Abstract
Leishmania secretes a large number of its effectors to the extracellular milieu. However, regulation of the secretory pathway in Leishmania is not well characterized. Here, we report the cloning, expression, and characterization of the Rab1 homologue from Leishmania. We have found that LdRab1 localizes in Golgi in Leishmania. To understand the role of LdRab1 in the secretory pathway of Leishmania, we have generated transgenic parasites overexpressing GFP-LdRab1:WT, GFP-LdRab1:Q67L (a GTPase-deficient dominant positive mutant of Rab1), and GFP-LdRab1:S22N (a GDP-locked dominant negative mutant of Rab1). Surprisingly, our results have shown that overexpression of GFP-LdRab1:Q67L or GFP-LdRab1:S22N does not disrupt the trafficking and localization of hemoglobin receptor in Leishmania. To determine whether the Rab1-dependent secretory pathway is conserved in parasites, we have analyzed the role of LdRab1 in the secretion of secretory acid phosphatase and Ldgp63 in Leishmania. Our results have shown that overexpression of GFP-LdRab1:Q67L or GFP-LdRab1:S22N significantly inhibits the secretion of secretory acid phosphatase by Leishmania. We have also found that overexpression of GFP-LdRab1:Q67L or GFP-LdRab1:S22N retains RFP-Ldgp63 in Golgi and blocks the secretion of Ldgp63, whereas the trafficking of RFP-Ldgp63 in GFP-LdRab1:WT-expressing cells is unaltered in comparison with control cells. Taken together, our results have shown that the Rab1-regulated secretory pathway is well conserved, and hemoglobin receptor trafficking follows an Rab1-independent secretory pathway in Leishmania.
Keywords: exocytosis, Leishmania, protein secretion, Rab, trafficking, hemoglobin receptor, Rab1, secretory pathway
Introduction
Small GTPases of the Rab family are master regulators of intracellular trafficking (1). These proteins are localized on specific compartments and regulate the transport through fusion between two compartments in a nucleotide-dependent process (2, 3). About 70 Rab proteins are identified in mammalian cells. In the endocytic pathway, Rab5 is present in the sorting endosome, whereas Rab4 and Rab11 are localized in recycling endosomes (4, 5). Rab7, Rab9, and Rab24 are associated with the late endosomal compartment. Rab7 mediates transport from the late endosomes to lysosomes, whereas Rab9 regulates the trafficking of lysosomal enzymes from the trans-Golgi network to lysosomes (6–9). In the secretory pathway, Rab1 regulates the anterograde transport from the endoplasmic reticulum (ER)3 to the Golgi (10), whereas retrograde transport from Golgi to ER is mediated by Rab2 (11). Rab6 localizes in the Golgi and is involved in intra-Golgi trafficking (12). Moreover, Rab18, Rab30, and Rab43 are found to be important for maintaining Golgi structure (13, 14). In addition, transport of cargo from the Golgi to the cell surface is regulated by different Rabs. For example, Rab3 plays a role in release of neurotransmitter, Rab27 regulates the trafficking of lytic granules and melanosomes toward the plasma membrane, and Rab8 is involved in regulating the traffic from the trans-Golgi network to the plasma membrane (15). However, the secretory pathway is not well characterized in unicellular pathogenic parasites.
Leishmania donovani, a pathogenic protozoa, causes visceral leishmaniasis, a fatal human disease that affects annually about 12 million people worldwide (16, 17). Drugs used for chemotherapy of leishmaniasis are toxic, and no licensed vaccine is available (18, 19). Incidentally, Leishmania lacks a complete heme biosynthetic pathway (20); therefore, a heme acquisition process from extracellular milieu is essential for the parasites (21). Previously, we have shown that Leishmania endocytosed hemoglobin (Hb) through a specific receptor located in the flagellar pocket (22–24). Bound Hb is rapidly internalized and degraded in the lysosomes via Rab5-dependent (25) and Rab7-dependent (26) processes. Finally, internalized Hb is degraded in the lysosomes to generate intracellular heme, and this process of Hb endocytosis is essential for the parasite (27). However, how newly synthesized hemoglobin receptor (HbR) is transported to the cell surface is not known.
In addition to HbR, Leishmania also secretes a large number of its effectors to the extracellular milieu (28, 29); however, regulation of the secretory pathway in Leishmania is not well characterized. Now there is convincing evidence that intracellular trafficking pathways, especially in trypanosomatid parasites, are regulated by various Rab GTPases (30). It has been shown that TbRab1 and TbRab2 are localized in the ER-Golgi complex in Trypanosoma brucei, and RNAi-mediated knockdown of these proteins partially inhibits the transport of variant surface glycoprotein to the cell surface (31), indicating that the Rab1-mediated secretory pathway is quite conserved in this group of parasites. Interestingly, it has been shown that export of newly synthesized proteins to the surface via secretory pathway is not actin-dependent, whereas trafficking in endocytic pathways is actin-dependent in Trypanosoma brucei (32). Although the secretory/endocytic pathway is not well characterized in Entamoeba histolytica, it has been shown that fibronectin receptor and Gal/GalNAc lectin trafficking to plasma membrane is brefeldin-A-dependent, whereas thiolproteinase secretion is brefeldin-A-independent in this parasite (33). Further characterization reveals that overexpression of a GTP-locked mutant of EhRabA alters ER morphology and mislocalizes Gal/GalNAc lectin without affecting the localization of other cell surface proteins, indicating the specific role of EhRabA in early secretory pathway (34). Bioinformatic analyses have shown the presence of a Rab1 homologue in Giardia lamblia (35), Plasmodium falciparum (36), and Leishmania major (37); however, the functional role of Rab1 in the secretory pathway of these parasites is not yet elucidated.
Here, we report the cloning, expression, and characterization of a Rab1 homologue from L. donovani. We have shown that overexpression of a dominant negative mutant of LdRab1 in Leishmania blocks the trafficking of glycosylphosphatidylinositol-anchored 63-kDa surface glycoprotein (gp63) and secretory acid phosphatase (SAP), whereas trafficking of HbR to the cell surface is unaffected, indicating that gp63 and SAP follow a Rab1-dependent conventional secretory pathway, whereas HbR trafficking to cell surface is a Rab1-independent process.
Experimental Procedures
Materials
Unless otherwise stated, all reagents were obtained from Sigma. M199 medium and gentamicin were purchased from Gibco. Luria-Bertani (LB) broth and LB-agar were supplied by Difco. Fetal calf serum (FCS) was procured from Biological Industries (Beit-Haemek, Israel). Platinum High Fidelity Taq polymerase and restriction enzymes were purchased from Invitrogen and Promega (Madison, WI), respectively. pGEX-4T-2 expression vector, Glutathione-Sepharose 4B beads, protein markers (RPN756 and RPN800), and ECL reagents were obtained from Amersham Biosciences. Alexa Fluor 594 succinimidyl ester, FM4-64, and LysoTracker Red were obtained from Molecular Probes, Inc. (Eugene, OR). The Leishmania expression vectors, pXG-GFP2+ and pNUS-mRFP-nD, were kindly provided by Dr. S. M. Beverley (Washington University, St. Louis, MO) and Dr. Jean-Paul di Rago (Institut de Biochimie et Génétique Cellulaires, Bordeaux, France), respectively. Geneticin and blasticidin were procured from Gibco and Calbiochem, respectively. [α-32P]GTP (800 Ci/mmol) was procured from PerkinElmer Life Sciences. All other reagents used were of analytical grade.
Cells
L. donovani (UR6) promastigotes were obtained from the Indian Institute of Chemical Biology (Kolkata, India). Cells were routinely maintained on blood agar slants containing glucose, peptone, sodium chloride, beef heart extract, rabbit blood, and gentamycin, as described previously (11). For experiments, cells were cultured in medium M199 (pH 7.4) supplemented with 10% FCS, 100 units/ml penicillin, 100 μg/ml streptomycin at 23 °C, and log phase cells were harvested in phosphate-buffered (10 mm, pH 7.2) saline (0.15 m).
Cloning and Expression of Rab1 from Leishmania (LdRab1)
To clone the Rab1 homologue from Leishmania, a putative Rab1-like sequence was identified from the L. major genome database having substantial homology with T. brucei Rab1 by BLAST analysis. Accordingly, appropriate forward (5′-GTGGATCCATGACCGCTGAGTACGACTACC-3′) and reverse primers (5′-GTGAATTCTCAGCAGCTGTCTTCCTTC-3′) were designed against start and stop codons of putative L. major Rab1 sequence with BamHI and EcoRI restriction sites (underlined), respectively. The ORF of the putative Rab1 sequence was amplified from L. donovani cDNA using these primers by RT-PCR. Briefly, mRNA isolated from Leishmania promastigotes using an Oligotex mRNA kit (Qiagen) was used for cDNA synthesis using a Thermo Script RT-PCR kit (Gibco) as per the manufacturer's instructions. Subsequently, PCR was performed using the above primers in a PerkinElmer Life Sciences thermocycler for 30 cycles under the following conditions: denaturation for 1 min at 94 °C followed by annealing at 60 °C for 30 s and extension at 68 °C for 1 min. A 603-bp fragment amplified by PCR was cloned into pGEM-T-easy vector and sequenced by using m13 universal primers in an automated sequencer. Finally, the PCR product was cloned into BamHI/EcoRI sites of pGEX-4T-2 expression vector and transformed into the XL-1 Blue strain of Escherichia coli.
Generation of Constitutively Active and Dominant Negative Mutants of LdRab1
To investigate the functions of Rab1 in Leishmania more precisely, two mutants (viz. LdRab1:Q67L and LdRab1:S22N) were generated by PCR-mediated site-directed mutagenesis as described previously (26). All mutants were generated using LdRab1:WT as a template. For the generation of LdRab1:Q67L mutant, a primer was designed having the Gln residue at position 67 changed to Leu (CAG codon was changed to CTG). In the first round of PCR, a megaprimer was amplified using the reverse mutant primer (5′-TGGCGGCCGGACCTCGCGAAG-3′) and the forward WT primer using LdRab1:WT as a template. PCR was carried out for 30 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s, and extension at 68 °C for 1 min. The amplified megaprimer (220 bp) was gel-purified using a Qiagen gel purification kit. Subsequently, a second PCR was set up to amplify the full-length mutant product (603 bp) with the reverse WT primer and megaprimer as a forward primer. The PCR was set up as follows: five cycles of denaturation at 94 °C for 1 min and extension at 68 °C for 1 min to allow synthesis of the megaprimer strand. While at 68 °C, 25 pmol/μl of the Rab1 forward primer was added, and PCR was resumed for 25 cycles of denaturation at 94 °C for 1 min, annealing at 70 °C for 30 s, and extension at 68 °C for 1 min. LdRab1:S22N was generated using the same procedure as described for LdRab1:Q67L except that the mutant primer (5′-CAGCCATTCTTGACGGACGAG-3′) used in the first PCR was designed such that the Ser residue at position 22 was changed to Asn (ACG codon was changed to AAT). The full-length PCR products (603 bp) were subsequently cloned into pGEM-T Easy and sequenced to confirm the respective mutations. Finally, mutants were subcloned into pGEX-4T-2 vector and transformed into E. coli.
Expression and Purification of LdRab1:WT and Mutant Proteins
To purify recombinant proteins, E. coli (BL21 strain) was transformed with respective constructs. Cells were induced with 0.2 mm isopropyl 1-thio-β-d-galactopyranoside for 3 h at 30 °C, and the respective GST fusion proteins were affinity-purified as per the manufacturer's instructions (Amersham Biosciences) using glutathione-Sepharose 4B. Briefly, bacterial cells were harvested by centrifugation and resuspended in PBS, pH 7.2, containing lysozyme (1 mg/ml) for 30 min at 4 °C. Subsequently, cell lysate were treated with DTT (1 mg/ml) in PBS, and unbroken cells were lysed by sonication at 4 °C. Finally, proteins were extracted with Triton X-100 (1%), and cell debris was separated by centrifugation at 18,500 × g for 10 min at 4 °C. Lysates were incubated with glutathione-Sepharose beads for 1 h at 4 °C. Following extensive washes with PBS, recombinant proteins were eluted from the beads in 50 mm Tris-HCl containing 30 mm glutathione, pH 9.0, and dialyzed against PBS. Purity of the proteins was checked by SDS-PAGE.
GTP Overlay Assay
GTP binding activity of purified LdRab1:WT and its mutants was detected by a GTP overlay assay (25). Briefly, 2 μg of GST-LdRab1:WT or its mutants was blotted onto nitrocellulose membrane, and membrane was incubated with 1 μCi/ml [α-32P]GTP in 50 mm phosphate buffer, pH 7.5, containing 5 mm MgCl2, 1 mm EGTA, and 0.3% Tween 20 for 3 h at 24 °C. Finally, the unbound radioactivity was removed by extensive washing and visualized by autoradiography.
GTPase Assay
The GTPase activities of LdRab1:WT and its mutant GTP were determined as described previously (25). Briefly, 5 μg of immobilized protein on glutathione beads was incubated with buffer A (20 mm Tris-HCl, pH 7.8, 100 mm NaCl, 5 mm MgCl2, 1 mm NaH2PO4, and 10 mm β-mercaptoethanol) for 20 min at 25 °C, and bound nucleotide was eluted with 1 m guanidine HCl. Immobilized nucleotide-free protein was then loaded with 2 pmol of [α-32P]GTP (800 Ci/mmol) in 20 μl of buffer A for 10 min at 0 °C. Subsequently, beads were washed and incubated for 1 h at 23 °C to allow the hydrolysis of bound GTP. Subsequently, the beads were washed, incubated in 8 μl of buffer B (0.2% SDS, 2 mm EDTA, 10 mm GDP, 10 mm GTP, pH 7.5), and heated at 70 °C for 2 min to elute the nucleotide from the protein. An aliquot was analyzed using thin-layer chromatography and visualized by autoradiography.
Generation of Antibodies against LdRab1
To generate antibody against LdRab1, immobilized GST-LdRab1 on beads was incubated with 10 units of thrombin (Pharmacia Corp.) in cleavage buffer (50 mm Tris-HCl, pH 7.5, containing 150 mm NaCl and 2.5 mm CaCl2) for 3 h at room temperature. Beads were removed by centrifugation, and the purity of the LdRab1 was checked by SDS-PAGE. Mice were immunized with LdRab1 to raise polyclonal antibodies by a standard method. The specificity of the antibody was determined by Western blot analysis using purified LdRab1, LdRab5, and LdRab7.
Overexpression of LdRab1 and Its Mutants in Leishmania
To overexpress LdRab1 and its mutants in Leishmania as a GFP fusion protein having GFP tag in the N terminus, respective clones were subcloned into the NotI/BamHI sites of the pXG-GFP2+ vector (38). Subsequently, Leishmania promastigotes were transfected with LdRab1:WT or its mutant constructs using the standard electroporation protocol (26). Briefly, cells were grown to the late log phase (1 × 107 cells/ml) at 23 °C in M199 medium supplemented with 10% FCS. Cells were harvested by centrifugation and resuspended at a density of 2 × 108 cells/ml in cytomix buffer (120 mm KCl, 0.15 mm CaCl2, 10 mm K2HPO4, 25 mm HEPES, 2 mm EDTA, and 5 mm MgCl2, pH 7.6). Cells (0.5 ml) were transferred to a precooled electroporation cuvette, and the appropriate plasmid DNA (40 μg) was added. Electroporation was carried out in a GenePulser (Bio-Rad) at 25-microfarad capacitance and 1500 V to facilitate DNA uptake by cells. Cells were then incubated on ice for 10 min and transferred into antibiotic-free M199 medium for 24 h at 23 °C. Subsequently, positive clones were selected in the presence of G418 antibiotic (30 μg/ml). Overexpression of respective protein was confirmed by Western blotting using anti-GFP and anti-Rab1 antibodies and confocal microscopy.
Localization of LdRab1 in Leishmania
To determine the localization of endogenous Rab1 in Leishmania, cells were harvested, washed, and fixed with 4% paraformaldehyde. Subsequently, cells were permeabilized with 0.4% saponin for 20 min and blocked with 10% FCS in PBS for 30 min. Finally, cells were probed with anti-LdRab1 antibody (1:200) in PBS containing 0.1% saponin and 10% FCS for 1 h at 23 °C. Cells were washed three times with PBS and incubated with Alexa Fluor 594-labeled goat anti-mouse secondary antibody in the same buffer for 1 h at 23 °C. Cells were washed and viewed in an LSM 510 Meta confocal microscope.
To identify the LdRab1-positive compartment, GFP-LdRab1:WT-expressing Leishmania were labeled for different compartment-specific markers. The early endosomes were labeled with anti-LdRab5 antibody and subsequently probed with goat anti-mouse secondary antibody labeled with Alexa Fluor 594. Similarly, a 5-min internalization of Alexa Fluor 594-labeled hemoglobin (Alexa594-Hb) was used to mark the early endocytic compartment. The lysosome-like compartments in Leishmania were labeled with 100 nm LysoTracker Red DND-99. Golgi complex was visualized by overexpressing LPG2-HA in Leishmania followed by immunostaining with Alexa546-labeled rabbit anti-HA antibody (1:500). The nucleus and kinetoplast of Leishmania were visualized by staining with Hoechst.
Estimation of Acid Phosphatase Secreted by Leishmania
Leishmania promastigotes secrete an acid phosphatase (SAP) into the culture medium (39). Therefore, to functionally characterize the role of LdRab1 in the secretory pathway of Leishmania, we determined the release of SAP in the spent medium by L. donovani promastigotes overexpressing LdRab1:WT or its mutant proteins. The enzymatic activity of SAP released in the spent medium was measured using the method described earlier (40). Briefly, 1 × 106 cells of each cell type were inoculated in 1 ml of sterile M199 medium supplemented with 10% fetal calf serum in a 24-well plate. Cells were incubated for different periods of time, after which cells were separated, and both medium and cell lysates were assayed for the enzyme activity by a standard assay. To measure the amount of SAP present in the culture supernatant, culture supernatant (138 μl) was incubated with 62 μl of 50 mm p-nitrophenyl phosphate as substrate in 50 mm Tris, pH 7.0, containing 0.1% (v/v) β-mercaptoethanol for 30 min at 37 °C. The enzyme released p-nitrophenol from p-nitrophenyl phosphate, which was converted to p-nitrophenolate by the addition of 0.25 m NaOH. The absorbance of p-nitrophenolate was measured at 410 nm. The amount of p-nitrophenolate ion released was calculated from a millimolar extinction coefficient of 17.8. Similarly, SAP activity was measured from cell lysates. Results were expressed as μm p-nitrophenol released in the spent medium/cell lysate by respective Leishmania.
Role of Rab1 in gp63 and HbR Trafficking in Leishmania
Release of gp63 of Leishmania is a spontaneous event (41). To determine the role of LdRab1 and its mutants in the trafficking of gp63, Leishmania promastigotes overexpressing GFP-LdRab1:WT, GFP-LdRab1:S22N, or GFP-LdRab1:Q67L were transfected with RFP-Ldgp63 using the same protocol as described previously (26). Transfected cells were then incubated on ice for 10 min and transferred into G418 (30 μg/ml) containing M199 medium for 24 h at 23 °C. Subsequently, positive clones expressing both proteins were selected in the presence of G418 (30 μg/ml) and blasticidin (15 μg/ml). Co-expression of GFP-LdRab1 and its mutants with RFP-Ldgp63 was examined by confocal microscopy.
Similarly, GFP-LdRab1:WT, GFP-LdRab1:S22N, or GFP-LdRab1:Q67L and LdHbR-RFP were co-expressed in Leishmania to determine the role of Rab1 in HbR trafficking in Leishmania. Endogenous localization of HbR in the flagellar pocket of GFP-LdRab1:WT-, GFP-LdRab1:S22N-, or GFP-LdRab1:Q67L-overexpressing cells was determined by anti-HbR-N antibody, which specifically recognized the extracellular N-terminal domain of HbR in unpermeabilized cells. Cells were visualized with goat anti-mouse Alexa546-labeled antibody using a confocal microscope.
Detection of Secreted gp63 in Spent Medium
To determine the amount of gp63 secreted by LdRab1 or its mutants overexpressing Leishmania, 1 × 107 parasites were grown in 1 ml of FCS-free M199 medium for 24 h at 23 °C. Subsequently, cells were pelleted by centrifugation (1500 × g for 10 min at 4 °C), and supernatant from the respective cultures was collected. The supernatants were further clarified by centrifugation at 18,000 × g for 1 h. Secreted proteins in the resultant supernatant were precipitated by the addition of 4 ml of chilled acetone and kept at −20 °C for 16 h. Protein precipitate was collected by centrifugation (18,000 × g for 30 min), and the presence of the respective proteins secreted by the indicated cells was analyzed by Western blot using specific antibody. The respective cell pellets were also analyzed by Western blot using specific antibody.
Electron Microscopy
To determine the ultrastructural changes in the morphology of the Golgi and secretory vesicles in GFP-LdRab1:S22N- or GFP-LdRab1:Q67L-overexpressing Leishmania in comparison with untransfected control parasites, the respective cells were harvested from freshly grown culture, washed three times with cold PBS, and fixed in modified Karnovsky's fixative, pH 7.3 (24). Fixed cells were washed twice with cacodylate buffer and treated with 1% OsO4 in cacodylate buffer for 2 h at 4 °C. Subsequently, the cells were rinsed and dehydrated in acetone and embedded in epoxy resin. Ultrathin sections were double-stained with uranyl acetate and lead citrate and examined on a JEOL JEM2100 transmission electron microscope.
To visualize the distribution of the Golgi marker protein, LPG2, cells co-expressing LdRab1 or its mutant and LPG2-HA were washed twice and fixed in 1% glutaraldehyde and 1% paraformaldehyde in PBS, pH 7.2, for 20 min at 4 °C. Cells were washed, dehydrated in ethanol, and embedded in LR White resin. Ultrathin sections of the LR White-embedded cells were blocked with 3% casein in 0.001% Tween 20 in PBS for 1 h at 37 °C. Sections were washed five times with PBS-Tween 20 and incubated with rabbit anti-HA antibody (1:200) for 15 min at 37 °C. Sections were washed five times in a similar manner, and they were incubated with protein A conjugated with 10-nm colloidal gold for 20 min at 37 °C to allow the detection of primary antibody binding sites. Finally, the cells were stained with uranyl acetate and viewed in a JEOL JEM2100 transmission electron microscope.
Results
Cloning and Expression of Rab1 Homolog from Leishmania
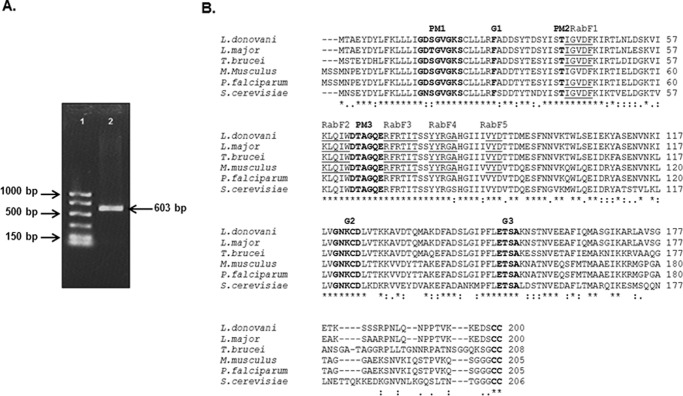
In order to clone a Rab1 homologue from L. donovani, a BLAST search was performed using the mouse Rab1 sequence as a query, which identified a putative Rab1-like sequence from the L. major genome showing 73% homology to mouse Rab1 sequence. Using appropriate forward and reverse primers, we amplified a 603-bp fragment from L. donovani cDNA by PCR (Fig. 1A). The PCR product was cloned, sequenced, and hypothetically translated into a 200-amino acid sequence. The sequence analysis of the cloned protein showed (Fig. 1B) the presence of five distinct Rab family motifs (RabF1–RabF5) as well as highly conserved guanine nucleotide binding regions, the effector loop, and the C-terminal isoprenylation motif (15). ClustalW multiple-sequence alignment of the cloned protein (LdRab1) revealed that cloned protein from L. donovani has 97% similarity with L. major YPT, 81% similarity with T. brucei Rab1, 64% similarity with S. cerevisiae Rab1, and 73% similarity with P. falciparum and mouse Rab1.
FIGURE 1.
Cloning of LdRab1 from L. donovani. A, a 603-bp fragment (lane 2) was amplified from L. donovani cDNA by PCR using appropriate forward and reverse primers as described under “Experimental Procedures.” Lane 1, low range DNA ladder. The sequence of LdRab1 has been submitted to the GenBankTM database under accession number KT003639. B, hypothetical translation of the obtained sequence into protein revealed that LdRab1 shows significant similarity with Rab1 from L. major, T. brucei, Mus musculus, P. falciparum, and S. cerevisiae. Residues implicated in guanine phosphate binding (PM1–PM3), GTP/GDP binding (G1–G3), and the isoprenylation motif (I) are marked in boldface type. Rab family-specific motifs are underlined.
Generation of Anti-LdRab1 Antibody to Determine the Endogenous Localization of Rab1 in Leishmania
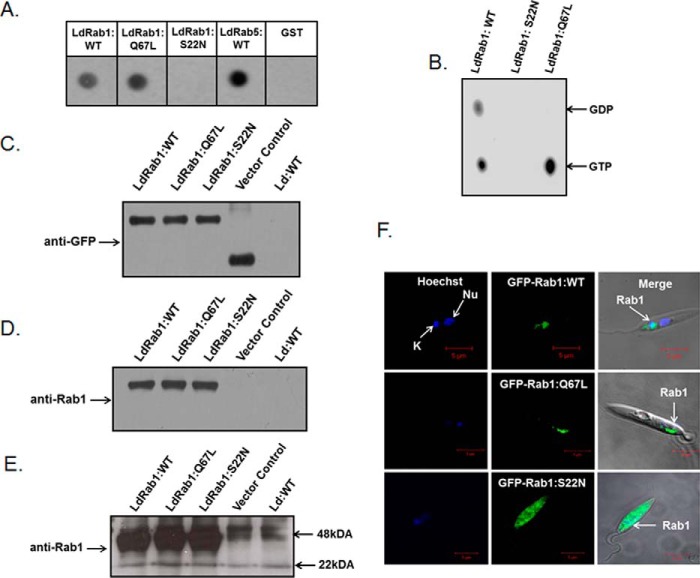
The pGEX-4T-2:LdRab1 plasmid was transformed into the BL-21 strain of E. coli for expression and purification of the GST-LdRab1 fusion protein. The cells were grown and induced with isopropyl 1-thio-β-d-galactopyranoside. Finally, GST-LdRab1 fusion protein was purified from the cell lysate using glutathione-Sepharose 4B. The results presented in Fig. 2A showed the purification of the expected size (48 kDa) of GST-LdRab1 to homogeneity. Subsequently, GST was removed from the purified protein by thrombin cleavage, and mice were immunized with LdRab1 to raise antibody. The specificity of anti-LdRab1 antibody was checked by Western blotting using purified GST-LdRab1, GST-LdRab5, and GST-LdRab7. Our results also showed that anti-LdRab1 antibody specifically recognizes GST-LdRab1 but does not cross-react with GST-LdRab5, GST-LdRab7, or free GST (Fig. 2B). This antibody was used to determine the endogenous localization of Rab1 in Leishmania, and our results showed that LdRab1 was localized into a discrete compartment near the apical region of the parasites (Fig. 2C).
FIGURE 2.
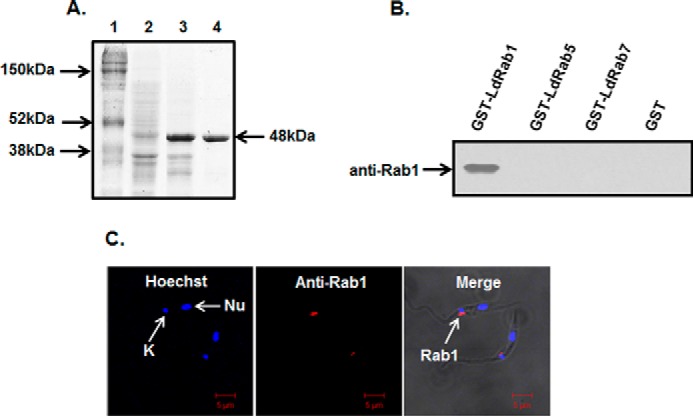
Purification and characterization of LdRab1. A, purification of GST-LdRab1. GST-LdRab1 was purified as described under “Experimental Procedures” and analyzed by SDS-PAGE. Lane 1, protein marker; lane 2, uninduced lysate of E. coli; lane 3, induced lysate of E. coli; lane 4, purified GST-LdRab1. B, specificity of LdRab1 antibody. To prepare anti-LdRab1 antibody, GST tag was removed by thrombin cleavage, and mice were immunized with LdRab1 as described under “Experimental Procedures.” The specificity of anti-LdRab1 antibody was checked by Western blotting using purified GST-LdRab1, GST-LdRab5, and GST-LdRab7. Results are representative of three independent preparations. C, to determine the endogenous localization of LdRab1 in Leishmania, cells were fixed and permeabilized with saponin as described under “Experimental Procedures.” Permeabilized cells were probed with LdRab1, washed, and visualized using Alexa Fluor 594-labeled goat anti-mouse secondary antibody. Red, localization of LdRab1; blue, nucleus (Nu). Cells were viewed in an LSM 510 Meta confocal microscope. Results are representative of three independent preparations.
Characterization of LdRab1 from Leishmania
To functionally characterize the role of LdRab1 in intracellular trafficking in Leishmania, two mutants were generated by site-directed mutagenesis based on the previous knowledge of analogous mutations in mammalian Rabs (42). The LdRab1:Q67L mutant was generated by substituting leucine residue for glutamine in the WDTAGQE, whereas asparagine was substituted for serine in the GK(T/S) region to generate the LdRab1:S22N mutant. Subsequently, GTP binding ability and GTPase activity of LdRab1:WT and its mutants were checked using LdRab5:WT as a control. Our results showed that both LdRab1:WT and LdRab1:Q67L bind with a comparable amount of [α-32P]GTP as observed with LdRab5:WT, whereas almost no binding of [α-32P]GTP was observed with LdRab1:S22N (Fig. 3A). This finding demonstrated that LdRab1:S22N mutant has reduced affinity to GTP. Analysis of GTPase activity revealed that LdRab1:WT hydrolyzes GTP to GDP, indicating that it is a functional GTPase. In contrast, GTP hydrolysis was found to be completely blocked in the LdRab1:Q67L mutant, indicating that this protein is a GTP-locked constitutively active form of LdRab1 (Fig. 3B).
FIGURE 3.
Characterization of LdRab1 and its mutants. A, GTP binding of purified LdRab1:WT and its mutants was detected using an [α-32P]GTP overlay assay. LdRab5:WT and GST proteins were used as control. B, GTPase activity of LdRab1 and its mutants was determined as described under “Experimental Procedures.” C, to determine the levels of overexpression of LdRab1:WT and its mutants as GFP fusion proteins in Leishmania, cell lysates were analyzed by Western blotting using anti-GFP antibody. Untransfected Leishmania was used as control. D, to determine the levels of overexpression of LdRab1:WT and its mutants as GFP fusion proteins in Leishmania, cell lysates were analyzed by Western blotting using anti-LdRab1 antibody. Untransfected Leishmania was used as control. E, the same membrane was exposed for a longer duration to detect endogenous Rab1. F, to determine the localization of Rab1:WT and its mutants in Leishmania, cells were transfected with indicated constructs to overexpress the respective protein in Leishmania as GFP fusion protein. Cells were visualized in a LSM 510 Meta confocal microscope. Green, localization of the indicated LdRab1; blue, nucleus (Nu). Results are representative of three independent preparations.
Subsequently, LdRab1 and its mutants were overexpressed in Leishmania as GFP fusion proteins using pXG-GFP+2 Leishmania expression vector to place GFP in the N terminus of the LdRab1 or its mutants so that the C terminus of LdRab1 remains free for prenylation and membrane attachment. Stable clones were selected in the presence of G418 antibiotic, and the overexpression of respective LdRab1s as GFP fusion proteins in Leishmania was confirmed by Western blot analysis of the cell lysates using anti-GFP antibody, anti-Rab1 antibody, and confocal microscopy. Western blot analysis with anti-GFP antibody showed comparable expression of GFP-Rab1:WT, GFP-Rab1:Q67L, and GFP-LdRab1:S22N protein in Leishmania (Fig. 3C). Similar results were obtained with anti-Rab1 antibody (Fig. 3D). However, anti-Rab1 antibody failed to detect endogenous Rab1 in Leishmania by Western blot analysis under these conditions. However, higher exposure of the same membrane detected the endogenous LdRab1 protein (Fig. 3E). Moreover, GFP-LdRab1:WT protein was found to be localized in the apical region of the cells like endogenous Rab1 in Leishmania (Fig. 3F), indicating that overexpression of LdRab1 as a GFP fusion protein does not alter its normal localization. In addition, our results also showed that GFP-LdRab1:Q67L is localized in the similar compartment like LdRab1:WT. However, GFP-LdRab1:S22N, the dominant negative mutant of LdRab1, failed to localize into a discrete intracellular compartment and dispersed throughout the cytoplasm (Fig. 3F).
Subcellular Localization of LdRab1 in Leishmania
To identify the LdRab1-positive compartment in Leishmania, cells overexpressing GFP-LdRab1:WT protein were stained with different compartment-specific markers. No co-localization of GFP-LdRab1 was observed with 5-min internalization of Alexa594-conjugated Hb, which labeled the early endosomal compartments in Leishmania. Similarly, LdRab5-positive early endosomal compartments were clearly separated from the GFP-LdRab1:WT-labeled structure. In addition, our results showed that GFP-LdRab1:WT is not localized with a LysoTracker Red-positive lysosome like the compartment in Leishmania. However, GFP-LdRab1 was found to be co-localized with LPG2-HA, a Golgi marker for Leishmania (Fig. 4), indicating that LdRab1 was localized in Golgi in Leishmania.
FIGURE 4.
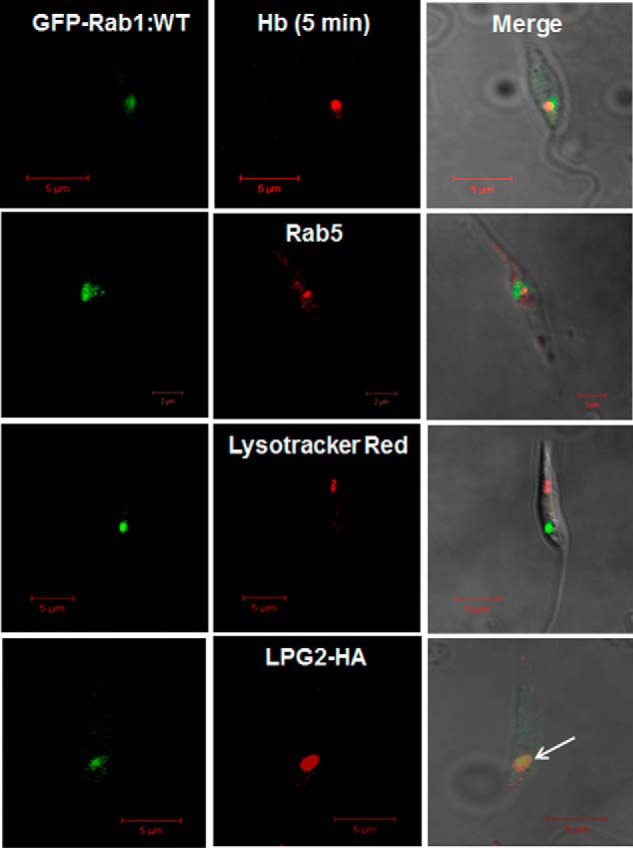
Identification of Rab1-positive compartment in Leishmania. To identify the LdRab1-positive compartment in Leishmania, Leishmania overexpressing GFP-LdRab1 was stained with various compartment-specific markers, such as 5-min internalized Alexa Fluor 594 Hb and anti-LdRab5 for early endosome, LysoTracker Red (100 nm) for lysosome, and LPG2-HA for Golgi, as described under “Experimental Procedures.” Finally, cells were visualized under a confocal microscope. Yellow (arrow), colocalization of LdRab1 with LPG2-HA-labeled Golgi in one plane after z-stack analysis by confocal microscopy. Results are representative of three independent observations.
Effect of LdRab1 Mutant Overexpression on the Morphology of Golgi in Leishmania
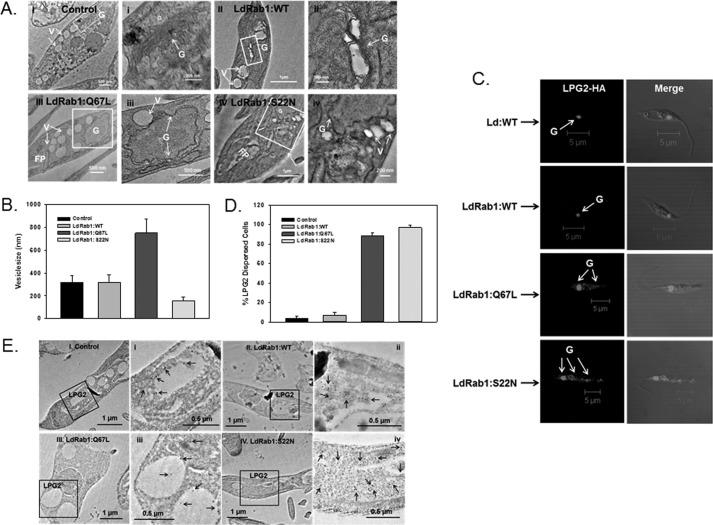
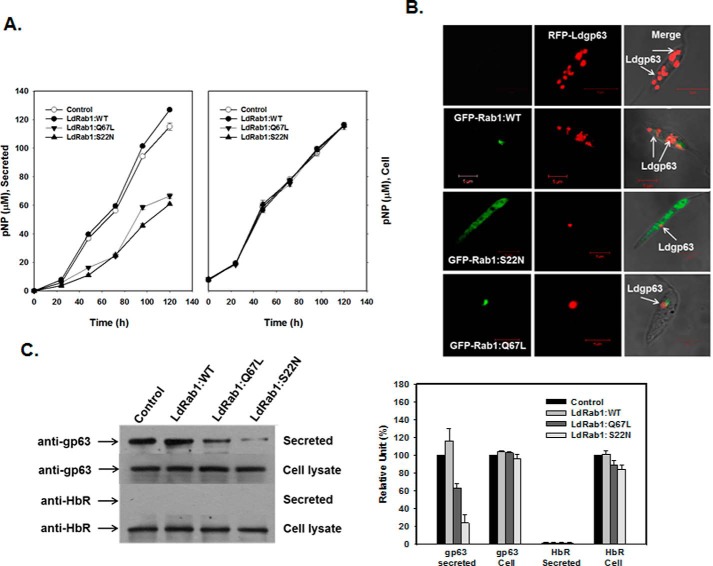
To determine whether the overexpression of LdRab1:WT, LdRab1:Q67L, or Rab1:S22N mutants altered the morphology of the Golgi and transport vesicles, morphological analysis was carried out from ultrathin sections of Leishmania promastigotes expressing respective Rab1 and its mutant proteins by electron microscopy. Our results showed that >80% of control untransfected Leishmania had well conserved Golgi morphology like mammalian cells (Fig. 5A, I). Similarly, Rab1:WT-overexpressing cells showed Golgi stacks together as in control cells; however, it appeared to be slightly inflated between the stacks in about 60% of cells (Fig. 5A, II). In contrast, we found very much dilated Golgi stacks along with fragmented Golgi in 90% of LdRab1:Q67L-overexpressing cells (Fig. 5A, III), whereas almost all Rab1:S22N-overexpressing Leishmania showed disintegrated Golgi stacks scattered in the cell cytoplasm (Fig. 5A, IV). Further quantitative analysis (Fig. 5B) of several electron micrographs (n = 50) revealed the presence of relatively larger transport vesicles (749 ± 121 nm) in close contact with Golgi in LdRab1:Q67L-overexpressing Leishmania in comparison with control (310 ± 55 nm) and LdRab1-overexpressing (318 ± 67 nm) cells. Conversely, clusters of relatively small vesicles (157 ± 32 nm) were observed in the close vicinity of disintegrated Golgi ribbon in Rab1:S22N-overexpressing Leishmania.
FIGURE 5.
Morphology of Golgi in Leishmania expressing GFP-LdRab1 or its mutants. A, transmission electron micrographs showing the ultrastructural morphology of Golgi. I, untransfected control cells; II, GFP-LdRab1:WT-overexpressing cells; III, GFP-LdRab1:Q67L-overexpressing cells; IV, Rab1:S22N-overexpressing cells. i, ii, iii, and iv, high magnification images of the Golgi region of the indicated cells. Images are representative of several independent observations. G, Golgi; FP, flagellar pocket; V, vesicles. B, quantitative analysis of several electron micrographs (n = 50) showing the size of the transport vesicles in the indicated cell types. Results are expressed as mean ± S.D. (error bars). C, distribution of the Golgi marker protein, LPG2, in Leishmania expressing LdRab1 and its mutants. LdRab1 and its mutant overexpressed cells were co-expressed with LPG2-HA as described under “Experimental Procedures.” Cells were stained with anti-HA antibody followed by secondary antibody labeling with Alexa546 and analyzed by confocal microscopy. Results are representative of three independent preparations. D, quantitative analysis of the percentage of cells showing dispersed distribution of LPG2 in the indicated cell types. Results are expressed as mean ± S.D. E, distribution of the Golgi marker protein, LPG2, in cells co-expressing LdRab1 or its mutant by immunoelectron microscopy.
To visualize the distribution of the Golgi marker protein, LPG2, in LdRab1 mutant-overexpressing cells, LdRab1 and its mutant-overexpressing cells were transfected with LPG2-HA construct to co-express LPG2-HA protein. Cells were stained with anti-HA antibody followed by secondary antibody labeled with Alexa546 and analyzed by confocal microscopy (Fig. 5C). Consistent with electron micrographs, our results showed the normal distribution of LPG2 predominantly into discrete Golgi compartment in more than 90% of control and LdRab1:WT-overexpressing Leishmania, whereas LPG2 was found to be redistributed in vesicular structures scattered in the cytoplasm in more than 90% of LdRab1:Q67L- and Rab1:S22N-overexpressing Leishmania (Fig. 5D), indicating the disassembly of Golgi stacks into dispersed vesicular structure. Similarly, immunoelectron microscopy results showed scattered distribution of LPG2 in the cell cytoplasm in LdRab1:Q67L and LdRab1:S22N cells, whereas LPG2 was found to be predominantly localized in a discrete Golgi region in control and LdRab1:WT-expressing cells (Fig. 5E).
Role of LdRab1 in HbR Trafficking in Leishmania
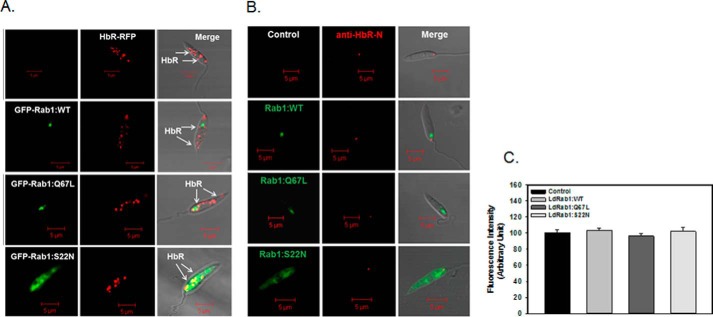
To determine whether the trafficking of newly synthesized HbR to its final destination follows the LdRab1-dependent conventional secretory pathway, LdHbR-RFP was co-expressed with GFP-LdRab1:WT, GFP-LdRab1:S22N, or GFP-LdRab1:Q67L in Leishmania. The results presented in Fig. 6A show that LdHbR-RFP is localized in the apical region of the parasite along with its localization in the discrete glycosomal compartment because HbR is a hexokinase. No alteration in the distribution of LdHbR-RFP was observed in parasites overexpressing GFP-LdRab1:WT, GFP-LdRab1:S22N, or GFP-LdRab1:Q67L, indicating that HbR trafficking was independent of the LdRab1-mediated conventional secretory pathway in Leishmania (Fig. 6A). In order to unequivocally prove that HbR trafficking to the flagellar pocket is independent of Rab1 function, GFP-LdRab1:WT-, GFP-LdRab1:S22N-, or GFP-LdRab1:Q67L-overexpressing cells were immunostained with anti-HbR N-terminal specific antibody without permeabilization because this antibody was shown to recognize the extracellular domain of HbR. We found that overexpression of GFP-LdRab1:WT, GFP-LdRab1:S22N, or GFP-LdRab1:Q67L did not affect the targeting of HbR to the flagellar pocket (Fig. 6B). Mean fluorescence intensity of HbR localized in the flagellar pocket appeared to be similar in all cell types (Fig. 6C). Moreover, we found that overexpression of GFP-LdRab1:WT, GFP-LdRab1:S22N, or GFP-LdRab1:Q67L did not interfere with the kinetics of Alexa Fluor 594-labeled Hb endocytosis and trafficking in these cells (data not shown). Taken together, these results indicate that overexpression of LdRab1 mutants does not alter the distribution of HbR on the cell surface as well as Hb trafficking.
FIGURE 6.
Determination of role of LdRab1 in HbR trafficking Leishmania. A, to determine the role of LdRab1 in the trafficking of HbR, Leishmania promastigotes overexpressing GFP-LdRab1:WT, GFP-LdRab1:S22N, or GFP-LdRab1:Q67L were transfected with HbR-RFP as described under “Experimental Procedures.” Cells were examined by confocal microscopy. Green, GFP-LdRab1; red, HbR-RFP. Results are representative of three independent experiments. B, to determine the endogenous HbR present in the flagellar pocket in respective Leishmania, GFP-LdRab1:WT-, GFP-LdRab1:S22N-, or GFP-LdRab1:Q67L-overexpressing cells were stained with anti-HBR-N antibody as described under “Experimental Procedures.” Cells were examined by confocal microscopy. Green, GFP-LdRab1; red, HbR. Results are representative of three independent experiments. C, quantitative analysis of mean fluorescence intensity of HbR in the indicated cell types. Results are expressed as mean ± S.D. (error bars).
Role of LdRab1 in Conventional Secretory Pathway in Leishmania
SAP of Leishmania could be an excellent marker for monitoring protein export in this parasite because this enzyme is constitutively secreted by Leishmania in the culture medium (39, 40). Therefore, we measured the secretion of secreted acid phosphatase by Leishmania promastigotes expressing LdRab1 or its mutant to determine the role of Rab1 in the conventional secretory pathway of Leishmania. The results presented in Fig. 7A (right) show that Leishmania promastigotes secreted SAP into culture media in a time-dependent way and that overexpression of LdRab1:WT in the parasites did not significantly alter the secretion of SAP. In contrast, about 50% inhibition of SAP activity in the spent culture was detected in the cells overexpressing LdRab1:S22N or LdRab1:Q67L compared with control parasites, indicating that both GTP binding and GTPase activity of LdRab1 are required for the secretion of SAP (Fig. 7A). However, intracellular SAP activities in all cell types were found to be similar, indicating that overexpression of LdRab1 or its mutants did not block the expression of SAP (Fig. 7A, left). Interestingly, no change in secretion of SAP was detected in the cells treated with brefeldin A (10 μg/ml) in comparison with control cells (data not shown).
FIGURE 7.
Determination of role of LdRab1 in conventional secretory pathway in Leishmania. A, to determine the role of LdRab1 in the secretion of SAP by Leishmania, the cell association or release of SAP in the spent media by L. donovani promastigotes overexpressing LdRab1:WT or its mutant proteins was determined as described under “Experimental Procedures.” The amount of p-nitrophenolate ion released or cell-associated by respective Leishmania was calculated, and results were expressed as μm p-nitrophenol ± S.D. (error bars) by respective Leishmania from three independent experiments. B, to determine the role of LdRab1 in the trafficking of gp63, Leishmania promastigotes overexpressing GFP-LdRab1:WT, GFP-LdRab1:S22N, or GFP-LdRab1:Q67L were transfected with RFP-Ldgp63 as described under “Experimental Procedures.” Cells were examined by confocal microscopy. Green, GFP-LdRab1; red, RFP-Ldgp63. Results are representative of three independent experiments. C, to determine the role of LdRab1 in the secretion of endogenous gp63 and HbR by Leishmania, the levels of gp63 or HbR associated with cells and secreted in the spent medium by respective L. donovani promastigotes were determined by Western blot analysis using specific antibodies as described under “Experimental Procedures.” Results are representative of three independent experiments.
GP63 is the major cell surface-associated glycosylphosphatidylinositol-anchored protein in Leishmania that is spontaneously secreted (41). Therefore, to determine the role of LdRab1 in the secretory pathway, we compared the trafficking of gp63 in LdRab1 and its mutant-overexpressing Leishmania. Accordingly, RFP-Ldgp63 was co-expressed with GFP-LdRab1:WT or its mutants in Leishmania. Our results showed (Fig. 7B) that RFP-Ldgp63 was localized into discrete punctate structures, probably the secretory vesicles in Leishmania when it was overexpressed alone. Overexpression of GFP-LdRab1:WT along with RFP-Ldgp63 did not alter the distribution of RFP-Ldgp63 in the parasites, whereas overexpression of GFP-LdRab1:S22N or GFP-LdRab1:Q67L completely blocked the trafficking of RFP-Ldgp63 in Leishmania (Fig. 7B), and all RFP-Ldgp63 was found to be retained in the LdRab1-positive Golgi compartment. Subsequently, we tried to detect the amount of endogenous gp63 released into Leishmania culture medium by LdRab1 and its mutant-overexpressing cells after 24 h of incubation at 23 °C. We found that LdRab1:WT-overexpressing parasites secreted an approximately 20% higher amount of gp63 than did untransfected control cells (Fig. 7C). In contrast, overexpression of GFP-LdRab1:Q67L or GFP-LdRab1:S22N inhibited secretion of gp63 by about 45 or 85%, respectively, in comparison with untransfected control cells (Fig. 7C), whereas no secreted HbR was detected in control as well as LdRab1:WT- and mutant-overexpressing cells. Intracellular content of gp63 and HbR was found to be unaltered in all cell types (Fig. 7C).
Discussion
About 70 Rab GTPases are reported in mammalian cells, whereas only 29 are present in C. elegans, 26 in Drosophila melanogaster, 16 in T. brucei, and 11 in Saccharomyces cerevisiae (43). Interestingly, six Rab proteins (Rab1, Rab2, Rab5, Rab7, Rab9, and Rab11) are found to be conserved across all species, indicating that these Rabs are possibly involved in the maintenance of basic functions in eukaryotic cell (44). Because Rab1 plays a key role in regulating the transport of newly synthesized proteins from the ER-Golgi network to the cell surface in mammalian cells (45), it will be interesting to determine the role of the Rab1 homologue in regulating the secretory pathway in Leishmania. Previously, we have characterized the role of Rab5 and Rab7 in the endocytic pathway in Leishmania (22–27). These results have shown that several components of intracellular trafficking machinery are well conserved in Leishmania. However, the role of Rab in the regulation of secretory pathway in Leishmania is not well characterized. Because this parasite provides a unique opportunity to determine the intricacies of membrane trafficking events in a whole organism, we have cloned and expressed Rab1 homologue from Leishmania to determine the role of Rab1 in the secretory pathway in this parasite. Using appropriate forward and reverse primers, we have amplified a 603-bp fragment from L. donovani cDNA by PCR, which codes for a ∼21-kDa protein. Sequence analysis of the cloned protein reveals the presence of distinct Rab family motifs as well as highly conserved guanine nucleotide binding regions, effector loop, and C-terminal isoprenylation motif. Moreover, cloned protein has a high degree of homology with Rab1 sequences reported from different organisms, indicating that the cloned protein is a Rab1 homologue from Leishmania (LdRab1).
It is well demonstrated that appropriate mutations within highly conserved regions of the Rab protein make the protein either a constitutively active GTP-bound form or a dominant negative GDP-bound conformation. Such mutant Rabs interfere with membrane traffic and are very useful to determine the function of Rabs in regulating intracellular trafficking. Similar mutants are also used to determine the role of Rab1 in regulating the early stages of the secretory pathway in mammalian cells (46, 47). Accordingly, we have generated the LdRab1:Q67L mutant, and our results have shown that LdRab1:Q67L binds GTP but is unable to hydrolyze GTP. We have also made the LdRab1:S22N mutant, and this mutant shows reduced affinity to GTP. This is consistent with the previous finding that Ser to Asn substitution reduces the affinity of Rab1 for GTP without altering its affinity to GDP (48). Therefore, this S22N mutant is restricted to a GDP-bound conformation. To characterize the role of LdRab1 in the secretory pathway of Leishmania, we have overexpressed LdRab1 and its mutant proteins in Leishmania as GFP fusion proteins. GFP-LdRab1:WT predominantly localizes in the LPG2-HA-labeled Golgi compartment in Leishmania. As expected, LdRab1:Q67L is also localized to the Golgi membranes like LdRab1:WT protein, whereas GDP-locked dominant negative LdRab1:S22N is distributed throughout the cytosol. In addition, we have observed similar localization of endogenous Rab1 in Leishmania using LdRab1-specific antibody, indicating that overexpression does not alter the localization of Rab1 in Leishmania. Our results are also consistent with previous reports that Rab1 is localized in the ER-Golgi network in mammalian cells (47) as well as in T. brucei (31). Interestingly, morphological analyses have shown that overexpression of LdRab1:Q67L or Rab1:S22N mutants disintegrate Golgi stacks in parasites. Consequently, we have found that LPG2, a Golgi marker in Leishmania, is redistributed in vesicular structures scattered in the cytoplasm. These results are consistent with previous findings in mammalian cells that microinjection of Rabl mutants causes vesiculation of the Golgi apparatus, and thereby Golgi enzymes are redistributed into small vesicles in the cell cytoplasm (49). Thus, our results have indicated that Rab1 function is required for the maintenance and assembly of Golgi complex in Leishmania. This is supported by the facts that Golgi integrity is coupled to Rab1 function in mammalian cells (48, 50) and that RNAi-mediated knockdown of Rab1 in T. brucei altered the morphology of Golgi (31). In addition, we have observed very large transport vesicles in close contact with Golgi ribbon in LdRab1:Q67L-overexpressing Leishmania possibly due the homotypic fusion of transport vesicles originating from Golgi, whereas clusters of relatively small vesicles in the close vicinity of disintegrated Golgi are detected in Rab1:S22N-overexpressing Leishmania. These results indicate that these mutants might block the transport of secretory vesicles.
The secretory pathway is a highly organized, multistep process, which delivers newly synthesized proteins to the cell surface through various intracellular compartments. It is now well demonstrated that the secretory pathway in higher eukaryotic cells is broadly classified into a classical conventional pathway and an unconventional pathway (51). In the conventional pathway, newly synthesized proteins are transported from the ER to their final destination via Golgi, and it is usually a COPII-mediated and Rab1-dependent process, whereas in the unconventional secretory pathway, newly synthesized proteins either exit ER in a COPII-independent process or bypass Golgi to reach their final destination (52). However, the secretory pathway in the parasitic protozoa is not well characterized.
In the present investigation, we have used three different molecules, namely HbR, SAP, and Ldgp63, to characterize the role of Rab1 in the secretory pathway of Leishmania. First, we have tried to understand the regulation of transport of newly synthesized HbR from the ER-Golgi network to the cell surface by co-expressing LdHbR-RFP with LdRab1 and its mutants. Surprisingly, we have found that overexpression of LdRab1:WT-GFP, LdRab1:S22N-GFP, or LdRab1:Q67L-GFP does not alter the distribution of LdHbR-RFP in Leishmania in comparison with control cells, and HbR is found to be localized in the flagellar pocket in similar way in both control and LdRab1 mutant-overexpressing parasites. This is supported by the fact that Hb endocytosis is found to be unaltered in these cells. These results are consistent with the fact that depletion of Rab1 by siRNA or overexpression of Rab1 dominant mutant does not affect the subcellular distribution of α2B-adrenergic receptor in mammalian cells (53). These results indicate that LdHbR traffic from the ER-Golgi network to the flagellar pocket is a Rab1-independent process in Leishmania.
These results prompted us to determine whether Rab1-dependent secretory pathway is present in Leishmania. Therefore, we have analyzed the role of LdRab1 in the trafficking of two secretory proteins of Leishmania, namely gp63 and SAP. Previous studies have shown that gp63 is a predominant cell surface-associated glycosylphosphatidylinositol-anchored protein in Leishmania (54). This protein is synthesized as an inactive precursor and targeted to the ER through a signal sequence present at the N terminus of the nascent protein (55). Subsequently, the majority of gp63 is N-glycosylated, and protein is secreted out through the cell surface (56). Similarly, it has been shown that Leishmania synthesize and secrete SAP outside the cells, possibly through a secretory pathway (57). However, the mechanism of regulation of secretion of gp63 and SAP in Leishmania is not known. Our results have shown that overexpression of LdRab1:Q67L or LdRab1:S22N mutant significantly inhibits the secretion of SAP and gp63 in Leishmania. This is supported by the fact that overexpression of these mutants of LdRab1 also blocks the trafficking of gp63 in Leishmania, and protein is found to be trapped in the Rab1-positive Golgi compartment. These results demonstrate that both GTP binding and hydrolysis of Rab1 are required for appropriate targeting of SAP and gp63 via the secretory pathway in Leishmania. These results are consistent with reports on the mammalian system, where expression of dominant negative Rab1 mutants or Rab1 siRNA significantly reduces cell surface expression of AT1R and β2-adrenergic receptor, and proteins are accumulated in perinuclear compartments positive for GM130, a Golgi marker (53). Therefore, our results have shown that Rab1 function in conventional secretory pathway is well conserved in Leishmania. Taken together, our results have shown that gp63 and SAP secretion follow a conventional Rab1-dependent secretory pathway, whereas trafficking of newly synthesized HbR to the cell surface is a Rab1-independent process. Thus, both Rab1-dependent and -independent secretory pathways are present in Leishmania.
Therefore, it is tempting to speculate that HbR trafficking in Leishmania might follow an unconventional protein secretory pathway. This is supported by the fact that the Rab1-independent unconventional protein secretary pathway in yeast and Drosophila is regulated by GRASP65 homologs. GRASP (Golgi reassembly stacking protein) is the single Drosophila homolog of mammalian GRASP55 and GRASP65. These two proteins have been shown to be localized only to the Golgi, and they play a role in Golgi organization (58, 59). Subsequently, it has been shown that αPS1 integrins in Drosophila are secreted in a Golgi-independent fashion but are dependent on GRASP. GRASP is a protein attached peripherally to the cytoplasmic surface of Golgi membranes, and it has been shown that in the absence of Drosophila GRASP, integrins are retained intracellularly, whereas secretion of other proteins is not affected (60). Similarly, the release of Dictyostelium AcbA (acyl-CoA-binding protein) during the development has also been found to be GRASP-dependent (61). Thus, the GRASP homolog is a major player in regulating the unconventional protein secretory pathway. However, the unconventional secretory pathway in trypanosomatid parasites is not yet characterized. We have recently identified the presence of a GRASP homologue in Leishmania, and currently, we are trying to characterize the role of GRASP in HbR trafficking through unconventional secretory pathway in Leishmania.
In conclusion, our results have shown that both conventional and unconventional protein secretory pathways are present in Leishmania. This is the first demonstration that gp63 and SAP secretion follow the Rab1-dependent Golgi-mediated conventional secretory pathway, whereas HbR trafficking to the cell surface is a Rab1-independent process and possibly follows an unconventional protein secretory pathway in the parasites.
Author Contributions
A. M. conceived and coordinated the study and wrote the paper. S. B. and S. P. performed experiments and analyzed results. H. M. and M. R. performed electron microscopy experiments.
These studies were supported by grants from the Department of Biotechnology, Government of India and a J.C. Bose Fellowship from the Department of Science and Technology, Government of India (to A. M.). The authors declare that they have no conflicts of interest with the contents of this article.
- ER
- endoplasmic reticulum
- HbR
- hemoglobin receptor
- SAP
- secretory acid phosphatase
- gp63
- glycosylphosphatidylinositol-anchored 63-kDa surface glycoprotein.
References
- 1.Zerial M., and McBride H. (2001) Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107–117 [DOI] [PubMed] [Google Scholar]
- 2.Deneka M., Neeft M., and van der Sluijs P. (2003) Regulation of membrane transport by rab GTPases. Crit. Rev. Biochem. Mol. Biol. 38, 121–142 [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer S. R. (2013) Rab GTPase regulation of membrane identity. Curr. Opin. Cell Biol. 25, 414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukhopadhyay A., Barbieri A. M., Funato K., Roberts R., and Stahl P. D. (1997) Sequential actions of Rab5 and Rab7 regulate endocytosis in the Xenopus oocyte. J. Cell Biol. 136, 1227–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sönnichsen B., De Renzis S., Nielsen E., Rietdorf J., and Zerial M. (2000) Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149, 901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhopadhyay A., Funato K., and Stahl P. D. (1997) Rab7 regulates transport from early to late endocytic compartments in Xenopus oocytes. J. Biol. Chem. 272, 13055–13059 [DOI] [PubMed] [Google Scholar]
- 7.Bucci C., Thomsen P., Nicoziani P., McCarthy J., and van Deurs B. (2000) Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11, 467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somsel Rodman J., and Wandinger-Ness A. (2000) Rab GTPases coordinate endocytosis. J. Cell Sci. 113, 183–192 [DOI] [PubMed] [Google Scholar]
- 9.Pfeffer S. R. (2001) Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11, 487–491 [DOI] [PubMed] [Google Scholar]
- 10.Plutner H., Cox A. D., Pind S., Khosravi-Far R., Bourne J. R., Schwaninger R., Der C. J., and Balch W. E. (1991) Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J. Cell Biol. 115, 31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tisdale E. J., and Balch W. E. (1996) Rab2 is essential for the maturation of pre-Golgi intermediates. J. Biol. Chem. 271, 29372–29379 [DOI] [PubMed] [Google Scholar]
- 12.Del Nery E., Miserey-Lenkei S., Falguières T., Nizak C., Johannes L., Perez F., and Goud B. (2006) Rab6A and Rab6A′ GTPases play non-overlapping roles in membrane trafficking. Traffic 7, 394–407 [DOI] [PubMed] [Google Scholar]
- 13.Dejgaard S. Y., Murshid A., Erman A., Kizilay O., Verbich D., Lodge R., Dejgaard K., Ly-Hartig T. B., Pepperkok R., Simpson J. C., and Presley J. F. (2008) Rab18 and Rab43 have key roles in ER-Golgi trafficking. J. Cell Sci. 121, 2768–2781 [DOI] [PubMed] [Google Scholar]
- 14.Kelly E. E., Giordano F., Horgan C. P., Jollivet F., Raposo G., and McCaffrey M. W. (2012) Rab30 is required for the morphological integrity of the Golgi apparatus. Biol. Cell 104, 84–101 [DOI] [PubMed] [Google Scholar]
- 15.Stenmark H., and Olkkonen V. M. (2001) The Rab GTPase family. Genome Biol. 2, REVIEWS3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kedzierski L., Sakthianandeswaren A., Curtis J. M., Andrews P. C., Junk P. C., and Kedzierska K. (2009) Leishmaniasis: current treatment and prospects for new drugs and vaccines. Curr. Med. Chem. 16, 599–614 [DOI] [PubMed] [Google Scholar]
- 17.Kaye P., and Scott P. (2011) Leishmaniasis: complexity at the host-pathogen interface. Nat. Rev. Microbiol. 9, 604–615 [DOI] [PubMed] [Google Scholar]
- 18.Croft S. L., and Coombs G. H. (2003) Leishmaniasis: current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19, 502–508 [DOI] [PubMed] [Google Scholar]
- 19.Evans K. J., and Kedzierski L. (2012) Development of vaccines against visceral leishmaniasis. J. Trop. Med. 2012, 892817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sah J. F., Ito H., Kolli B. K., Peterson D. A., Sassa S., and Chang K. P. (2002) Genetic rescue of Leishmania deficiency in porphyrin biosynthesis creates mutants suitable for analysis of cellular events in uroporphyria and for photodynamic therapy. J. Biol. Chem. 277, 14902–14909 [DOI] [PubMed] [Google Scholar]
- 21.Kelly J. X., Ignatushchenko M. V., Bouwer H. G., Peyton D. H., Hinrichs D. J., Winter R. W., and Riscoe M. (2003) Antileishmanial drug development: exploitation of parasite heme dependency. Mol. Biochem. Parasitol. 126, 43–49 [DOI] [PubMed] [Google Scholar]
- 22.Sengupta S., Tripathi J., Tandon R., Raje M., Roy R. P., Basu S. K., and Mukhopadhyay A. (1999) Hemoglobin endocytosis in Leishmania is mediated through a 46-kDa protein located in the flagellar pocket. J. Biol. Chem. 274, 2758–2765 [DOI] [PubMed] [Google Scholar]
- 23.Krishnamurthy G., Vikram R., Singh S. B., Patel N., Agarwal S., Mukhopadhyay G., Basu S. K., and Mukhopadhyay A. (2005) Hemoglobin receptor in Leishmania is a hexokinase located in the flagellar pocket. J. Biol. Chem. 280, 5884–5891 [DOI] [PubMed] [Google Scholar]
- 24.Agarwal S., Rastogi R., Gupta D., Patel N., Raje M., and Mukhopadhyay A. (2013) Clathrin-mediated hemoglobin endocytosis is essential for survival of Leishmania. Biochim. Biophys. Acta 1833, 1065–1077 [DOI] [PubMed] [Google Scholar]
- 25.Singh S. B., Tandon R., Krishnamurthy G., Vikram R., Sharma N., Basu S. K., and Mukhopadhyay A. (2003) Rab5-mediated endosome-endosome fusion regulates hemoglobin endocytosis in Leishmania donovani. EMBO J. 22, 5712–5722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel N., Singh S. B., Basu S. K., and Mukhopadhyay A. (2008) Leishmania requires Rab7-mediated degradation of endocytosed hemoglobin for their growth. Proc. Natl. Acad. Sci. U.S.A. 105, 3980–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guha R., Gupta D., Rastogi R., Vikram R., Krishnamurthy G., Bimal S., Roy S., and Mukhopadhyay A. (2013) Vaccination with Leishmania hemoglobin receptor-encoding DNA protects against visceral leishmaniasis. Sci. Transl. Med. 5, 202ra121. [DOI] [PubMed] [Google Scholar]
- 28.McConville M. J., Mullin K. A., Ilgoutz S. C., and Teasdale R. D. (2002) Secretory pathway of trypanosomatid parasites. Microbiol. Mol. Biol. Rev. 66, 122–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman J. M., Chan S. K., Robinson D. P., Dwyer D. M., Nandan D., Foster L. J., and Reiner N. E. (2008) Proteomic analysis of the secretome of Leishmania donovani. Genome Biol. 9, R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overath P., and Engstler M. (2004) Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol. Microbiol. 53, 735–744 [DOI] [PubMed] [Google Scholar]
- 31.Dhir V., Goulding D., and Field M. C. (2004) TbRAB1 and TbRAB2 mediate trafficking through the early secretory pathway of Trypanosoma brucei. Mol. Biochem. Parasitol. 137, 253–265 [DOI] [PubMed] [Google Scholar]
- 32.Nolan D. P., and Garcia-Salcedo J. A. (2008) Loss of actin does not affect export of newly synthesized proteins to the surface of Trypanosoma brucei. Mol. Biochem. Parasitol. 157, 233–235 [DOI] [PubMed] [Google Scholar]
- 33.Manning-Cela R., Marquez C., Franco E., Talamas-Rohana P., and Meza I. (2003) BFA-sensitive and insensitive exocytic pathways in Entamoeba histolytica trophozoites: their relationship to pathogenesis. Cell. Microbiol. 5, 921–932 [DOI] [PubMed] [Google Scholar]
- 34.Welter B. H., and Temesvari L. A. (2009) Overexpression of a mutant form of EhRabA, a unique Rab GTPase of Entamoeba histolytica, alters endoplasmic reticulum morphology and localization of the Gal/GalNAc adherence lectin. Eukaryot. Cell 8, 1014–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langford T. D., Silberman J. D., Weiland M. E., Svärd S. G., McCaffery J. M., Sogin M. L., and Gillin F. D. (2002) Giardia lamblia: identification and characterization of Rab and GDI proteins in a genome survey of the ER to Golgi endomembrane system. Exp. Parasitol. 101, 13–24 [DOI] [PubMed] [Google Scholar]
- 36.Elias M., Patron N. J., and Keeling P. J. (2009) The RAB family GTPase Rab1A from Plasmodium falciparum defines a unique paralog shared by chromalveolates and rhizaria. J. Eukaryot. Microbiol. 56, 348–356 [DOI] [PubMed] [Google Scholar]
- 37.Cappai R., Osborn A. H., Gleeson P. A., and Handman E. (1993) Cloning and characterization of a Golgi-associated GTP-binding protein homologue from Leishmania major. Mol. Biochem. Parasitol. 62, 73–82 [DOI] [PubMed] [Google Scholar]
- 38.Ha D. S., Schwarz J. K., Turco S. J., and Beverley S. M. (1996) Use of the green fluorescent protein as a marker in transfected Leishmania. Mol. Biochem. Parasitol. 77, 57–64 [DOI] [PubMed] [Google Scholar]
- 39.Ilg T., Stierhof Y. D., Etges R., Adrian M., Harbecke D., and Overath P. (1991) Secreted acid phosphatase of Leishmania mexicana: a filamentous phosphoglycoprotein polymer. Proc. Natl. Acad. Sci. U.S.A. 88, 8774–8778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakalara N., Seyfang A., Baltz T., and Davis C. (1995) Trypanosoma brucei and Trypanosoma cruzi: life cycle-regulated protein tyrosine phosphatase activity. Exp. Parasitol. 81, 302–312 [DOI] [PubMed] [Google Scholar]
- 41.Isnard A., Shio M. T., and Olivier M. (2012) Impact of Leishmania metalloprotease GP63 on macrophage signaling. Front. Cell. Infect. Microbiol. 2, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Press B., Feng Y., Hoflack B., and Wandinger-Ness A. (1998) Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J. Cell Biol. 140, 1075–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bock J. B., Matern H. T., Peden A. A., and Scheller R. H. (2001) A genomic perspective on membrane compartment organization. Nature 409, 839–841 [DOI] [PubMed] [Google Scholar]
- 44.Armstrong J. (2000) Membrane traffic between genomes. Genome Biol. 1, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tisdale E. J., Bourne J. R., Khosravi-Far R., Der C. J., and Balch W. E. (1992) GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 119, 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pind S. N., Nuoffer C., McCaffery J. M., Plutner H., Davidson H. W., Farquhar M. G., and Balch W. E. (1994) Rab1 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J. Cell Biol. 125, 239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filipeanu C. M., Zhou F., Claycomb W. C., and Wu G. (2004) Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum-to-Golgi transport in cardiac myocytes. J. Biol. Chem. 279, 41077–41084 [DOI] [PubMed] [Google Scholar]
- 48.Nuoffer C., Davidson H. W., Matteson J., Meinkoth J., and Balch W. E. (1994) A GDP-bound of rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J. Cell Biol. 125, 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson B. S., Nuoffer C., Meinkoth J. L., McCaffery M., Feramisco J. R., Balch W. E., and Farquhar M. G. (1994) A Rab1 mutant affecting guanine nucleotide exchange promotes disassembly of the Golgi apparatus. J. Cell Biol. 125, 557–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu S., and Storrie B. (2012) Are Rab proteins the link between Golgi organization and membrane trafficking? Cell Mol. Life Sci. 69, 4093–4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonifacino J. S., and Glick B. S. (2004) The mechanisms of vesicle budding and fusion. Cell 116, 153–166 [DOI] [PubMed] [Google Scholar]
- 52.Grieve A. G., and Rabouille C. (2011) Golgi bypass: skirting around the heart of classical secretion. Cold Spring Harb. Perspect. Biol. 10.1101/cshperspect.a005298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu G., Zhao G., and He Y. (2003) Distinct pathways for the trafficking of angiotensin II and adrenergic receptors from the endoplasmic reticulum to the cell surface: Rab1-independent transport of a G protein-coupled receptor. J. Biol. Chem. 278, 47062–47069 [DOI] [PubMed] [Google Scholar]
- 54.Chang K. P. (1983) Cellular and molecular mechanisms of intracellular symbiosis in leishmaniasis. Int. Rev. Cytol. Suppl. 14, 267–305 [PubMed] [Google Scholar]
- 55.Fong D., and Chang K. P. (1982) Surface antigenic change during differentiation of a parasitic protozoan, Leishmania mexicana: identification by monoclonal antibodies. Proc. Natl. Acad. Sci. U.S.A. 79, 7366–7370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ellis M., Sharma D. K., Hilley J. D., Coombs G. H., and Mottram J. C. (2002) Processing and trafficking of Leishmania mexicana GP63: analysis using GP18 mutants deficient in glycosylphosphatidylinositol protein anchoring. J. Biol. Chem. 277, 27968–27974 [DOI] [PubMed] [Google Scholar]
- 57.Bates P. A., Hermes I., and Dwyer D. M. (1989) Leishmania donovani: immunochemical localization and secretory mechanism of soluble acid phosphatase. Exp. Parasitol. 68, 335–346 [DOI] [PubMed] [Google Scholar]
- 58.Xiang Y., and Wang Y. (2010) GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J. Cell Biol. 188, 237–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vinke F. P., Grieve A. G., and Rabouille C. (2011) The multiple facets of the Golgi reassembly stacking proteins. Biochem. J. 433, 423–433 [DOI] [PubMed] [Google Scholar]
- 60.Schotman H., Karhinen L., and Rabouille C. (2008) dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev. Cell 14, 171–182 [DOI] [PubMed] [Google Scholar]
- 61.Kinseth M. A., Anjard C., Fuller D., Guizzunti G., Loomis W. F., and Malhotra V. (2007) The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell 130, 524–534 [DOI] [PubMed] [Google Scholar]