Abstract
The aim of this study was to test the effects of B-group vitamin supplements on wound healing in diabetic mice. The mice in the experimental group were treated daily with 1 g/L B6, 1.25 mg/L B12, and 62.5 mg/L folic acid in their drinking water. Full-thickness excision wounds were created with 6-mm skin biopsy punches. Each wound closure was digitally photographed. Beginning on day 3 after wounding, the wound area in the diabetic mice was statistically larger than that of normal mice (p<0.05 vs diabetic mice). The diabetic mice treated with B vitamins displayed accelerated wound closure on day 3 (wound area 42.8 ± 11.3%, p<0.05). On day 9 after wounding, the wound area in the diabetic mice was also statistically larger than that of normal mice (p<0.05 vs diabetic mice). The diabetic mice treated with B vitamins displayed accelerated wound closure on day 3 (wound area 13.2 ± 16.8%, p<0.05). In addition, the high glucose level in the diabetic animals decreased significantly in response to B vitamin treatment. In conclusion, the results of this study indicate that B vitamin supplementation may improve wound healing in diabetic mice.
Keywords: B vitamin supplementation, wound healing, diabetic mice, glucose level, TNF-α
Introduction
Diabetes mellitus (DM) is characterized by a host of complications that can affect many organs. Delayed wound healing is a major complication of diabetes that can enhance overall morbidity and mortality in diabetics.(1,2) Impaired wound healing is characterized by delayed cellular infiltration and granulation tissue formation, decreased collagen organization, and reduced angiogenesis.(3,4) The process of wound healing is a dynamic series of events involving inflammation, proliferation, maturation, and remodeling. In diabetes, there is a delayed influx of inflammatory cells into the wound site initially, but when inflammatory conditions become established, inflammatory cells prevent the deposition of matrix components and remodeling. It has been suggested that ‘receptor for advanced glycation end-products’ (RAGE) is involved in this sustained inflammatory response. Indeed, there is enhanced expression of RAGE in slow-healing wounds in diabetic mice.(5)
Nutritional status has profound effects on immune responses.(6) Strategies to prevent complications of diabetes, including nutritional and therapeutic support related to B vitamin supplementation, have emerged recently.(7) Several studies have shown protective effects of B vitamins in diabetic patients.(8) A previous study showed that the circulating and tissue concentrations of water-soluble vitamins were significantly decreased in diabetic subjects.(9) In animal models, diabetes lowered the folate, vitamin B6, and vitamin B12 levels significantly in kidney, heart, liver, and muscle.(10) These results indicate that diabetes causes depression B vitamin in sufficiency, and combinational supplementation of B vitamins may be beneficent for wound healing in diabetes. The aim of this study was to test the effects of B-group vitamin supplements on wound healing in diabetic mice. Some studies showed the association of decreased serum levels of B vitamin and hyperhomocysteinemia. Hyperhomocysteinemia is associated with several microvascular diseases, such as coronary artery disease, cerebrovascular disease, peripheral arterial disease, and deep-vein thrombosis.(11–13) Folic acid, vitamin B12 and vitamin B6 are cofactors for the enzymes involved in homocysteine metabolism. Folic acid, vitamin B12 and vitamin B6 deficiency and reduced enzyme activities inhibit the breakdown of homocysteine.(14) Supplementation with these nutrients was shown to lower homocysteine levels in a number of clinical trials.(15)
Cystathionine β-synthase (CBS) catalyzes the first step of homocysteine transsulfuration as a rate-limiting enzyme. Genetically increased CBS expression would benefit from the protection due to the low homocysteine levels.(16) We also examined CBS expression in liver.
Materials and Methods
Animals
Male 6-week-old KKAy (diabetic) and C57BL/6 (normal) mice were obtained from CLEA Japan (Tokyo, Japan). In this study, 15 and 17 mice of each strain were used. Type 2 diabetes is characterized by impaired insulin sensitivity and the resulting dysregulation of glucose and lipid metabolism. KKAy mice exhibit morbid obesity and metabolic abnormalities, including hyperglycemia, and are known to serve as an excellent model of type 2 diabetes mellitus.(17) The mice were housed individually at a constant temperature (23 ± 2°C) and 55 ± 5% relative humidity, under a 12-h/12-h light/dark cycle (lights on at 07:00), and had free access to food and water. The body weight and blood glucose level of the mice were measured weekly. At the age of 10 weeks, the mice were used in the following experiments. The mice were separated randomly into four groups, as follows: control normal mice, experimental normal mice, control diabetic mice and experimental diabetic mice. The mice in the experimental group were treated daily with 1 g/L B6 (Sigma-Aldrich, St. Louis, MO), 1.25 mg/L B12 (Sigma-Aldrich), and 62.5 mg/L folic acid (Sigma-Aldrich) in their drinking water and studied 4 weeks after exposure to the B vitamins to determine the effects on wound healing. Intake of B6, B12 and folic acid from food were 0.05 mg, 0.33 µg and 0.01 mg, respectively. Intake of B6, B12 and folic acid from supplemented drinking water were 8 mg, 0.01 mg and 0.5 mg, respectively. At 2 weeks after B vitamin exposure (at the age of 12 weeks), the mice were anesthetized by an intraperitoneal injection of sodium pentobarbital (Somnopentyl; 50 mg/kg body weight; Schering-Plough, Munich, Germany). An intraperitoneal injection of 0.1 ml of lidocaine (Xylocaine, 1:8 dilution; Astra Zeneca, Osaka, Japan) was administered to control bleeding and provide additional anesthesia. The animal’s dorsal hair was shaved and two full-thickness excision wounds were created with 6-mm skin biopsy punches (Kai Medical, Solingen, Germany). Each wound closure was digitally photographed at the indicated time points (0, 1, 3, 7, 9, 11 and 14 days). The wound areas were quantified using Adobe Photoshop Elements 2.0. All procedures were approved by the Animal Experimentation Committee at Nihon University School of Dentistry, Tokyo, Japan.
Body weight and blood glucose
We observed the general condition of the mice and measured their body weights and blood glucose levels. Peripheral venous blood was taken from the tail of each mouse every week after 12 h of fasting. The fasting plasma glucose level was measured with a glucose measuring device (Bayer Medical, Tuttlingen, Germany).
Enzyme-linked immunosorbent assay (ELISA)
The levels of TNF-α and IL-6 in the serum of the mice were determined by ELISAs using commercially available kits (BioSource International, Inc., Camarillo, CA), according to the manufacturer’s instructions.
Real-time PCR
For RNA isolation, liver was fixed immediately in RNA stabilization reagent (RNAlater; Qiagen, Valencia, CA), and the samples were homogenized. Total RNA was extracted using an RNeasy Mini kit (Qiagen). Complementary DNA was synthesized using a Ready-To-Go T-Primed First-Strand kit (Amersham Biosciences, Tokyo, Japan). The primer and probe sets for TNF-α, IL-6 and cystathionine β-synthase (CBS) were from Applied Biosystems (Foster City, CA). Real-time PCR was performed on an ABI PRISM 7700 Sequence Detector (Applied Biosystems) using the following parameters: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and primer extension at 60°C for 1 min. The expression level of each gene was first normalized to that of the glyceraldehyde-3-phosphate dehydrogenase gene in the same sample. The data are shown as means ± SD.
Statistical analysis
The data were analyzed using SPSS software (ver. 16.0 for Windows; SPSS Inc., Chicago, IL). Statistical analyses were performed using Tukey-Kramer test.
Results
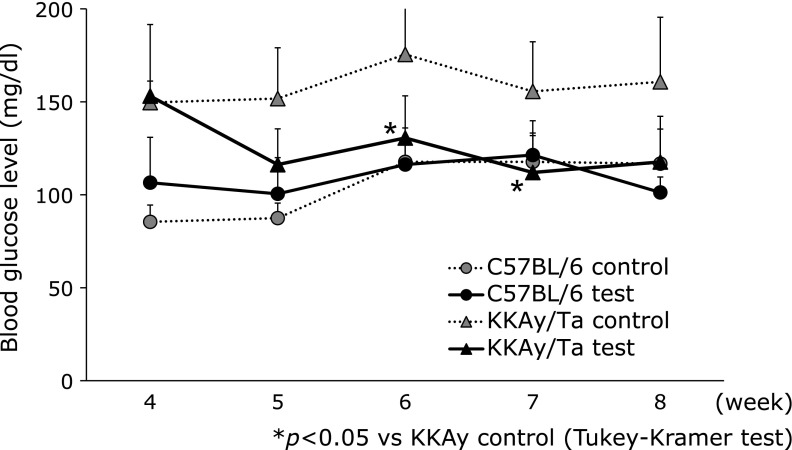
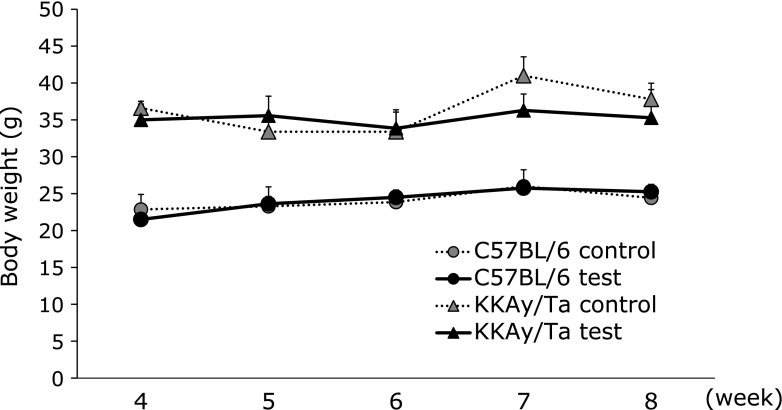
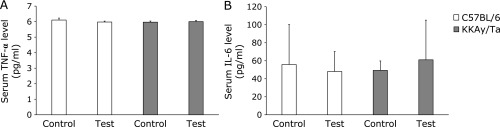
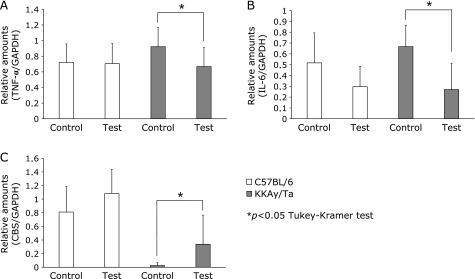
Fig. 1 shows the effects of B vitamins on glucose levels. The high glucose level in the diabetic animals decreased significantly in response to B vitamin treatment. B vitamin therapy showed no apparent effect on body weight in normal or diabetic mice (Fig. 2). We examined serum TNF-α and IL-6 levels using ELISAs on day 14. However, no significant change in serum TNF-α or IL-6 levels was observed in the B vitamin-treated diabetic mice (Fig. 3). Next, real-time PCR was used to measure the levels of TNF-α and IL-6 mRNA expression in liver. A significant reduction in the expression of these genes was observed in the B vitamin-treated diabetic mice (Fig. 4A and B). The levels of cystathionine β-synthase (CBS) mRNA expression in liver significantly increased in the B vitamin-treated diabetic mice compared with control diabetic mice (Fig. 4C).
Fig. 1.
Effect of B vitamin supplementation on blood glucose level in C57BL/6 and KKAy/Ta mice. Peripheral blood was taken from the tail vein and the fasting blood glucose level was measured on every weeks. *p<0.05 (Tukey-Kramer test), C57BL/6 control, control C57BL/6 mice (n = 7); KKAy/Ta control, control KKAy/Ta mice (n = 8); C57BL/6 test, C57BL/6 mice treated with B-vitamins (n = 8); KKAy/Ta test, KKAy/Ta mice treated with B-vitamins (n = 9).
Fig. 2.
Effect of B vitamin supplementation on body weight in C57BL/6 and KKAy/Ta mice. Body weight was measured on every weeks. C57BL/6 control, control C57BL/6 mice (n = 7); KKAy/Ta control, control KKAy/Ta mice (n = 8); C57BL/6 test, C57BL/6 mice treated with B-vitamins (n = 8); KKAy/Ta test, KKAy/Ta mice treated with B-vitamins (n = 9).
Fig. 3.
Effect of B vitamin supplementation on serum levels of cytokines in C57BL/6 and KKAy/Ta mice. Blood was taken from cardiac puncture and the serum levels of (A) tumor necrosis factor-α (TNF-α) and (B) interleukin-6 (IL-6) were measured by enzyme-linked immunosorbent assay. C57BL/6 control, control C57BL/6 mice (n = 7); KKAy/Ta control, control KKAy/Ta mice (n = 8); C57BL/6 test, C57BL/6 mice treated with B-vitamins (n = 8); KKAy/Ta test, KKAy/Ta mice treated with B-vitamins (n = 9).
Fig. 4.
Effect of B vitamin supplementation on mRNA expression in liver of C57BL/6 and KKAy/Ta mice. (A) tumor necrosis factor-α (TNF-α), (B) interleukin-6 (IL-6), (C) cystathionine β-synthase (CBS) mRNA expression in liver were measured using real-time polymerase chain reaction. The results were normalized by reference to the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). *p<0.05 (Tukey-Kramer test), C57BL/6 control, control C57BL/6 mice (n = 7); KKAy/Ta control, control KKAy/Ta mice (n = 8); C57BL/6 test, C57BL/6 mice treated with B-vitamins (n = 8); KKAy/Ta test, KKAy/Ta mice treated with B-vitamins (n = 9).
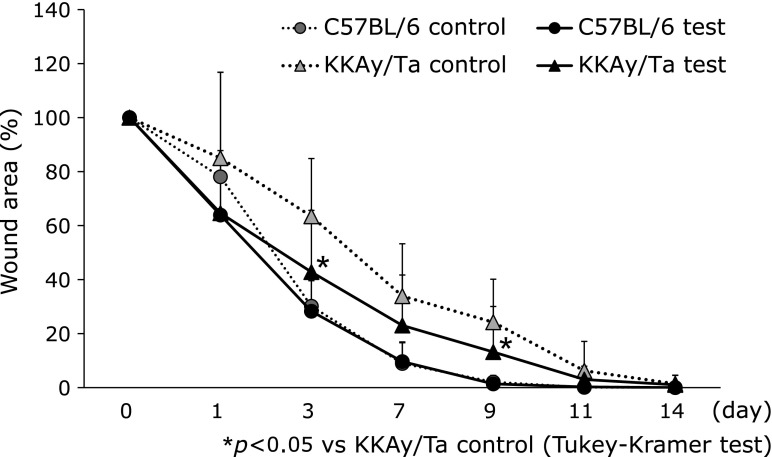
Full-thickness punch biopsy wounds (6 mm) were created on two sites of the shaved back of normal and diabetic mice, and the wounded areas were monitored serially (Fig. 5). Beginning on day 3 after wounding, the wound area in the diabetic mice was 63.4 ± 21.4%, compared with 30.2 ± 12.9% in the normal mice (p<0.05). The diabetic mice treated with B vitamins displayed accelerated wound closure on day 3 (wound area 42.8 ± 11.3%, p<0.05). On day 9 after wounding, the wound area in the diabetic mice was 24.2 ± 15.9%, compared with 2.1 ± 2.4% in the normal mice (p<0.05). The diabetic mice treated with B vitamins displayed accelerated wound closure on day 3 (wound area 13.2 ± 16.8%, p<0.05). The area under the curves (AUC) of control normal mice, experimental normal mice, control diabetic mice and experimental diabetic mice were 20.6 ± 7.7%, 18.7 ± 5.3%, 38.3 ± 11.9% and 27.0 ± 12.3%, respectively. It was found that the AUC of experimental diabetic mice was significantly lower than that of control diabetic mice (p<0.05).
Fig. 5.
Effect of B vitamin supplementation on wound healing in C57BL/6 and KKAy/Ta mice. At 2 weeks after B vitamin exposure, two full-thickness excision wounds were created with 6-mm skin biopsy punches. Each wound closure was digitally photographed at the indicated time points (0, 1, 3, 7, 9, 11 and 14 days). The wound areas were quantified using Adobe Photoshop Elements 2.0. *p<0.05 vs C57BL/6 control Day 3 (Tukey-Kramer test), C57BL/6 control, control C57BL/6 mice (n = 7); KKAy/Ta control, control KKAy/Ta mice (n = 8); C57BL/6 test, C57BL/6 mice treated with B-vitamins (n = 8); KKAy/Ta test, KKAy/Ta mice treated with B-vitamins (n = 9).
Discussion
This study investigated the effects of B vitamins on wound healing in diabetic mice. The concentration of B vitamins in the drinking water has been described previously.(18–20) Some studies found that the combinatorial supplementation of B vitamins may be more effective.(18) Several studies have shown the potential role of vitamin B6 supplementation against the effects of oxidative stress.(21) Increased plasma and tissue lipid oxidation has also been reported in rats receiving a B6-deficient diet.(22) Vitamin B6 inhibits the formation of advanced glycation end products (AGEs). Vitamin B6 appears to act by a mechanism analogous to that of AGE breakers (i.e., by reaction with dicarbonyl intermediates in AGE formation).(23) These observations indicate that vitamin B6 may be beneficial for wound healing in diabetics.
In patients with diabetes, elevated homocysteine (Hcy) levels have been reported to be associated with endothelial dysfunction.(24–26) Elevated Hcy levels can decrease the endothelium-derived signaling molecule nitric oxide, which, in turn, regulates vascular function. It has been reported that hyperhomocysteinemia is associated with microvascular failure.(27) Hcy can be methylated to form methionine, catalyzed by the enzyme methionine synthase and folic acid and vitamin B12 cofactors.(28) The conversion of Hcy to cystathionine is catalyzed by systathionine β-synthase in the liver.(29,30) Our results also showed that cystathionine β-synthase mRNA expression in liver of diabetic mice was increased by B vitamin supplementation. One possible explanation is that B vitamin supplementation may improve microcirculation in the wound healing process via Hcy reduction.
In this study, blood glucose levels were reduced significantly by B vitamin supplementation at weeks 2 and 3. No significant difference was observed in body weight. A previous human study also showed that B vitamin supplementation for 8 weeks in adults with metabolic syndrome resulted in improved fasting blood glucose and insulin levels.(31) Although the mechanisms by which B vitamins decrease blood glucose are not clearly understood, several hypotheses have been suggested. One possible mechanism is that Hcy may inhibit insulin-stimulated tyrosine phosphorylation of the insulin receptor subunit.(32) Another possibility is that B vitamins lower oxidative stress, which, in turn, reduces inflammatory activity such as TNF-α production in the liver. In this study, a significant reduction in the expression of TNF-α mRNA was observed in the B vitamin-treated diabetic mice liver. However, no significant change in serum TNF-α level was observed. This indicates that B vitamin supplementation has limited effectiveness against inflammatory cytokine expression. In this study, the high glucose level in the diabetic animals decreased significantly in response to B vitamin treatment. However, wound closure in the B vitamin-treated diabetic mice was delayed compared to normal mice. Hyperglycemia could directly contribute to poor wound healing in diabetes as well as indirectly by glycation. (33) Long-term administration of B vitamins might be beneficial to improve diabetic complications.
In conclusion, the results of this study indicate that B vitamin supplementation may improve wound healing in diabetic mice. B vitamins may also stimulate glucose utilization. Furthermore, B vitamins may reduce oxidative stress and result in TNF-α expression in liver. Various questions remain about the connection between B vitamins and diabetic complications. In addition, dose of supplementation of this study was extremely higher than reference nutrient intake. Further study is needed to determine the mechanisms underlying such effects and appropriate dose of supplementation.
Acknowledgments
This study was supported by the JSPS KAKENHI (Grant-in-Aid for Scientific Research B) Grant Number 25862058 (to M. T.) and a special research grant from the Promotion and Mutual Aid Corporation for Private Schools of Japan and Sato Fund, Nihon University School of Dentistry.
Abbreviations
- CBS
cystathionine β-synthase
- DNA
deoxyribonucleic acid
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- Hcy
homocysteine
- IL
interleukin
- RNA
ribonucleic acid
- TNF
tumor necrosis factor
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 2.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 3.Goodson WH, 3rd, Hunt TK. Studies of wound healing in experimental diabetes mellitus. J Surg Res. 1977;22:221–227. doi: 10.1016/0022-4804(77)90137-8. [DOI] [PubMed] [Google Scholar]
- 4.Bohlen HG, Niggl BA. Adult microvascular disturbances as a result of juvenile-onset diabetes in Db/Db mice. Blood Vessels. 1979;16:269–276. doi: 10.1159/000158215. [DOI] [PubMed] [Google Scholar]
- 5.Goova MT, Li J, Kislinger T, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Patholo. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcos A, Nova E, Montero A. Changes in the immune system are conditioned by nutrition. Eur J Clin Nutr. 2003;57 Suppl 1:S66–S69. doi: 10.1038/sj.ejcn.1601819. [DOI] [PubMed] [Google Scholar]
- 7.Martini LA, Catania AS, Ferreira SR. Role of vitamins and minerals in prevention and management of type 2 diabetes mellitus. Nutr Rev. 2010;68:341–354. doi: 10.1111/j.1753-4887.2010.00296.x. [DOI] [PubMed] [Google Scholar]
- 8.Sudchada P, Saokaew S, Sridetch S, Incampa S, Jaiyen S, Khaithong W. Effect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2012;98:151–158. doi: 10.1016/j.diabres.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Mooradian AD, Failla M, Hoogwerf B, Maryniuk M, Wylie-Rosett J. Selected vitamins and minerals in diabetes. Diabetes Care. 1994;17:464–479. doi: 10.2337/diacare.17.5.464. [DOI] [PubMed] [Google Scholar]
- 10.Reddi AS, Jyothirmayi GN, DeAngelis B, Frank O, Baker H. Tissue concentrations of water-soluble vitamins in normal and diabetic rats. Int J Vitam Nutur Res. 1993;63:140–144. [PubMed] [Google Scholar]
- 11.Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc. 2008;83:1203–1212. doi: 10.4065/83.11.1203. [DOI] [PubMed] [Google Scholar]
- 12.Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 13.Khandanpour N, Loke YK, Meyer FJ, Jennings B, Armon MP. Homocysteine and peripheral arterial disease: systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2009;38:316–322. doi: 10.1016/j.ejvs.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Stanger O, Herrmann W, Pietrzik K, et al. Clinical use and rational management of homocysteine, folic acid, and B vitamins in cardiovascular and thrombotic disease. Z Kardiol. 2004;93:439–453. doi: 10.1007/s00392-004-0075-3. [DOI] [PubMed] [Google Scholar]
- 15.Clarke R, Armitage J. Vitamin supplements and cardiovascular risk: review of the randomized trials of homocysteine-lowering vitamin supplements. Semin Thromb Hemost. 2000;26:341–348. doi: 10.1055/s-2000-8101. [DOI] [PubMed] [Google Scholar]
- 16.Zhao JY, Yang XY, Shi SN, et al. A functional variant in the cystathionine β-synthase gene promoter significantly reduces congenital heart disease susceptibility in a Han Chinese population. Cell Res. 2013;23:242–253. doi: 10.1038/cr.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki H, Shino A, Suzuoki Z. General survey of diabetic features of yellow KK mice. Endocrinology. 1970;17:23–35. doi: 10.1507/endocrj1954.17.23. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Li Y, Tang Y, et al. The maternal combined supplementation of folic acid and Vitamin B12 suppresses ethanol-induced developmental toxicity in mouse fetuses. Reprod Toxicol. 2006;22:56–61. doi: 10.1016/j.reprotox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Chang KC, Liang JT, Tsai PS, Wu MS, Hsu KL. Prevention of arterial stiffening by pyridoxamine in diabetes is associated with inhibition of the pathogenic glycation on aortic collagen. Br J Pharmacol. 2009;157:1419–1426. doi: 10.1111/j.1476-5381.2009.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roe ND, He EY, Wu Z, Ren J. Folic acid reverses nitric oxide synthase uncoupling and prevents cardiac dysfunction in insulin resistance: role of Ca2+/calmodulin-activated protein kinase II. Free Radic Biol Med. 2013;65:234–243. doi: 10.1016/j.freeradbiomed.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benderitter M, Hadj-Saad F, Lhuissier M, Maupoil V, Guilland JC, Rochette L. Effects of exhaustive exercise and vitamin B6 deficiency on free radical oxidative process in male trained rats. Free Radic Biol Med. 1996;21:541–549. doi: 10.1016/0891-5849(96)00047-0. [DOI] [PubMed] [Google Scholar]
- 22.Cabrini L, Bergami R, Fiorentini D, Marchetti M, Landi L, Tolomelli B. Vitamin B6 deficiency affects antioxidant defences in rat liver and heart. Biochem Mol Biol Int. 1998;46:689–697. doi: 10.1080/15216549800204222. [DOI] [PubMed] [Google Scholar]
- 23.Metz TO, Alderson NL, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: a novel therapy for treatment of diabetic complications. Arch Biochem Biophys. 2003;419:41–49. doi: 10.1016/j.abb.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–881. [PubMed] [Google Scholar]
- 25.Perry IJ, Refsum H, Morris RW, Ebrahim SB, Ueland PM, Shaper AG. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet. 1995;346:1395–1398. doi: 10.1016/s0140-6736(95)92407-8. [DOI] [PubMed] [Google Scholar]
- 26.van den Berg M, Stehouwer CD, Bierdrager E, Rauwerda JA. Plasma homocysteine and severity of atherosclerosis in young patients with lower-limb atherosclerotic disease. Arterioscler Thromb Vasc Biol. 1996;16:165–171. doi: 10.1161/01.atv.16.1.165. [DOI] [PubMed] [Google Scholar]
- 27.Kamburoğlu HO, Uzun H, Bitik O, et al. The effects of hyperhomocysteinemia on the microcirculation of skin flaps. Plast Reconstr Surg. 2011;128:124e–130e. doi: 10.1097/PRS.0b013e318221db4d. [DOI] [PubMed] [Google Scholar]
- 28.Strain JJ, Dowey L, Ward M, Pentieva K, Mcnulty H. B-vitamins, homocysteine metabolism and CVD. Proc Nutr Soc. 2004;63:597–603. doi: 10.1079/pns2004390. [DOI] [PubMed] [Google Scholar]
- 29.Ratnam S, Maclean KN, Jacobs RL, Brosnan ME, Kraus JP, Brosnan JT. Hormonal regulation of cystathionine β-synthase expression in liver. J Biol Chem. 2002;277:42912–42918. doi: 10.1074/jbc.M206588200. [DOI] [PubMed] [Google Scholar]
- 30.Schalinske KL. Interrelationship between diabetes and homocysteine metabolism: hormonal regulation of cystathionine β-synthase. Nutr Rev. 2003;61:136–138. doi: 10.1301/nr.2003.apr.136-138. [DOI] [PubMed] [Google Scholar]
- 31.Setola E, Monti LD, Galluccio E, et al. Insulin resistance and endothelial function are improved after folate and vitamin B12 therapy in patients with metabolic syndrome: relationship between homocysteine levels and hyperinsulinemia. Eur J Endocrinol. 2004;151:483–489. doi: 10.1530/eje.0.1510483. [DOI] [PubMed] [Google Scholar]
- 32.Najib S, Sánchez-Margalet V. Homocysteine thiolactone inhibits insulin signaling, and glutathione has a protective effect. J Mol Endocrinol. 2001;27:85–91. doi: 10.1677/jme.0.0270085. [DOI] [PubMed] [Google Scholar]
- 33.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006;23:594–608. doi: 10.1111/j.1464-5491.2006.01773.x. [DOI] [PubMed] [Google Scholar]