Abstract
Anaplasma phagocytophilum, the etiologic agent of human anaplasmosis, is a bacterial pathogen that specifically colonizes neutrophils. Neutrophils utilize the NADPH oxidase complex to generate superoxide (O2−) and initiate oxidative killing of microorganisms. A. phagocytophilum's unique tropism for neutrophils, however, indicates that it subverts and/or avoids oxidative killing. We therefore examined the effects of A. phagocytophilum infection on neutrophil NADPH oxidase assembly and reactive oxygen species (ROS) production. Following neutrophil binding, Anaplasma invasion requires at least 240 min. During its prolonged association with the neutrophil plasma membrane, A. phagocytophilum stimulates NADPH oxidase assembly, as indicated by increased cytochrome b558 mobilization to the membrane, as well as colocalization of Rac and p22phox. This initial stimulation taxes the host neutrophil's finite oxidase reserves, as demonstrated by time- and bacterial-dose-dependent decreases in secondary activation by N-formyl-methionyl-leucyl-phenylalanine (FMLP) or phorbol myristate acetate (PMA). This stimulation is modest, however, and does not diminish oxidase stores to nearly the extent that Escherichia coli, serum-opsonized zymosan, FMLP, or PMA do. Despite the apparent activation of NADPH oxidase, no change in ROS-dependent chemiluminescence is observed upon the addition of A. phagocytophilum to neutrophils, indicating that the bacterium may scavenge exogenous O2−. Indeed, A. phagocytophilum rapidly detoxifies O2− in a cell-free system. Once internalized, the bacterium resides within a protective vacuole that excludes p22phox and gp91phox. Thus, A. phagocytophilum employs at least two strategies to protect itself from neutrophil NADPH oxidase-mediated killing.
Human anaplasmosis (formerly called human granulocytic ehrlichiosis) is an emerging vector-borne infection in the United States and Europe (8, 10, 15). The causative agent is Anaplasma phagocytophilum, an obligate intracellular bacterium that naturally cycles among ticks of the Ixodes ricinus complex and mammalian hosts (8, 15, 16). Humans are occasional inadvertent hosts. Following tick inoculation, human anaplasmosis initially presents with nonspecific symptoms, including fever, chills, malaise, and myalgia. More distinguishing manifestations include leukopenia, thrombocytopenia, and elevated levels of C-reactive protein and hepatic transaminases. The hallmark manifestation, however, is A. phagocytophilum colonization of polymorphonuclear leukocytes. Severe, potentially fatal complications can result, including prolonged fever, shock, pneumonitis, and increased susceptibility to opportunistic infections (8, 15).
Neutrophils represent an initial line of defense against invading microorganisms. Individuals suffering from deficiencies in neutrophil numbers or function suffer from recurring, life-threatening infections and demonstrate the important role these phagocytes play in combating microbial pathogens (4). A primary means by which neutrophils destroy bacteria and fungi is through the production of toxic oxygen intermediates derived from superoxide (O2−) (34). Furthermore, the pH change within the phagosome resulting from the O2− influx triggers a potassium-dependent release of proteases that, in cooperation with hydrogen peroxide (H2O2) and hypochlorous acid, destroy ingested microorganisms (33, 34). The tightly controlled, multicomponent NADPH oxidase complex is responsible for most of the inducible O2− production in activated neutrophils. The oxidase is unassembled in resting neutrophils and consists of membrane and cytosolic components. Cytochrome b558 is a membrane-spanning heterodimer composed of two subunits, gp91phox and p22phox, localized in the plasma membrane (∼5%) and in the membranes of mobilizable specific granules and secretory vesicles (∼95%) (12, 34). Cytochrome b558 becomes expressed on the phagolysosome membrane and on the cell surface upon vesicular-granular fusion with the larger membrane systems during enzyme activation. Concurrent with this event, the cytosolic components, Rac2, p40phox, p47phox, and p67phox, translocate and unite with cytochrome b558 in the membrane to form the active enzyme complex, which transfers electrons from cytosolic NADPH to intraphagosomal or extracellular molecular oxygen, thereby generating O2− (34).
A. phagocytophilum's unique ability to invade and flourish within the hostile intraneutrophil environment suggests that it employs mechanisms for avoiding and/or subverting the normal microbicidal strategies of its host cell. Indeed, neutrophils from patients with human anaplasmosis, experimentally infected mice, or long-term-infected HL-60 cells exhibit deficiencies in NADPH oxidase activation (2, 7, 37). This functional defect associated with established A. phagocytophilum infection is attributable to the fact that the bacterium inhibits the transcription of genes encoding two key oxidase components, gp91phox and Rac2 (2, 7). However, neutrophils emerge from the bone marrow fully armed, with each of the oxidase components preformed and ready to assemble upon stimulation (34). As such, studies have demonstrated that neutrophils exhibit diminished production of reactive oxygen species (ROS) following A. phagocytophilum infection, although the time frame in which this occurs is a matter of dispute (11, 28). Furthermore, though possible mechanisms by which this phenomenon occurs have been postulated (29), the evidence remains inconclusive. We therefore correlated the effects of A. phagocytophilum on neutrophil ROS production, as well as the assembly and membrane localization of NADPH oxidase components, at various time points during infection. We also determined whether the bacterium is capable of directly scavenging O2− in a cell-free system. This study provides further insight into the means by which A. phagocytophilum combats O2−-mediated killing by its target mammalian cell.
MATERIALS AND METHODS
Cultivation of A. phagocytophilum strains and isolation of host cell-free bacteria.
Unless otherwise noted, A. phagocytophilum strain NCH-1 (7) was used in these studies. A. phagocytophilum strain HZ was kindly provided by Ralph Horowitz of New York Medical College (Valhalla) and Yasuko Rikihisa of Ohio State University (Columbus). A. phagocytophilum strains MD, 96HE 158, and 99HE 4 were generously provided by J. Stephen Dumler of Johns Hopkins University. Each strain was cultivated in HL-60 cells, and host cell-free A. phagocytophilum was prepared as previously described (7).
Isolation of neutrophils.
Human neutrophils were isolated from peripheral blood from healthy donors by centrifugation through an equal volume of Polymorphprep (Axis-Shield; Greiner Bio-One, Frickenhausen, Germany) at 470 × g for 30 min. The resulting neutrophil band was removed by aspiration and mixed with equal volumes of 0.45% NaCl and RPMI 1640 (Invitrogen, Carlsbad, Calif.)-0.5 mM EDTA to reestablish isotonic conditions. The cells were centrifuged at 210 × g for 10 min, washed twice with RPMI 1640- 0.5 mM EDTA, resuspended in RPMI 1640, and enumerated using a hemacytometer.
Assessment of A. phagocytophilum binding and invasion.
Five-hundred-microliter suspensions of 106 neutrophils per ml of RPMI 1640 were added to individual wells of a 24-well Ultra Low Attachment plate (Corning Inc., Corning, N.Y.). To these were added suspensions of host cell-free A. phagocytophilum that had been liberated from 2.5 × 106 highly (≥90%) infected HL-60 cells. At 5, 15, 30, 60, 120, and 240 min postinfection (p.i.), aliquots were removed and washed twice with RPMI 1640- 0.5 mM EDTA. Two hundred microliters of 5 × 105 neutrophils/ml in RPMI 1640- 20% fetal calf serum (FCS) was centrifuged onto glass slides at 70 × g for 2 min using a Cytospin 4 centrifuge (Thermo Electron, Pittsburgh, Pa.). The slides were fixed in 2% paraformaldehyde (PFA)-phosphate-buffered saline (PBS) and screened using an indirect-immunofluorescence assay to determine the percentage of infected neutrophils and the number of bacteria per infected cell as previously described (7).
To assess the rate at which bound A. phagocytophilum cells are internalized, viable host cell-free bacteria were fluoresceinated with Celltracker Green (Molecular Probes, Eugene, Oreg.) as previously described (6, 39). Celltracker Green-labeled bacteria were added to neutrophils at ratios of 1, 5, and 50 per cell. The cells were incubated at 37°C in 5% CO2 for 1, 2, or 4 h. At the appropriate time p.i., the cells were washed twice with RPMI 1640- 0.5 mM EDTA, resuspended in 500 μl of 1× PBS- 1% FCS- 1% PFA, and analyzed by flow cytometry using a FACScalibur (Becton Dickinson, San Diego, Calif.). Data were analyzed using Cellquest software (Becton Dickinson). Bacterial internalization was inferred as a decrease in CellTracker Green fluorescence over time.
Assessment of neutrophil O2− production.
Suspensions of 100,000 neutrophils in 100 μl of RPMI 1640 were added to luminometer cuvettes (Promega, Madison, Wis.). To these was added 100 μl of RPMI 1640 alone or RPMI 1640 containing host cell-free A. phagocytophilum, followed by incubations for 0, 60, 120, or 240 min at 37°C in 5% CO2. In some cases, A. phagocytophilum cells were incubated with either 20% autologous serum or rabbit polyclonal antiserum against A. phagocytophilum prior to being added to the neutrophils. In some cases, zymosan A particles from Saccharomyces cerevisiae (Sigma) that had been opsonized with autologous serum (OpZ) or Escherichia coli organisms were added to neutrophils at ratios of 10 particles or 10 bacteria per cell, respectively. At the appropriate time p.i. or post-addition of stimuli, 100 μl of Diogenes (National Diagnostics, Atlanta, Ga.) was added. Diogenes is a chemiluminescent substrate that is highly specific for O2− and is unaffected by the presence of H2O2. In some cases, this was followed by the addition of either phorbol myristate acetate (PMA) (100 ng/ml; Sigma) or N-formyl-methionyl-leucyl-phenylalanine (FMLP) (1 μM; Sigma). For uninduced samples, vehicle alone was added. Changes in Diogenes chemiluminescence (DCL) were recorded in total relative light units (RLU) using a TD-20/20 luminometer (Turner Designs; Promega).
Assessment of A. phagocytophilum infection, surface-associated gp91phox, and host cell size by flow cytometry.
Celltracker Green-labeled bacteria were added to neutrophils at ratios of 0.3, 3, and 30 per cell in a final volume of 1 ml of RPMI 1640 in individual wells of a 24-well Ultra Low Attachment plate and incubated at 37°C in 5% CO2 for 1, 2, or 4 h. At the appropriate time p.i., the cells were washed twice with RPMI 1640- 0.5 mM EDTA and incubated with 5 μg of anti-CD16-CD32/ml and 1 μg of mouse immunoglobulin G1 isotype control/ml (both from BD Pharmingen, San Diego, Calif.) in 100 μl of 1× PBS- 1% fetal calf serum for 30 min on ice. The cells were successively screened with 3 μg of monoclonal antibody (MAb) 7D5 (a kind gift from Dirk Roos of the University of Amsterdam [34])/ml and Cy5-conjugated anti-mouse immunoglobulin G1 (BD Pharmingen), each in 1× PBS-1% fetal calf serum, for 30 min on ice. After each antibody incubation, the cells were washed with 1 ml of 1× PBS- 1% FCS. The cells were resuspended in 500 μl of 1× PBS- 1% FCS- 1% PFA and analyzed by flow cytometry.
Double labeling and confocal laser scanning microscopy.
Neutrophils were isolated and incubated in the presence or absence of A. phagocytophilum as described above. To ensure that conditions were optimal for infection, an equivalent aliquot of bacteria was labeled with CellTracker Green and used to infect a parallel sample of neutrophils, which was subsequently examined by flow cytometry. HL-60 lysate was added as a control. PMA or vehicle alone was added. Cytsopins were prepared and fixed as described above and double labeled for Rac and p22phox using anti-Rac MAb (Transduction Laboratories, BD Biosciences) and anti-p22phox MAb 44.1 (kindly provided by Mark Quinn, Montana State University, Bozeman), respectively. Labeled samples were examined, and images were collected as previously described (23, 24).
Assessment of cell-free O2− and urate production.
Xanthine (100 μM; Sigma) in 50 mM KH2PO4 was incubated in the presence or absence of 0.125 mg of superoxide dismutase (SOD; Sigma)/ml. Titrated concentrations of host cell-free A. phagocytophilum in Hank's balanced salt solution were added. In some cases, A. phagocytophilum cells were either heated to 60°C or treated with Pronase E (Sigma) at 37°C for 20 min prior to being added. HL-60 lysate components were added to control samples. Diogenes was added, followed by the addition of xanthine oxidase (25 μM; Sigma). O2− was indirectly measured as the change in DCL every 10 s for a period of 3 min. For measuring uric acid production, the assay conditions were repeated, except that the change in A290 was recorded.
Electron microscopy.
Neutrophils were incubated with host cell-free A. phagocytophilum for 240 min as described above, followed by washing with PBS to removed unattached bacteria. Cells were initially fixed for 1 h at room temperature with 4% PFA in 0.25 M HEPES, pH 7.4, followed by fixation overnight at 4°C with 8% PFA in 0.25 M HEPES, pH 7.4. Samples were imbedded in gelatin, sectioned, and screened as previously described (18). The final concentrations of MAbs 7D5 and 44.1 were 30 and 3.25 μg/ml, respectively.
Statistical analysis.
Statistical analysis was performed using the Prism version 4.0 software package (Graphpad, San Diego, Calif.). If analysis of variance (ANOVA) indicated a group difference (P < 0.05), then Tukey's test was used to test for a significant difference among groups.
Study approval.
The Human Investigation Committee at Yale University approved these studies.
RESULTS
A. phagocytophilum exhibits prolonged association with the neutrophil outer membrane during invasion.
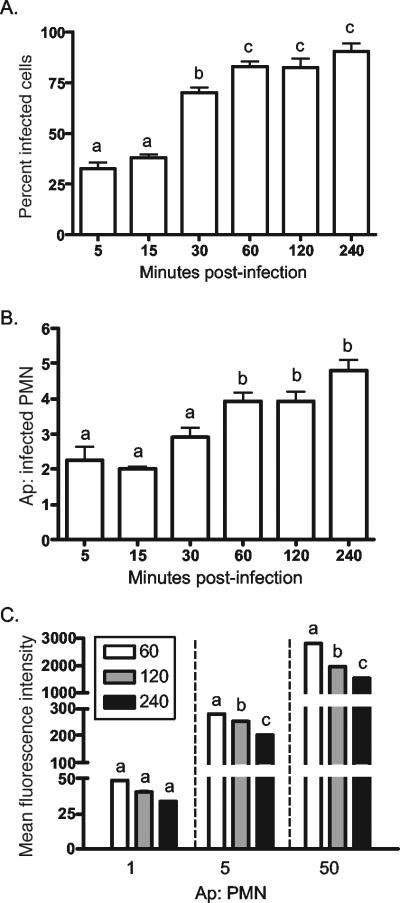
To properly assess the effects of A. phagocytophilum infection on neutrophil ROS production, it was first essential to determine the kinetics by which the bacterium binds and invades its host cell. Neutrophils were incubated with host cell-free A. phagocytophilum that had been liberated from a fivefold excess of highly infected HL-60 cells. At 5, 15, 30, 60, 120, and 240 min p.i., the neutrophils were examined by immunofluorescence microscopy for the percentage of infected cells and the number of A. phagocytophilum organisms per infected cell. Under the conditions employed, 30 to 60 min of incubation was required for at least 70.5 ± 4.6% of the neutrophils to be bound with a minimum of 2.9 ± 0.6 bacteria per cell (Fig. 1A and B). At earlier time points, <38.3 ± 2.8% of the cells were infected with <2 bacteria per cell. Achieving >90% infection, which would be necessary to reliably assess the effect of A. phagocytophilum infection on NADPH oxidase function, required an incubation period of 240 min. The relatively slow binding kinetics are likely due to the cells being in suspension and are not likely to be reflective of the bacterium's avidity for its host cell, as it is well established that A. phagocytophilum tenaciously binds neutrophils (6, 19, 20, 39).
FIG. 1.
A. phagocytophilum binding and invasion of neutrophils. (A and B) Host cell-free A. phagocytophilum (Ap) cells were added to neutrophils, as described in Materials and Methods. At the indicated times p.i., aliquots of neutrophils were examined by immunofluorescence microscopy for the percentage of infected cells (A) and the number of A. phagocytophilum cells per infected neutrophil (polymorphonuclear leukocyte [PMN]) (B). The results are presented as the mean plus standard deviation of five independent counts of at least 100 cells per time point. (C) To indirectly assess the rate at which bound A. phagocytophilum cells enter neutrophils, host cell-free A. phagocytophilum cells were fluoresceinated with CellTracker Green, added to neutrophils at approximate ratios of 1, 5, and 50 bacteria per neutrophil for 60, 120, or 240 min, and examined by flow cytometry. The results are presented as the means of assays performed in triplicate. Statistical significance (P < 0.05) was determined using ANOVA, followed by Tukey's test. The mean values indicated by different letters are significantly different. The results are representative of three independent studies.
To assess the rate at which bound A. phagocytophilum enters neutrophils, host cell-free A. phagocytophilum cells were fluoresceinated using the viable dye Celltracker Green, which has proven effective for tracing A. phagocytophilum binding and infection (6, 20, 39), and added to neutrophils at ratios of 1, 5, and 50 bacteria per cell for periods of 60, 120, or 240 min. When assessed by flow cytometry, the Celltracker Green fluorescence intensity correlated directly with the bacterial load (Fig. 1C). Since the fluorescence intensity of CellTracker Green-labeled A. phagocytophilum is stable for at least 48 h (J. A. Carlyon and E. Fikrig, unpublished observation), the degree of Anaplasma internalization was indirectly assessed as a decrease in fluorescence over the time course. Maximal internalization was not achieved until 240 min p.i. This indicates that internalization of bound A. phagocytophilum is a lengthy process. Based on our observations, neutrophils were infected for periods of 60, 120, or 240 min for subsequent NADPH oxidase assays.
A. phagocytophilum-infected neutrophils exhibit reduced ROS production.
To study the effects of A. phagocytophilum infection on NADPH oxidase activity, neutrophils were incubated with titrated concentrations of host cell-free A. phagocytophilum strain NCH-1 or with HL-60 lysate as a negative control for periods of 60, 120, or 240 min. At the appropriate time postincubation, Diogenes substrate was added, followed by the addition of FMLP or PMA. ROS production was monitored kinetically as the change in DCL. In the absence of exogenous stimuli, A. phagocytophilum elicited little or no increase in DCL regardless of the concentration. In contrast to controls, cells incubated with NCH-1 demonstrated decreases in PMA activation that correlated directly with the bacterial load and duration of infection (Fig. 2A). The greatest inhibition, which was marked by a 35.1% reduction in ROS production, was observed at 240 min p.i. and occurred with a ratio of 30 A. phagocytophilum cells per neutrophil.
FIG. 2.
A. phagocytophilum-infected neutrophils (polymorphonuclear leukocytes [PMN]) exhibit reduced ROS production. Host cell-free A. phagocytophilum (Ap) cells were added to 100,000 neutrophils at approximate ratios of 10 and 30 A. phagocytophilum cells/neutrophil and incubated for 60, 120, or 240 min. Neutrophils incubated with HL-60 lysate served as a control (ctrl). At various numbers of minutes p.i. (mpi), PMA (A) or FMLP (B) was added, and ROS production was indirectly measured as the change in DCL over time. The data are presented in RLU. The results are representative of at least 10 independent experiments.
Interestingly, neutrophils that had been incubated with 30 bacteria per cell displayed a 57.5% increase in ROS when stimulated with FMLP 60 min p.i., while those incubated for 120 and 240 min demonstrated decreases of 66.6 and 25.2%, respectively, upon stimulation (Fig. 2B). A similar but delayed response to FMLP was observed for neutrophils incubated with 10 Anaplasma organisms per cell. While there was little difference in ROS production between infected and uninfected control cells at 60 min p.i., a 33.1% increase was observed at 120 min p.i., which had nearly subsided by 240 min p.i. Ratios of ≤1 bacterium per neutrophil exhibited no difference in either PMA- or FMLP-mediated ROS from uninfected controls (data not shown). Also, results observed at 30 min p.i. exhibited little difference from those observed at 60 min p.i. (data not shown). Collectively, these data demonstrate that A. phagocytophilum infection reduces NADPH oxidase responsiveness in a time- and dose-dependent manner and suggest that Anaplasma may even stimulate oxidase activity during the early phase of infection.
The initial induction and subsequent abrogation of the neutrophil respiratory burst by A. phagocytophilum infection that we observed are consistent with a report by Choi and Dumler (11) but differ from the rapid, absolute inhibition suggested by Mott and Rikihisa (28). Because of this inconsistency, we extended our analyses to include A. phagocytophilum strain HZ, which was used in their study (28). In addition, we also performed our analyses using strains MD, 96HE 158, and 99HE 4. The results obtained with all four strains reproduced those observed for NCH-1 (data not shown). Due to the consistency in our observations obtained using five different A. phagocytophilum strains, we chose strain NCH-1 for the remaining studies.
Exposure to A. phagocytophilum yields little or no change in detectable ROS levels.
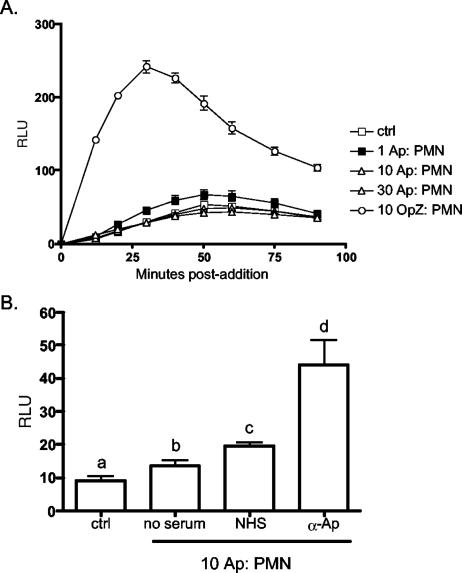
Neutrophils exhibit reduced NADPH oxidase responsiveness post-A. phagocytophilum infection. However, our data also suggest that initial interaction with the bacterium might elicit such activity. Moreover, though several studies have examined O2− production by A. phagocytophilum-infected myelocytic cells (2, 7, 11, 28, 37), none have assessed the immediate effect the bacterium has on NADPH oxidase activity. We therefore examined neutrophil ROS production immediately following the addition of A. phagocytophilum at ratios of 1, 10, or 30 bacteria per cell. Relative to uninfected controls, very little change in DCL was observed for neutrophils incubated with A. phagocytophilum, regardless of the bacterial concentration (Fig. 3A). In sharp contrast, however, neutrophils yielded significantly higher ROS levels upon exposure to OpZ, which presumably would have activated NADPH oxidase by Fc receptor-mediated phagocytosis (1).
FIG. 3.
Induction of neutrophil ROS production by A. phagocytophilum. (A) Host cell-free A. phagocytophilum (Ap; ratios of ∼1, 10, or 30 per cell) or OpZ (10 per cell) were added to samples of 100,000 neutrophils (polymorphonuclear leukocytes [PMN]), and ROS production was indirectly assessed as the change in DCL at 10-min intervals over a 90-min time course. (B) Nonopsonsized host cell-free A. phagocytophilum or bacteria that had been incubated with either normal human serum (NHS) or rabbit polyclonal anti-A. phagocytophilum antiserum (α-Ap) were added to neutrophils at 10 A. phagocytophilum cells/neutrophil. ROS production was recorded as described for panel A. Neutrophils alone served as controls (ctrl) for both experiments. Data are presented as the mean (plus standard deviation) maximum change in RLU per time point. Assays were performed in triplicate. Statistical significance (P < 0.05) was determined using ANOVA, followed by Tukey's test. The mean values indicated by different letters are significantly different. The results are representative of three independent experiments.
Current evidence suggests that A. phagocytophilum, unlike many other bacterial species, is not internalized into neutrophils or HL-60 cells by phagocytosis. Instead, the bacteria actively enter their mammalian host cells via a process involving caveolae and endocytosis (3, 25, 27, 38, 40). Consistent with this, Anaplasma opsonized by rabbit polyclonal A. phagocytophilum antiserum, which presumably would have been internalized by Fc receptor-mediated phagocytosis, elicited an oxidase response severalfold higher than that of Anaplasma preincubated with either normal human serum or no serum (Fig. 3B).
Initial exposure to A. phagocytophilum mobilizes neutrophil NADPH oxidase reserves.
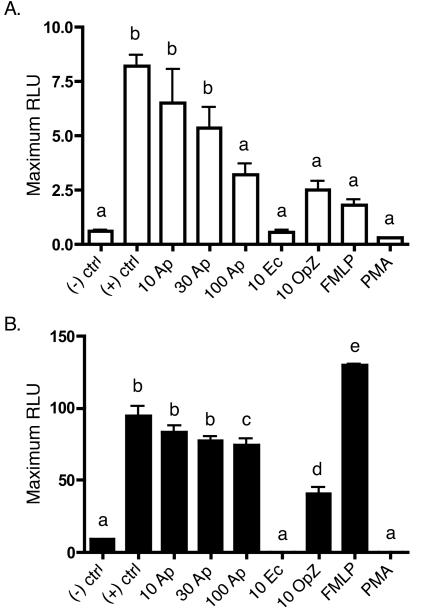
Secondary activation of neutrophils results in reduced O2− generation, as their NADPH oxidase stores are finite and cannot be replenished (1, 12, 34). A. phagocytophilum-infected neutrophils demonstrate lowered NADPH oxidase responsiveness to both FMLP and PMA stimulation that is maximal 240 min p.i. We therefore assessed whether inhibition of ROS generation is specific for A. phagocytophilum infection or merely the consequence of the bacterium stimulating oxidase mobilization during binding and invasion. Neutrophils were initially stimulated with host cell-free A. phagocytophilum, E. coli, OpZ, FMLP, or PMA. Four hours poststimulation, the neutrophils were restimulated with either FMLP or PMA, and the resulting change in DCL was observed. Uninfected cells receiving no stimuli at either time point served as negative controls, while those exposed to stimuli at 4 h only served as positive controls. E. coli, OpZ, and PMA each elicited respiratory bursts substantial enough to exhaust neutrophil oxidase reserves, as little or no ROS were released upon secondary stimulation by FMLP or PMA (Fig. 4). Consistent with our previous observations, A. phagocytophilum-infected neutrophils displayed a dose-dependent reduction in ROS release upon secondary activation by FMLP (Fig. 4A). However, this reduction was not very pronounced. Only at an excessively high concentration of 100 Anaplasma organisms per cell did the drop in secondary FMLP stimulation begin to approach that yielded by preincubation with E. coli, OpZ, FMLP, or PMA.
FIG. 4.
Neutrophil restimulation assay. Samples of 100,000 neutrophils were initially exposed to host cell-free A. phagocytophilum (Ap; 10, 30, or 100 per cell), E. coli (Ec; 10 per cell), OpZ (10 per cell), FMLP, or PMA. Four hours later, the neutrophils were restimulated with either FMLP (A) or PMA (B), and ROS production was indirectly measured as the change in DCL over 60 min. Neutrophils receiving no stimulation at either 0 or 4 h served as negative controls [(−) ctrl], while those receiving stimulation only at 4 h served as positive controls [(+) ctrl]. The data are presented as the mean (plus standard deviation) maximum change in RLU. Assays were performed in triplicate. Statistical significance (P < 0.05) was determined using ANOVA, followed by Tukey's test. The mean values indicated by different letters are significantly different. The results are representative of three independent experiments.
Restimulation by PMA was modestly affected by A. phagocytophilum infection for all bacterial concentrations (Fig. 4B). The discrepancy in secondary activation profiles between FMLP and PMA is likely due to the difference in the duration over which they activate NADPH oxidase. While FMLP activation is rapid and transient, PMA irreversibly activates proteins containing a C1 domain, principally protein kinase C (1). Subjecting Anaplasma to either heat or pronase treatment eliminated bacterial binding to neutrophils, which consequently did not alter the NADPH oxidase response upon secondary PMA activation (data not shown). Consistent with published data, initial stimulation with FMLP primed neutrophils for a strong secondary response to PMA (21). The secondary activation profiles of A. phagocytophilum-infected neutrophils strongly suggest that physical interaction with the bacterium stimulates NADPH oxidase activation, though to a considerably lesser degree than E. coli, phagocytic particles, or soluble stimuli. However, A. phagocytophilum elicits very little or no change in DCL, which presents an interesting paradox warranting further investigation.
A. phagocytophilum stimulates cytochrome b558 aggregation at the neutrophil plasma membrane and cell size increase.
Our restimulation data suggest that A. phagocytophilum interaction results in neutrophil activation and NADPH oxidase mobilization. This is in contrast, however, to our observation that addition of the bacterium to neutrophils results in little to no change in DCL. Therefore, we shifted our focus to directly gauge NADPH oxidase assembly in the context of A. phagocytophilum infection. We first assessed the localization of cytochrome b558 to the neutrophil plasma membrane in response to A. phagocytophilum. Celltracker Green-labeled A. phagocytophilum was added to neutrophils at ratios of 0.3, 3, and 30 bacteria per cell for periods of 60, 120, and 240 min. The infected neutrophils were gated according to CellTracker Green fluorescence and examined for levels of plasma membrane-associated cytochrome b558 based on their ability to be recognized by MAb 7D5, which targets a surface-exposed epitope of gp91phox. This epitope is accessible only when gp91phox is complexed with p22phox, forming the cytochrome b558 heterodimer (5, 30). A. phagocytophilum stimulated cytochrome b558 association with the neutrophil plasma membrane in a time- and dose-dependent manner (Fig. 5A).
FIG. 5.
A. phagocytophilum interaction results in increased cytochrome b558 association with the neutrophil plasma membrane and increased host cell size. Viable host cell-free A. phagocytophilum cells were fluorescently labeled using CellTracker Green and added to neutrophils at approximate A. phagocytophilum cell/neutrophil ratios of 0.3 (open bars), 3 (shaded bars), and 30 (solid bars) for periods of 60, 120, and 240 min. (A) At the appropriate time post-addition of bacteria, infected neutrophils were assessed by flow cytometry for plasma membrane levels of cytochrome (cyto) b558 as determined by MAb 7D5 recognition. (B) Infected cells were also examined for changes in their forward scatter properties as an indication of cell size variation. Statistical significance (P < 0.05) was determined for each set of means per p.i. time point using ANOVA, followed by Tukey's test. The mean values indicated by different letters are significantly different. The results are representative of three independent experiments.
Another phenomenon associated with neutrophil activation is an increase in cell size (14, 17, 22, 31), which can be directly measured by flow cytometry as an increase in forward scatter. The forward scatter of infected cells increased directly with the bacterial load and duration of infection (Fig. 5B), providing further evidence that A. phagocytophilum interaction leads to host cell activation. Neutrophils incubated with HL-60 lysate demonstrated no increase in either cytochrome b558 or forward scatter (data not shown). These data confirm that A. phagocytophilum activates its host cell during infection and stimulates the localization of cytochrome b558 to the neutrophil plasma membrane, which is an integral step in NADPH oxidase assembly.
A. phagocytophilum stimulates colocalization of p22phox and Rac2.
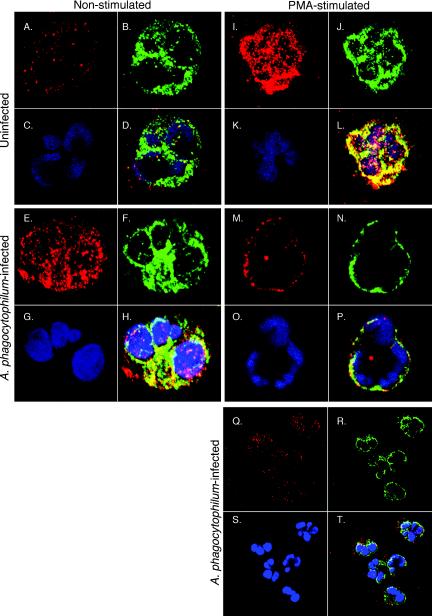
Our flow cytometric data suggest that interaction of A. phagocytophilum with its host neutrophil elicits plasma membrane association of cytochrome b558, with the highest degree of association occurring at 240 min p.i. As a means of determining the bacterium's effect on the assembly of additional oxidase components in the presence and absence of secondary stimulation, A. phagocytophilum-infected neutrophils were directly visualized for intracellular levels and colocalization of p22phox and Rac using confocal microscopy following the addition of PMA or vehicle alone. Neutrophils were incubated with A. phagocytophilum at a ratio of 3.3 ± 0.6 bacteria per cell (as determined by immunofluorescence microscopy) for 240 min. Higher ratios resulted in considerable irreversible neutrophil clumping, which precluded our examination of individual cells. Neutrophils incubated with HL-60 lysate components served as a negative control.
Control neutrophils exhibited very diffuse staining for both p22phox and Rac and demonstrated no colocalization (Fig. 6A to D). Upon PMA stimulation, the staining patterns for each changed dramatically, becoming considerably more intense and patchy (Fig. 6I to L). Such patterns are consistent with NADPH oxidase assembly (23, 24). Moreover, significant colocalization occurred, as indicated by a high distribution of yellow to yellow-orange areas throughout the cells, providing further evidence of oxidase formation. A. phagocytophilum-infected cells exhibited considerable degrees of patchy p22phox and Rac staining, although the patterns were not as intense as those associated with PMA stimulation (Fig. 6E to H). Also, at least some degree of Rac and p22phox colocalization occurred in infected cells, indicating that the bacterium evokes at least minor levels of oxidase assembly. These data are consistent with our flow cytometric studies. Upon PMA stimulation, however, infected neutrophils demonstrated complete redistribution of both Rac and p22phox to the plasma membrane, with minute patches of colocalization (Fig. 6M to P). This is not surprising, as PMA promotes plasma membrane enrichment of NADPH oxidase components (13). Therefore, the combination of stimuli would be expected to induce greater oxidase mobilization than either alone. The results presented for individual cells in Fig. 6 are representative of the majority of cells for each population examined (Fig. 6Q to T). Furthermore, Z-stack analyses revealed that the colocalization and plasma membrane redistribution of Rac and p22phox observed for infected and PMA-activated infected cells, respectively, were not restricted to a single focal plane (Fig. 7). Collectively, our flow cytometric and confocal studies illustrate that A. phagocytophilum stimulates the aggregation and assembly of at least two oxidase components, Rac and cytochrome b558.
FIG. 6.
Confocal analysis of Rac and p22phox colocalization in uninfected and A. phagocytophilum-infected neutrophils. Neutrophils were incubated in the presence of host cell-free A. phagocytophilum for 240 min. Control neutrophils were incubated with HL-60 lysate components. At 240 min p.i., infected neutrophils had 3.3 ± 0.6 bacteria per cell. Infected and control neutrophils were stimulated with PMA for 5 min. Cytospins of each sample were double labeled for Rac and p22phox, followed by DAPI nuclear counterstain (blue) (C, G, K, O, and S). Rac immunofluorescence (A, E, I, M, and Q), shown in red, was overlaid with green p22phox immunofluorescence (B, F, J, N, and R) to determine colocalization, as indicated by a yellow-orange color (D, H, L, P, and T). (A to D) Uninfected, nonstimulated neutrophils. (E to H) A. phagocytophilum-infected, nonstimulated neutrophils. (I to L) Uninfected, PMA-stimulated neutrophils. (M to P) A. phagocytophilum-infected, PMA-stimulated neutrophils. (Q to T) Lower magnification of infected, PMA-stimulated cells showing a population of cells in one field. Magnification: panels A to P, ×40; panels Q to T, ×10. The results are indicative of two separate experiments.
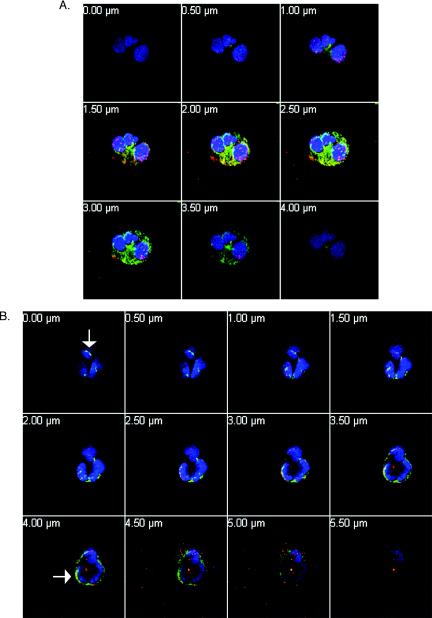
FIG. 7.
Z sections of A. phagocytophilum-infected cells. Cells from Fig. 6E to H and M to P are shown in 0.5-μm-thick Z sections to show membrane localization of Rac (red) and p22phox (green), with blue indicating nuclei. (A) Neutrophils infected with A. phagocytophilum. (B) A. phagocytophilum-infected, PMA-stimulated neutrophils. The cells were treated as described for Fig. 6. The sections are shown from top to bottom of cells on cytospins. The number in each panel refers to the distance traversed (in 0.5-μM sections) in progressing from the uppermost to the lowermost focal plane. The arrows indicate colocalization of Rac and p22phox on the membranes of infected, PMA-stimulated cells. Magnification, ×40.
A. phagocytophilum reduces O2− produced in a cell-free system.
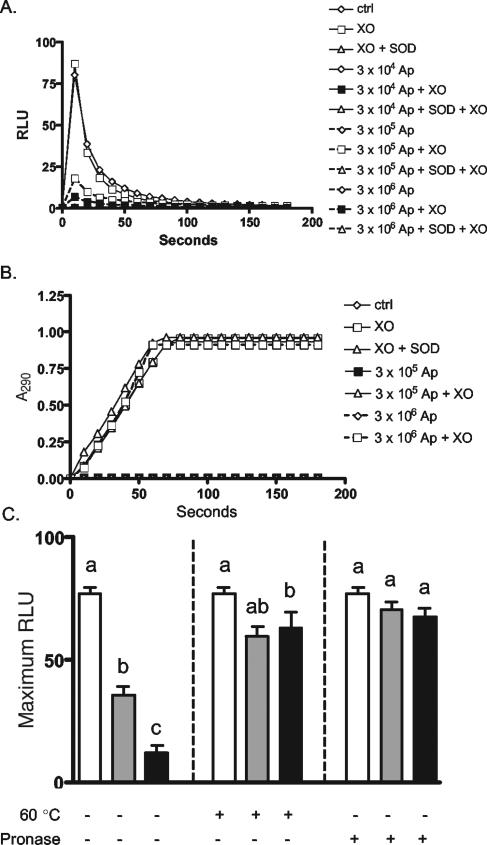
Upon interaction with A. phagocytophilum, neutrophils exhibit very little to no change in DCL despite the assembly of at least two oxidase components. This raises the likelihood that A. phagocytophilum is able to directly detoxify O2−. We therefore investigated this possibility using a cell-free system in which xanthine oxidase is added to xanthine to rapidly generate uric acid, with O2− and H2O2 as by-products. As previously stated, the DCL assay employed is highly specific for O2− and is unaffected by the presence of H2O2. Prior to the addition of xanthine oxidase, host cell-free A. phagocytophilum or HL-60 lysate components were added to xanthine at concentrations ranging from 3 × 104 to 3 × 106 bacteria per sample. The total numbers of A. phagocytophilum organisms added were identical to the numbers added to neutrophils for the oxidase stimulation and colocalization studies described above. As a positive control for O2− detoxification, samples were also incubated in the presence of SOD. The addition of xanthine oxidase to xanthine rapidly produced O2−, as inferred from a sharp rise in DCL that peaked 10 s postaddition (Fig. 8A). This system, however, yielded no change in DCL in the presence of SOD. A. phagocytophilum inhibited cell-free O2− production in a dose-dependent manner. Concentrations of 3 × 105 and 3 × 106 bacteria markedly reduced, but did not abolish, O2− generation, while a concentration of 3 × 104 exhibited little or no effect. This inhibition was specific for O2−, as neither SOD nor A. phagocytophilum affected urate production (Fig. 8B). The A. phagocytophilum mechanism responsible for rapidly scavenging exogenous O2− involves a heat-labile surface protein(s), as both pronase E digestion and heat treatment eliminated the bacterium's ability to detoxify O2− (Fig. 8C). Thus, while A. phagocytophilum apparently stimulates the production of O2− by its target neutrophil, it is able to rapidly scavenge the anion, which likely serves as a means of protecting itself during host cell binding and invasion.
FIG. 8.
A. phagocytophilum detoxifies O2− produced in a cell-free system. Host cell-free A. phagocytophilum (Ap) cells were added to xanthine at concentrations of 3 × 104, 3 × 105, and 3 × 106. HL-60 lysate components were added as controls (ctrl). SOD was added to duplicate samples. (A) Upon the addition of xanthine oxidase (XO), cell-free O2− production was measured as the resulting change in DCL every 10 s over a 3-min period. The results are representative of 10 experiments with similar results. (B) Uric acid production was measured as the change in A290. The results are representative of four separate experiments. (C) The assay was repeated using 0 (open bars), 3 × 105 (shaded bars), or 3 × 106 (solid bars) untreated host-cell-free A. phagocytophilum cells or cells that had either been heated to 60°C (+) or incubated with pronase E (+) at 37°C for 20 min. Statistical significance (P < 0.05) was determined for each set of means per treatment using ANOVA, followed by Tukey's test. Mean values indicated by different letters are significantly different. The results are representative of five independent experiments. The error bars indicate standard deviations.
A. phagocytophilum vacuole excludes gp91phox and p22phox.
A. phagocytophilum binding and invasion stimulates cytochrome b558 recruitment to the neutrophil plasma membrane. The bacterium protects itself by scavenging released O2−. Two hundred and forty minutes p.i., when the majority of Anaplasma cells would have been internalized, neutrophils retain the capability to mobilize NADPH oxidase to the plasma membrane and produce ROS. The intracellular fate of A. phagocytophilum with regard to NADPH oxidase mobilization is unknown. To assess whether NADPH oxidase components localize to the A. phagocytophilum vacuole, frozen sections of infected neutrophils were examined 240 min p.i. by immunoelectron microscopy using antibodies targeting gp91phox and p22phox. Consistent with previous observations, A. phagocytophilum infection results in the host cell's cytoplasm appearing more electron lucent (38). As shown in Fig. 9, gold particles associated predominantly with secretory vesicles but not with the A. phagocytophilum parasitophorous vacuole. This is especially evident in Fig. 9B, in which two p22phox-labeled vesicles reside in close proximity to, but fail to fuse with, the Anaplasma vacuolar membrane. Thus, once internalized, A. phagocytophilum resides within a protective compartment that excludes NADPH oxidase, thereby providing it with a safe haven from intracellularly derived ROS.
FIG. 9.
Neither gp91phox nor p22phox colocalizes to the A. phagocytophilum (Ap) vacuolar membrane. Neutrophils were examined by immunoelectron microscopy 240 min post-A. phagocytophilum infection as described in Materials and Methods using MAbs directed against gp91phox (A) and p22phox (B). The arrowheads highlight representative gold particles. Bar, 500 nm. The results are representative of several micrographs.
DISCUSSION
NADPH oxidase-mediated O2− production is a major means by which neutrophils kill bacteria. A. phagocytophilum, however, specifically invades and flourishes within these soldier phagocytes, indicating that it uses strategies for avoiding and/or combating the respiratory burst. Indeed, the bacterium scavenges extracellular O2−. This likely serves to protect A. phagocytophilum during the initial steps of neutrophil invasion, which proceeds at a rather inefficient rate. During its prolonged association with the neutrophil plasma membrane, A. phagocytophilum stimulates cellular activation, assembly, and membrane localization of at least two oxidase components and subsequent O2− release in a dose-dependent manner.
Oxidase mobilization in response to A. phagocytophilum is contact dependent, as association with neutrophils initially primed host cells for a more robust response to FMLP within the first 60 min of infection. As infection proceeds, however, more oxidase stores are mobilized. This is supported by a dose-dependent decrease in the respiratory burst of infected cells upon secondary activation with FMLP or PMA. Also, cytochrome b558 membrane localization increases over the course of invasion, as does its association with Rac. These data underline the importance of A. phagocytophilum's requirement for a means of protecting itself from released ROS.
Exogenous O2− is rapidly scavenged by A. phagocytophilum in a dose-dependent manner. The precise mechanism by which this occurs is unclear. A common strategy employed by a wide array of bacteria to rapidly detoxify O2− is the use of a periplasm-localized SOD (26). Transcription of an A. phagocytophilum sodB homolog has been demonstrated in infected HL-60 cells and in the blood of patients with human anaplasmosis (32). However, sodB genes typically encode Fe-cofactored SODs involved in protection from intracellularly derived oxidative radicals, such as those arising as metabolic by-products, while the Cu-Zn-cofactored SodC proteins protect bacteria from extracellular O2− (26). An A. phagocytophilum sodC homolog has yet to be demonstrated. Furthermore, recombinant SOD added to the xanthine-xanthine oxidase system completely inhibited O2− generation, while only partial reduction was observed in the presence of A. phagocytophilum, even at high bacterial concentrations. Therefore, A. phagocytophilum either expresses SOD at a lower concentration relative to the amount of recombinant SOD used as an experimental control or utilizes an undefined, less efficient mechanism for detoxifying exogenous O2−.
Still, the efficiency with which the A. phagocytophilum O2−-scavenging mechanism proceeds is likely substantial enough to protect it from the levels of ROS it elicits during binding and invasion. For instance, neutrophils incubated with Anaplasma exhibited reduced ROS release upon secondary stimulation. This indicated that exposure to the bacterium stimulated some degree of NADPH oxidase mobilization and O2− production. However, no change in DCL was observed over 90 min immediately following the addition of Anaplasma to neutrophils, which confirms that the bacterium is able to successfully scavenge released ROS while invading its target cell. Furthermore, A. phagocytophilum, whether alone or preincubated with autologous serum, stimulates almost no respiratory-burst activity. This is even more evident when compared to ROS levels generated in response to other bacteria, such as E. coli, or to phagocytic particles, such as OpZ or A. phagocytophilum opsonized with anti-A. phagocytophilum serum. Herron and Goodman provided evidence for a SOD-independent mechanism by proposing that A. phagocytophilum expresses an outer membrane-tethered cytochrome c oxidase that accepts electrons from O2−, thereby converting it to harmless O2 (M. J. Herron and J. Goodman, Abstr. Am. Soc. Rickettsiol.-Bartonella Emerg. Pathog. Grp. 2001 Joint Conf., abstr. 50, 2001). Consistent with our observations, the O2− detoxification mechanism characterized by Herron and Goodman involves at least one heat-labile protein expressed on the A. phagocytophilum surface.
Results obtained from our O2− detection studies differ from those presented by Mott and Rikihisa, which suggested that A. phagocytophilum abolishes neutrophil NADPH oxidase activity within 30 min (28). Their studies relied primarily on the reduction of cytochrome c, which can be measured spectrophotometrically as an indicator of extracellular O2− production. Rather than kinetically recording the change in absorbance following the addition of cytochrome c, they performed a single measurement after 2 h. A likely pitfall of these conditions is that reduced cytochrome c can, with time, be reoxidized by oxidants such as H2O2 (35), which would certainly have been generated by activated neutrophils. Alternatively, if A. phagocytophilum does employ a surface-exposed cytochrome c oxidase, this would likely have reoxidized cytochrome c. Thus, at 2 h, it is probable that reoxidized cytochrome c was assayed, which would have falsely indicated that no O2− had been produced. In addition, results from their chemiluminescence studies reported ROS production as a percent change in luminol-dependent chemiluminescence. This can be problematic, as it does not take into account the resting state of control neutrophils. In fact, changing the presentation of data from our chemiluminescence studies from RLU to percent change in DCL falsely suggests that A. phagocytophilum eliminates neutrophil ROS production (data not shown).
Flow cytometric and confocal microscopic studies, which allow a true assessment of oxidase colocalization, confirmed that neutrophil exposure to A. phagocytophilum results in oxidase assembly and cellular activation. It has been suggested that inhibition of neutrophil O2− production following A. phagocytophilum infection is due to the bacterium actively degrading p22phox (29). Loss of p22phox would prohibit proper formation of the cytochrome b558 heterodimer (34), which would effectively prevent O2− formation. This hypothesis, however, is not supported by results obtained from these studies. Confocal analysis demonstrated considerably increased p22phox staining of A. phagocytophilum-infected cells. Western analyses confirmed that p22phox levels are not reduced during A. phagocytophilum infection (data not shown). Moreover, surface recognition by MAb 7D5, which binds to gp91phox only when complexed with p22phox, directly increases with the A. phagocytophilum burden. This confirms high levels of p22phox and, more importantly, demonstrates assembly of functional cytochrome b558 in response to A. phagocytophilum infection.
Following neutrophil invasion, A. phagocytophilum resides within a vacuole that does not acquire gp91phox and p22phox, despite its immediate proximity to cytochrome b558-carrying secretory vesicles. This suggests that the vacuolar membrane excludes NADPH oxidase and protects the bacterium from intracellularly directed ROS. The A. phagocytophilum vacuole is a privileged site indeed, as it also fails to accumulate late endosomal markers and prevents luminal acidification and phagolysosomal fusion (27, 38). Excluding the phagocyte NADPH oxidase from its parasitophorous vacuole may be a common virulence theme among intracellular bacteria, as it was first identified as an intramacrophage survival strategy of Salmonella enterica serovar Typhimurium. The ability of S. enterica serovar Typhimurium to exclude p22phox and p47phox (36), as well as vesicles containing inducible nitric oxide synthase (9), from its vacuole is conferred by the presence of a type III secretion system encoded by Salmonella pathogenicity island II. A. phagocytophilum, however, is the only pathogen for which exclusion of neutrophil NADPH oxidase from the parasitophorous vacuole has been demonstrated. The means by which it does so is not known.
A. phagocytophilum's tropism for neutrophils makes it highly unusual among microorganisms and indicates that it effectively protects itself from the oxidative killing capacity of its host cell. The data presented here demonstrate that Anaplasma entry into its host neutrophil is a prolonged process, during which it stimulates NADPH oxidase assembly and plasma membrane redistribution. This indicates that the bacterium would be exposed to O2− upon binding and invasion. Based on our data, we hypothesize that A. phagocytophilum is able to protect itself by directly detoxifying O2− in its local environment by using a currently undefined mechanism. Additionally, following neutrophil invasion, A. phagocytophilum resides within a protective vacuole that excludes NADPH oxidase. Future studies will seek to define the molecular means by which A. phagocytophilum directly scavenges O2− and prevents the fusion of oxidase-carrying vesicles with its protective parasitophorous compartment. Such efforts will provide a thorough understanding of how this unique bacterium combats the neutrophil respiratory burst.
Acknowledgments
We thank Ralph Horwitz of New York Medical College and Yasuko Rikihisa of Ohio State University for providing A. phagocytophilum strain HZ; J. Stephen Dumler of Johns Hopkins University for providing A. phagocytophilum strains MD, 96HE 158, and 99HE 4; Dirk Roos of the University of Amsterdam for providing MAb 7D5 and for helpful discussions; Mark Quinn of Montana State University for providing MAb 44.1; Bindu Sukumaran and Nandhini Ramamoorthi for assisting with the O2− assays; Jacob Ijdo of the University of Iowa for helpful discussions regarding the cell-free O2− system and for discussing his data prior to publication; and Hannah Gould for assistance with statistical analysis.
This work was supported by grants AI041440 (E.F.) and AI10554 and KO1DK065039 (J.A.C.) from the National Institutes of Health; the Burroughs Wellcome Fund (E.F.); and the Canadian Institutes of Health Research (P.L.).
Editor: F. C. Fang
REFERENCES
- 1.Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1464-1476. [PubMed] [Google Scholar]
- 2.Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J. Immunol. 164:3946-3949. [DOI] [PubMed] [Google Scholar]
- 3.Barnewall, R. E., N. Ohashi, and Y. Rikihisa. 1999. Ehrlichia chaffeensis and Ehrlichia sennetsu, but not the human granulocytic ehrlichiosis agent, colocalize with transferrin receptor and up-regulate transferrin receptor mRNA by activating iron-responsive protein 1. Infect. Immun. 67:2258-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burg, N. D., and M. H. Pillinger. 2001. The neutrophil: function and regulation in innate and humoral immunity. Clin. Immunol. 99:7-17. [DOI] [PubMed] [Google Scholar]
- 5.Burritt, J. B., F. R. DeLeo, C. L. McDonald, J. R. Prigge, M. C. Dinauer, M. Nakamura, W. M. Nauseef, and A. J. Jesaitis. 2001. Phage display epitope mapping of human neutrophil flavocytochrome b558. Identification of two juxtaposed extracellular domains. J. Biol. Chem. 276:2053-2061. [DOI] [PubMed] [Google Scholar]
- 6.Carlyon, J. A., M. Akkoyunlu, L. Xia, T. Yago, T. Wang, R. D. Cummings, R. P. McEver, and E. Fikrig. 2003. Murine neutrophils require alpha 1,3-fucosylation but not PSGL-1 for productive infection with Anaplasma phagocytophilum. Blood 102:3387-3395. [DOI] [PubMed] [Google Scholar]
- 7.Carlyon, J. A., W. T. Chan, J. Galan, D. Roos, and E. Fikrig. 2002. Repression of rac2 mRNA expression by Anaplasma phagocytophila is essential to the inhibition of superoxide production and bacterial proliferation. J. Immunol. 169:7009-7018. [DOI] [PubMed] [Google Scholar]
- 8.Carlyon, J. A., and E. Fikrig. 2003. Invasion and survival strategies of Anaplasma phagocytophilum. Cell Microbiol. 5:743-754. [DOI] [PubMed] [Google Scholar]
- 9.Chakravortty, D., I. Hansen-Wester, and M. Hensel. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J. Exp. Med. 195:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, K. S., and J. S. Dumler. 2003. Early induction and late abrogation of respiratory burst in A. phagocytophilum-infected neutrophils. Ann. N. Y. Acad. Sci. 990:488-493. [DOI] [PubMed] [Google Scholar]
- 12.Dahlgren, C., and A. Karlsson. 1999. Respiratory burst in human neutrophils. J. Immunol. Methods 232:3-14. [DOI] [PubMed] [Google Scholar]
- 13.DeLeo, F. R., L. A. Allen, M. Apicella, and W. M. Nauseef. 1999. NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 163:6732-6740. [PubMed] [Google Scholar]
- 14.Demaurex, N., G. P. Downey, T. K. Waddell, and S. Grinstein. 1996. Intracellular pH regulation during spreading of human neutrophils. J. Cell Biol. 133:1391-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumler, J. S., and J. S. Bakken. 1998. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu. Rev. Med. 49:201-213. [DOI] [PubMed] [Google Scholar]
- 16.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Segura, E., J. M. Garcia, J. L. Santos, and A. Campos. 1995. Shape, F-actin, and surface morphology changes during chemotactic peptide-induced polarity in human neutrophils. Anat. Rec. 241:519-528. [DOI] [PubMed] [Google Scholar]
- 18.Folsch, H., M. Pypaert, P. Schu, and I. Mellman. 2001. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol. 152:595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman, J. L., C. M. Nelson, M. B. Klein, S. F. Hayes, and B. W. Weston. 1999. Leukocyte infection by the granulocytic ehrlichiosis agent is linked to expression of a selectin ligand. J. Clin. Investig. 103:407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herron, M. J., C. M. Nelson, J. Larson, K. R. Snapp, G. S. Kansas, and J. L. Goodman. 2000. Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science 288:1653-1656. [DOI] [PubMed] [Google Scholar]
- 21.Karnad, A. B., K. L. Hartshorn, J. Wright, J. B. Myers, J. H. Schwartz, and A. I. Tauber. 1989. Priming of human neutrophils with N-formyl-methionyl-leucyl-phenylalanine by a calcium-independent, pertussis toxin-insensitive pathway. Blood 74:2519-2526. [PubMed] [Google Scholar]
- 22.Klut, M. E., B. A. Whalen, and J. C. Hogg. 1997. Activation-associated changes in blood and bone marrow neutrophils. J. Leukoc. Biol. 62:186-194. [DOI] [PubMed] [Google Scholar]
- 23.Lacy, P., D. Abdel-Latif, M. Steward, S. Musat-Marcu, S. F. Man, and R. Moqbel. 2003. Divergence of mechanisms regulating respiratory burst in blood and sputum eosinophils and neutrophils from atopic subjects. J. Immunol. 170:2670-2679. [DOI] [PubMed] [Google Scholar]
- 24.Lacy, P., S. Mahmudi-Azer, B. Bablitz, M. Gilchrist, P. Fitzharris, D. Cheng, S. F. Man, G. M. Bokoch, and R. Moqbel. 1999. Expression and translocation of Rac2 in eosinophils during superoxide generation. Immunology 98:244-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, M., and Y. Rikihisa. 2003. Obligatory intracellular parasitism by Ehrlichia chaffeensis and Anaplasma phagocytophilum involves caveolae and glycosylphosphatidylinositol-anchored proteins. Cell Microbiol. 5:809-820. [DOI] [PubMed] [Google Scholar]
- 26.Lynch, M., and H. Kuramitsu. 2000. Expression and role of superoxide dismutases (SOD) in pathogenic bacteria. Microbes Infect. 2:1245-1255. [DOI] [PubMed] [Google Scholar]
- 27.Mott, J., R. E. Barnewall, and Y. Rikihisa. 1999. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect. Immun. 67:1368-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mott, J., and Y. Rikihisa. 2000. Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils. Infect. Immun. 68:6697-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mott, J., Y. Rikihisa, and S. Tsunawaki. 2002. Effects of Anaplasma phagocytophila on NADPH oxidase components in human neutrophils and HL-60 cells. Infect. Immun. 70:1359-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura, M., M. Murakami, T. Koga, Y. Tanaka, and S. Minakami. 1987. Monoclonal antibody 7D5 raised to cytochrome b558 of human neutrophils: immunocytochemical detection of the antigen in peripheral phagocytes of normal subjects, patients with chronic granulomatous disease, and their carrier mothers. Blood 69:1404-1408. [PubMed] [Google Scholar]
- 31.O'Flaherty, J. T., D. L. Kreutzer, and P. A. Ward. 1977. Neutrophil aggregation and swelling induced by chemotactic agents. J. Immunol. 119:232-239. [PubMed] [Google Scholar]
- 32.Ohashi, N., N. Zhi, Q. Lin, and Y. Rikihisa. 2002. Characterization and transcriptional analysis of gene clusters for a type IV secretion machinery in human granulocytic and monocytic ehrlichiosis agents. Infect. Immun. 70:2128-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291-297. [DOI] [PubMed] [Google Scholar]
- 34.Roos, D., R. van Bruggen, and C. Meischl. 2003. Oxidative killing of microbes by neutrophils. Microbes Infect. 5:1307-1315. [DOI] [PubMed] [Google Scholar]
- 35.Tarpey, M. M., and I. Fridovich. 2001. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ. Res. 89:224-236. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 37.Wang, T., S. E. Malawista, U. Pal, M. Grey, J. Meek, M. Akkoyunlu, V. Thomas, and E. Fikrig. 2002. Superoxide anion production during Anaplasma phagocytophila infection. J. Infect. Dis. 186:274-280. [DOI] [PubMed] [Google Scholar]
- 38.Webster, P., J. W. IJdo, L. M. Chicoine, and E. Fikrig. 1998. The agent of Human Granulocytic Ehrlichiosis resides in an endosomal compartment. J. Clin. Investig. 101:1932-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yago, T., A. Leppanen, J. A. Carlyon, M. Akkoyunlu, S. Karmakar, E. Fikrig, R. D. Cummings, and R. P. McEver. 2003. Structurally distinct requirements for binding of PSGL-1 and sialyl Lewis x to Anaplasma phagocytophilum and P-selectin. J. Biol. Chem. 278:37987-37997. [DOI] [PubMed] [Google Scholar]
- 40.Yoshiie, K., H. Y. Kim, J. Mott, and Y. Rikihisa. 2000. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect. Immun. 68:1125-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]