Abstract
Obesity has reached epidemic proportions worldwide. Obesity results in reduced serum testosterone levels, which causes many disorders in men. Lifestyle modifications (increased physical activity and calorie restriction) can increase serum testosterone levels. However, it is unknown whether increased physical activity or calorie restriction during lifestyle modifications has a greater effects on serum testosterone levels. Forty-one overweight and obese men completed a 12-week lifestyle modification program (aerobic exercise training and calorie restriction). We measured serum testosterone levels, the number of steps, and the total energy intake. We divided participants into two groups based on the median change in the number of steps (high or low physical activities) or that in calorie restriction (high or low calorie restrictions). After the program, serum testosterone levels were significantly increased. Serum testosterone levels in the high physical activity group were significantly higher than those in the low activity group. This effect was not observed between the groups based on calorie restriction levels. We found a significant positive correlation between the changes in serum testosterone levels and the number of steps. Our results suggested that an increase in physical activity greatly affected the increased serum testosterone levels in overweight and obese men during lifestyle modification.
Keywords: testosterone, obesity, lifestyle modification, aerobic exercise training, calorie restriction
Introduction
Testosterone is an androgen that is primarily produced by the Leydig cells of the testes. Obesity results in reduced serum testosterone levels in men.(1) Recent studies have shown that low serum testosterone levels were associated with endothelial dysfunction,(2) insulin resistance,(3) and cognitive dysfunction,(4) which are independent risk factors for cardiovascular diseases,(5,6) type 2 diabetes mellitus,(7,8) and dementia.(9) Therefore, it is important for men to prevent a decline in serum testosterone levels.
Obesity has reached epidemic proportions worldwide and is an increasing public health problem.(10,11) Increased physical activity levels with habitual aerobic exercise and/or calorie restriction by dietary modifications are effective means for achieving weight loss, especially the combination of aerobic exercise training and calorie restriction.(12–15) Foresta et al.(16) have reported that the serum testosterone levels in obese males were lower than those in age-matched nonobese males. On the other hand, we have reported that lifestyle modification-induced weight reduction (combination of aerobic exercise training and dietary modification) increases serum testosterone level in overweight and obese men.(14) However, it had not been compared the effect of physical activity level and calorie restriction on the testosterone levels during weight-loss in overweight and obese men.
Thus, the purpose of this study was to investigate which lifestyle modification, increased physical activity or calorie restriction, had a greater effect on serum testosterone levels. For this purpose, we measured serum testosterone levels, the number of steps taken per day, and the total energy intake for overweight and obese men before and after a 12-week lifestyle modification program.
Materials and Methods
Participants
A total of 41 overweight and obese men (mean age: 49 ± 2 years; mean body mass index, BMI: 29 ± 1 kg/m2) completed a lifestyle modification program. Two participants were current smokers and nineteen participants were taking hypertensive, hypercholesterolemia or hypoglycemic medication. The participants’ smoking habits and medication use did not change during the study period. This study was reviewed and approved by the institutional review board of the University of Tsukuba. All study procedures and potential risks were explained to the participants and each provided written informed consent to participate in this study.
Experimental design
All participants were examined before and after a 12-week lifestyle modification program that included aerobic exercise training and dietary modification. Before and after this program, we measured serum testosterone levels, the number of steps taken per day, and total energy intake for all participants. We assessed the effects of increased physical activity levels or reduced energy intake on serum testosterone levels based on two examinations.
Study 1: Participants were divided into two groups based on the median change in the number of steps taken per day after the lifestyle modification program (median: 3,579 steps/day) into a low physical activity group (LPA; range: 1,110–3,579 steps/day; n = 20) and a high physical activity group (HPA; range: 3,586–10,486 steps/day; n = 21).
Study 2: Participants were divided into two groups based on the median change in total energy intake after the lifestyle modification program (median: –494 kcal/day) into a low calorie restriction group (LCR; range: –189 to –494 kcal/day; n = 20) and a high calorie restriction group (HCR; range: –539 to –2,735 kcal/day; n = 21).
All measurements were made after abstaining from caffeine and an overnight fast. None of the participants exercised on the day before these measurements. Measurements were made while participants were in a quiet, temperature-controlled room (24–26°C). All measurements were made after a rest period of at least 20 min.
Aerobic exercise training
The subjects participated in an aerobic exercise class for up to 90 min/day, three times per week for 12 weeks, as previously described with minor modifications.(13) In addition, the participants were instructed to perform exercise training by themselves at least once each week. Therefore, participants performed aerobic exercise 4–7 days/week. The exercise program included a 15–20 min warm-up session followed by an approximately 40–60 min walking and/or jogging session, and concluded with a 15–20 min cool-down session.
Exercise intensity was set so that it increased a participant’s heart rate to between 60% and 85% of his maximum heart rate. Exercise intensity was monitored using a heart rate monitor (Polar RS400TM; Polar Electro Oy, Kempele, Finland) and an activity monitor with a uniaxial accelerometer (Kenz Lifecorder GS; Suzuken Co., Ltd., Nagoya, Japan). Total daily steps taken were determined using the uniaxial accelerometer every day, from 2 weeks prior to the intervention period and throughout the 12-week program.
Dietary modifications
Participants were provided with a dietary restriction of 1,680 kcal per day that was maintained through a nutritionally balanced diet restriction program consisting of 12 weekly lectures, as previously described.(12) During the 12-week intervention period, participants kept food diaries that were monitored by dieticians who provided recommendations at the weekly lectures. Individual counseling was provided after these classes to assist the participants in adhering to the calorie consumption guidelines. At baseline and at week 12, the participants maintained daily food intake records for 3 days. A dietician used the food intake records to estimate the total daily energy intake using Excel Eiyo-Kun ver. 4 software (Kenpakusya, Tokyo, Japan).
Anthropometric measurements
Anthropometric measurements were made before and after the 12-week lifestyle modification program. During each measurement, body mass was measured once to the nearest 0.1 kg using a digital scale (WB-150; TANITA, Tokyo, Japan) and height was measured once to the nearest 0.1 cm using a wall-mounted stadiometer (YG-200; Yagami, Nagoya, Japan) with the subjects in their underwear and barefoot prior to eating in the morning. BMI values were determined by weight (kg) divided by height (m) squared. Waist circumference was directly measured on the skin at the level of the umbilicus while in a standing position. Waist circumference measurements were made in duplicate to the nearest 0.1 cm.
Serum testosterone levels and blood biochemistry tests
Blood samples were collected from each subject before and after the program. Each blood sample was placed in a serum separator tube, clotted for 2 h, and then centrifuged at 3,000 rpm at 4°C for 15 min. Serum was stored at –80°C until assayed. Total serum testosterone concentrations were determined using standard radioimmunoassay methods.(17) Serum concentrations of total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, insulin, and homeostasis model of insulin resistance (HOMA-IR) were determined using standard enzymatic methods.
Peak oxygen uptake
Before and after the 12-week lifestyle modification program, the peak oxygen uptake (V̇O2peak) was determined during a graded exercise test using a cycle ergometer (828E; Monark, Stockholm, Sweden), as previously described.(14) After a 2-min warm-up at 30 W, a subject started with a workload of 15 W/min until he felt exhausted or reached 85% of his age-predicted maximal heart rate. Individual’s V̇O2peak was calculated by regression analysis for the slopes of CO2 production, O2 uptake, and minute ventilation plots. Pulmonary ventilation and gas exchange were also measured, breath-by-breath, using an online data acquisition system (Oxycon Alpha System; Mijnhardt, Breda, The Netherlands).
Blood pressure and heart rate
Resting supine arterial blood pressure and heart rate were determined using the right arm of each subject with an automated device (form PWV/ABI; Colin Medical Technology, Komaki, Japan). We measured arterial blood pressure three times, and the mean values were used for analysis.
Statistical analysis
The Shapiro-Wilk test was used to assess the normality of all parameters. Unpaired Student’s t test and Wilcoxon rank sum test were used to assess the group differences between all parameters at baseline. Paired Student’s t test and Wilcoxon signed-rank test were used to assess the group differences between data before and after the 12-week lifestyle modification program. Two-way analysis of variance with repeated measures was used to identify group (HPA or LPA) × time (before or after) or group (HCR or LCR) × time (before or after) interaction or main effects. When a significant main effect or interaction was identified, specific mean comparisons were made by paired Student’s t test to identify significant differences with each intervention. For intergroup comparisons, an unpaired Student’s t test was used. Possible associations between the changes in serum testosterone levels and other measurements were assessed using Pearson correlation analysis and Spearman’s rank correlation coefficient. All data are reported as means ± SEs. Statistical significance was set at p<0.05.
Results
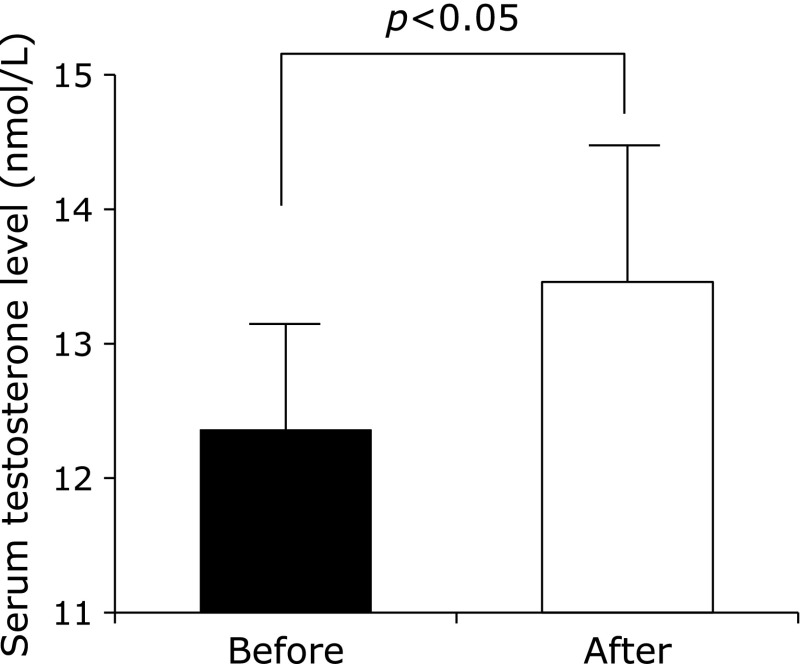
Table 1 shows the physical and clinical characteristics of all the overweight and obese men before and after the 12-week lifestyle modification program (aerobic exercise training and dietary modification). The decrease in body mass, BMI and total energy intake were similar to our previous studies.(14,15) Serum testosterone levels were significantly increased after the 12-week lifestyle modification program (Fig. 1). In addition, the percentage changes in serum testosterone levels were significantly correlated with that in the total number of steps taken per day (r = 0.40, p<0.01). On the other hand, there were no significant correlations between the percentage changes in serum testosterone levels and that in total energy intake, body mass and BMI.
Table 1.
Characteristics of overweight and obese men before and after the lifestyle modification
| Before | After | |
|---|---|---|
| Age (years) | 49 ± 2 | — |
| Height (cm) | 171 ± 1 | — |
| Body mass (kg) | 85 ± 2 | 73 ± 2.1** |
| Body mass index (kg/m2) | 29 ± 1 | 25 ± 1** |
| Waist circumference (cm) | 99 ± 2 | 87 ± 2** |
| SBP (mmHg) | 136 ± 4 | 119 ± 3** |
| DBP (mmHg) | 87 ± 3 | 76 ± 2** |
| MAP (mmHg) | 105 ± 3 | 91 ± 2** |
| Heart rate (beat/min) | 65 ± 2 | 56 ± 2** |
| Total cholesterol (mmol/L) | 5.2 ± 0.3 | 4.6 ± 0.2** |
| HDL cholesterol (mmol/L) | 1.3 ± 0.1 | 1.4 ± 0.1* |
| LDL cholesterol (mmol/L) | 3.2 ± 0.2 | 2.7 ± 0.2** |
| Triglyceride (mmol/L) | 1.9 ± 0.3 | 1.0 ± 0.3** |
| Insulin (µU/ml) | 10.3 ± 2.0 | 6.1 ± 2.3** |
| HOMA-IR (IU) | 2.5 ± 0.6 | 1.4 ± 0.6** |
| V̇O2peak (ml/min/kg) | 28.9 ± 1.5 | 35.9 ± 1.8** |
| Steps (steps/day) | 7,334 ± 611 | 11,076 ± 723** |
| Total energy intake (kcal/day) | 2,094 ± 123 | 1,435 ± 57** |
SBP, systolic blood pressure; DBP, diastoloc blood pressure; MAP, mean arterial pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein. Date are expressed as mean ± SE. Significant difference vs before intervention, **p<0.01, *p<0.05.
Fig. 1.
Serum testosterone levels before and after the 12-week lifestyle modification program. Results are shown as means ± SEs.
Study 1
We divided the participants into two groups based on the median change in the number of steps taken per day (LPA: 1,110–3,579 steps/day; HPA: 3,586–10,486 steps/day). Table 2 shows the physical and clinical characteristics of the participants before and after the intervention in these two groups. At baseline, there were no differences between these two groups. In particular, there were no differences in body mass and total energy intake. In addition, before and after the lifestyle modification program, there were no significant interactions between the HPA and LPA groups for all the tested variables.
Table 2.
Characteristics of overweight and obese men before and after the lifestyle modification in LPA and HPA groups
| Low physical activity |
High physical activity |
||||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Age (years) | 50 ± 2 | — | 47 ± 2 | — | |
| Height (cm) | 169 ± 1 | — | 172 ± 1 | — | |
| Body mass (kg) | 83 ± 2 | 71 ± 2** | 87 ± 2 | 74 ± 2** | |
| Body mass index (kg/m2) | 29 ± 1 | 25 ± 1** | 29 ± 1 | 25 ± 1** | |
| Waist circumference (cm) | 100 ± 2 | 88 ± 2** | 99 ± 1 | 86 ± 1** | |
| SBP (mmHg) | 141 ± 4 | 120 ± 2** | 132 ± 4 | 118 ± 3** | |
| DBP (mmHg) | 89 ± 3 | 76 ± 2** | 84 ± 2 | 76 ± 2** | |
| MAP (mmHg) | 108 ± 3 | 91 ± 2** | 101 ± 3 | 91 ± 2** | |
| Heart rate (beat/min) | 65 ± 2 | 55 ± 2** | 65 ± 2 | 57 ± 2** | |
| Total cholesterol (mmol/L) | 5.2 ± 0.2 | 4.5 ± 0.2** | 5.3 ± 0.2 | 4.7 ± 0.2** | |
| HDL cholesterol (mmol/L) | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | |
| LDL cholesterol (mmol/L) | 3.1 ± 0.1 | 2.6 ± 0.2** | 3.2 ± 0.2 | 2.7 ± 0.2* | |
| Triglyceride (mmol/L) | 1.8 ± 0.2 | 0.8 ± 0.1** | 2.0 ± 0.4 | 1.3 ± 0.3* | |
| Insulin (µU/ml) | 9.5 ± 1.7 | 4.5 ± 0.4** | 11.0 ± 1.9 | 7.7 ± 2.9** | |
| HOMA-IR (IU) | 2.3 ± 0.4 | 1.0 ± 0.1** | 2.8 ± 0.6 | 1.9 ± 0.8** | |
| V̇O2peak (ml/min/kg) | 30 ± 1 | 35 ± 1** | 28 ± 1 | 37 ± 2** | |
| Total energy intake (kcal/day) | 2,060 ± 95 | 1,470 ± 56** | 2,126 ± 119 | 1,400 ± 41** | |
SBP, systolic blood pressure; DBP, diastoloc blood pressure; MAP, mean arterial pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein. Date are expressed as mean ± SE. Significant difference vs before intervention, **p<0.01, *p<0.05.
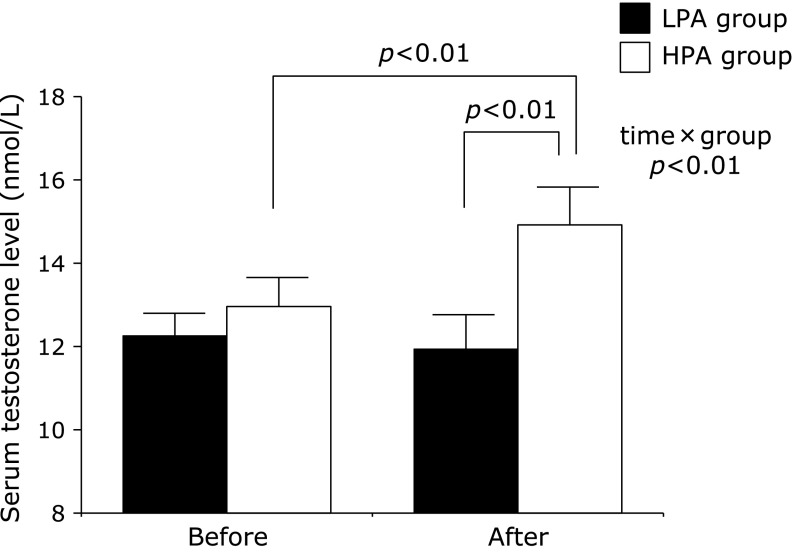
Fig. 2 shows the changes in serum testosterone levels and after the intervention in these two groups. After the 12-week intervention, serum testosterone levels significantly increased only in the HPA group but not in the LPA group. Furthermore, there was a significant interaction for serum testosterone levels between the HPA and LPA groups (p<0.01).
Study 2
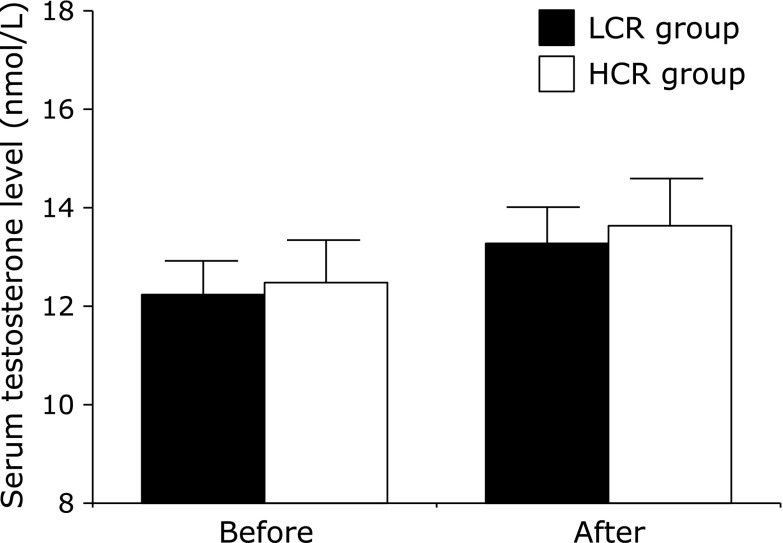
We divided the participants into two groups based on the median change in total energy intake (LCR: –189 to –494 kcal/day; HCR: –539 to –2,735 kcal/day). Table 3 shows the physical and clinical characteristics of the participants before and after the intervention in these two groups. At baseline, there were no differences between these two groups. In particular, there were no differences in body mass and the number of steps taken per day. In addition, there were no significant interactions between the HCR and LCR groups for all the tested variables before and after the lifestyle modification. Unlike the results of Study 1, there was no significant interaction for serum testosterone levels between the HCR and LCR groups (Fig. 3).
Table 3.
Characteristics of overweight and obese men before and after the lifestyle modification in LCR and HCR groups
| Low calorie restriction |
High calorie restriction |
||||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Age (years) | 50 ± 2 | — | 47 ± 2 | — | |
| Height (cm) | 172 ± 1 | — | 170 ± 1 | — | |
| Body mass (kg) | 84 ± 2 | 72 ± 1** | 86 ± 2 | 73 ± 2** | |
| Body mass index (kg/m2) | 29 ± 1 | 25 ± 1** | 29 ± 1 | 25 ± 1** | |
| Waist circumference (cm) | 99 ± 1 | 86 ± 1** | 100 ± 2 | 88 ± 2** | |
| SBP (mmHg) | 132 ± 4 | 119 ± 2** | 140 ± 3 | 119 ± 3** | |
| DBP (mmHg) | 87 ± 3 | 77 ± 2** | 86 ± 2 | 74 ± 3** | |
| MAP (mmHg) | 104 ± 3 | 92 ± 2** | 105 ± 2 | 90 ± 2** | |
| Heart rate (beat/min) | 63 ± 2 | 55 ± 2** | 67 ± 2 | 58 ± 2** | |
| Total cholesterol (mmol/L) | 5.4 ± 0.3 | 4.6 ± 0.2** | 5.1 ± 0.2 | 4.5 ± 0.2** | |
| HDL cholesterol (mmol/L) | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1* | |
| LDL cholesterol (mmol/L) | 3.3 ± 0.2 | 2.7 ± 0.1* | 3.0 ± 0.2 | 2.6 ± 0.1** | |
| Triglyceride (mmol/L) | 1.9 ± 0.3 | 1.0 ± 0.2** | 2.0 ± 0.3 | 1.0 ± 0.3** | |
| Insulin (µU/ml) | 9.3 ± 1.0 | 4.8 ± 0.9** | 11.2 ± 2.4 | 7.4 ± 2.8** | |
| HOMA-IR (IU) | 2.3 ± 0.3 | 1.1 ± 0.2** | 2.8 ± 0.7 | 1.8 ± 0.8** | |
| V̇O2peak (ml/min/kg) | 29 ± 1 | 37 ± 1** | 28 ± 2 | 35 ± 2** | |
| Steps (steps/day) | 7,156 ± 553 | 11,163 ± 665** | 7,521 ± 537 | 10,986 ± 625** | |
SBP, systolic blood pressure; DBP, diastoloc blood pressure; MAP, mean arterial pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein. Date are expressed as mean ± SE. Significant difference vs before intervention, **p<0.01, *p<0.05.
Fig. 3.
Serum testosterone levels before and after the intervention program in the LCR and HCR groups. Results are shown as means ± SEs.
Discussion
In this study, we investigated whether increased physical activity or calorie restriction during a lifestyle modification program had a greater effect on serum testosterone levels in men. The changes in physical activity level and calorie restriction were the similar to our previous studies.(14,15) Our results showed that serum testosterone levels were significantly increased after the 12-week lifestyle modification program. In addition, as shown in Study 1, serum testosterone levels were significantly increased only in the HPA group. The changes in serum testosterone levels were also positively correlated with the total number of steps taken per day and independent of age, smoking, medication use, and changes in body mass after the 12-week lifestyle modification. In contrast, as shown in Study 2, there were no significant changes in serum testosterone levels in the groups that were based on the calorie restriction levels. These results suggest that an increase in physical activity levels has a greater effect on increasing serum testosterone levels in overweight and obese men during this 12-week lifestyle modification program. In our knowledge, there were no studies investigated the effect of physical activity level or calorie restriction on serum testosterone levels. For the first time, we have shown that an increase of physical activity level has greater effects on circulating testosterone levels than calorie restriction during lifestyle modification-induced weight reduction (combination of aerobic exercise and calorie restriction) in the present study.
In Study 1, there were no significant differences in the average weight reductions between the LPA and HPA groups (LPA: –12 kg; HPA: –13 kg). However, after the 12-week intervention, serum testosterone levels were significantly increased in the HPA group but not in the LPA group. In a previous study, Kaukua et al.(18) showed that a calorie restriction-induced weight reduction (approximately –21 kg) was associated with increased serum testosterone levels in obese men. Furthermore, Facchiano et al.(19) reported that bariatric surgery-induced weight reductions (BMI: 44 to 35 kg/m2) resulted in increased serum testosterone levels. These studies implied that a large weight reduction was necessary to increase serum testosterone levels in obese men. In the present study, although the average weight reduction was similar in both groups, serum testosterone levels did not significantly increase in the LPA group. In our LPA group, it was possible that the average weight reduction achieved was insufficient to increase serum testosterone levels compared with the results in previous studies. In comparison, although the average weight reduction in the HPA group was similar to that in the LPA group, serum testosterone levels significantly increased only in the HPA group. Thus, a significant increase in physical activity possibly contributed to the increased serum testosterone levels. For obese individuals, there may be too much of a burden placed on them to achieve a weight loss using the means reported by Kaukua et al.(18) or Facchiano et al.(19) In contrast, we used a moderate weight loss method in the present study, which was less burdensome for obese men than those previously reported. In our previous study, we demonstrated that a combination of aerobic exercise training and dietary modification reduced body mass (approximately –12 kg) and increased serum testosterone levels due to an increased physical activity at the same level as that in the HPA group in the present study.(14,15) Taken together, the results of this study suggest that serum testosterone levels were increased by increasing physical activity accompanied by a moderate weight reduction, even if obese men did not achieve a large weight reduction as that with bariatric surgery or very low calorie restriction.
Previous studies have suggested that aerobic exercise training resulted in increased serum testosterone levels in men. Grandys et al.(20) have reported that a 5-week aerobic exercise training program (4 times/week) increased plasma testosterone levels in healthy young men. However, Hiruntrakul et al.(21) reported that there were no significant changes in serum testosterone levels after a 12-week aerobic exercise training program (1 time/week) in sedentary young men. In addition, Baillot et al.(22) reported that there were no significant changes in serum testosterone levels after an 8-week aerobic exercise training program (2 times/week) in patients with type 2 diabetes mellitus. On the other hand, in animal studies, aerobic exercise training (5 times/week) significantly increased plasma and muscular sex steroid hormones via increase in steroidogenesis-related enzymes.(23,24) Taken together, it has been implied that regular aerobic exercise (>4 times/week) resulted in increase serum testosterone levels in men. In the present study, our participants performed aerobic exercise training >4 days per week. We suggest that habitual aerobic exercise of >4 times per week may be needed to achieve increased serum testosterone levels in overweight and obese men.
In conclusion, in this study we demonstrated that a 12-week lifestyle modification program that combined aerobic exercise training and dietary modification resulted in increased serum testosterone levels in overweight and obese men. Furthermore, based on changes in physical activity, serum testosterone levels only significantly increased in our HPA group. Moreover, we found that the changes in serum testosterone levels were positively correlated with the changes in the number of steps taken per day and independent of changes in body mass. These results suggest that an increase in physical activity greatly affected the serum testosterone levels in overweight and obese men during lifestyle modification.
Limitations
The lack of an appropriate control/observational group is clear limitations in this study. The control group is important to demonstrate the reliability of the data. However, in this study, all overweight and obese men underwent the 12-week lifestyle modification program and we did not employee the control group; as the previous study suggested that the control group could be considered unethical in that it would deny participants either the experimental or usual care treatment likely to lower body mass, the risk of cardiovascular disease, type 2 diabetes mellitus and dementia.(25)
Fig. 2.
Serum testosterone levels before and after the intervention program in the LPA and HPA groups. Results are shown as means ± SEs.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (24590654).
Abbreviations
- LPA
low physical activity
- HPA
high physical activity
- LCR
low calorie restriction
- HCR
high calorie restriction
Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- 2.Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007;30:1029–1034. doi: 10.1291/hypres.30.1029. [DOI] [PubMed] [Google Scholar]
- 3.Osuna JA, Gómez-Pérez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl. 2006;52:355–361. doi: 10.1080/01485010600692017. [DOI] [PubMed] [Google Scholar]
- 4.Maggio M, Dall'Aglio E, Lauretani F, et al. The hormonal pathway to cognitive impairment in older men. J Nutr Health Aging. 2012;16:40–54. doi: 10.1007/s12603-012-0002-7. [DOI] [PubMed] [Google Scholar]
- 5.Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210:232–236. doi: 10.1016/j.atherosclerosis.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Rosano GM, Leonardo F, Pagnotta P, et al. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–1670. doi: 10.1161/01.cir.99.13.1666. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30:911–917. doi: 10.2337/dc06-1426. [DOI] [PubMed] [Google Scholar]
- 8.Zietz B, Cuk A, Hügl S, et al. Association of increased C-peptide serum levels and testosterone in type 2 diabetes. Eur J Intern Med. 2000;11:322–328. doi: 10.1016/s0953-6205(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 9.Moffat SD, Zonderman AB, Metter EJ, et al. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 12.Miyaki A, Maeda S, Yoshizawa M, et al. Effect of weight reduction with dietary intervention on arterial distensibility and endothelial function in obese men. Angiology. 2009;60:351–357. doi: 10.1177/0003319708325449. [DOI] [PubMed] [Google Scholar]
- 13.Miyaki A, Maeda S, Yoshizawa M, et al. Effect of habitual aerobic exercise on body weight and arterial function in overweight and obese men. Am J Cardiol. 2009;104:823–828. doi: 10.1016/j.amjcard.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai H, Miyaki A, Higashino R, et al. Lifestyle modification-induced increase in serum testosterone and SHBG decreases arterial stiffness in overweight and obese men. Artery Res. 2014;8:80–87. [Google Scholar]
- 15.Kumagai H, Zempo-Miyaki A, Yosikawa T, Tsujimoto T, Tanaka K, Maeda S. Lifestyle modification increases serum testosterone level and decrease central blood pressure in overweight and obese men. Endocr J. 2015;62:423–430. doi: 10.1507/endocrj.EJ14-0555. [DOI] [PubMed] [Google Scholar]
- 16.Foresta C, Di Mambro A, Pagano C, Garolla A, Vettor R, Ferlin A. Insulin-like factor 3 as a marker of testicular function in obese men. Clin Endocrinol (Oxf) 2009;71:722–726. doi: 10.1111/j.1365-2265.2009.03549.x. [DOI] [PubMed] [Google Scholar]
- 17.Goebelsmann U, Horton R, Mestman JH, et al. Male pseudohermaphroditism due to testicular 17-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 1973;36:867–879. doi: 10.1210/jcem-36-5-867. [DOI] [PubMed] [Google Scholar]
- 18.Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Sex hormones and sexual function in obese men losing weight. Obes Res. 2003;11:689–694. doi: 10.1038/oby.2003.98. [DOI] [PubMed] [Google Scholar]
- 19.Facchiano E, Scaringi S, Veltri M, et al. Age as a predictive factor of testosterone improvement in male patients after bariatric surgery: preliminary results of a monocentric prospective study. Obes Surg. 2013;23:167–172. doi: 10.1007/s11695-012-0753-6. [DOI] [PubMed] [Google Scholar]
- 20.Grandys M, Majerczak J, Duda K, Zapart-Bukowska J, Sztefko K, Zoladz JA. The effect of endurance training on muscle strength in young, healthy men in relation to hormonal status. J Physiol Pharmacol. 2008;59:89–103. [PubMed] [Google Scholar]
- 21.Hiruntrakul A, Nanagara R, Emasithi A, Borer KT. Effect of endurance exercise on resting testosterone levels in sedentary subjects. Cent Eur J Public Health. 2010;18:169–172. doi: 10.21101/cejph.a3589. [DOI] [PubMed] [Google Scholar]
- 22.Baillot A, Vibarel-Rebot N, Amiot V, Emy P, Collomp K. Effects of an 8-week aerobic exercise training on saliva steroid hormones, physical capacity, and quality of life in diabetic obese men. Horm Metab Res. 2012;44:146–151. doi: 10.1055/s-0031-1297262. [DOI] [PubMed] [Google Scholar]
- 23.Sato K, Iemitsu M, Aizawa K, Mesaki N, Fujita S. Increased muscular dehydroepiandrosterone levels are associated with improved hyperglycemia in obese rats. Am J Physiol Endocrinol Metab. 2011;301:E274–E280. doi: 10.1152/ajpendo.00564.2010. [DOI] [PubMed] [Google Scholar]
- 24.Aizawa K, Iemitsu M, Maeda S, Mesaki N, Ushida T, Akimoto T. Endurance exercise training enhances local sex steroidogenesis in skeletal muscle. Med Sci Sports Exerc. 2011;43:2072–2080. doi: 10.1249/MSS.0b013e31821e9d74. [DOI] [PubMed] [Google Scholar]
- 25.Seals DR, Tanaka H, Clevenger CM, et al. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol. 2001;38:506–513. doi: 10.1016/s0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]