Abstract
Ascorbic acid is an important antioxidant that plays an essential role in the biosynthesis of numerous bioactive substances. The detection of ascorbic acid has traditionally been achieved using high-performance liquid chromatography and absorption spectrophotometry assays. However, the development of fluorescence probes for this purpose is highly desired because they provide a much more convenient and highly sensitive technique for the detection of this material. OFF-ON-type fluorescent probes have been developed for the detection of non-fluorescent compounds. Photo-induced electron transfer and fluorescence resonance energy transfer are the two main fluorescence quenching mechanisms for the detection of ascorbic acid, and several fluorescence probes have been reported based on redox-responsive metals and quantum dots. Profluorescent nitroxide compounds have also been developed as non-metal organic fluorescence probes for ascorbic acid. These nitroxide systems have a stable unpaired electron and can therefore react with ascorbic acid and a strong fluorescence quencher. Furthermore, recent synthetic advances have allowed for the synthesis of α-substituted nitroxides with varying levels of reactivity towards ascorbic acid. In this review, we have discussed the design strategies used for the preparation of fluorescent probes for ascorbic acid, with particular emphasis on profluorescent nitroxides, which are unique radical-based redox-active fluorescent probes.
Keywords: ascorbic acid, fluorescence, probe, nitroxide, antioxidant
Introduction
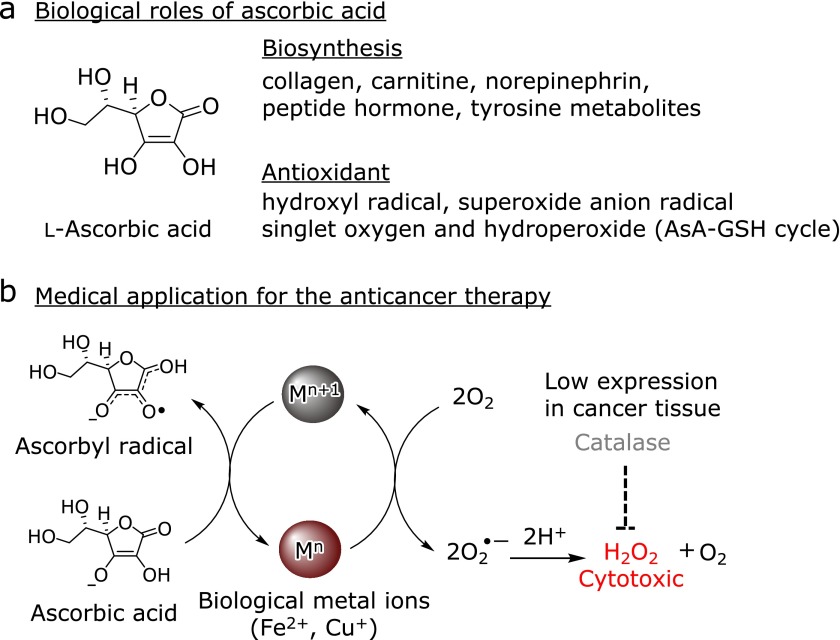
Ascorbic acid is a small bioactive molecule that performs a variety of different physiological functions in vivo (Fig. 1a).(1) For example, ascorbic acid plays an important role in the biosynthesis of several bioactive substances, including collagen, carnitine, norepinephrine, peptide hormones, and tyrosine metabolites.(2,3) Furthermore, ascorbic acid acts as an essential antioxidant in humans, where it is involved in the scavenging of reactive oxygen species (ROS), such as hydroxyl radicals, superoxide anion radicals, and singlet oxygen.(4,5) Ascorbic acid also functions as an electron donor in the single-electron reduction of α-tocopheroxyl radicals to α-tocopherol in the inhibition process of lipid peroxidation.(6) The resulting oxidized ascorbic acid (dehydroascorbic acid) is subsequently converted back to ascorbic acid though the ascorbic acid-glutathione cycle.(7,8) Ascorbic acid is therefore considered to be an essential molecule for the maintenance of human health and the normal biological function of our bodies. Notably, reduced levels of ascorbic acid have been reported to be closely related to the pathogenesis and exacerbation of several disease states, including diabetes mellitus,(9) cognitive impairment,(10) coronary heart disease,(11) and age-related cataracts(12). Furthermore, research conducted in Europe has revealed a significant correlation between the level of ascorbic acid in the plasma samples of 19,068 British men and women and their fat distribution.(13)
Fig. 1.
Biological roles and medical applications of ascorbic acid. (a) Roles of ascorbic acid in vivo as an enzymatic cofactor in numerous biosynthetic pathways and an antioxidant for reactive oxygen species. (b) Anticancer therapy based on ascorbic acid and biological metal ions.
Ascorbic acid has also attracted considerable attention as an anticancer agent (Fig. 1b).(14,15) This strategy is based on the generation of hydroperoxides using biologically derived Fe and Cu ions following the intravenous injection of ascorbic acid. Notably, the use of this strategy in conjunction with conventional chemotherapeutic approaches has been reported to enhance the effects of these treatments and suppresses the occurrence of side effects.(16,17) The intravenous administration of ascorbic acid has also been reported to exhibit remarkable curative effects in several diseases.(18) For this type of therapeutic strategy to be successful, the level of ascorbic acid in systemic circulation has to be kept at a high concentration. Therefore, quantitative analysis methods of ascorbic acid used for clinical samples such as blood and urine are required.
A variety of different methods have been reported for the quantitative analysis of ascorbic acid, including high-performance liquid chromatography with electrochemical detection (HPLC-ECD)(19) and a colorimetric assay based on 2,6-dichlorophenol-indophenol (DCPIP).(20) Fluorometric analysis is recognized as a convenient and highly sensitive technique for the detection of bioactive small molecules.(21,22) However, the measurement of a non-fluorescent compound such as ascorbic acid using a fluorometric assay would require the use of a “fluorescent probe”. In this way, the reaction of a fluorescent probe with a non-fluorescent target molecule would lead to a change in the optical properties of the probe, which could be detected by fluorometric analysis. In this review, we have introduced several fluorescent probes for the detection of ascorbic acid. Notably, we have placed particular emphasis on the use of profluorescent nitroxide, which is a unique radical-based redox-active fluorescent probe.
Fluorometric Analysis Method of Ascorbic Acid using Fluorescent Probes
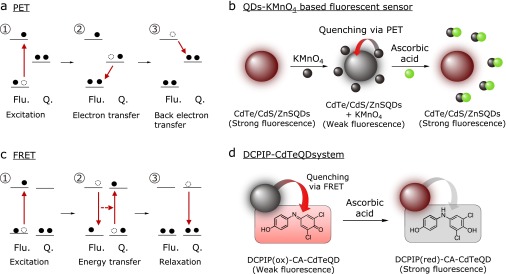
Molecular detection systems based on fluorescent probes are promising analytical methods that can be used to develop a deeper understanding of complex biological processes.(21,22) In particular, “OFF-ON-type fluorescent probes” can interact with target molecules to significantly enhance their fluorescent emission characteristics. In most cases, OFF-ON-type fluorescent probes consist of a “fluorescent sensor” and a “fluorescent switching” moiety. When these systems are in the resting state, the emission of the fluorescent sensor moiety is usually quenched by the fluorescent switching moiety. However, the reaction of the fluorescent switching moiety with a target molecule can lead to a change in its electrochemical structure, causing the fluorescent sensor to switch from its “OFF” state to its “ON” state. To improve the quenching efficacy of these OFF-ON-type fluorescence probes, we need to develop a better understanding of the quenching mechanism. The process of fluorescence quenching involves a decrease in the fluorescence emission of a material by inducing a relaxation in the energy of the fluorophore through a non-radiative transition such as heat. Photo-induced electron transfer (PET) and fluorescence resonance energy transfer (FRET) are widely accepted principles in fluorescence quenching processes (Fig. 2a and c).(23–25) In PET, an excited electron in the fluorescent sensor moiety (Flu) is transferred to the fluorescence switching moiety (Q), leading to formation of a charge-separated state. This process typically occurs when the energy of the molecular orbital of Q sits somewhere in between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of Flu (Fig. 2a). The resulting charge-separated state can then relax to the ground state without any luminescence emission. In contrast, FRET processes require a broad overlap between the emission and absorption spectra of the sensor and switching moieties, respectively. Compared with PET, this phenomenon can occur over much longer distances than those typically encountered between the Flu and Q groups (Fig. 2c). To design an effective fluorescent probe, it is therefore important to develop an effective switching moiety that shows selective reactivity towards a target molecule. Furthermore, any newly designed fluorescent probe would need to be compatible with the electrochemical and optical properties of the sensor. With this in mind, the characteristic redox properties of ascorbic acid could be a useful reaction target for the development of a fluorescent switching moiety for ascorbic acid. Several fluorometric methods have been developed during the last decade for the detection of ascorbic acid using redox-responsive metals and quantum dots (QDs).(26–34) For example, Huang et al.(27) reported the development of a fluorometric detection method using CdTe/CdS/ZnS core/shell/shell QDs and KMnO4 (Fig. 2b). In this system, the fluorescence emission of the QDs was efficiently quenched by KMnO4 via a PET process from KMnO4 to the QDs. Furthermore, the fluorescence intensity was recovered following the reduction of KMnO4 with ascorbic acid. This analytical method was used for the direct detection of ascorbic acid at concentrations in the range of 8.0 × 10−9 to 1.0 × 10−7 M, and gave a limit of detection of 1.8 × 10−9 M. Huang’s group also reported the utility of this system for determining the concentration of ascorbic acid in human urine and vitamin C tablets. In a separate study, Kong et al.(28) reported the development of a FRET-based DCPIP-CdTe QD system (Fig. 2d). In this system, DCPIP quenched the fluorescence emission of the CdTe QDs via a FRET process. Following the two-electron reduction of DCPIP by ascorbic acid, there was a significant reduction in its absorbance band at 600 nm, which led to a significant increase in the fluorescence emission of the QDs. In a separate study, Zhai et al.(29) conducted a systematic investigation of the quenching mechanism of an organic fluorophore with single-layered MnO2 nanosheets. Based on this mechanistic information, they reported a novel sensing method for measuring the level of ascorbic acid in rat brain microdialysates. Several other fluorescent methods have also been developed for the detection of ascorbic acid, including Cu-containing fluorescent labeling DNA-Au systems,(30) and protein-modified Au(31) and Ag nanoparticles.(32)
Fig. 2.
Principles of (a) PET and (c) FRET for controlling the fluorescence characteristics of fluorophores and redox-active metal-based fluorescent probes for ascorbic acid. (b) PET-based QDs-KMnO4 probe, (d) FRET-based DCPIP-CA-CdTeQD system.
Profluorescent Nitroxide for the Detection of Ascorbic Acid
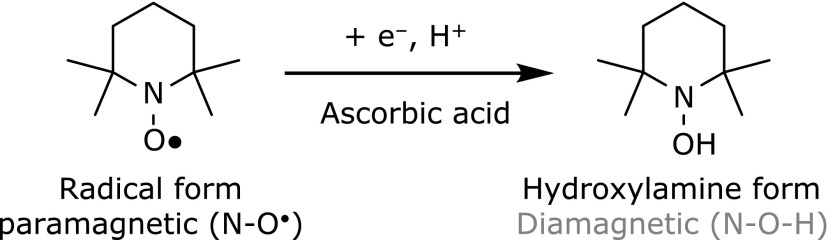
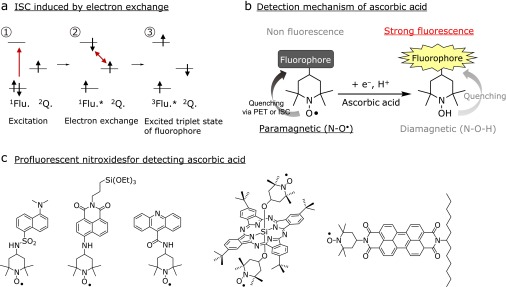
Although fluorescence probes based on redox-responsive metals exhibited high levels of selectivity and sensitivity for ascorbic acid, we will introduce interesting compounds “nitroxide”, which are non-metallic organic molecules with a stable unpaired electron on their N-O moiety. These compounds have unique reaction properties, such as their tendency towards oxidation-reduction reactions. Based on their interesting properties, nitroxides have been widely used as spin probes,(35,36) spin labels,(37) contrast agents,(38,39) and antioxidants.(40) In terms of their reducing properties, nitroxides can react with ascorbic acid through a single-electron reduction process when they are mixed together in polar protic solvents (Fig. 3).(41) In these types of reaction, the N-O moiety of the nitroxide is immediately converted to its diamagnetic hydroxylamine form (N-OH). Piperidine-based nitroxides, in particular, exhibit relatively high levels of reactivity towards ascorbic acid compared with most other nitroxide derivatives. Nitroxides also possess unique physicochemical properties that allow them to function as a strong fluorescence quenching agents towards fluorescent molecules.(42) It is generally believed that the quenching of fluorophores by nitroxides occur via a PET process or an intersystem crossing (ISC) induced by an electron exchange process. Fig. 4a shows a schematic of an ISC induced by an electron exchange pathway. Photo-induced intersystem crossing (1Flu→3Flu) events hardly ever occur because they are spin-forbidden transitions. However, in the presence of a nitroxide, the electrons in the LUMO orbital of the excited fluorophore and single occupied molecular orbital (SOMO) of the nitroxide moiety can be efficiently exchanged, resulting in the generation of the excited triplet state of the fluorophore. Several physicochemical studies have been conducted to develop a better understanding of the quenching mechanism of nitroxides. For example, Colvin et al.(43) reported that the interaction between 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO) and perylene-3,4:9,10-bis(dicarboximide) (PDI) in tetrahydrofuran resulted in a photo-induced electron transfer from TEMPO to PDI, which led to the formation of a charge-separated state with transient optical absorption properties. In contrast, Ishii et al.(44) reported that TEMPO induced the intersystem crossing of phthalocyanine silicon (SiPc) in its excited singlet state and promoted the generation of triplet state SiPc. These papers therefore suggest that the quenching mechanism of nitroxides can vary depending on the nature of the fluorophore and the surrounding environment. Taken together, these results show that nitroxides are highly reactive and selective for ascorbic acid in terms of their quenching capacity. These compounds are therefore suitable for use as fluorescent switching moieties in probes designed for the detection of ascorbic acid. During the last three decades, a wide variety a radical-based profluorescent nitroxide probes have been developed as organic OFF-ON-type fluorescent probes for the detection of ascorbic acid (Fig. 4b and c).(45–49) Lozinsky et al.(46) proposed a novel detection method for the quantitative analysis of ascorbic acid using a profluorescent nitroxide probe, which consisted of TEMPO and a dansyl fluorophore. More recently, Ishii et al.(48) reported the development of profluorescent nitroxide probe linked to SiPc, which they used for the detection of ascorbic acid in living cells by confocal microscopy. These results therefore demonstrate that profluorescent nitroxides can be used convenient and sensitive assays for the detection and quantification of ascorbic acid without using heavy metals and strong redox substances to accelerate the reactions.
Fig. 3.
One electron reduction of nitroxide with ascorbic acid.
Fig. 4.
(a) Principles of intersystem crossing (ISC) induced by electron exchange between nitroxide and fluorophore, (b) Detection mechanism of ascorbic acid using profluorescent nitroxide. (c) Previously reported profluorescent nitroxides for the detection of ascorbic acid.
Novel Strategy for Controlling the Reactivity of Nitroxides by Varying the Nature of the Substituent at the α-Position
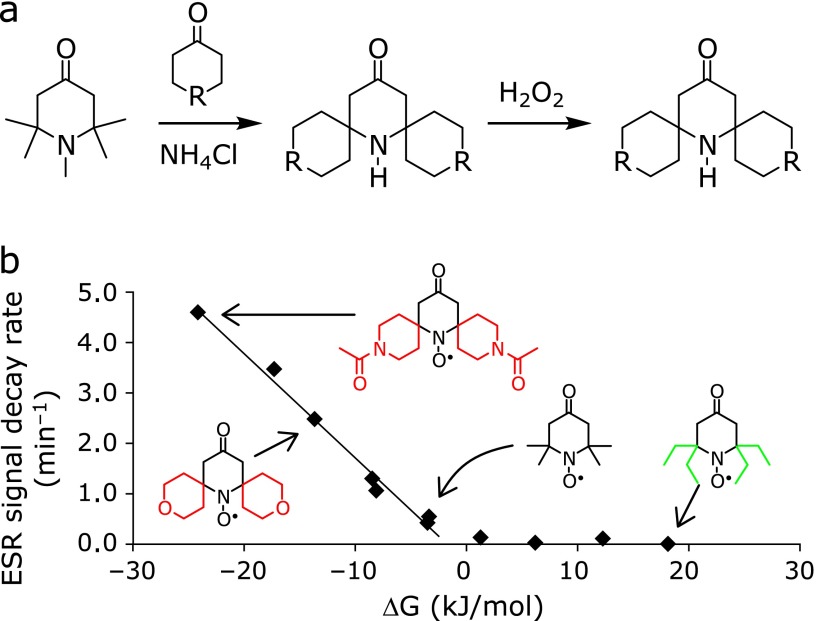
The mechanism of the reaction between a nitroxide species and ascorbic acid involves a hydrogen atom transfer.(50–52) Given that the two ascorbyl radicals generated during this reaction would be converted to ascorbic acid and dehydroascorbic acid via a disproportionation step, the stoichiometric ratio of this reaction should be 1:2. However, this reaction is an equilibrium process because the reverse reaction between dehydroascorbic acid and diamagnetic hydroxylamine can lead to the formation of the starting nitroxide. Moreover, piperidine-based nitroxides can be readily oxidized to the corresponding oxoammonium cations by ROS such as superoxide anion radicals. An improvement in the reactivity of the nitroxide is therefore required to increase its selectivity and sensitivity towards ascorbic acid. It was envisaged that the use of substituted nitroxides would provide a better understanding of the reactivity of nitroxides towards ascorbic acid, as well as providing ideas for the design of new compounds. For example, the phosphorylation of pyrrolidinyl nitroxide led to an increase in its reactivity towards ascorbic acid compared with the corresponding tetramethyl-based system.(53) In contrast, tetraethyl-based nitroxides have been reported to show low levels of reactivity towards biological reductants because of the steric hindrance around their reactive site, which resulted in an increase in their biological half life.(54–59) The substitution of nitroxides at their α-position can therefore be used as an effective strategy for controlling their reactivity. In a recent study, we developed an alternative method for the facile synthesis of α-substituted nitroxides (Fig. 5a).(60) Notably, this new method involved the use of 1,2,2,6,6-pentamethyl-4-piperidone and carbonyl compounds under mild reaction conditions. Interestingly, the redox potentials of the α-substituted nitroxides synthesized in this way changed significantly compared with those of the tetramethyl-based nitroxides.(61) In particular, the introduction of substituents bearing heteroatoms led to a positive shift in the single-electron reduction potentials of these material based on the electron-withdrawing inductive effect of the heteroatom. These changes led to a significant increase in the reduction rates of these nitroxides compared with those of the corresponding tetramethyl-based nitroxide systems without a heteroatom at the α-position. These results therefore indicate that changes in the electronic environment surrounding the N-O moiety of a nitroxide can have a significant impact on its ability to reduce ascorbic acid, as well as its steric properties. Moreover, these changes in the reduction rate correlated well with the calculated Gibbs free energy values for the reactions of the α-substituted nitroxides with ascorbic acid (Fig. 5b). Based on these results, it is clear that the α-substitution of nitroxides represents a promising approach for regulating the reactivity of these systems towards ascorbic acid.
Fig. 5.
(a) Synthetic route for the preparation of 2,6-substituted piperidinyl nitroxide. (b) Relationship between the ESR signal decay rates and the Gibbs free energy calculated from the redox potentials of ascorbate and the nitroxides. Reprinted with permission from Ref (61) (Yamasaki et al., J Org Chem 2011; 76: 435). Copyright (2012) American Chemical Society.
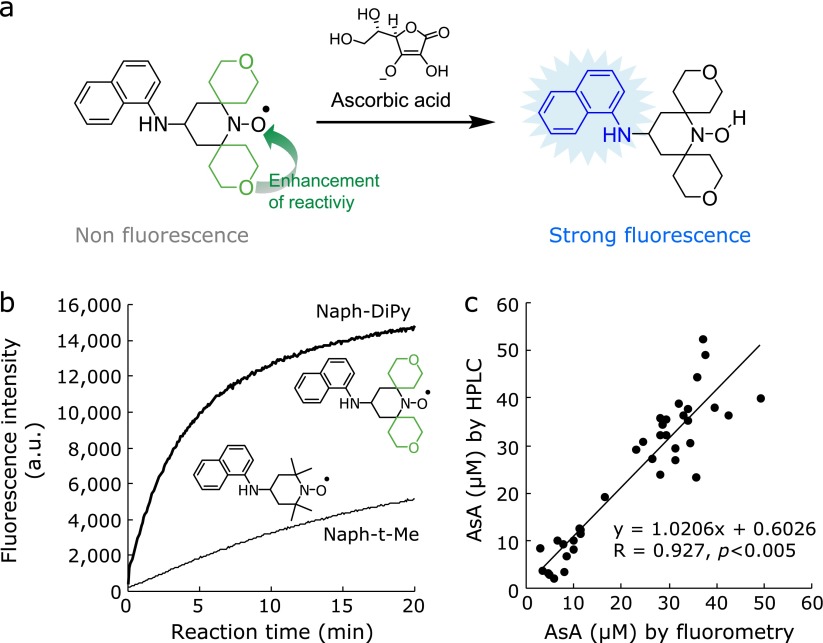
We previously reported the preparation of 15-(naphthyl-1-amino)-3,11-dioxa-7-azadispiro[5.1.58.36]hexadecan-7-oxyl (Naph-DiPy) as an α-substituted profluorescent nitroxide probe for ascorbic acid. This probe consists of an α-substituted nitroxide bearing an electron-withdrawing group with 1-naphthylamine as a fluorophore (Fig. 6a).(62) This probe showed higher levels of reactivity and selectivity towards ascorbic acid than 2,2,6,6-tetramethyl-4-(naphthalene-1-ylamino)piperidin-1-oxyl (Naph-Me), which used TEMPO as a nitroxide moiety. Furthermore, this probe allowed for a reduction in the time requirement for the measurement of ascorbic acid and led to a 80-fold improvement in the detection limit compared with Naph-Me (Fig. 6b). Although Naph-DiPy rapidly reacted with ascorbic acid, it did not respond to any other biological redox substances, such as glutathione, nicotinamides, and ROS. In terms of its application, we demonstrated that Naph-DiPy could be used for the quantitative analysis of ascorbic acid in plasma samples taken from osteogenic disorder Shionogi rats, which lack the ability to synthesize ascorbic acid because of a single mutation in their l-gulonolactone oxidase enzyme. The concentration of plasma ascorbic acid measured by Naph-DiPy and HPLC-ECD analyses showed a good correlation (Fig. 6c). Furthermore, the fluorescence emission intensity of Naph-DiPy was not affected by the many different substances present in the rat plasma. Ito et al.(63) also reported the measurement of the ascorbic acid levels in the liver and plasma of aldehyde reductase (Akr1a)-knockout mice using Naph-DiPy as a fluorescent probe. Moreover, Naph-DiPy fluorometric assays can be used to provide a quick measurement of ascorbic acid levels and can be used to measure multiple samples at the same time using a micro-plate reader.
Fig. 6.
(a) AsA highly reactive detection probe, Naph-DiPy. (b) Reactivity of Naph-DiPy and Naph-t-Me towards ascorbic acid. The data represent the fluorescence intensities at 430 nm with excitation at 310 nm. (c) Measurement of the ascorbic acid levels in the plasma of ODS rats using Naph-DiPy nitroxide and HPLC. Reprinted with permission from Ref (62) (Matsuoka et al., Free Radic Biol Med 2012; 53: 2112). Copyright (2012) Elsevier Inc.
Conclusion
Ascorbic acid is an essential small molecular antioxidant, and its levels in the human body can be used to inform clinicians with regard to the diagnosis and treatment of certain diseases. For this reason, the development of highly sensitive methods for the detection of ascorbic acid has attracted considerable interest. Compared with conventional analytical methods based on HPLC and absorption spectrophotometry, fluorometric assays using fluorescent probes represent promising approaches for the detection of ascorbic acid because they are convenient, sensitive, and selective. In this review, we have focused on design strategies for the development of fluorescent probes for ascorbic acid. In particular, organic radical-based fluorescent probes based on α-substituted profluorescent nitroxides have shown selective reactivity towards ascorbic acid and could therefore provide increasingly facile and sensitive tools for the detection of ascorbic acid in clinical samples such as blood.
Acknowledgments
This work was supported in part by Japan Science and Technology Agency PRESTO, Japan Society of the Promotion of Science KAKENHI (Grant Numbers 24390011 and 24659020), and the Platform for Drug Discovery, Informatics and Structural Life Science from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- DCPIP
2,6-dichlorophenol-indophenol
- ECD
electrochemical detection
- FRET
fluorescence resonance energy transfer
- HOMO
highest occupied molecular orbital
- HPLC
high-performance liquid chromatography
- ISC
intersystem crossing
- LUMO
lowest unoccupied molecular orbital
- Naph-DiPy
15-(naphthyl-1-amino)-3,11-dioxa-7-azadispiro[5.1.58.36]hexadecan-7-oxyl
- PDI
perylene-3,4:9,10-bis(dicarboximide)
- PET
photo-induced electron transfer
- QDs
quantum dots
- ROS
reactive oxygen species
- SiPc
phthalocyanine silicon
- SOMO
single occupied molecular orbital
- TEMPO
2,2,6,6-tetramethyl-1-piperidinyloxyl
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Eggersdorfer M, Laudert D, Létinois U, et al. One hundred years of vitamins-a success story of the natural sciences. Angew Chem Int Engl. 2012;51:12960–12990. doi: 10.1002/anie.201205886. [DOI] [PubMed] [Google Scholar]
- 2.Peterkofsky B, Udenfriend S. Enzymatic hydroxylation of proline in microsomal polypeptide leading to formation of collagen. Proc Natl Acad Sci U S A. 1965;53:335–342. doi: 10.1073/pnas.53.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–1423. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 4.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human-blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci. 1992;669:7–20. doi: 10.1111/j.1749-6632.1992.tb17085.x. [DOI] [PubMed] [Google Scholar]
- 6.Naziroğlu M, Kilinç F, Uğuz AC, et al. Oral vitamin C and E combination modulates blood lipid peroxidation and antioxidant vitamin levels in maximal exercising basketball players. Cell Biochem Funct. 2010;28:300–305. doi: 10.1002/cbf.1657. [DOI] [PubMed] [Google Scholar]
- 7.Basu S, Som S, Deb S, Mukherjee D, Chatterjee IB. Dehydroascorbic acid reduction in human erythrocytes. Biochem Biophys Res Commun. 1979;90:1335–1340. doi: 10.1016/0006-291x(79)91182-3. [DOI] [PubMed] [Google Scholar]
- 8.Bánhegyi G, Braun L, Csala M, Puskás F, Mandl J. Ascorbate metabolism and its regulation in animals. Free Radic Biol Med. 1997;23:793–803. doi: 10.1016/s0891-5849(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 9.Sun F, Iwaguchi K, Shudo R, et al. Change in tissue concentrations of lipid hydroperoxides, vitamin C and vitamin E in rats with streptozotocin-induced diabetes. Clin Sci (Lond) 1999;96:185–190. [PubMed] [Google Scholar]
- 10.Dixit S, Bernardo A, Walker JM, et al. Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in App/Psen1 and normally aging mice. ACS Chem Neurosci. 2015;6:570–581. doi: 10.1021/cn500308h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osganian SK, Stampfer MJ, Rimm E, et al. Vitamin C and risk of coronary heart disease in women. J Am Coll Cardiol. 2003;42:246–252. doi: 10.1016/s0735-1097(03)00575-8. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida M, Takashima Y, Inoue M, et al. Prospective study showing that dietary vitamin C reduced the risk of age-related cataracts in a middle-aged Japanese population. Eur J Nutr. 2007;46:118–124. doi: 10.1007/s00394-006-0641-8. [DOI] [PubMed] [Google Scholar]
- 13.Canoy D, Wareham N, Welch A, et al. Plasma ascorbic acid concentrations and fat distribution in 19,068 British men and women in the European Prospective Investigation into Cancer and Nutrition Norfolk cohort study. Am J Clin Nutr. 2005;82:1203–1209. doi: 10.1093/ajcn/82.6.1203. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Espey MG, Krishna MC, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Espey MG, Sun AY, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2005;102:13604–13609. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6:222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 17.Monti DA, Mitchell E, Bazzan AJ, et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS ONE. 2012;7:e29794. doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem. 2013;28:314–328. doi: 10.1007/s12291-013-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behrens WA, Madère R. A highly sensitive high-performance liquid-chromatography method for the estimation of ascorbic and dehydroascorbic acid in tissues, biological-fluids, and foods. Anal Biochem. 1987;165:102–107. doi: 10.1016/0003-2697(87)90206-5. [DOI] [PubMed] [Google Scholar]
- 20.Vanderjagt DJ, Garry PJ, Hunt WC. Ascorbate in plasma as measured by liquid-chromatography and by dichlorophenolindophenol colorimetry. Clin Chem. 1986;32:1004–1006. [PubMed] [Google Scholar]
- 21.Li XH, Gao XH, Shi W, Ma H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem Rev. 2014;114:590–659. doi: 10.1021/cr300508p. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem Rev. 2010;110:2620–2640. doi: 10.1021/cr900263j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka K, Miura T, Umezawa N, et al. Rational design of fluorescein-based fluorescence probes. Mechanism-based design of a maximum fluorescence probe for singlet oxygen. J Am Chem Soc. 2001;123:2530–2536. doi: 10.1021/ja0035708. [DOI] [PubMed] [Google Scholar]
- 24.Miura T, Urano Y, Tanaka K, Nagano T, Ohkubo K, Fukuzumi S. Rational design principle for modulating fluorescence properties of fluorescein-based probes by photoinduced electron transfer. J Am Chem Soc. 2003;125:8666–8671. doi: 10.1021/ja035282s. [DOI] [PubMed] [Google Scholar]
- 25.Algar WR, Krull UJ. Quantum dots as donors in fluorescence resonance energy transfer for the bioanalysis of nucleic acids, proteins, and other biological molecules. Anal Bioanal Chem. 2008;391:1609–1618. doi: 10.1007/s00216-007-1703-3. [DOI] [PubMed] [Google Scholar]
- 26.Chen YJ, Yan XP. Chemical redox modulation of the surface chemistry of Cdte quantum dots for probing ascorbic acid in biological fluids. Small. 2009;5:2012–2018. doi: 10.1002/smll.200900291. [DOI] [PubMed] [Google Scholar]
- 27.Huang S, Zhu F, Xiao Q, et al. A Cdte/Cds/Zns core/shell/shell Qds-based “OFF-ON” fluorescent biosensor for sensitive and specific determination of L-ascorbic acid. RSC Adv. 2014;4:46751–46761. [Google Scholar]
- 28.Kong C, Li DW, Li Y, et al. Reversible electrochemical modulation of fluorescence and selective sensing of ascorbic acid using a DCIP-CA-CdTe QD system. Analyst. 2012;137:1094–1096. doi: 10.1039/c2an15921j. [DOI] [PubMed] [Google Scholar]
- 29.Zhai W, Wang C, Yu P, Wang Y, Mao L. Single-layer MnO2 nanosheets suppressed fluorescence of 7-hydroxycoumarin: mechanistic study and application for sensitive sensing of ascorbic acid in vivo. Anal Chem. 2014;86:12206–12213. doi: 10.1021/ac503215z. [DOI] [PubMed] [Google Scholar]
- 30.Malashikhina N, Pavlov V. DNA-decorated nanoparticles as nanosensors for rapid detection of ascorbic acid. Biosens Bioelectron. 2012;33:241–246. doi: 10.1016/j.bios.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Chen Y, Huang L, Ma L, Lin Q, Chen G. A fluorescent sensor based on ovalbumin-modified au nanoclusters for sensitive detection of ascorbic acid. Anal Methods. 2015;7:4123–4129. [Google Scholar]
- 32.Park HW, Alam SM, Lee SH, et al. Optical ascorbic acid sensor based on the fluorescence quenching of silver nanoparticles. Luminescence. 2009;24:367–371. doi: 10.1002/bio.1119. [DOI] [PubMed] [Google Scholar]
- 33.Yang SS, Ren CL, Zhang ZY, Hao JJ, Hu Q, Chen XG. Aqueous synthesis of CdTe/CdSe core/shell quantum dots as pH-sensitive fluorescence probe for the determination of ascorbic acid. J Fluoresc. 2011;21:1123–1129. doi: 10.1007/s10895-010-0788-9. [DOI] [PubMed] [Google Scholar]
- 34.Ma Q, Li Y, Lin ZH, Tang G, Su XG. A novel ascorbic acid sensor based on the Fe3+/Fe2+ modulated photoluminescence of CdTe quantum dots@Sio2 nanobeads. Nanoscale. 2013;5:9726–9731. doi: 10.1039/c3nr03060a. [DOI] [PubMed] [Google Scholar]
- 35.Kuppusamy P, Li H, Ilangovan G, et al. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002;62:307–312. [PubMed] [Google Scholar]
- 36.Yamada K, Yamamiya I, Utsumi H. In vivo detection of free radicals induced by diethylnitrosamine in rat liver tissue. Free Radic Biol Med. 2006;40:2040–2046. doi: 10.1016/j.freeradbiomed.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Borbat PP, Costa-Filho AJ, Earle KA, Moscicki JK, Freed JH. Electron spin resonance in studies of membranes and proteins. Science. 2001;291:266–269. doi: 10.1126/science.291.5502.266. [DOI] [PubMed] [Google Scholar]
- 38.Davis RM, Matsumoto S, Bernardo M, et al. Magnetic resonance imaging of organic contrast agents in mice: capturing the whole-body redox landscape. Free Radic Biol Med. 2011;50:459–468. doi: 10.1016/j.freeradbiomed.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emoto MC, Yamada K, Yamato M, Fujii HG. Novel ascorbic acid-resistive nitroxide in a lipid emulsion: an efficient brain imaging contrast agent for MRI of small rodents. Neurosci Lett. 2013;546:11–15. doi: 10.1016/j.neulet.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 40.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saphier O, Silberstein T, Shames AI, et al. The reduction of a nitroxide spin label as a probe of human blood antioxidant properties. Free Radic Res. 2003;37:301–308. doi: 10.1080/1071576021000050410. [DOI] [PubMed] [Google Scholar]
- 42.Green SA, Simpson DJ, Zhou G, Ho PS, Blough NV. Intramolecular quenching of excited singlet-states by stable nitroxyl radicals. J Am Chem Soc. 1990;112:7337–7346. [Google Scholar]
- 43.Colvin MT, Giacobbe EM, Cohen B, Miura T, Scott AM, Wasielewski MR. Competitive electron transfer and enhanced intersystem crossing in photoexcited covalent TEMPO-perylene-3,4:9,10-bis(dicarboximide) dyads: unusual spin polarization resulting from the radical-triplet interaction. J Phy Chem A. 2010;114:1741–1748. doi: 10.1021/jp909212c. [DOI] [PubMed] [Google Scholar]
- 44.Ishii K, Hirose Y, Fujitsuka H, Ito O, Kobayashi N. Time-resolved EPR, fluorescence, and transient absorption studies on phthalocyaninatosilicon covalently linked to one or two tempo radicals. J Am Chem Soc. 2001;123:702–708. doi: 10.1021/ja002780h. [DOI] [PubMed] [Google Scholar]
- 45.Morrow BJ, Keddie DJ, Gueven N, Lavin MF, Bottle SE. A novel profluorescent nitroxide as a sensitive probe for the cellular redox environment. Free Radic Biol Med. 2010;49:67–76. doi: 10.1016/j.freeradbiomed.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Lozinsky E, Martin VV, Berezina TA, Shames AI, Weis AL, Likhtenshtein GI. Dual fluorophore-nitroxide probes for analysis of vitamin C in biological liquids. J Biochem Biophys Methods. 1999;38:29–42. doi: 10.1016/s0165-022x(98)00029-3. [DOI] [PubMed] [Google Scholar]
- 47.Maki T, Soh N, Nakano K, Imato T. Flow injection fluorometric determination of ascorbic acid using perylenebisimide-linked nitroxide. Talanta. 2011;85:1730–1733. doi: 10.1016/j.talanta.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 48.Ishii K, Kubo K, Sakurada T, Komori K, Sakai Y. Phthalocyanine-based fluorescence probes for detecting ascorbic acid: phthalocyaninatosilicon covalently linked to TEMPO radicals. Chem Commun (Camb) 2011;47:4932–4934. doi: 10.1039/c1cc10817d. [DOI] [PubMed] [Google Scholar]
- 49.Yang T, Zheng B, Liang H, Wan Y, Du J, Xiao D. A sensitive and selective chemosensor for ascorbic acid based on a fluorescent nitroxide switch. Talanta. 2015;132:191–196. doi: 10.1016/j.talanta.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 50.Sajenko I, Pilepić V, Brala CJ, Ursić S. Solvent dependence of the kinetic isotope effect in the reaction of ascorbate with the 2,2,6,6-tetramethylpiperidine-1-oxyl radical: tunnelling in a small molecule reaction. J Phy Chem A. 2010;114:3423–3430. doi: 10.1021/jp911086n. [DOI] [PubMed] [Google Scholar]
- 51.Warren JJ, Mayer JM. Surprisingly long-lived ascorbyl radicals in acetonitrile: concerted proton-electron transfer reactions and thermochemistry. J Am Chem Soc. 2008;130:7546–7547. doi: 10.1021/ja802055t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warren JJ, Mayer JM. Tuning of the thermochemical and kinetic properties of ascorbate by its local environment: solution chemistry and biochemical implications. J Am Chem Soc. 2010;132:7784–7793. doi: 10.1021/ja102337n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathieu C, Mercier A, Witt D, Debmkowski L, Tordo P. Beta-phosphorylated nitroxides in the pyrrolidine series: reduction by ascorbate. Free Radic Biol Med. 1997;22:803–806. doi: 10.1016/s0891-5849(96)00424-8. [DOI] [PubMed] [Google Scholar]
- 54.Marx L, Chiarelli R, Guiberteau T, Rassat A. A comparative study of the reduction by ascorbate of 1,1,3,3-tetraethylisoindolin-2-yloxyl and of 1,1,3,3-tetramethylisoindolin-2-yloxyl. J Chem Soc, Perkin Trans 1. 2000:1181–1182. [Google Scholar]
- 55.Kirilyuk IA, Bobko AA, Grigor’ev IA, Khramtsov VV. Synthesis of the tetraethyl substituted pH-sensitive nitroxides of imidazole series with enhanced stability towards reduction. Org Biomol Chem. 2004;2:1025–1030. doi: 10.1039/b400252k. [DOI] [PubMed] [Google Scholar]
- 56.Kinoshita Y, Yamada K, Yamasaki T, et al. In vivo evaluation of novel nitroxyl radicals with reduction stability. Free Radic Biol Med. 2010;49:1703–1709. doi: 10.1016/j.freeradbiomed.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Emoto M, Sugimoto A, et al. Synthesis of 15N-labeled 4-oxo-2,2,6,6-tetraethylpiperidine nitroxide for EPR brain imaging. Tetrahedron Lett. 2014;55:2146–2149. [Google Scholar]
- 58.Paletta JT, Pink M, Foley B, Rajca S, Rajca A. Synthesis and reduction kinetics of sterically shielded pyrrolidine nitroxides. Org Lett. 2012;14:5322–5325. doi: 10.1021/ol302506f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahn HY, Fairfull-Smith KE, Morrow BJ, et al. Two-photon fluorescence microscopy imaging of cellular oxidative stress using profluorescent nitroxides. J Am Chem Soc. 2012;134:4721–4730. doi: 10.1021/ja210315x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakai K, Yamada K, Yamasaki T, Kinoshita Y, Mito F, Utsumi H. Effective 2,6-substitution of piperidine nitroxyl radical by carbonyl compound. Tetrahedron. 2010;66:2311–2315. [Google Scholar]
- 61.Yamasaki T, Mito F, Ito Y, et al. Structure-reactivity relationship of piperidine nitroxide: electrochemical, ESR and computational studies. J Org Chem. 2011;76:435–440. doi: 10.1021/jo101961m. [DOI] [PubMed] [Google Scholar]
- 62.Matsuoka Y, Yamato M, Yamasaki T, Mito F, Yamada K. Rapid and convenient detection of ascorbic acid using a fluorescent nitroxide switch. Free Radic Biol Med. 2012;53:2112–2118. doi: 10.1016/j.freeradbiomed.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 63.Ito J, Otsuki N, Zhang X, et al. Ascorbic acid reverses the prolonged anesthetic action of pentobarbital in Akr1a-knockout mice. Life Sci. 2014;95:1–8. doi: 10.1016/j.lfs.2013.12.004. [DOI] [PubMed] [Google Scholar]