Abstract
Purpose
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a devastating disease that is associated with multiple symptoms, and is usually diagnosed on the basis of pain, urgency and frequency in the absence of other known causes. To date there is no diagnostic test.
Methods
In vivo diagnostic contrast-enhanced magnetic resonance imaging (CE-MRI) was used, involving intravesical administration of Gd-DTPA (gadolinium diethylene triamine penta acetic acid) contrast via a catheter, for visualization of increased permeability of the bladder urothelium, and i.v. administration of Gd-DTPA was used to visualize secondary tissue effects to the colon, in a rat model undergoing intravesical exposure to protamine sulfate (PS).
Results
Both bladder urothelilium and colonic mucosa were assessed 24 hours following bladder PS exposure. Enhanced contrast MR images were able to establish bladder urothelium leakage of Gd-DTPA (399.7±68.7 % change in MRI signal intensity (SI) for PS-exposed rats, compared to 39.2±12.2 for controls; p<0.0001), and colonic-related uptake in contrast agent (65.2±17.1 % for PS-exposed rats, compared to 20.8±9.8 for controls; p<0.01) following bladder PS exposure. Kinetics of Gd-DTPA uptake and excretion were also obtained over 20 min in the bladder, and over 30 min in the colon, indicating increased signal intensities at 7 and 12 min, respectively.
Conclusions
These preliminary studies indicate that CE-MRI is capable of monitoring primary bladder urothelium loss of impermeability and secondary enhanced contrast in colon mucosa. This method can be considered a potential clinical diagnostic method for IC/PBS that involves loss of the permeability barrier, and assessing visceral organ cross-talk.
Keywords: Bladder permeability, bladder-colon cross interaction, CE-MRI (contrast-enhanced magnetic resonance imaging), rats, Gd-DTPA, interstitial cystitis, painful bladder syndrome
Introduction
IC/BPS is a chronic painful condition of the bladder that affects up to 7% of females in the U.S.A.1, and over 30% of female patients with chronic pelvic pain2. The prevalence of IC/PBS is thought to be markedly higher (100–300/100,000 women) than in previous years3. Frequency, urgency and pain during bladder filling are the most commonly reported symptoms of IC/BPS4. Associated conditions include psychological distress, depression, sexual assault, irritable bowel syndrome and fibromyalgia4. The bladder is normally impermeable to urinary wastes, and any disruption of this permeability barrier would allow leakage of urine constituents into underlying cell layers, resulting in inflammation5,6. There are four main processes that appear to produce IC/PBS symptoms, including disruption of the bladder glycosaminoglycan (GAG)/proteoglycan layer, upregulation of the immune/inflammatory response, neural upregulation, and pelvic floor dysfunction7.
Magnetic Resonance Imaging (MRI) has been used successfully to image bladder carcinoma using morphological MRI8–11 and diffusion-weighted imaging11, and should have the ability to image the bladder to detect increased permeability. Other applications of MRI used to understand bladder tissue physiology/pathophysiology include a study by Shi et al. (2013), who used MRI to assess different texture features in order to differentiate bladder carcinoma from the bladder wall9. In another study by Bains et al. (2010), they used dynamic contrast enhanced (DCE) MRI and tracer kinetic analysis to study the magnitude of water exchange effects in bladder cancer patients8. MRI has also been used to study bladder wall dynamics, such as thickness12 and contours13. The method has superior image resolution (100–200 µm in-plane resolution on high field MRI systems, and >300 µm resolution for clinical MRI systems), and MRI is wide available clinically. It has not, to date, been used to diagnose or classify IC patients.
In this study we describe the use of MRI to detect increased bladder permeability in an animal model. Protamine sulfate (PS) is commonly used in experimental models of cystitis as this compound increases urothelial permeability [6]. The concentration and exposure time of PS is crucial, because high concentrations essentially denude the urothelium14,15. Lower concentrations will remove only the apical cell layer16. We used an even lower concentration that produces increased permeability without producing extensive tissue damage to more closely mimic what is seen in IC17. IC and IBS have been shown to have a high co-morbidity, up to 75%18, and experimental animal studies have indicated that communication occurs between the bladder and bowel, and that irritation in one organ produces a concomitant response in the other19,20.
DCE-MR imaging is commonly performed by intravenous administration of a low-molecular-weight contrast agent (e.g. Gd-DTPA) that readily diffuses from the blood into the extravascular–extracellular space (also termed leakage space [νe]), at a rate determined by the permeability of the microvessels, their surface area and blood flow. Measurement of the tissue signal intensity changes can be done with fast T1-weighted imaging21. In this study, we introduce the contrast agent directly into the bladder to visualize leakage into the urothelium and also apply it conventionally to visualize secondary bowel cross-talk from the increased bladder permeability. The ultimate aim is to demonstrate the feasibility of using contrast-enhanced MRI as a diagnostic tool for lower pelvic pain syndromes.
Methods
Animals
Female rats (ovarectimized (7 weeks old) SAS Sprague-Dawley; 250–300 g; n=10) were anesthetized with isofluorane (1.5–3.0% with 800–1,000 mL O2) for MRI experiments. Bladder permeability assessments were made 24 hours following exposure to PS (n=5). Sham controls (n=5) were administered saline (400 µL) instead of PS. PS was administered at a concentration of 1 mg/mL (in saline) in a total volume of 400 µL via an intravesical catheter (18G×1¼” Surflo i.v. catheter; Therumo Medical Corp., Elkton, MD, USA). A lubricated 18G cannula from an angiocath over a lubricated guide wire was used to transurethally catheterize each animal. PS was instilled in the bladder for 10min. PS was removed from the bladder using abdominal pressure and followed by three saline flushes (400 µL per flush).
Magnetic resonance imaging (MRI)
MRI experiments were conducted on a 7 Tesla 30 cm-bore Bruker Biospec MRI system. MRI scans were obtained before exposure to protamine sulfate (PS) (pre-scan image), and 24 hours following PS (pre-contrast image, bladder contrast images, and colon contrast images). Specifically, the in vivo diagnostic contrast-enhanced magnetic resonance imaging (CE-MRI) approach involved administration of Gd-DTPA (0.034 mM Gd-DTPA diluted to 800 µl in saline), administered via an intravesical catheter, for visualization of loss of impermeability of the bladder urothelium (bladder contrast images). Pre-PS exposure image datasets were obtained. Bladder contrast images were obtained every 3 min. 43 sec. min. for a total period of 20 min. For colon contrast images, Gd-DTPA was subsequently administered (0.08 mM diluted to 200 µl in saline) i.v. via a tail-vein catheter (24G×0.75” BD Insyte Autoguard shielded i.v. catheter; Becton, Dickinson Infusion Therapy Systems, Sandy, UT, USA), and images were obtained over a 30 min. period. For all MR images, a T1-weighted RARE (rapid acquisition with relaxation enhancement) MRI pulse sequence was used with the following parameters: repetition time (TR) of 1200 ms, echo time (TE) of 9 ms, a RARE factor of 4, 4 averages, an image slice thickness of 1 mm, a matrix of 256×256, a field-of-view (FOV) of 6.5 × 6.5 cm2, and with both motion and fat suppression.
Histology
After animals were completed imaging, the animals were euthanized and bladders and sections of colon were removed and placed in 10% formalin. The tissues were then embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Stained sections were observed with a Nikon Optiphot microscope. Images were taken using a Canon EOS Rebel T3i. Control tissues were obtained from un-manipulated animals.
Data analysis and Statistics
MRI signal intensities were measured from regions-of-interest (ROIs) within images (4–5 ROIs were taken in high-intensity regions in the bladder periphery, colon mucosa, adipose body surrounding the bladder, surrounding colon tissues, and medial thigh muscle, along with corresponding regions in sham animal datasets) displayed on Paravision (v 5.0, Bruker Biospin). Statistical analyses were done using ANOVA with a post Tukey’s multiple comparison test for evaluating differences in treatment groups (Instat; GraphPad Software, San Diego, CA, USA). Data are represented as mean ± S.D., and p-values < 0.05, < 0.01 or < 0.001 were considered statistically significant.
Results
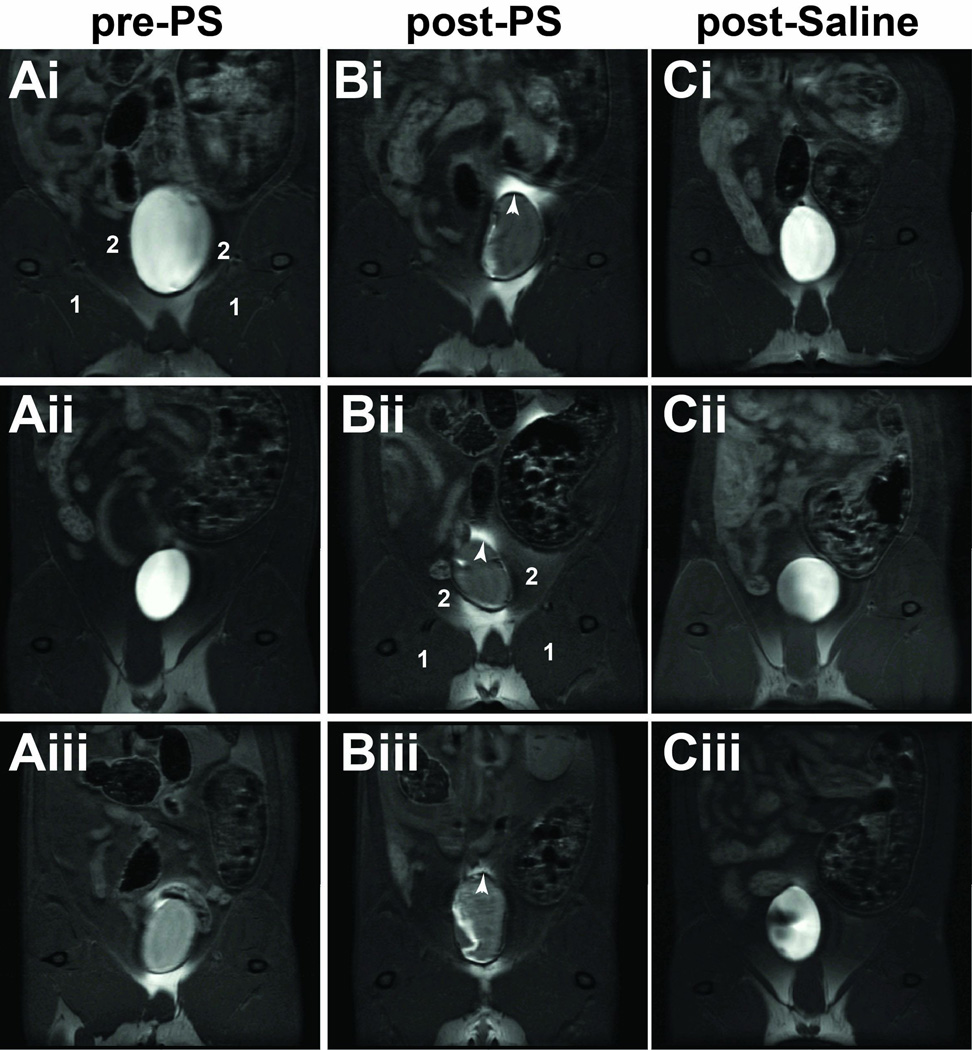
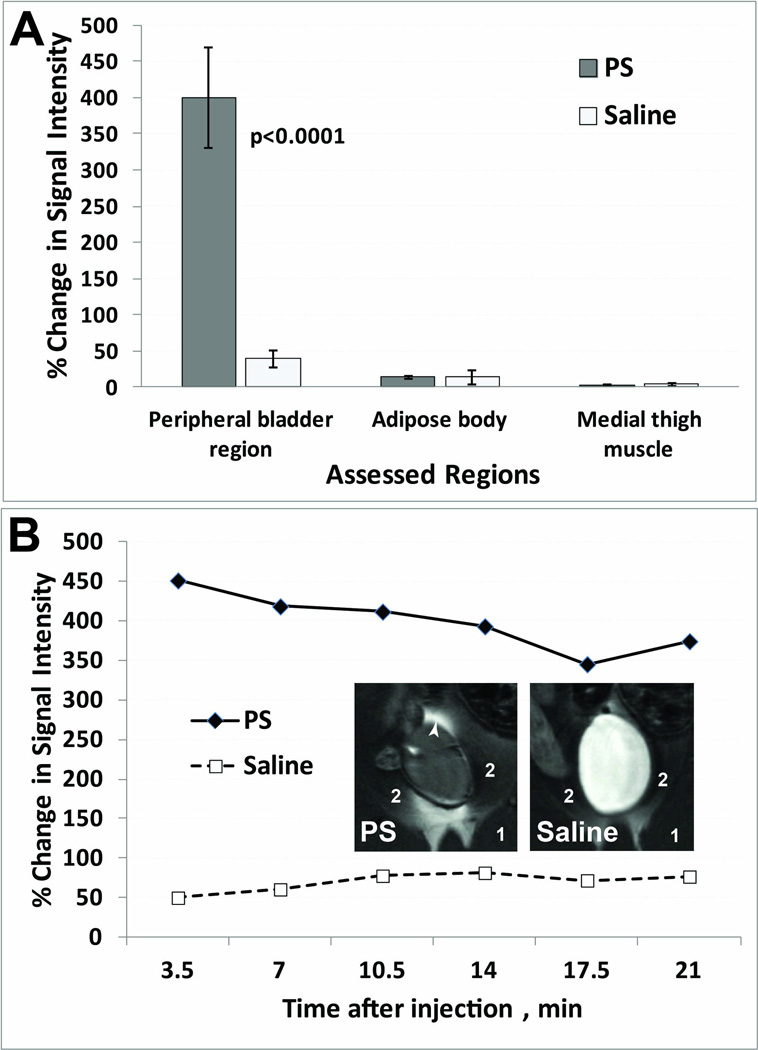
As proof-of-concept, we developed an approach to study alterations in bladder wall permeability by assessing the leakage of a widely used MRI contrast agent in a rat pre-clinical model where bladder permeability was altered by exposure to protamine sulfate (PS). Bladder permeability kinetics of Gd-DTPA contrast-enhancement were initially obtained over 20 min in the bladder (following intravesical administration). The enhanced contrast MR images (7 min. following Gd-DTPA administration) were able to establish that there was bladder urothelium leakage of the Gd-DTPA contrast agent following PS-induced neutralization of the glycosaminoglycan (GAG) layer in T1-weighted (T1w) horizontal MR images of rat bladders (see several examples of representative MR images of rat bladder regions in Fig. 1). In T1-weighted MR images, leakage of the Gd-DTPA contrast agent is illustrated by an increase in contrast signal intensity within the peritoneal cavity. Comparative sham controls are also shown in Fig. 1, which demonstrate no leakage of the Gd-DTPA contrast agent through the bladder urothelium in these animals. Quantitative assessment of percent change in MRI signal intensity between post-contrast and pre-contrast images, 24 hours after PS exposure, are shown in Fig. 2A. For consistency, these data were obtained 7 min. post-Gd-DTPA administration via an intravesical catheter. There was a significant increase in % change in MRI signal intensity (p<0.0001) for the PS exposed rats (399.7±68.7) compared to the saline-treated sham controls (39.2±12.2). Fig. 2B illustrates the kinetic % change in MRI signal intensity over 20 min, with representative MR images for each treatment group. Non-high-intensity regions were also assessed in the medial thigh muscle (labeled 1 in Figs. 1 and 2B) and adipose body (labeled 2 in Figs. 1 and 2B) surrounding the bladder, which showed no differences between PS-administered animals compared to shams.
Figure 1.
CE-MRI visualization of increased bladder permeability in PS exposed rats. (A) Pre-PS exposure contrast-enhanced (CE) images (i–iii). (B) Post-contrast images (i–iii) obtained 24 h post-PS exposure. Gd-DTPA leakage observed through the urothelium in the peroteneal cavity (white arrow heads). (C) Contrast images 24 h post-saline (shams; i–iii). Medial thigh muscle (1), and adipose body surrounding the bladder (2).
Figure 2.
(A) Quantitative assessment of increased bladder permeability in PS exposed rats. Percent (%) change in MRI signal intensity 24 h following either exposure to PS or saline (n=5 for each treatment group). Regions assessed: peripheral bladder area (white arrowhead), medial thigh muscle (1), and adipose body (2). (B) Representative % change in MRI signal intensity post-contrast for PS (closed diamonds) and saline (open squares) rats. Insets: Representative MR images of PS and saline-treated rat bladders.
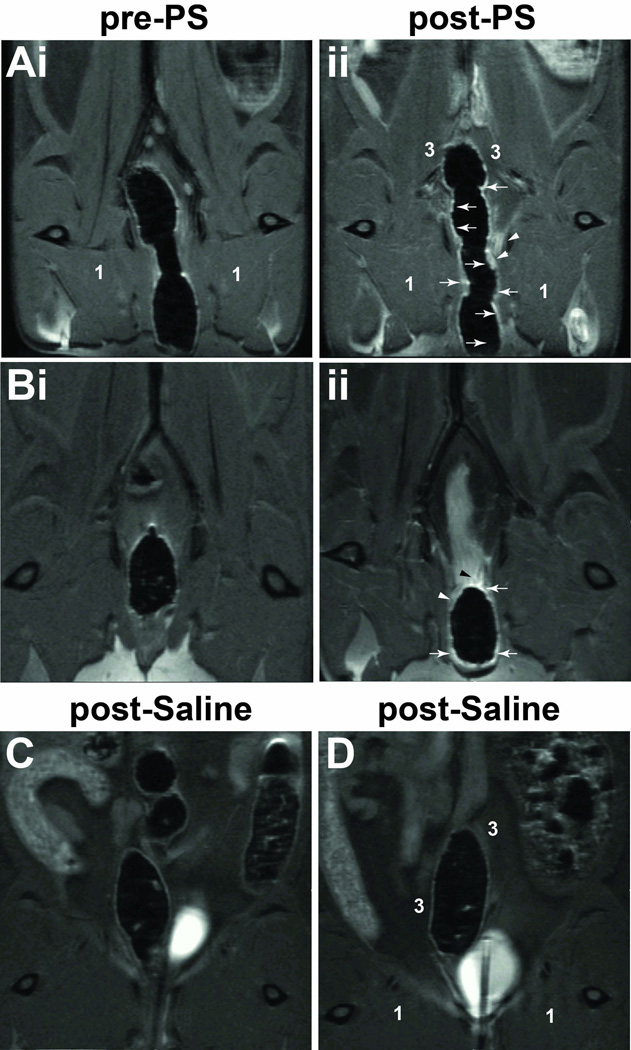
In addition we also administered Gd-DTPA (0.08 mM diluted to 200 µl in saline) intravenously via a tail-vein catheter to visualize secondary contrast enhancement in the colonic mucosa. We were able to detect an increase in contrast-enhanced signal intensity in rat colon regions at 24 hours following PS exposure of the bladder. Figure 3 presents some representative CE-MR images (horizontal orientation; 12 min. following Gd-DTPA administration) pre-PS exposure, 24 hours post-PS exposure and 24 hours following saline administration. Increases in MRI signal intensities were noted in the colonic mucosa, and in some cases within close peripheral tissues (for example, see Fig. 3Aii), 24 hours following PS exposure in the bladder. Comparatively, we did not observe any contrast-enhanced signal intensities in the saline sham controls (Figs. 3C and D). Quantitative data on % changes in MRI signal intensities, at a consistent time-point of 12 min post-administration of Gd-DTPA, at 24 hours post PS exposure to the bladder, is shown in Fig. 4A. There was a significant increase in % change in MRI signal intensities associated with the colon (p<0.01) for PS exposed rats (65.2±17.1) compared to saline sham controls (20.8±9.8) (Fig. 4B). There were no significant differences in signal intensities in the surrounding colonic tissues (region 3 in Fig. 3Aii) or the medial thigh muscle for PS-administered animals compared to shams (Fig. 4A).
Figure 3.
CE-MRI visualization of increased contrast-related signal intensity in the colon following exposure of the bladder to PS. (A,B) Pre-PS bladder exposure CE images (i). (ii) Post-contrast images obtained 24 h post-PS bladder exposure. Increased Gd-DTPA contrast in colon mucosa (white arrows) and surrounding tissue (white arrow heads) is observed. (C,D) Contrast images 24 h post-saline administration (shams). Medial thigh muscle (1) and tissues surrounding the colon (3).
Figure 4.
(A) Quantitative assessment of increased contrast-enhancement in the colon following PS exposed bladders in rats. Percent (%) change in MRI signal intensity 24 h following either bladder exposure to PS or saline (n=5 for each group). Regions assessed: colon mucosa (white arrowhead), medial thigh muscle (1), and tissues surrounding the colon (3). (B) Representative % change in MRI signal intensity post-contrast for PS (closed diamonds) and saline (open squares) rats. Insets: Representative MR images of PS and saline-treated rat colons.
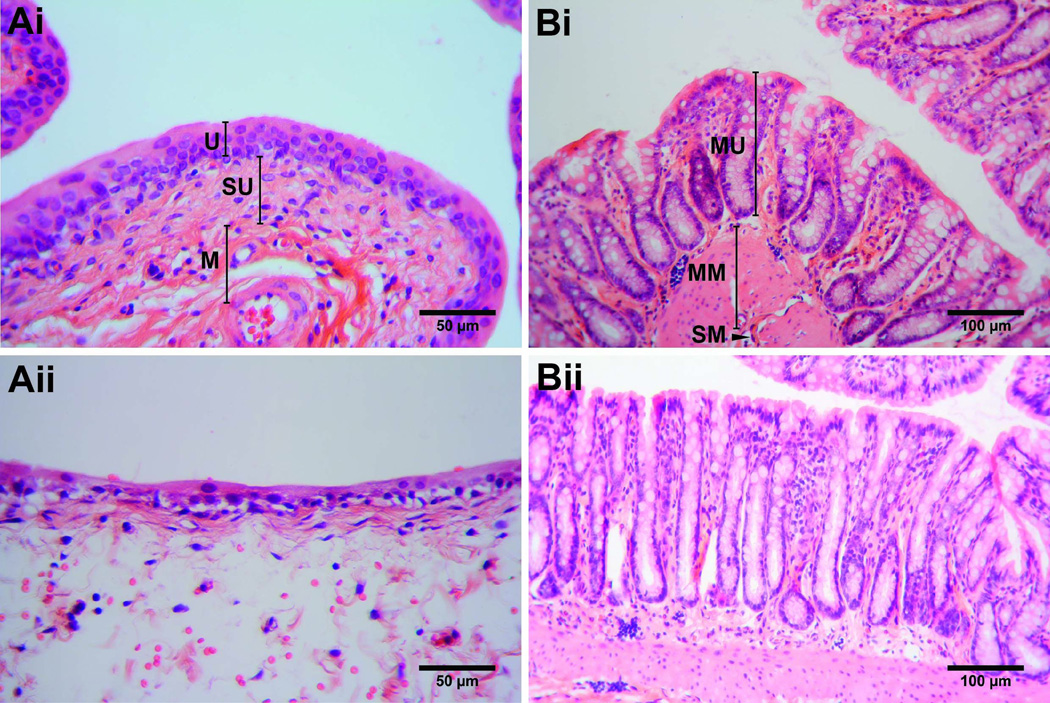
Regarding histological assessment, animals in which the bladders were treated with PS exhibited localized edema in the bladder (Fig. 5A). The sections of edema were mostly within the submucosa with intracellular edema in the urothelium. Minor infiltrates of eosinophils, neutrophils, and lymphoid cells were observed within areas of edema. This edematous response was localized to one area with the majority of the bladder appearing normal. While the urothelium of the PS-treated animals was thinned in the area of edema the tissue layer appeared continuous and intact. The colons from animals with PS-treated bladders appeared normal with no extraneous signs of damage or inflammation (Fig. 5B). The bladders (Fig. 5Ai) and colons (Fig. 5Bi) from animals with saline treated bladders appeared comparable to control tissues.
Figure 5.
Histology slides of rat bladders (A) either saline-treated (i) or PS-treated (24 h post treatment) (ii), and the colon (B) either saline-treated (i) or from animals with bladders exposed to PS (24 h post-treatment) (ii). Control bladder tissue obtained from a saline-treated mouse (40× magnification, 50 µm scale bar). Labels: Urothelium (U), Suburothelium (SU), and Muscle layer (M). Example of PS-treated bladder shown with localized edema in the suburothelium, and intracellular edema in the urothelium (40× magnification, 50 µm scale bar). Control colon tissue obtained from animal treated with saline in the bladder (20× magnification, 100 µm scale bar). Labels: Mucosa (MU), Muscularis mucosae (MM), and Submucosa (SM). Example of colon region of PS-treated bladder shown (20× magnification, 100 µm scale bar).
Discussion
In this study we have demonstrated that dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) can detect altered permeability in the bladder and bowel in an animal model. We suggest this could have two, important uses. First, MRI could be used to determine the degree to which increased bladder permeability is a factor in bladder disorders including IC/BPS, overactive bladder, neurogenic bladder and other bladder-related diseases. In our study we used a concentration of 0.034 mM (800 µL volume) Gd-DTPA which resulted in a hypo-intense MRI signal in the bladder in most cases (see Figs. 1Aiii, 1Bi-iii and 1Cii and iii), however subsequent leakage through the bladder urothelium resulted in a hyper-intense MRI signal (see Fig. 1Bi–iii for examples). This concentration of Gd-DTPA therefore allowed an enhanced contrast effect that was readily observed in a T1-weighted image following leakage of the contrast agent outside the bladder wall (Fig. 1B), which could also be quantified (Fig. 2A). Histological sections confirmed the presence of an edematous response in the bladder following treatment with PS (Fig. 5A). The kinetics data (Fig. 2B), which measured MR image signal intensities in selected ROIs outside the bladder wall, indicated a slight decrease in MRI image signal intensity change due to the dissipation of the Gd-DTPA contrast agent in the peritoneal cavity (see Fig. 1B and inset in Fig. 2B). MRI could also be used simultaneously to assess increased cross-talk between the bladder and the bowel, as measured by a secondary contrast-enhancement in the colon, indicating a breakdown of the colon’s impermeability barrier. A particular advantage is that this method will allow individual animals to be studied sequentially. In this study, we were able to obtain the colon-related data, following exposure of the bladder to PS, with the use of a subsequent administration of Gd-DTPA intravenously to monitor the uptake of the contrast agent into the colonic tissue (see Figs. 3Aii and Bii for visual examples and Fig. 4A for quantitative data). There seemed to be no evidence of colonic tissue damage from histological sections (Fig. 5B), however our group has evidence of increased permeability in the colon following bladder exposure to PS (data to be published elsewhere). Kinetic data over a 30 min time-course (Fig. 4B), of ROIs taken predominantly in the colon, was characterized by a rapid uptake of Gd-DTPA into the colon and approximately 50% elimination of the contrast agent approximately 25 min post-contrast injection.
Although the pathophysiological mechanism of IC/BPS is still unknown, it has been noted that the bladder urothelium is thinner2,22 and ulcerated23, has abnormal cell-to-cell adhesion, and impaired barrier function in these patients22. Urinary frequency and urgency has also been noted4. Alterations at the molecular level for IC/PBS patients seem to indicate a bladder epithelium cell defect and specifically altered epithelial cell gene expression (e.g. increased E-cadherin, inducible nitric oxide synthase (iNOS), and P2×2 and P2×3 receptors; and decreased uroplakin III (UPIII), zonula occludens type 1 (ZO-1), occluding and vimentin)23–26. Overexpression of microRNA miR-199a-5p, a regulator of intercellular junctions, in urothelial cells from IC/PBS patients, is thought to impair tight junction formation and subsequently lead to increased permeability27. It is known that chondroitin sulfate plays an important role in bladder barrier function28. In support of the role of chondroitin sulfate/GAG in IC/PBS, it has been shown that intravesical GAG replenishment therapy (chondroitin sulfate) reduced the recruitment of inflammatory cells in an acute, damaged bladder model (rat bladders exposed to 10 mM hydrochloric acid)29. However, IC/PBS is a heterogeneous disorder, and clinical trials show a notoriously high placebo effect as well as a significant fraction of non-responders30. Being able to separate patients into “leakers” and “non-leakers” by objective criteria could identify subcategories of IC/BPS that differ according to underlying etiology, natural history and responsiveness to various therapies. In other words, use of DCE-MRI may allow for the individualization of therapy leading to optimized outcomes for people with IC/BPS. Other bladder disorders including overactive bladder are suspected of involving increased permeability. MRI could therefore prove useful in validating this, which could identify targetable etiologies.
Conclusions
This study indicates that CE-MRI is capable of monitoring permeability alterations associated with the bladder urothelium, and might be considered as a clinical diagnostic method for IC/PBS. In addition, The CE-MRI method could also detect cross-talk associated between the bladder and the colon following bladder wall permeability alterations, and may provide a useful tool to further understand how bladder-related problems may also affect the colon.
Acknowledgements
Funding for the project was obtained from the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant no. P20DK097799 (REH).
References
- 1.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J. Urol. 2011;186:540. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cervigni M, Natale F. Gynecological disorders in bladder pain syndrome/interstitial cystitis patients. Int. J. Urol. 2014;21(Suppl 1):85–88. doi: 10.1111/iju.12379. [DOI] [PubMed] [Google Scholar]
- 3.Chrysanthopoulou EL, Doumouchtsis SK. Challenges and current evidence on the management of bladder pain syndrome. Neurol. Urodyn. 2013 doi: 10.1002/nau.22475. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Offiah I, McMahon SB, O’Reilly BA. Insterstitial cystitis/bladder pain syndrome: diagnosis and management. Int. Urogynecol. J. 2013;24:1243–1256. doi: 10.1007/s00192-013-2057-3. [DOI] [PubMed] [Google Scholar]
- 5.Teichman JM, Moldwin R. The role of the bladder surface in interstitial cystitis/painful bladder syndrome. Can. J. Urol. 2007;14:3599–3607. [PubMed] [Google Scholar]
- 6.Soler R, Bruschini H, Freire MP, et al. Urine is necessary to provoke bladder inflammation in protamine sulfate induced urothelial injury. J. Urol. 2008;180:1527–1531. doi: 10.1016/j.juro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Dyer AJ, Twiss CO. Painful bladder syndrome: an update and review of current management strategies. Curr. Urol. Rep. 2014;15:384. doi: 10.1007/s11934-013-0384-z. [DOI] [PubMed] [Google Scholar]
- 8.Bains LJ, McGrath DM, Naish JH, et al. Tracer kinetic analysis of dynamic contrast-enhanced MRI and CT bladder cancer data: A preliminary comparison to assess the magnitude of water exchange effects. Magn. Reson. Med. 2010;64:595–603. doi: 10.1002/mrm.22430. [DOI] [PubMed] [Google Scholar]
- 9.Shi Z, Yang Z, Zhang G, et al. Characterization of texture features of bladder carcinoma and the bladder wall on MRI: initial experience. Acad. Radiol. 2013;20:930–938. doi: 10.1016/j.acra.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghafoori M, Shakiba M, Ghiasi A, et al. Value of MRI in local staging of bladder cancer. Urol. J. 2013;10:866–872. [PubMed] [Google Scholar]
- 11.Wu LM, Chen XX, Xu JR, et al. Clinical value of T2-weighted imaging combined with diffusion-weighted imaging in preoperative T staging of urinary bladder cancer: a large-scale, multiobserver prospective study on 3.0-T MRI. Acad. Radiol. 2013;20:939–946. doi: 10.1016/j.acra.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Liang Z, Zhu H, et al. Bladder wall thickness mapping for magnetic resonance cystography. Phys. Med. Biol. 2013;58:5173–5192. doi: 10.1088/0031-9155/58/15/5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggio A, Carillo V, Cozzarini C, et al. Impact of the radiotherapy technique on the correlation between dose-volume histograms of the bladder wall defined on MRI imaging and dose-volume/surface histograms in prostate cancer patients. Phys. Med. Biol. 2013;58:N115–N123. doi: 10.1088/0031-9155/58/7/N115. [DOI] [PubMed] [Google Scholar]
- 14.Niku SD, Stein PC, Scherz HC, et al. A new method for cytodestruction of bladder epithelium using protamine sulfate and urea. J..Urol. 1994;152:1025–1028. doi: 10.1016/s0022-5347(17)32648-4. [DOI] [PubMed] [Google Scholar]
- 15.Soler R, Bruschini H, Freire MP, et al. Urine is necessary to provoke bladder inflammation in protamine sulfate induced urothelial injury. J. Urol. 2008;180:1527–1531. doi: 10.1016/j.juro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Stemler KM, Crock LW, Lai HH, et al. Protamine sulfate induced bladder injury protects from distention induced bladder pain. J. Urol. 2013;189:343–351. doi: 10.1016/j.juro.2012.08.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser PJ, Dozmorov MG, Bane BL, et al. Abnormal expression of differentiation related proteins and proteoglycan core proteins in the urothelium of patients with interstitial cystitis. J. Urol. 2008;179:764–769. doi: 10.1016/j.juro.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bullones Rodriguez MA, Afari N, Buchwald DS. Evidence for overlap between urological and nonurological unexplained clinical conditions. J. Urol. 2013;189:S66–S74. doi: 10.1016/j.juro.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malykhina AP, Qin C, Greenwood-Van MB, et al. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol. Motil. 2006;18:936–948. doi: 10.1111/j.1365-2982.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald JJ, Ustinova E, Koronowski KB, et al. Evidence for the role of mast cells in colon-bladder cross organ sensitization. Auton. Neurosci. 2013;173:6–13. doi: 10.1016/j.autneu.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fennessy FM, Fedorov A, Gupta SN, et al. Practical considerations in T1 mapping of prostate for dynamic contrast enhancement pharmacokinetic analyses. Magn. Reson. Imaging. 2012;30:1224–1233. doi: 10.1016/j.mri.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birder LA, Hanna-Mitchell AT, Mayer E, et al. Cystitis, co-morbid disorders and associated epithelial dysfunction. Neurourol Urodyn. 2011;30:668–672. doi: 10.1002/nau.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keay S, Leitzell S, Ochrzcin A, et al. A mouse model for interstitial cystitis/painful bladder syndrome based on APF inhibition of bladder epithelial repair: a pilot study. BMC Urology. 2012;12:17. doi: 10.1186/1471-2490-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keay S, Zhang C-O, Shoenfelt JL, et al. Decreased in vitro proliferation of bladder epithelial cells from patients with interstitial cystitis. Urology. 2003;61:1278–1284. doi: 10.1016/s0090-4295(03)00005-0. [DOI] [PubMed] [Google Scholar]
- 25.Hurst RE, Moldwin RM, Mulholland SG. Bladder defense molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology. 2007;69(Suppl 4A):17–23. doi: 10.1016/j.urology.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Chai TC. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J. Urol. 2004;17:448–452. doi: 10.1097/01.ju.0000099660.46774.3c. [DOI] [PubMed] [Google Scholar]
- 27.Monastyrskaya K, Sánchez-Freire V, Hashemi Gheinani A, et al. miR-199a-5p regulates urothelial permeability and may play a role in bladder pain syndrome. Am J Pathol. 2013;182:431–448. doi: 10.1016/j.ajpath.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Janssen DA, van Wijk XM, Jansen KC, et al. The distribution and function of chondroitin sulfate and other sulfated glycosaminoglycans in the human bladder and their contribution to the protective bladder barrier. J. Urol. 2013;189:336–342. doi: 10.1016/j.juro.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Engles CD, Hauser PJ, Abdullah SN, et al. Intravesical chondroitin sulfate inhibits recruitment of inflammatory cells in an acute acid damage “leaky Bladder” model of cystitis. Urology. 2012;79:483.e13–483.e17. doi: 10.1016/j.urology.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theoharides TC, Whitmore K, Stanford E, et al. Interstitial cystitis: bladder pain and beyond. Expert Opin. Pharmacother. 2008;9:2979–2994. doi: 10.1517/14656560802519845. [DOI] [PubMed] [Google Scholar]