Abstract
Introduction
Youth with Type 1 diabetes and lower family income typically have poorer glycemic control. This post hoc analysis examines whether a family-oriented behavioral intervention for this population is differentially effective across income levels.
Methods
Families of youth aged 9–15 years with Type 1 diabetes (N=390; 49.2% female; age, 12.4 [1.7] years; hemoglobin A1c [HbA1c], 8.4 [1.2]; pump, 33.8%) at four U.S. pediatric endocrinology clinics participated in a 2-year RCT (data collected 2006–2011) of a clinic-integrated behavioral intervention designed to improve diabetes management by facilitating problem-solving skills, communication skills, and responsibility sharing. HbA1c was analyzed centrally. Family income was categorized as <$50,000 (low), $50,000 to <$100,000 (middle), and ≥$100,000 (high). Treatment effect was defined as the change in HbA1c from baseline to 2-year follow-up. A linear model tested the interaction of intervention treatment effect with family income, controlling for race, treatment regimen, and site (analyzed in 2014).
Results
Baseline HbA1c was significantly poorer (p=0.004) in the low-income group. There was a significant overall effect of treatment group on change in HbA1c from baseline to follow-up (p=0.04). The interaction term for treatment by income group was not significant (p=0.44). Within each income category, a smaller deterioration in glycemic control was observed for the treatment group relative to controls.
Conclusions
This clinic-integrated behavioral intervention was similarly effective in improving glycemic control among youth with Type 1 diabetes across income levels. This family-oriented problem-solving approach offers flexibility in addressing families’ needs, and may optimize impact on health outcomes across income groups.
Introduction
Consistent with general trends in health disparities research, youth with Type 1 diabetes who experience lower family income have poorer glycemic control,1,2 increasing risk for long-term diabetes complications.3 Behavioral interventions have demonstrated efficacy in improving diabetes management.4–8 Extending from the inverse equity hypothesis,9 people experiencing higher income may be better equipped to benefit from such interventions, inadvertently exacerbating health disparities. However, the impact of socioeconomic factors on behavioral intervention effectiveness is rarely examined.
“WE-CAN manage diabetes” is a clinic-integrated behavioral intervention designed to improve families’ Type 1 diabetes management by facilitating problem-solving skills, communication skills, and appropriate responsibility sharing. This intervention targeted families of preadolescents and adolescents, who typically experience deterioration in glycemic control.10,11 The intervention was effective in improving glycemic control relative to standard care.8 The objective of this post hoc analysis is to examine whether the intervention effect differs across income levels.
Methods
Participants
Child inclusion criteria included: age 9–14.9 years; Type 1 diabetes diagnosis ≥3 months; daily insulin usage ≥0.5 μ/kg/day for those diagnosed ≥1 year or 0.2 μ/kg/day for those diagnosed <1 year, with two or more injections or insulin pump use; most recent hemoglobin A1c (HbA1c) >6.0% and <12.0% for those diagnosed ≥1 year and >6.0% for those diagnosed <1 year at any time post-diagnosis; and no other major chronic disease (except well-controlled thyroid disease, asthma, and celiac), cognitive impairments, or psychiatric diagnosis. Additional parent/family inclusion criteria included home telephone access, English fluency, attendance of two or more clinic visits in the past year, and no psychiatric diagnoses in participating parents. Sample size was based on detecting meaningful differences in HbA1c between intervention and control conditions, and has been reported previously.8
Design and Procedures
This clinical trial employed a multicenter, parallel-group study with equal randomization. Participants were recruited during routine clinic visits from four large, geographically dispersed, pediatric endocrinology clinics in the U.S.; data were collected from 2006 to 2011. Families were randomized to intervention or usual care, stratified by age (≥9 to <12 years and ≥12 to <15 years) and HbA1c (≤8.3% and >8.3%). A system of random permuted blocks within strata was prepared by the study coordinating center by a person uninvolved with data collection. A separate randomization list was prepared for each stratum; lists were transferred to a sequence of sealed envelopes, each containing the assignment of intervention or usual care. Families were enrolled in the study for 2 years; brief questionnaire and biomedical assessments were administered at each clinic visit (typically every 3–4 months). Intervention contacts occurred at each clinic visit for 21 months, with a final assessment at the following visit. The study protocol was approved by the IRBs of each participating institution.
Behavioral Intervention
The intervention was designed to improve diabetes management by facilitating constructive collaboration between youth and parents and enhancing individual and family problem-solving skills. Grounded in social cognitive theory,12 self-regulation models,13,14 and systems theory,15 the WE-CAN manage diabetes intervention was delivered by specially trained nonprofessionals at each routine clinic visit for approximately 21 months (described in the Appendix). Briefly, at each visit, families identified a specific diabetes management problem and developed a behavioral plan targeting this issue. Sessions were structured by the WE-CAN problem-solving approach, a pneumonic representing the steps in the problem-solving process.
Measures
Blood samples were obtained at each visit and shipped to a central laboratory for HbA1c assay (Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer™, Tosoh Medics, South San Francisco, CA), reference range, 4%–6%. Simultaneous samples were processed with the DCA-2000 (Siemens Healthcare Diagnostics, Deerfield, IL) on site. These results were used to impute replacement values if samples were lost or damaged (1.2% of values).16
Data on demographic and disease-related characteristics were obtained from the electronic medical record and from parent report. Family income was reported by parents and categorized as <$50,000 (low), $50,000 to <$100,000 (middle), and ≥$100,000 (high).
Statistical Analysis
The primary outcome was HbA1c (a biomarker of glycemic control; lower values indicate better control). For this analysis, conducted in 2014, treatment effect was defined as the change in HbA1c from baseline to 2-year follow-up. ANOVA was used to test for differences between income groups in baseline HbA1c. To determine whether the treatment effect differed by income group, a linear model was used to test the interaction of treatment effect with income group, controlling for site, regimen, and race/ethnicity, and using multiple imputation for missing data.
Results
A total of 390 families participated in the trial. Participant flow from recruitment through follow-up is shown in Figure 1. Seventy percent of eligible participants enrolled and completed baseline assessments; subject retention through study completion was 92%. Participant withdrawal did not differ significantly between income groups. No study-related adverse events were reported.
Figure 1.
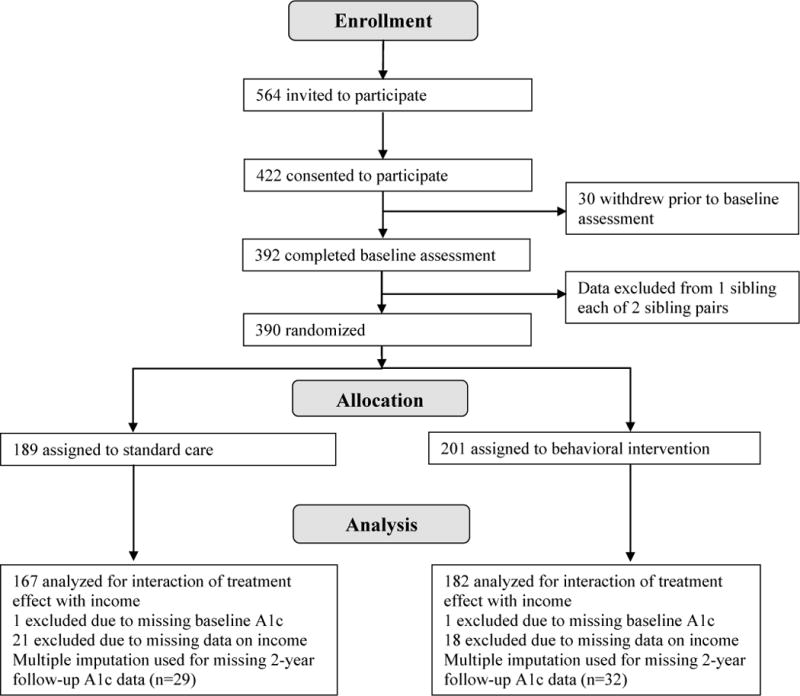
Participant flow through study.
Baseline characteristics were well balanced by treatment assignment (Table 1). Mean number of clinic visits was lower (p=0.02), and baseline HbA1c was poorer (p=0.004) in the low-income group compared with the higher-income groups. The low-income group included a higher proportion of ethnic/racial minorities (p<0.001) and a lower proportion using insulin pump therapy (p<0.001).
Table 1.
Baseline Characteristics by Treatment Assignment and Family Income Levela
| Treatment Group | Annual Family Income | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control N=167 | Intervention N=182 | <$50,000 N=86 | $50,000–$99,999 N=137 | $100,000+ N=126 | p-value | |
| Age (years); mean±SD | 12.3±1.7 | 12.4±1.7 | 12.5±1.7 | 12.4±1.6 | 12.2±1.8 | 0.47 |
| Gender; N(%) | 0.05 | |||||
| Female | 77 (46.1%) | 89 (48.9%) | 31 (36.1%) | 69 (49.6%) | 66 (52.4%) | |
| Male | 90 (53.9%) | 93 (51.1%) | 55 (63.9%) | 68 (50.4%) | 60 (47.6%) | |
| Race/ethnicity; | <0.001 | |||||
| N(%)b | 120 | 137 (75.3%) | 37 (43.0%) | 109 | 111 | |
| White | (73.2%) | 21 (11.5%) | 16 (18.6%) | (80.7%) | (88.8%) | |
| Hispanic | 16 (9.8%) | 13 (7.1%) | 23 (26.7%) | 13 (9.6%) | 8 (6.4%) | |
| Black | 18(11.0%) | 11 (6.1%) | 10 (11.7%) | 6 (4.4%) | 2 (1.6%) | |
| Other | 10 (6.0%) | 7 (5.3%) | 4 (3.2%) | |||
| Duration of diabetes (years); mean±SD | 4.8 (3.2) | 4.8 (3.3) | 4.6±3.3 | 4.9±3.2 | 4.9±3.3 | 0.72 |
| Regimen; N(%) | <0.001 | |||||
| Pump | 53 (31.7%) | 63 (34.6%) | 15 (17.4%) | 45 (32.8%) | 56 (44.4%) | |
| Injection | 114 (68.3%) | 119 (65.4%) | 71 (82.6%) | 92 (67.2%) | 70 (55.6%) | |
| HbA1c; mean±SD | 8.3±1.2 | 8.4±1.2 | 8.7±1.4 | 8.3±1.0 | 8.2±1.1 | 0.004 |
| Total # of visits | 7.4±1.8 | 7.2±2.1 | 6.8±2.5 | 7.4±1.8 | 7.5±1.7 | 0.02 |
Test for group differences using ANOVA for continuous and chi-square for categorical variables. Data reported for 349 families with income data reported
Race/ethnicity not reported by three families, all assigned to control group
Note: Boldface indicates statistical significance (p<0.05)
There was an overall effect of treatment group on change in HbA1c from baseline to follow-up (p=0.04). The interaction of treatment and income was not significant (p=0.44); findings were unchanged when including baseline HbA1c as a covariate. A smaller deterioration in glycemic control was observed for the treatment group relative to the control group in the low- (0.52 vs 1.09), middle- (0.42 vs 0.51), and high- (0.38 vs 0.71) income groups (Figure 2).
Figure 2.
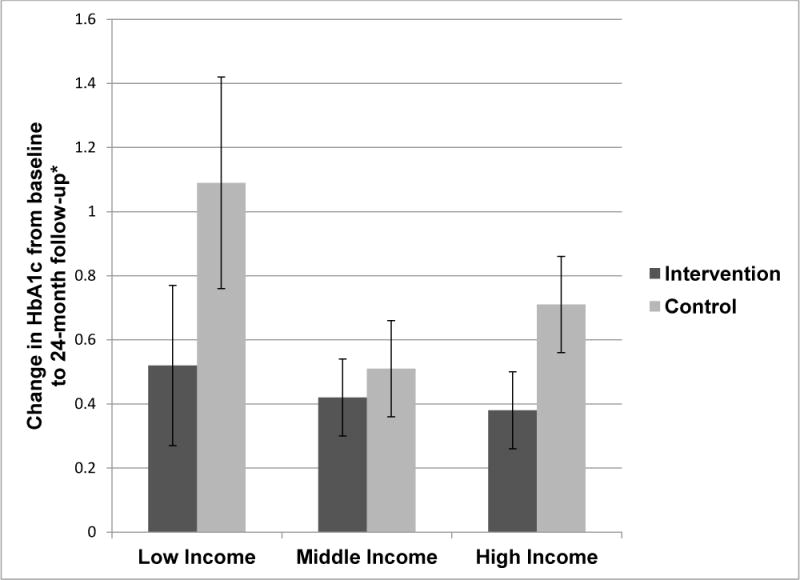
Change in HbA1c from baseline to follow-up by family income level.
Discussion
Glycemic control is known to worsen during adolescence due to developmental and physiologic processes.17,18 In this study, those in the low-income group demonstrated poorer baseline glycemic control and attended fewer clinic visits on average. Nevertheless, this clinic-integrated behavioral intervention was similarly effective across income levels in reducing the expected deterioration in glycemic control among youth with Type 1 diabetes. Thus, although the intervention did not amend existing income disparities, it also did not increase those disparities. In low- and high-income groups, the deterioration in glycemic control in the intervention group was approximately half that observed in the control group. Across a variety of health-related behaviors, findings on the effect of behavioral interventions in low-income groups and the moderation of intervention effects by income level are inconsistent.19–21 To our knowledge, no previous behavioral interventions in youth with Type 1 diabetes have examined intervention effects by SES. Considering the potential financial, time, and stress burden associated with diabetes management, this research question is of considerable clinical utility. The effectiveness across income levels observed in this study may be attributable in part to the adaptable and individualized nature of the intervention approach.
Findings should be interpreted in light of study limitations. This was a post-hoc analysis of treatment effects by subgroups; such analyses must be interpreted accordingly.22 However, the sample included families from four geographically dispersed clinical sites, and was large relative to other studies of behavioral interventions in this population, supporting the internal and external validity of the findings. Importantly, these findings demonstrate the first evidence of comparable efficacy of a behavioral intervention across income groups in youth with Type 1 diabetes, and suggest income may not necessarily impede benefits of health behavior interventions.
Supplementary Material
Acknowledgments
This research was supported by the intramural research program of the NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development, contract numbers N01-HD-4-3364, N01-HD-4-3361, N01-HD-4-3362, N01-HD-4-3363, and N01-HD-3-3360. The authors wish to acknowledge the contributions of the research staff at the participating clinical sites and the families who participated in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov identifier: NCT00273286 (registration date, January 05, 2006)
No financial disclosures were reported by the authors of this paper.
References
- 1.Carter PJ, Cutfield WS, Hofman PL, et al. Ethnicity and social deprivation independently influence metabolic control in children with type 1 diabetes. Diabetologia. 2008;51(10):1835–1842. doi: 10.1007/s00125-008-1106-9. http://dx.doi.org/10.1007/s00125-008-1106-9. [DOI] [PubMed] [Google Scholar]
- 2.Deladoëy J, Henderson M, Geoffroy L. Linear association between household income and metabolic control in children with insulin-dependent diabetes mellitus despite free access to health care. J Clin Endocrinol Metab. 2013;98(5):E882–E885. doi: 10.1210/jc.2013-1212. http://dx.doi.org/10.1210/jc.2013-1212. [DOI] [PubMed] [Google Scholar]
- 3.Secrest AM, Costacou T, Gutelius B, Miller RG, Songer TJ, Orchard TJ. Associations Between Socioeconomic Status and Major Complications in Type 1 Diabetes: The Pittsburgh Epidemiology of Diabetes Complication (EDC) Study. Ann Epidemiol. 2011;21(5):374–381. doi: 10.1016/j.annepidem.2011.02.007. http://dx.doi.org/10.1016/j.annepidem.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. J Pediatr. 2000;137(1):107–113. doi: 10.1067/mpd.2000.106568. http://dx.doi.org/10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- 5.Laffel LMB, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. J Pediatr. 2003;142(4):409–416. doi: 10.1067/mpd.2003.138. http://dx.doi.org/10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- 6.Wysocki T, Harris MA, Buckloh LM, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. J Pediatr Psychol. 2006;31(9):928–938. doi: 10.1093/jpepsy/jsj098. http://dx.doi.org/10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- 7.Nansel TR, Iannotti RJ, Simons-Morton BG, Plotnick LP, Clark LM, Zeitzoff L. Long-term maintenance of treatment outcomes: Diabetes personal trainer intervention for youth with type 1 diabetes. Diabetes Care. 2009;32(5):807–809. doi: 10.2337/dc08-1968. http://dx.doi.org/10.2337/dc08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nansel TR, Iannotti RJ, Liu A. Clinic-integrated behavioral intervention for families of youth with type 1 diabetes: randomized clinical trial. Pediatrics. 2012;129(4):e866–e873. doi: 10.1542/peds.2011-2858. http://dx.doi.org/10.1542/peds.2011-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Victora CG, Vaughan JP, Barros FC, Silva AC, Tomasi E. Explaining trends in inequities: Evidence from Brazilian child health studies. Lancet. 2000;356(9235):1093–1098. doi: 10.1016/S0140-6736(00)02741-0. http://dx.doi.org/10.1016/S0140-6736(00)02741-0. [DOI] [PubMed] [Google Scholar]
- 10.Group DCaCTR. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125(2):177–188. doi: 10.1016/s0022-3476(94)70190-3. http://dx.doi.org/10.1016/S0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 11.Helgeson VS, Siminerio L, Escobar O, Becker D. Predictors of metabolic control among adolescents with diabetes: A 4-year longitudinal study. J Pediatr Psychol. 2009;34(3):254–270. doi: 10.1093/jpepsy/jsn079. http://dx.doi.org/10.1093/jpepsy/jsn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 13.Leventhal H, Leventhal EA, Contrada RJ. Self-regulation, health and behavior: a perceptual-cognitive approach. Psychol Health. 1998;13:717–733. http://dx.doi.org/10.1080/08870449808407425. [Google Scholar]
- 14.Leventhal H, Leventhal EA, Cameron L. Representations, procedures, and affect in illness self-regulations: a perceptual-cognitive model. In: Baum A, Revenson TA, Singer JE, editors. Handbook of Health Psychology. Mahwah, NJ: Erlbaum; 2001. pp. 19–47. [Google Scholar]
- 15.Bateson G. Steps toward an ecology of mind. New York: Ballantine; 1972. [Google Scholar]
- 16.Tamborlane W, Xing D, Steffes M, et al. Performance of the DCA2000 for Measurement of Hemoglobin A1c Levels in Children with T1DM in a DirecNet Outpatient Clinical Trial. 2004 Pediatric Academic Societies’ Annual Meeting. 2004 [Google Scholar]
- 17.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty: A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315(4):215–219. doi: 10.1056/NEJM198607243150402. http://dx.doi.org/10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 18.Susman-Stillman A, Hyson DM, Anderson FS, Colllins WA. Adolescent psychosocial development and adherence to treatment for insulin-dependent diabetes mellitus. In: McNamara JA Jr, Trotman CA, editors. Creating the Compliant Patient. Ann Arbor, MI: Center for Human Growth and Development. The University of Michigan; 1997. pp. 73–101. [Google Scholar]
- 19.Kavanagh J, Oliver S, Lorenc T, et al. School-based cognitive-behavioural interventions: A systematic review of effects and inequalities. Health Psychol Rev. 2009;18(1):61–78. http://dx.doi.org/10.5172/hesr.18.1.61. [Google Scholar]
- 20.Magnée T, Burdorf A, Brug J, et al. Equity-specific effects of 26 dutch obesity-related lifestyle interventions. Am J Prev Med. 2013;44(6):e57–e66. doi: 10.1016/j.amepre.2012.11.041. http://dx.doi.org/10.1016/j.amepre.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 21.Michie S, Jochelson K, Markham WA, Bridle C. Low-income groups and behaviour change interventions: A review of intervention content, effectiveness and theoretical frameworks. J Epidemiol Community Health. 2009;63(8):610–622. doi: 10.1136/jech.2008.078725. http://dx.doi.org/10.1136/jech.2008.078725. [DOI] [PubMed] [Google Scholar]
- 22.Petticrew M, Tugwell P, Kristjansson E, Oliver S, Ueffing E, Welch V. Damned if you do, damned if you don’t: Subgroup analysis and equity. J Epidemiol Community Health. 2012;66(1):95–98. doi: 10.1136/jech.2010.121095. http://dx.doi.org/10.1136/jech.2010.121095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


