAC-3′ |
| SALNR_R | 5′-AAATTATCTGGGCATAGTGTCA-3′ |
| lncRNAU1_F | 5′-GCATTAGAAGGTTGTTGAGAC-3′ |
| lncRNAU1_R | 5'-GCAAAGGAAGTAGAAGGCTC-3′ |
| lncRNAU2_F | 5′-GTCCCAAACCTGCTATCACTT-3′ |
| lncRNAU2_R | 5′-CCATTTCCTTCTGCCTACATT-3′ |
| lncRNAU3_F | 5′-AAGGTTTGGGTAAGGGAGGAG-3′ |
| lncRNAU3_R | 5′-TTGGCGTGCTCTGTGTAAGTG-3′ |
| lncRNAU4_F | 5′-GCTGTTTGTGATGCCCTAAGA-3′ |
| lncRNAU4_R | 5′-TCCAAGTTGCGTGAGATAAGA-3′ |
| lncRNAU5_F | 5′-AAGAAAAACGCTATGGCAAAG-3′ |
| lncRNAU5_R | 5′-CAAACTTGGATACAGCATGGT-3′ |
| lncRNAD1_F | 5′-ATCACTTCAAGATGGCTAAC-3′ |
| lncRNAD1_R | 5′-AAGGAAATGAACCACATAGA-3′ |
| lncRNAD2_F | 5′-TTCACACCCTTCCGAGAGTTC-3′ |
| lncRNAD2_R | 5′-CACAAGACACCACGCCAGAC-3′ |
| lncRNAD3_F | 5′-GATCTAACTTTTTTTCCTCCT-3′ |
| lncRNAD3_R | 5′-GAAGTTCTGAGACAACACAAC-3′ |
| lncRNAD4_F | 5′-TCCCCTCTAACACCCCTTCTC-3′ |
| lncRNAD4_R | 5′-AACCCCCCAGCACTTACCACT-3′ |
| lncRNAD5_F | 5′-CTACTCCCAGCCCTGTTCCTA-3′ |
| lncRNAD5_R | 5′-TGTTGTGGGAGCAGAGGTTGT-3′ |
| GAPDH_F | 5′-ACGGATTTGGTCGTATTGGG-3′ |
| GAPDH_R | 5′-TGATTTTGGAGGGATCTCGC-3′ |
| SALNRFISH_F | 5′-AGTTTCACTTCGTCACCTGG-3′ |
| SALNRFISH_R | 5′-ATGCAGGCTTTTCAAGAAGGTA-3′ |
| NF90F_F | 5′-ATGCGTCCAATGCGAATTTTTG-3′ |
| NF90F_R | 5′-CTTTTAGACGCTCTAGGAAGACCCA-3′ |
| KRAS_F | 5′-GGCCTGCTGAAAATGACTGA-3′ |
| KRAS_R | 5′-GTTGGATCATATTCGTCCAC-3′ |
| BRAF_F | 5′-TCATAATGCTTGCTCTGATAGGA-3′ |
| BRAF_R | 5′-CTTTCTAGTAACTCAGCAGC-3′ |
| pri-miR-181a_F | 5′-ATCCATCAGCGGTGGTCT-3′ |
| pri-miR-181a_R | 5′-TTCTGGTGAACTCATGTGACA-3′ |
| pri-miR-22_F | 5′-GCTGAGCCGCAGTAGTTCT-3′ |
| pri-miR-22_R | 5′-CCATTTCTGTCACCTTCCAG-3′ |
| pri-miR-1233_F | 5′-CAGCGTCTGAGCCCTGTCCTC-3′ |
| pri-miR-1233_R | 5′-TGCCACAAGCCGCAACACCAT-3′ |
TestCode
TestCode was developed to identify potential protein coding regions in nucleic acid sequences by plotting a measure of the non-randomness of the composition at every third base (19). A score of >0.95 means that the sequence is probably coding. A score of <0.74 means that the sequence is probably non-coding. Scores between 0.74 and 0.95 mean that it is uncertain whether the sequence is coding or not.
Vector Constructs
The following viral vectors were used in this study. pWZL-Hygro-Ras (H-RasV12) and pZsG-Puro were generously provided by Drs. Lixiang Xue and Xiaowei Zhang (Peking University Health Science Center), respectively. SALNR-pZSG was prepared as follows. The full-length SALNR sequence was amplified by PCR on the cDNA of 2BS cells with primers SALNR_F and SALNR_R (Table 1). The PCR fragment was cloned into the NotI/BamHI sites of pZsG-Puro vector. The full-length SALNR was also cloned into the NotI/BamHI sites of the mammalian expression vector pcDNA3.1(+) (Invitrogen) to generate sense SALNR-pcDNA3.1 vector. Antisense SALNR-pcDNA3.1 was constructed by subcloning the antisense of SALNR into BamHI/NotI sites of the pcDNA3.1(−) vector (Invitrogen).
RNA Interference
RNA interference was used to knock down SALNR and NF90 in young 2BS cells. The vector used for generating lentivirus was remolded pLL3.7, whose sequence expressing GFP was replaced by sequence expressing G418. The sense strands of shRNAs were as follows: shSALNR, 5′-GTGCTTGATAACTATATCT-3′; shNF90#1, 5′-GGTCTTCCTAGAGCGTCTAAA-3′; shNF90#2, 5′-CAGACTGCTACGGCTATCA-3′; shControl, 5′-TTCTCCGAACGTGTCACGT-3′.
Transfection and Virus Packaging
All transfections were carried out with LipofectamineTM 2000 (Invitrogen) according to the manufacturer's instructions. To produce infectious viruses, the 293T packaging cell line was co-transfected with viral backbone plasmids and packaging plasmids (pMD2.G and psPAX2). After 48 h of transfection, the medium was collected, and the virus particles were concentrated by centrifugation. 2BS cells were plated on 60-mm plates 16–18 h before infection. Cells were infected with 5 × 104 viral particles/plate in the presence of 8 μg/ml Polybrene (Sigma). After 12 h of incubation, the virus-containing medium was replaced with fresh medium, and on the next day, 2 μg/ml puromycin (for pZsG-Puro) or 500 μg/ml G418 (for pLL3.7-G418) was added to select stably infected cell populations. After 5 days of selection, stable cell pools were established, and expression was verified by quantitative RT-PCR (qRT-PCR) or Western blot analysis.
RNA Extract and qRT-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. qRT-PCR was performed using SuperscriptTM III transcriptase (Invitrogen) and THUNDERBIRD SYBR qPCR Mix (Toyobo, Osaka, Japan) according to the manufacturer's instructions. The expression of lncRNAU1 to -U5, lncRNAD1 to -D5, and microRNA primary transcript (pri-miRNA) was analyzed by qRT-PCR with the use of the primers shown in Table 1. GAPDH was used as an internal control. The mirVanaTM qRT-PCR miRNA detection kit and primer sets were purchased from Ambion (Foster City, CA). The cDNA was synthesized from total RNA using gene-specific primers according to the TaqMan microRNA assay protocol (Ambion). qRT-PCR was performed on cDNA generated from total miRNA using the mirVana qRT-PCR miRNA detection kit protocol (Ambion). Amplification and detection of specific products were performed using the ABI Prism 7300 sequence detection system (Applied Biosystems) with a cycle profile according to the instructions of the mirVana qRT-PCR miRNA detection kit. As an internal control, U6 snRNA was used for RNA template normalization.
Cell Extracts and Western Blotting
The isolation of nucleoli fractionation was described previously (20). The primary anti-β-actin, anti-lamin B, and anti-fibrillarin antibodies were from Cell Signaling Technology (Beverly, MA). Anti-NF90 antibody was from BD Biosciences. Anti-NF45 antibody was from Abcam (Cambridge, UK). Anti-p16 and anti-p53 antibodies were from Santa Cruz Biotechnology, Inc. Anti-FLAG antibody was from Sigma.
Senescent Assay and Cell Proliferation Assay
Detection of senescence-associated β-galactosidase (SA-β-gal) activity and SAHF staining were performed as described previously (21). BrdU was detected using a BrdU incorporation assay kit according to the manufacturer's instructions (Roche Applied Science). For the cell proliferation assay, 4 × 105 cells were plated in 35-mm tissue dishes. Cells were cultured for up to the indicated days and then fixed and stained with crystal violet. Stain was extracted by treatment with 95% ethanol. Subsequently, A570 values were measured.
Cell Cycle Analysis
Cells were washed with PBS, detached with 0.25% trypsin, and fixed with 75% ethanol overnight. After treatment with 1 mg/ml RNase A (Sigma), cells were resuspended in PBS and stained with propidium iodide in the dark. Fluorescence was measured with a FACScan flow cytometry system (BD Biosciences).
Immunofluorescence (IF)
Cells were fixed with 4% formaldehyde for 15 min and then incubated with PBS containing 0.3% Triton X-100 and 5% normal goat serum for 1 h at room temperature, followed by incubation with antibodies specific for NF90 (BD Biosciences), fibrillarin (Cell Signaling Technology), or NF45 (Abcam) overnight at 4 °C. Cells were then washed with PBS plus 0.3% Triton X-100 and incubated with a fluorochrome-conjugated secondary antibody. Nuclei were stained with DAPI. Cells were imaged on a Zeiss LSM 410 confocal laser-scanning microscope.
The human colon adenoma and control non-adenoma samples were fixed in 10% formalin, routinely embedded in paraffin, and sectioned at 5 μm. Serial sections were deparaffinized in xylene and hydrated through graded concentrations of ethanol. Epitopes were retrieved using microwave in boiling Tris-EDTA buffer, pH 9.0, for 20 min. Blocking, incubations with primary antibodies, and incubations with secondary antibodies were carried out in the same manner as that of culture cells.
RNA-FISH/IF
To label the RNA probes, partial cDNA fragments of SALNR were amplified by PCR using the sequence-specific primers SALNR FISH_F and SALNR FISH_R (Table 1). The PCR product was inserted into pcDNA3.1(+) or pcDNA3.1(−) vector. The plasmid DNA was then linearized and used for transcription with T7 RNA polymerases to generate sense and antisense probes with the FISH tag RNA multicolor kit (Invitrogen) in accordance with the manufacturer's protocol. Detection of RNA-FISH/IF was performed as described previously (22).
RNA Pull-down Assay
Sense SALNR-pcDNA3.1(+) and antisense SALNR-pcDNA3.1(−) were linearized with the corresponding restriction enzymes used to clone the vector at the 3′-end to obtain the template DNAs for in vitro transcription. An RNA pull-down assay was performed as described previously (23). The retrieved protein was detected by silver staining or Western blotting. Protein bands differently visualized in sense SALNR compared with antisense were excised and analyzed by mass spectrometry.
RNA Immunoprecipitation (RIP) Assay
An RIP assay was carried out using the Magna RIPTM RNA-binding protein immunoprecipitation kit (Millipore, Billerica, MA). The antibody used for RIP was anti-NF90 (BD Biosciences). The co-precipitated RNAs were purified with phenol/chloroform/isoamyl alcohol and detected by qRT-PCR.
Detection of RNA Half-life
Cells were treated with 5 μg/ml actinomycin D. After the indicated incubation period, total RNA was isolated for qRT-PCR as described before. The ratio of the RNA level for SALNR relative to that for GAPDH before actinomycin D treatment was defined as 100%, and the half-life of lncRNA SALNR was calculated from the time period required for the SALNR RNA to undergo a reduction to one-half of its initial abundance.
NF90-FLAG Vector Construct
FLAG-NF90 was prepared as follows. The full-length NF90 sequence was amplified by PCR on the cDNA of 2BS cells with primers NF90F_F and NF90F_R (Table 1). The PCR fragment was then cloned into the BamHI/EcoRI sites of pCMV-Tag2B vector.
Mutational Analysis
Genomic DNA was extracted from colon adenoma and non-adenoma samples using the QIAamp DNA microkit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. KRAS mutation analysis at codons 12 and 13 was performed using direct sequencing of a fragment containing codons 12 and 13 of the KRAS gene using primers KRAS_F and KRAS_R (Table 1). These cover the length of the KRAS gene and derive a DNA fragment product of 116 base pairs. BRAF mutation analysis at codon 600 was performed using direct sequencing of a fragment containing codon 600 of the BRAF gene using primers BRAF_F and BRAF_R (Table 1). These cover the length of BRAF gene and derive a DNA fragment product of 251 base pairs.
Statistical Analysis
Results are expressed as means ± S.D., and the error bars represent S.D. values from three independent experiments unless otherwise indicated. The statistical significance of differences in the experimental data were analyzed using the two-sample Student's t test (p < 0.05 (*) was regarded as significant).
Results
Genome-wide Screen for Senescence-associated lncRNAs in Human Fibroblasts
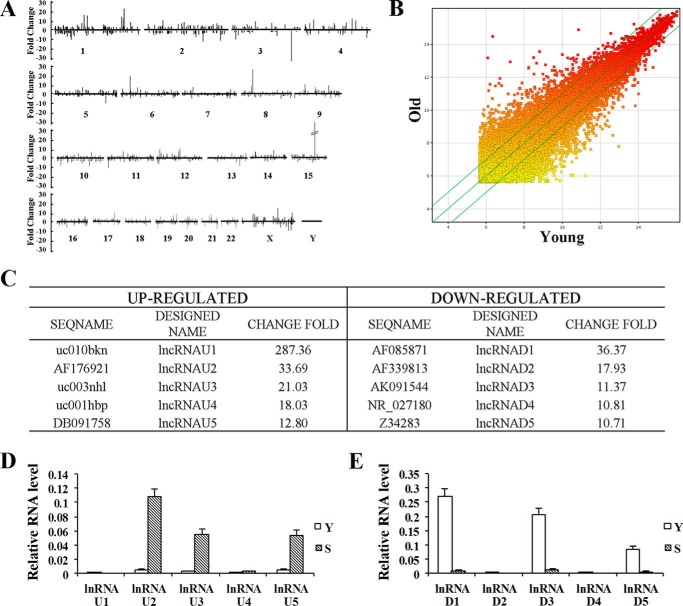
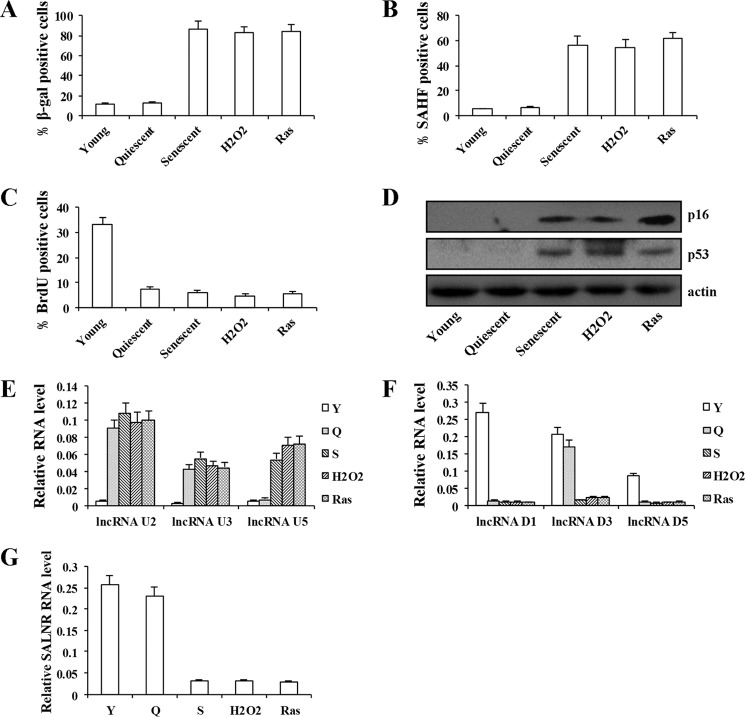
To search for lncRNAs that are associated with senescence, we used the lncRNA microarray to profile lncRNA expression in replicative senescent (divided more than 60 times) and young human 2BS fibroblast. We identified 1,867 lncRNAs (with a ≥2.0-fold change and p < 0.05) in replicative senescent cells compared with young cells, among which 899 were up-regulated and 968 were down-regulated in senescent cells (Fig. 1, A and B). We then picked the five most up-regulated (U1–U5) or down-regulated (D1–D5) lncRNAs for validation (Fig. 1C). As shown in Fig. 1, D and E, the results from real-time PCR were consistent with microarray data. Because of their low abundance, lncRNAU1, lncRNAU4, lncRNAD2, and lncRNAD4 were not studied further. To ensure that the expression change of lncRNAs was senescence-specific, we analyzed the expression of lncRNAs in quiescent and premature senescent cells. The senescence in quiescent and premature senescent cells was quantified and validated by measurement of SA-β-gal activity, SAHF formation, cells incorporating BrdU, and the expression of p16 and p53 (Fig. 2, A–D). For the six selected lncRNAs, similar expression changes were observed in Ras- or H2O2-induced premature senescent cells (Fig. 2, E and F). Interestingly, serum-deprived quiescent cells exhibited a similar change of lncRNAU2, -U3, -D1, and -D5 expression, suggesting that expression change in these four lncRNAs is not senescence-specific (Fig. 2, E and F). Both lncRNAU5 and -D3 expressions did not change in serum-deprived quiescent cells compared with young cells, suggesting that these two lncRNA expression changes were associated with senescence rather than growth arrest (Fig. 2, E and F). In this study, we focused on lncRNAD3 and designated it as SALNR (senescence-associated long non-coding RNA). Decreased expression of SALNR also was observed in senescent WI-38 cells (Fig. 2G), suggesting that SALNR down-regulation is not unique to 2BS cells. To obtain the complete structure of the SALNR gene, we performed 5′-RACE and 3′-RACE using gene-specific primers (Fig. 3A). The 3,788-bp SALNR cDNA contains a putative TATA box (TCTTTTAAAT) at −29 bp upstream of the putative transcription start site, without any introns. The full-length cDNA is mapped to 10q23.33 positive strand (NCBI36/hg18). By using the NCBI ORF finder program, we predicted multiple stop codons and several short open reading frames (ORFs) (<201 nucleotides) in SALNR full-length transcript (Fig. 3B). The TestCode value of SALNR is 0.386, which suggests an ncRNA. Furthermore, phylogenetic conservation analysis showed that SALNR was not conservative among humans, dogs, horses, mice, rats, and chickens (Fig. 3C), which appears to be a common feature for most lncRNAs.
FIGURE 1.
Genome-wide screening of senescence-related lncRNAs and validation. A, lncRNA microarrays identified 1,867 senescence-related lncRNAs comparing senescent and young 2BS fibroblasts. The threshold was set as 2.5-fold changes. Numbers 1–22 and X and Y represent chromosome number. B, a scatter plot is used for assessing lncRNA expression variation. The values of the x and y axes in the scatter plot are the averaged normalized signal values of groups of samples (log2-scaled). C, the five most significantly up-regulated (designated as lncRNAU1 to -U5) or down-regulated (designated as lncRNAD1 to -D5) lncRNAs listed according to lncRNA microarrays. D, qRT-PCR analysis of lncRNAU1 to -U5 expression in young (Y) and replicative senescent (S) 2BS cells. E, qRT-PCR analysis of lncRNAD1 to -D5 expression in young (Y) and replicative senescent (S) 2BS cells. Levels are normalized to GAPDH expression and are represented as means ± S.D. (error bars) unless otherwise indicated.
FIGURE 2.
Expression of lncRNAs in senescent fibroblasts. The activity of SA-β-gal (A), SAHF formation (B), percentage of cells incorporating BrdU (C), and expression of p16 and p53 protein (D) were detected in young, quiescent, replicative senescent, H2O2-induced senescent, and Ras-induced senescent 2BS cells. Quiescent cells were incubated in medium containing 0.2% serum for 48 h. H2O2-induced senescent cells were treated with a sublethal dose (200 μm H2O2) for 2 h. E, qRT-PCR analysis of lncRNAU2, -U3, and -U5 expression in young (Y), quiescent (Q), replicative senescent (S), H2O2-induced senescent, and Ras-induced senescent 2BS cells. F, qRT-PCR analysis of lncRNAD1, -D3, and -D5 expression in young (Y), quiescent (Q), replicative senescent (S), H2O2-induced senescent, and Ras-induced senescent 2BS cells. G, qRT-PCR analysis of SALNR expression in young (Y), quiescent (Q), replicative senescent (S), H2O2-induced senescent, and Ras-induced senescent WI-38 cells. Levels are normalized to GAPDH expression and represented as means ± S.D. (error bars) unless otherwise indicated.
FIGURE 3.
Molecular cloning of SALNR and analysis of the ORF and phylogenetic conservation of SALNR. A, molecular cloning of SALNR. 5′- and 3′-ends of SALNR were extended from 2BS cells using the RACE technique (bottom). A schematic diagram of the full-length SALNR genomic structure is shown at the top. B, the ORF of SALNR was analyzed with the NCBI ORF Finder program. Blue boxes, predicted ORFs. C, phylogenetic conservation of the SALNR transcript was analyzed using VISTA. The graph is a plot of nucleotide identity for a 100-bp sliding window centered at a given position.
SALNR Overexpression Delays Cellular Senescence
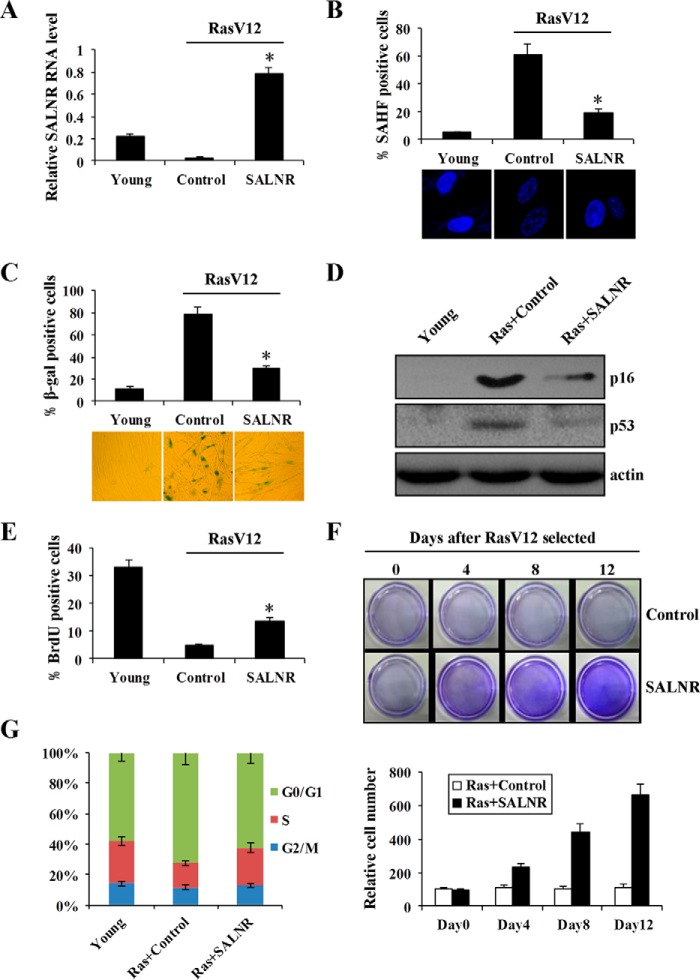
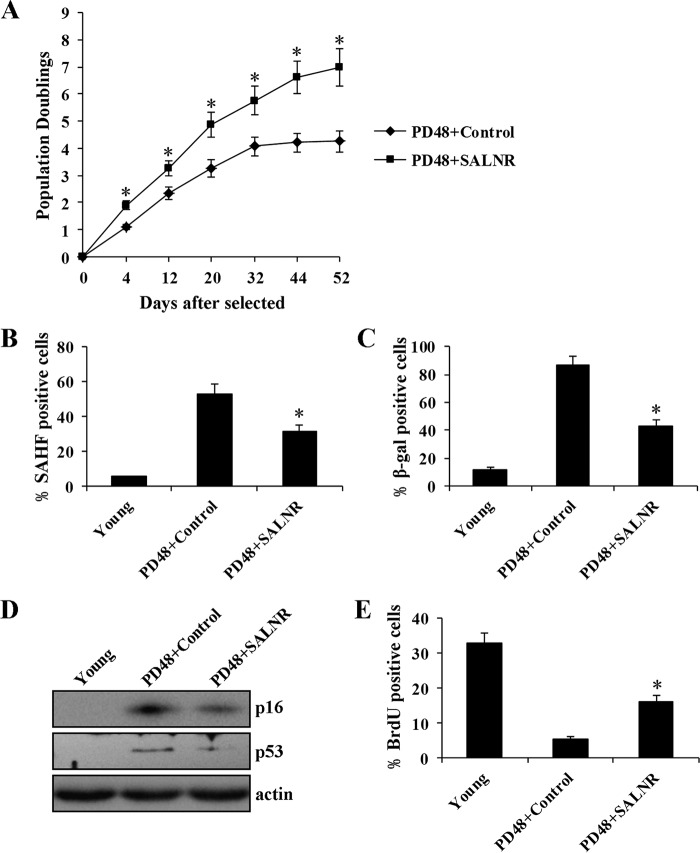
To test the significance of SALNR alterations in cellular senescence, we overexpressed SALNR in young cells, followed by RasV12 infection to induce senescence (Fig. 4A). SALNR overexpression resulted in a decrease of SAHF formation (Fig. 4B), a decreased activity of SA-β-gal (Fig. 4C), decreased expression of p16 and p53 protein (Fig. 4D), and an increase in the percentage of cells incorporating BrdU (Fig. 4E). The cell proliferation assay showed that SALNR overexpression prevented cells from entering Ras-induced senescence, and cells maintained proliferation (Fig. 4F). In addition, SALNR overexpression resulted in an increase of S compartment and a decrease in G0/G1 compartment compared with control (Fig. 4G). Furthermore, we found that SALNR overexpression extended the life span of replicative senescent cells (Fig. 5A), accompanied by decreased SAHF formation (Fig. 5B), decreased SA-β-gal activity (Fig. 5C), decreased expression of p16 and p53 protein (Fig. 5D), and an increase in the percentage of cells incorporating BrdU (Fig. 5E). These findings suggest that SALNR plays an important role in cellular senescence.
FIGURE 4.
SALNR overexpression delays oncogene-induced senescence. A, qRT-PCR detects the SALNR level in oncogenically stressed (RasV12-expressing) 2BS cells with SALNR overexpression. pZSG was used as control, and levels are normalized to GAPDH expression. Shown are SAHF formation (B), SA-β-gal activity (C), expression of p16 and p53 protein (D), and BrdU incorporation (E) assays of Ras-induced senescent 2BS cells with SALNR overexpression. F, cell proliferation assay of oncogenically stressed (RasV12-expressing) 2BS cells with SALNR overexpression. Cells were fixed and stained 0, 4, 8, and 12 days after selected. Crystal violet was extracted and quantified (bottom). G, the 2BS cell cycle was measured upon SALNR overexpression. *, p < 0.05. Error bars, S.D.
FIGURE 5.
SALNR overexpression delays replicative senescence. 48PD replicative senescent 2BS cells were infected with SALNR-pZSG or control. Growth curves (A), SAHF formation (B), activity of SA-β-gal (C), expression of p16 and p53 protein (D), and percentage of cells incorporating BrdU (E) were detected after SALNR overexpression. *, p < 0.05. Error bars, S.D.
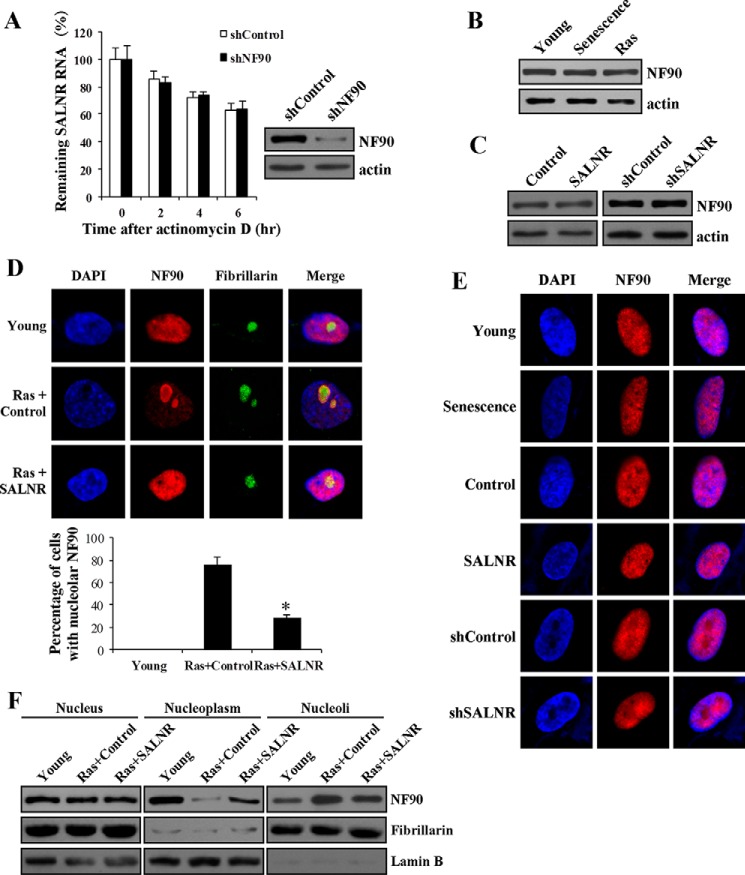
SALNR Interacts with NF90 and Regulates NF90 Nuclear Localization
To explore the molecular mechanism underlying SALNR regulation in cellular senescence, we sought to identify SALNR-associated target proteins in young cells by an RNA pull-down assay. First, we found that the normal subcellular localization of SLANR is in the nucleus and excluded from nucleoli (Fig. 6A). Then an RNA pull-down experiment revealed a direct binding of SALNR and a 90-kDa nuclear protein, which was identified as NF90 (nuclear factor of activated T-cells, 90 kDa) by mass spectrometry (Fig. 6B). The interaction between SALNR and NF90 was further confirmed by Western blotting (Fig. 6C). Afterward, we performed RIP to determine whether SALNR binds NF90 in vivo. As expected, NF90 antibody could effectively enrich SALNR RNA compared with nonspecific IgG (Fig. 6D).
FIGURE 6.
SALNR interacts with NF90. A, subcellular location of SALNR. The antisense of SALNR was used for a control. Fibrillarin is the nucleolar marker, and DAPI staining was used to indicate the cell nucleus. B, identification of SALNR-associated target proteins in young 2BS cells by RNA pull-down assay. The bands specific to sense SALNR were detected with mass spectrometry. C, Western blot analysis of the interaction of SALNR with NF90. Proteins pulled down were detected by NF90 antibody. D, RIP analysis of NF90 binding to SALNR in vivo. RNA was immunoprecipitated using anti-NF90 antibody in young 2BS cells, with IgG as a negative control, and analyzed by qRT-PCR. Error bars, S.D.
NF90 is a double-stranded RNA binding protein and plays an important role in RNA metabolism, including degradation (24, 25). Thus, we proposed two possibilities for the significance of SALNR-NF90 interaction: 1) NF90 regulates SALNR turnover, which probably leads to decreased expression of SALNR during senescence, or 2) SALNR regulates senescence by modulating NF90 function. On one hand, NF90 knockdown in young cells did not affect the half-life of SALNR (Fig. 7A), suggesting that NF90 does not affect SALNR turnover. On the other hand, SALNR did not seem to affect NF90 expression because the NF90 level was unchanged during replicative or Ras-induced senescence despite the decrease of SALNR expression (Fig. 7B). In addition, SALNR overexpression or knockdown in young cells did not affect NF90 protein level (Fig. 7C). This led us to investigate whether SALNR affects NF90 subcellular distribution. In young 2BS cells, NF90 diffusely distributed throughout the nucleus (Fig. 7D, first row). NF90 was enriched in the nucleolus in Ras-induced senescent (Fig. 7D, second row) but not replicative senescent cells (Fig. 7E, first and second rows), suggesting that NF90 cellular distribution changes upon Ras stress. Overexpression or knockdown of SALNR alone in young cells did not affect NF90 location (Fig. 7E, third to sixth rows). However, overexpressing SALNR was able to antagonize NF90 translocation into the nucleolus upon Ras stress (Fig. 7D, third row), which was validated by quantification (Fig. 7D, fourth row) and Western blots (Fig. 7F). Our data suggest that SALNR regulates NF90 translocation upon oncogenic stress.
FIGURE 7.
SALNR affects NF90 cellular distribution. A, effect of NF90 knockdown on the stability of endogenous SALNR RNA. Western blotting showed NF90 protein expression in young 2BS cells after knockdown of NF90 (right). B, NF90 expression does not change in replicative senescent and Ras-induced senescent 2BS cells. C, SALNR overexpression or knockdown in young cells does not affect NF90 protein level. The level of NF90 was determined using Western blotting. D, immunofluorescence of Ras-induced senescent 2BS cells with SALNR overexpression. Young cells were infected with SALNR-pZSG or control and then were induced to senescence by RasV12. Fibrillarin is the nucleolar marker, and DAPI staining was used to indicate the cell nucleus. The percentage of cells with nucleolar NF90 in Ras-induced senescent 2BS cells with SALNR overexpression was analyzed (fourth row). E, the localization of NF90 does not change in replicative senescent cells, SALNR-overexpressing cells, or SALNR knockdown cells. DAPI staining was used to indicate the cell nucleus. F, Western blot analysis of NF90 in Ras-induced senescent 2BS cells with SALNR overexpression. Fibrillarin is the nucleolar marker, and lamin B is the nucleoplasmic marker. *, p < 0.05. Error bars, S.D.
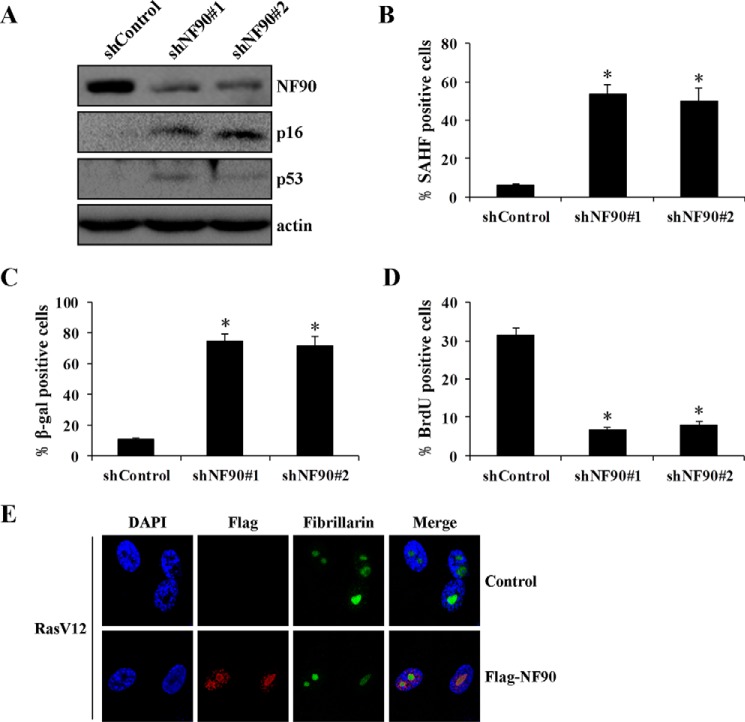
NF90 Functions as a Repressor of Senescence
NF90 has been shown to coordinately repress the senescence-associated secretory phenotype (26). Infection of NF90 shRNA in young cells decreased NF90 levels and resulted in an increase of p16 and p53 expression levels (Fig. 8A), SAHF formation (Fig. 8B), and activity of SA-β-gal (Fig. 8C) and a decrease in the percentage of cells incorporating BrdU (Fig. 8D). Similar results were achieved with two different shRNAs, suggesting that NF90 might contribute to repress or delay cellular senescence, consistent with another report with WI-38 and IDH4 fibroblasts (26). However, NF90 overexpression had little, if any, inhibitory effect on Ras-induced senescence (data not shown). This phenomenon may be due to the fact that NF90 is extinguished in nucleoli upon Ras-induced stress (Fig. 8E).
FIGURE 8.
NF90 regulates cellular senescence. A, effect of NF90 knockdown on the expression of senescence-associated protein. Young 2BS cells were infected with shNF90-pLL3.7 or shControl-pLL3.7. The level of protein was determined by Western blotting. SAHF formation (B), SA-β-gal activity (C), and BrdU incorporation (D) were detected in NF90 knockdown cells. E, ectopic expression of NF90 accumulates in nucleoli in Ras-induced senescent cells. Shown is immunofluorescence analysis of Ras-induced senescent 2BS cells with FLAG-NF90 overexpression. Fibrillarin is the nucleolar marker and DAPI staining was used to indicate the cell nucleus. *, p < 0.05. Error bars, S.D.
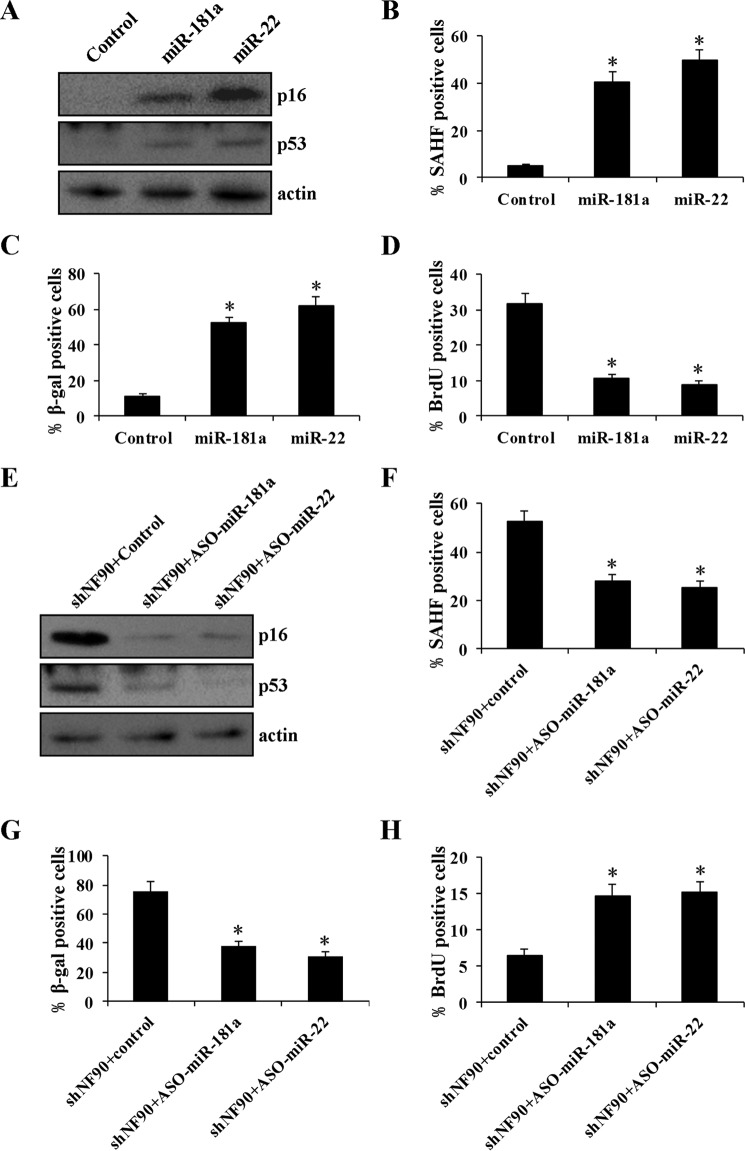
In the replicative senescence model, NF90 may repress the translation of the senescence-associated secretory phenotype factors via binding to their mRNA 3′-UTR; thus, it is presumed to suppress cellular senescence (26). However, this does not seem to be the case in the Ras-induced senescent model, because NF90 was located in the nucleus in Ras-induced senescent cells. A recent report has shown that NF90 acts as a negative regulator of miRNA biogenesis, binding pri-miRNA and blocking miRNA processing (25). We surmised that NF90 might regulate cellular senescence through miRNA pathways. We first used TaqMan human microRNA arrays to screen NF90-regulated miRNAs in 2BS fibroblasts. Silencing NF90 by shRNAs resulted in a change (≥2-fold) in the expression of 149 miRNAs (Fig. 9A and supplemental Table S1). A large number of these miRNAs (133 of 149) displayed elevated expression, consistent with the inhibitory role of NF90 in miRNA biogenesis. These up-regulated miRNAs include several previously characterized miRNAs, such as miR-181a (27) and miR-22 (28), which play a positive role in senescence induction. Using qRT-PCR, we validated our findings of up-regulated miR-181a and miR-22 upon NF90 silencing (Fig. 9B). Interestingly, despite the up-regulation of miRNA levels, their corresponding pri-miRNAs showed a decreased expression in 2BS cells with NF90 knockdown (Fig. 9C), suggesting that these miRNAs are post-transcriptionally inhibited by NF90. To further confirm whether these miRNAs were directly regulated by NF90, we performed RIP analysis using an anti-NF90 antibody. As shown in Fig. 9D, these pri-miRNAs were effectively enriched in NF90 IP, suggesting that NF90 directly regulates the expression of these miRNAs. NF90 did not bind pri-miRNA of control miR-1233 (Fig. 9D), whose expression is unchanged upon NF90 knockdown (Fig. 9, B and C), suggesting that NF90 specifically binds to pri-miR-181a and pri-miR-22. Finally, we found that miR-181a and miR-22 promote senescence in young 2BS cells (Fig. 10, A–D), and suppression of miR-181a or miR-22 attenuated shNF90-induced senescence (Fig. 10, E–H), which suggests that shNF90-induced senescence is dependent on miR-181a and miR-22. Taken together, these data indicate that NF90 delays senescence through inhibiting miRNA expression.
FIGURE 9.
NF90 inhibits miRNA biogenesis. A, analysis of differently expressed miRNAs by the TaqMan® human microRNA array after knockdown of NF90. The values of the x and y axes are negative Ct values of each group. B, qRT-PCR analysis of the expression of miR-181a, miR-22, and miR-1233 after NF90 knockdown. miR-1233 was used as a negative control, and U6 was used as an internal control. C, qRT-PCR analysis of the expression of primary transcript of miR-181a, miR-22, and miR-1233 after NF90 knockdown with GAPDH as the internal control. D, RIP analysis of NF90 binding to primary transcript of miRNA in young 2BS cells. Immunoprecipitation enrichment is determined as the amount of RNA associated with NF90 relative to IgG control. *, p < 0.05. Error bars, S.D.
FIGURE 10.
miR-181a and miR-22 promote senescence in young 2BS cells, and inhibition of miR-181a and miR-22 delays NF90 knockdown-induced senescence. Young 2BS cells were transfected with a control scrambled oligonucleotide or synthetic miR-181a or miR-22, and expression of p16 and p53 protein (A), SAHF formation (B), activity of SA-β-gal (C), and percentage of cells incorporating BrdU (D) were analyzed. Young 2BS cells infected with NF90 shRNA were transfected with control oligonucleotide or the antisense oligonucleotide miR-181a (ASO-miR-181a) or ASO-miR-22. Expression of p16 and p53 protein (E), SAHF formation (F), and SA-β-gal activity (G) and the percentage of cells incorporating BrdU (H) were detected. *, p < 0.05. Error bars, S.D.
NF90 Is Required for SALNR to Delay Cellular Senescence
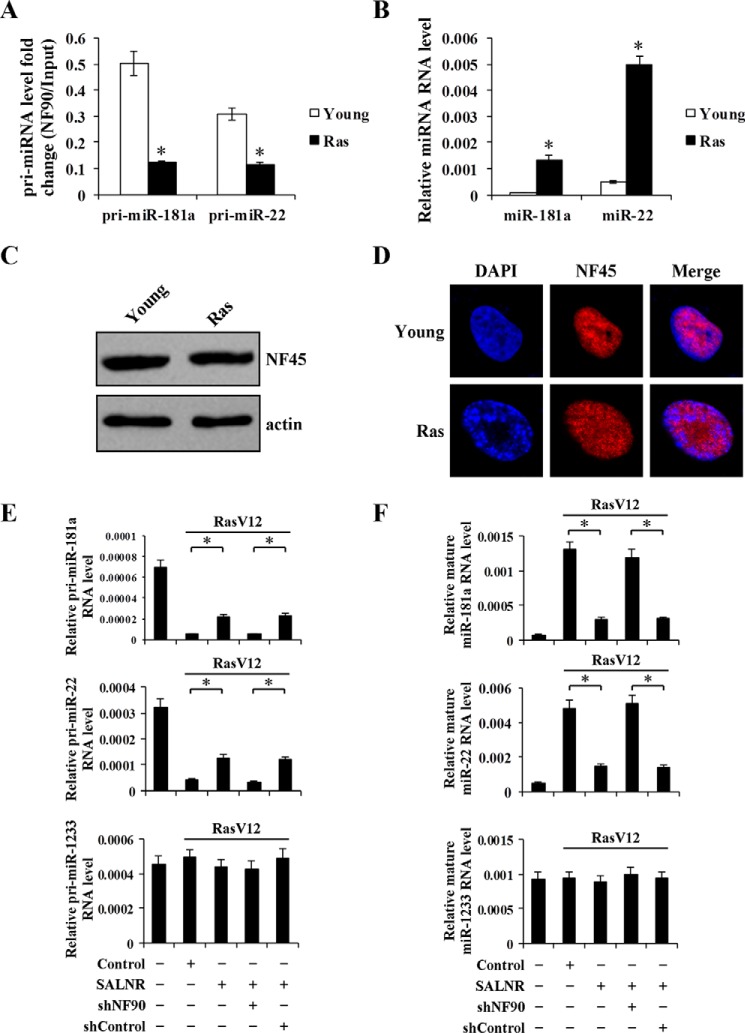
NF90 nucleoli translocation in Ras-induced senescent cells prompts us to investigate whether its localization altered its activity. Here, we chose NF90 inhibition of miRNA expression as an indicator of NF90 activity. An RIP assay showed that NF90 was not able to bind these pri-miRNAs in senescent cells compared with young cells (Fig. 11A). Consistently, NF90-targeting miRNAs displayed increased expression in Ras-induced senescent cells (Fig. 11B), suggesting loss of the inhibitory effect of NF90 on pri-miRNA processing in senescent cells. Because NF90 binds pri-miRNAs in the form of NF90-NF45 complex, in addition to NF90 itself, NF45 also affects NF90 binding of pri-miRNAs (25). To rule out the possibility that the inability of NF90 to bind pri-miRNAs results from NF45 changes, we analyzed NF45 protein expression level and cellular distribution. Senescent cells displayed protein levels and cellular distribution of NF45 similar to that in young cells (Fig. 11, C and D), suggesting that translocation of NF90 nucleoli is associated with its activity in Ras-induced senescent cells. Indeed, SALNR overexpression blocked NF90 nucleoli translocation and promoted the interaction between NF90 and its targeting pri-miRNAs, leading to decreased expression of targeting miRNAs in senescent cells (Fig. 11, E and F). The inhibitory effect of SALNR on expression of senescence-associated miRNAs is dependent on NF90 because NF90 shRNA antagonized not only NF90 interaction with these pri-miRNAs but also the suppression of two target miRNAs in senescent cells (Fig. 11, E and F). Overexpressing SALNR did not change the NF90 level, which rules out the possibility that SALNR suppression of miRNAs is due to increased expression of NF90 (Fig. 7C, left). Our findings indicate that translocation of NF90 into nucleoli is associated with NF90 activities, exemplified by loss of its suppressive effect on senescence-associated miRNAs, thus weakening its inhibitory role in senescence.
FIGURE 11.
MicroRNA processing is regulated by NF90 independent of NF45 in senescent cells. A, RIP analysis of NF90 binding to primary transcript of miRNAs in Ras-induced 2BS cells. B, qRT-PCR quantification of miR-181a and miR-22 expression in Ras-induced senescent 2BS cells with U6 as an internal control. C, Western blot analysis of expression of NF45 in Ras-induced senescent cells with β-actin as an internal control. D, cellular localization of NF45 in Ras-induced senescent cells. DAPI staining was used to indicate the cell nucleus. E, qRT-PCR analysis of the expression of primary transcript of miR-181a, miR-22, and miR-1233 with GAPDH as the internal control. F, qRT-PCR analysis of the expression of mature miR-181a, miR-22, and miR-1233 with U6 as an internal control. Young 2BS cells were infected with control, SALNR-pZSG, shControl-pLL3.7, or shNF90-pLL3.7, followed by senescence induction with activated RasV12. *, p < 0.05. Error bars, S.D.
Then we investigated whether NF90 is required for SALNR to delay cellular senescence. 2BS cells with SALNR overexpression were infected with NF90 shRNA or control lentivirus, followed by senescence induction with activated RasV12. NF90 knockdown attenuated SALNR-induced decrease of p16 and p53 expression (Fig. 12A), SAHF formation (Fig. 12B), and SA-β-gal activity (Fig. 12C) and increase of cells incorporating BrdU (Fig. 12D) and cellular proliferation (Fig. 12E). This suggests that NF90 contributes to SALNR delay of cellular senescence.
FIGURE 12.
NF90 is required for SALNR to delay cellular senescence. 2BS cells were treated as indicated, and expression of p16 and p53 protein (A), SAHF formation (B), SA-β-gal activity (C), BrdU incorporation (D), and cell proliferation (E) were analyzed. *, p < 0.05. Error bars, S.D.
Finally, we tested whether miR-181a and miR-22 are involved in SALNR delaying oncogene-induced senescence. Young 2BS cells with SALNR overexpression were transfected with a control scrambled oligonucleotide or synthetic miR-181a or miR-22, followed by infection with RasV12 to induce senescence. Overexpression of miR-181a or miR-22 attenuated SALNR-induced decrease of p16 and p53 expression (Fig. 13A), SAHF formation (Fig. 13B), and SA-β-gal activity (Fig. 13C) and increase of cells incorporating BrdU (Fig. 13D).
FIGURE 13.
miR-181a and miR-22 are involved in SALNR delaying oncogene-induced senescence. Young 2BS cells with SALNR overexpression were transfected with a control scrambled oligonucleotide or synthetic miR-181a or miR-22, followed by senescence induction with activated RasV12. Expression of p16 and p53 protein (A), SAHF formation (B), SA-β-gal activity (C), and percentage of cells incorporating BrdU (D) were detected. *, p < 0.05. Error bars, S.D.
The Expression of SALNR Decreased in Preneoplastic Lesions
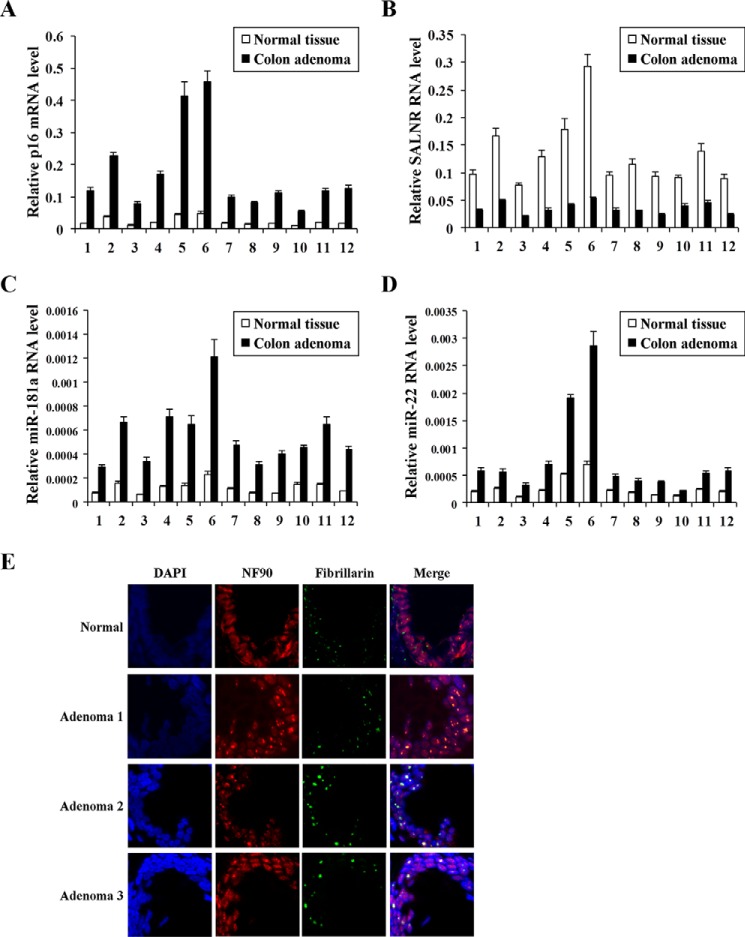
SALNR down-regulation during Ras-induced senescence prompted us to ask whether it might also be decreased in preneoplastic lesions associated with senescent cells. Colon adenomas are preneoplastic lesions associated with telomere shortening-induced replicative senescence (29) and oncogene-induced premature senescence (30). The expression of p16INK4A, an in vivo senescent marker (31), was much more abundant in colon adenomas than in normal colon tissues (Fig. 14A and Table 2), which was consistent with a previous report (32). Compared with adjacent normal tissues, SALNR level was also significantly down-regulated in colon adenomas (Fig. 14B). Consistently, the expression of miR-181a and miR-22 was elevated in colon adenoma tissues compared with normal colon tissues (Fig. 14, C and D). Moreover, we performed immunofluorescence staining of colon adenomas to identify the location of NF90. As illustrated in Fig. 14E, NF90 diffusely distributed throughout the nucleus of normal colon cells (first row) but was enriched in the nucleolus of colon adenoma cells (second to fourth rows), which is in accordance with the location of NF90 in Ras-induced senescent cells. These findings show that the signature of SALNR expression occurs not only in vitro but also in vivo.
FIGURE 14.
The expression of p16INK4A, SALNR, miR-181a, and miR-22 and location of NF90 in colon adenomas. qRT-PCR analysis of p16INK4A (A), SALNR (B), miR-181a (C), and miR-22 (D) expression in colon adenomas and adjacent normal colon tissues. Levels are normalized to GAPDH (p16INK4A and SALNR) or U6 (miR-181a and miR-22) expression and represented as means ± S.D. (error bars). E, immunofluorescence localization of NF90 in colon adenomas. Fibrillarin is the nucleolar marker, and DAPI staining was used to indicate the cell nucleus.
TABLE 2.
Information on 12 colon adenoma samples
| Case number | Histopathological diagnosis | KRAS | BRAF |
|---|---|---|---|
| 1 | Tubular adenoma | MUT 12 (GGT to GAT) | WT |
| 2 | Villous adenoma | MUT 12 (GGT to GTT) | WT |
| 3 | Tubular adenoma | WT | WT |
| 4 | Tubulovillous adenoma | WT | WT |
| 5 | Tubulovillous adenoma | MUT 12 (GGT to GAT) | WT |
| 6 | Tubular adenoma | WT | WT |
| 7 | Tubular adenoma | WT | WT |
| 8 | Tubulovillous adenoma | MUT 13 (GGC to GAC) | WT |
| 9 | Tubular adenoma | WT | WT |
| 10 | Villous adenoma | MUT 12 (GGT to GAT) | WT |
| 11 | Tubulovillous adenoma | MUT 12 (GGT to GTT) | WT |
| 12 | Tubulovillous adenoma | WT | WT |
| 13 | Tubulovillous adenoma | NAa | NA |
| 14 | Tubular adenoma | NA | NA |
| 15 | Tubular adenoma | NA | NA |
a NA, not applicable.
Discussion
lncRNAs play crucial roles in various biological processes, but little is known about the significance of lncRNAs in cellular senescence. A large number of lncRNAs by RNA sequencing differently expressed during replicative senescence (8), suggesting that lncRNAs are involved in cell senescence. In this study, we also found that a set of lncRNAs displayed different expression during Ras-induced premature senescence by lncRNA microarray and identified a 3.8-kb lncRNA, SALNR, regulating cellular senescence. We revealed that SALNR regulation of senescence is achieved by its binding and blocking of NF90 nucleolus translocation. Also, NF90 knockdown may antagonize SALNR delay of cellular senescence.
Here we provide evidence to show that NF90 acts as a repressor of cellular senescence. Although the mechanisms underlying NF90 repression of senescence are unclear, a recent report (26) indicated that NF90 may interact with 3′-UTR of numerous senescence-associated secretory phenotype mRNAs, including two important senescent regulators, IL6 and IL8, and inhibit their expression, thus contributing to suppression of senescence. Here, we showed that NF90 suppresses senescence by inhibiting expression of senescence-associated miRNAs, such as miR-22 and -181a. In the Ras-induced senescent model, NF90 is sequestered into nucleoli, which leads to the loss of its inhibitory activity on miRNA biogenesis. It also contributes to increased expression of target miRNAs and induction of cellular senescence. SALNR antagonized NF90 translocation into nucleoli and rescued its inhibitory activity on senescence-associated miRNA expression. Thus, SALNR delays cellular senescence at least partly via suppressing NF90-mediated miRNA expression. This also reveals a cross-talk between lncRNAs and miRNAs.
NF90 is predominantly expressed in the nucleus and can regulate transcription in mammalian cells (33). However, it has been shown that some stimuli are able to cause its translocation to the cytoplasm, where NF90 controls mRNA turnover or translation via binding mRNA 3′UTR (24, 34). In this study, we found that NF90 nucleoli translocation takes place upon exposure of normal fibroblasts to Ras stress. On one hand, nucleoli translocation leads to loss of inhibitory activity of NF90 on miRNA biogenesis or likely NF90 transcription regulation function. On the other hand, as an RNA-binding protein, NF90 translocates in nucleoli binding pre-rRNAs and inhibiting rRNA biogenesis, thus slowing protein synthesis in senescent cells. SALNR regulates cellular senescence, probably by multiple NF90-dependent pathways. Interestingly, SALNR knockdown alone might not be sufficient to induce nucleolar translocation of NF90 (Fig. 7E, fifth and sixth rows), and NF90 was not enriched in the nucleolus in replicative senescent cells (Fig. 7E, first and second rows), suggesting the presence of an additional mechanism in favor of NF90 nucleolar localization in the absence of SALNR, such as NF90 modification or interaction with nucleolar protein.
It appears that ectopic SALNR may substantially extend the replicative life span (Fig. 5A), whereas NF90 is not enriched in nucleoli during replicative senescence (Fig. 7E, first and second rows), which suggests that SALNR extends the replicative life span through an NF90-independent pathway. Replicative senescence was first attributed to telomere attrition caused by the successive DNA replications because overexpression of human telomerase reverse transcriptase corrects telomere erosion and could delay replicative senescence (35). When telomeres shorten below a certain threshold, they are recognized as double strand breaks of DNA and trigger the DNA damage response, which ultimately activates the mechanisms of cellular arrest (36). Intriguingly, oncogenes also induce telomeric lesions and stochastic telomere attrition in primary fibroblasts (37). However, oncogene-induced senescence was initially reported to be independent of telomere length and telomerase activity (38). In human cells, aberrant oncogenic signaling initially causes cells to hyperproliferate, which leads to abnormal high DNA replication rates, leading to frequent DNA replication fork stalling events, including improperly or prematurely terminated replication forks. As a result, single strand DNA breaks and double strand breaks are generated in the vicinity of collapsed replication forks and activate a robust DNA damage response and induce cell senescence (39, 40).
Oncogene caused a high degree of non-telomeric and telomeric DNA damage response foci (37), but replicative senescence may be triggered only by telomeric DNA damage response. Therefore, we think that SALNR may participate in telomere maintenance in replicative and oncogene-induced senescence independent of NF90 and participate in non-telomeric replication fork repair in oncogene-induced senescence dependent of NF90. NF90 is also involved in DNA break repair, which supports this hypothesis (41). Moreover, a recent study compared gene expression levels between replicative and oncogene-induced senescence (42). In this study, gene set enrichment analysis showed that the gene ontology sets most enriched in replicative senescence included “Extracellular structure organization & biogenesis,” “Synapse organization & biogenesis,” “Female pregnancy,” “Synaptogenesis,” and “Hematopoietin interferon class D2000 cytokine receptor activity,” but none is scored highly in oncogene-induced senescence. lncRNA SALNR may also participate in these processes to delay senescence. The precise mechanism of SALNR in regulating replicative senescence will need to be explored later.
Author Contributions
Z.-B. M., B. J., C.-L. W., H. C., and Y. W. were responsible for design of the work. B. J., Y. W., C.-L. W., H. C., B.-S. X., and Z.-B. M. were responsible for acquisition, analysis, or interpretation of data. Z.-B. M., C.-L. W., and Y. W. drafted the paper.
Supplementary Material
Acknowledgments
We thank Drs. Lixiang Xue and Xiaowei Zhang (Peking University Health Science Center) for kindly providing viral vector pWZL-Hygro-Ras (H-RasV12) and pZsG-Puro, respectively.
This work was supported by National Basic Research Programs of China Grants 2013CB530801 and 2011CB966203 and Natural Science Foundation of China Grant 81471406. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Table S1.
- SAHF
- senescence-associated heterochromatic foci
- lncRNA
- long non-coding RNA
- SALNR
- senescence-associated lncRNA
- NF90
- nuclear factor of activated T-cells, 90 kDa
- qRT-PCR
- quantitative reverse transcriptase PCR
- SA-β-gal
- senescence-associated β-galactosidase
- RIP
- RNA immunoprecipitation
- RACE
- rapid amplification of cDNA ends
- miRNA
- microRNA
- pri-miRNA
- microRNA primary transcript
- IF
- immunofluorescence.
References
- 1.Hayflick L. (1965) The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 [DOI] [PubMed] [Google Scholar]
- 2.Kuilman T., Michaloglou C., Mooi W. J., and Peeper D. S. (2010) The essence of senescence. Genes Dev. 24, 2463–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sikora E., Arendt T., Bennett M., and Narita M. (2011) Impact of cellular senescence signature on ageing research. Ageing Res. Rev. 10, 146–152 [DOI] [PubMed] [Google Scholar]
- 4.Lanigan F., Geraghty J. G., and Bracken A. P. (2011) Transcriptional regulation of cellular senescence. Oncogene 30, 2901–2911 [DOI] [PubMed] [Google Scholar]
- 5.Pont A. R., Sadri N., Hsiao S. J., Smith S., and Schneider R. J. (2012) mRNA decay factor AUF1 maintains normal aging, telomere maintenance, and suppression of senescence by activation of telomerase transcription. Mol. Cell 47, 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W., Yang X., Cristofalo V. J., Holbrook N. J., and Gorospe M. (2001) Loss of HuR is linked to reduced expression of proliferative genes during replicative senescence. Mol. Cell. Biol. 21, 5889–5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F. J., Wen T., and Liu L. (2012) MicroRNAs as a novel cellular senescence regulator. Ageing Res. Rev. 11, 41–50 [DOI] [PubMed] [Google Scholar]
- 8.Abdelmohsen K., Panda A., Kang M. J., Xu J., Selimyan R., Yoon J. H., Martindale J. L., De S., Wood W. H. 3rd, Becker K. G., and Gorospe M. (2013) Senescence-associated lncRNAs: senescence-associated long noncoding RNAs. Aging Cell 12, 890–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J. T. (2012) Epigenetic regulation by long noncoding RNAs. Science 338, 1435–1439 [DOI] [PubMed] [Google Scholar]
- 10.Gong C., and Maquat L. E. (2011) lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature 470, 284–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon J. H., Abdelmohsen K., and Gorospe M. (2013) Posttranscriptional gene regulation by long noncoding RNA. J. Mol. Biol. 425, 3723–3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., Tramontano A., and Bozzoni I. (2011) A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147, 358–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wapinski O., and Chang H. Y. (2011) Long noncoding RNAs and human disease. Trends Cell Biol. 21, 354–361 [DOI] [PubMed] [Google Scholar]
- 14.Harries L. W. (2012) Long non-coding RNAs and human disease. Biochem. Soc. Trans. 40, 902–906 [DOI] [PubMed] [Google Scholar]
- 15.Yan B., and Wang Z. (2012) Long noncoding RNA: its physiological and pathological roles. DNA Cell Biol. 31, S34–S41 [DOI] [PubMed] [Google Scholar]
- 16.Tang Z., Zhang Z., Zheng Y., Corbley M. J., and Tong T. (1994) Cell aging of human diploid fibroblasts is associated with changes in responsiveness to epidermal growth factor and changes in HER-2 expression. Mech. Ageing Dev. 73, 57–67 [DOI] [PubMed] [Google Scholar]
- 17.Sui W., Yan Q., Li H., Liu J., Chen J., Li L., and Dai Y. (2013) Genome-wide analysis of long noncoding RNA expression in peripheral blood mononuclear cells of uremia patients. J. Nephrol. 26, 731–738 [DOI] [PubMed] [Google Scholar]
- 18.Yu J., Li A., Hong S. M., Hruban R. H., and Goggins M. (2012) MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin. Cancer Res. 18, 981–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fickett J. W. (1982) Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 10, 5303–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotoglou P., Kalaitzakis A., Vezyraki P., Tzavaras T., Michalis L. K., Dantzer F., Jung J. U., and Angelidis C. (2009) Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress Chaperones 14, 391–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuo de X., Niu X. H., Chen Y. C., Xin D. Q., Guo Y. L., and Mao Z. B. (2010) Vitamin D3 up-regulated protein 1(VDUP1) is regulated by FOXO3A and miR-17–5p at the transcriptional and post-transcriptional levels, respectively, in senescent fibroblasts. J. Biol. Chem. 285, 31491–31501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias A. P., Dufu K., Lei H., and Reed R. (2010) A role for TREX components in the release of spliced mRNA from nuclear speckle domains. Nat. Commun. 1, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai M. C., Manor O., Wan Y., Mosammaparast N., Wang J. K., Lan F., Shi Y., Segal E., and Chang H. Y. (2010) Long noncoding RNA as modular scaffold of histone modification complexes. Science 329, 689–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwano Y., Kim H. H., Abdelmohsen K., Pullmann R. Jr., Martindale J. L., Yang X., and Gorospe M. (2008) MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol. Cell. Biol. 28, 4562–4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto S., Aoki K., Higuchi T., Todaka H., Morisawa K., Tamaki N., Hatano E., Fukushima A., Taniguchi T., and Agata Y. (2009) The NF90-NF45 complex functions as a negative regulator in the microRNA processing pathway. Mol. Cell. Biol. 29, 3754–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tominaga-Yamanaka K., Abdelmohsen K., Martindale J. L., Yang X., Taub D. D., and Gorospe M. (2012) NF90 coordinately represses the senescence-associated secretory phenotype. Aging 4, 695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancini M., Saintigny G., Mahé C., Annicchiarico-Petruzzelli M., Melino G., and Candi E. (2012) MicroRNA-152 and -181a participate in human dermal fibroblasts senescence acting on cell adhesion and remodeling of the extra-cellular matrix. Aging 4, 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu D., Takeshita F., Hino Y., Fukunaga S., Kudo Y., Tamaki A., Matsunaga J., Takahashi R. U., Takata T., Shimamoto A., Ochiya T., and Tahara H. (2011) miR-22 represses cancer progression by inducing cellular senescence. J. Cell Biol. 193, 409–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Sullivan J., Risques R. A., Mandelson M. T., Chen L., Brentnall T. A., Bronner M. P., Macmillan M. P., Feng Z., Siebert J. R., Potter J. D., and Rabinovitch P. S. (2006) Telomere length in the colon declines with age: a relation to colorectal cancer? Cancer Epidemiol. Biomarkers Prev. 15, 573–577 [DOI] [PubMed] [Google Scholar]
- 30.Kuilman T., Michaloglou C., Vredeveld L. C., Douma S., van Doorn R., Desmet C. J., Aarden L. A., Mooi W. J., and Peeper D. S. (2008) Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 [DOI] [PubMed] [Google Scholar]
- 31.Krishnamurthy J., Torrice C., Ramsey M. R., Kovalev G. I., Al-Regaiey K., Su L., and Sharpless N. E. (2004) Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai C. Y., Furth E. E., Mick R., Koh J., Takayama T., Niitsu Y., and Enders G. H. (2000) p16(INK4a) expression begins early in human colon neoplasia and correlates inversely with markers of cell proliferation. Gastroenterology 119, 929–942 [DOI] [PubMed] [Google Scholar]
- 33.Kiesler P., Haynes P. A., Shi L., Kao P. N., Wysocki V. H., and Vercelli D. (2010) NF45 and NF90 regulate HS4-dependent interleukin-13 transcription in T cells. J. Biol. Chem. 285, 8256–8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shim J., Lim H., R Yates J., and Karin M. (2002) Nuclear export of NF90 is required for interleukin-2 mRNA stabilization. Mol. Cell 10, 1331–1344 [DOI] [PubMed] [Google Scholar]
- 35.Counter C. M., Meyerson M., Eaton E. N., Ellisen L. W., Caddle S. D., Haber D. A., and Weinberg R. A. (1998) Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene 16, 1217–1222 [DOI] [PubMed] [Google Scholar]
- 36.d'Adda di Fagagna F., Reaper P. M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N. P., and Jackson S. P. (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 [DOI] [PubMed] [Google Scholar]
- 37.Suram A., Kaplunov J., Patel P. L., Ruan H., Cerutti A., Boccardi V., Fumagalli M., Di Micco R., Mirani N., Gurung R. L., Hande M. P., d'Adda di Fagagna F., and Herbig U. (2012) Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J. 31, 2839–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei S., Wei W., and Sedivy J. M. (1999) Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 59, 1539–1543 [PubMed] [Google Scholar]
- 39.Courtois-Cox S., Jones S. L., and Cichowski K. (2008) Many roads lead to oncogene-induced senescence. Oncogene 27, 2801–2809 [DOI] [PubMed] [Google Scholar]
- 40.Di Micco R., Fumagalli M., Cicalese A., Piccinin S., Gasparini P., Luise C., Schurra C., Garre' M., Nuciforo P. G., Bensimon A., Maestro R., Pelicci P. G., and d'Adda di Fagagna F. (2006) Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638–642 [DOI] [PubMed] [Google Scholar]
- 41.Shamanna R. A., Hoque M., Lewis-Antes A., Azzam E. I., Lagunoff D., Pe'ery T., and Mathews M. B. (2011) The NF90/NF45 complex participates in DNA break repair via nonhomologous end joining. Mol. Cell. Biol. 31, 4832–4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson D. M., McBryan T., Jeyapalan J. C., Sedivy J. M., and Adams P. D. (2014) A comparison of oncogene-induced senescence and replicative senescence: implications for tumor suppression and aging. Age 36, 9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.