Abstract
Background.
Total brain volume is an integrated measure of health and may be an independent indicator of mortality risk independent of any one clinical or subclinical disease state. We investigate the association of brain volume to total and cause-specific mortality in a large nondemented stroke-free community-based cohort.
Methods.
The analysis includes 3,543 men and women (born 1907–1935) participating in the Age, Gene, Environment Susceptibility—Reykjavik Study. Participants with a known brain-related high risk for mortality (cognitive impairment or stroke) were excluded from these analyses. Quantitative estimates of total brain volume, white matter, white matter lesions, total gray matter (GM; cortical GM and subcortical GM separately), and focal cerebral vascular disease were generated from brain magnetic resonance imaging. Brain atrophy was expressed as brain tissue volume divided by total intracranial volume, yielding a percentage. Mean follow-up duration was 7.2 (0–10) years, with 647 deaths. Cox regression was used to analyze the association of mortality to brain atrophy, adjusting for demographics, cardiovascular risk factors, and cerebral vascular disease.
Results.
Reduced risk of mortality was significantly associated with higher total brain volume (hazard ratio, 95% confidence interval = 0.71, 0.65–0.78), white matter (0.85, 0.78–0.93), total GM (0.74, 0.68–0.81), and cortical GM (0.78, 0.70-0.87). Overall, the associations were similar for cardiovascular and noncardiovascular-related deaths.
Conclusions.
Independent of multiple risk factors and cerebral vascular damage, global brain volume predicts mortality in a large nondemented stroke-free community-dwelling older cohort. Total brain volume may be an integrated measure reflecting a range of health and with further investigation could be a useful clinical tool when assessing risk for mortality.
Key Words: Brain aging, Epidemiology, Neuroimaging , Mortality risking.
Identifying global predictors of mortality that are easily accessible for measurement provides a means to identify at-risk older persons and disentangle the physiological processes of aging. The brain is a prime regulator of physiologic processes and motor and cognitive function. At older age, loss of brain tissue (atrophy) is used as an indicator of cerebrovascular disease and neurodegeneration in the assessment of stroke and dementia. However, brain atrophy may reflect a range of subclinical disease states and metabolic dysregulation, much of which has not been adequately characterized. Therefore, beside the use of brain volume in the assessment of dementia and stroke, brain atrophy, measured on magnetic resonance (MR) images, may provide a useful indicator of risk for mortality.
There are few studies on the association of mortality and brain atrophy (1–4). In the Cardiovascular Health Study, ventricular widening, a marker for brain atrophy, was significantly associated with an increased risk for mortality (2). In a birth cohort study, the Scottish Mental Survey, 1932, a correlation between computed tomography-assessed brain atrophy and survival (3) was reported. Most of these studies excluded prevalent dementia which could contribute to any observed associations of brain atrophy and mortality risk. However, fewer excluded prevalent cases of stroke. Further, some of these studies were performed in restricted age groups, had limited follow-up time, or limited number of deaths. Most of the studies did not examine cause-specific differences in mortality (eg, cardiovascular [CV], non-CV death). Differences in the associated risk of brain atrophy in these groups would have relevant clinical implications for patient care. Additionally, only some of these studies examined whether regional differences in brain atrophy had differential effects with respect to mortality risk.
Given the limitations of prior studies, a comprehensive examination that more fully characterizes the relationship of brain tissue volume, a quantitative measure of atrophy, and mortality in the elderly is needed. We investigate in a large population-based cohort born 1907–1935, and free of prevalent stroke and dementia, the association of brain tissue volumes to cause-specific and all-cause mortality. Further, we assess whether total or more specific regional brain tissue volumes are an independent predictor of mortality in old age.
Methods
Study Design
This analysis is based on the cohort of men and women who participated in the Reykjavik Study (RS) and its follow-up the Age Gene Environment Susceptibility—RS (AGES—RS). The RS (1967–1996) was initiated by the Icelandic Heart Association to study CV disease and risk factors in middle age. The cohort of men and women were born between 1907 and 1935, and living in Reykjavik in 1967. The AGES—RS was initiated in 2002 to examine the contribution of genetic susceptibility and gene/environment interaction to conditions common in old age (5). From February 2002 to February 2006, a random sample of survivors of the original RS cohort was invited to the AGES—RS, and 5,764 subjects (72% response rate) participated in the study. All participants in the AGES—RS underwent extensive evaluation, including a standard clinical evaluation, brain MR imaging (MRI), and neuropsychological testing. The protocol was approved by the Icelandic National Bioethics Committee (VSN 00-063), the Icelandic Data Protection Authority, and by the institutional review board of the National Institute of Aging and all participants gave written informed consent.
Assessment of Dementia and Stroke
The assessment of dementia in AGES—RS has been described previously (6). Briefly, cognitive function of all subjects was assessed with the Mini-Mental State Examination and Digit Symbol Substitution Test. Those with scores on either test that were below a prespecified cut-point were administered a diagnostic battery of neuropsychological tests. Those who performed below a prespecified cut-point on either the Rey Verbal Memory Test or the Trails test, were examined by a neurologist and a proxy interview was administered. A consensus diagnosis of dementia and mild cognitive impairment based on international guidelines was made by a panel that included a geriatrician, neurologist, neuropsychologist, and neuroradiologist. Stroke history was ascertained by standard questions about a physician’s diagnosis and presence of symptoms for at least 24 hours.
Brain MRI
All AGES—RS participants with no MR contraindications were eligible for brain MRI. MR images were acquired on a study dedicated 1.5 T Signa Twinspeed system (General Electric Medical Systems, Waukesha, WI). The imaging protocol has been described previously (7). Briefly, the following 20-minute protocol produced the data used in this analysis: T1-weighted three-dimensional spoiled gradient echo sequence (voxel size 1.5 × 0.94 × 0.94mm), and proton density/T2-weighted fast spin echo sequence, and fluid-attenuated inversion recovery sequence both with a voxel size of 3 × 0.86 × 0.86mm. Full brain coverage and slices were angled parallel to the anterior commissure–posterior commissure line.
Brain parenchyma compartments were segmented automatically with a previously described AGES—RS-modified algorithm based on the Montreal Neurological Institute pipeline (7). The volumes of gray matter (GM), white matter (WM), WM lesions, and cerebral spinal fluid were estimated for each subject. Total brain volume (TBV) was estimated by summing GM, WM, and WM lesion volumes. GM volume was further delineated as cortical (frontal, parietal, lateral, and occipital lobar GM structures) and subcortical (nucleus caudate, putamen, nucleus accumbens, globus pallidus, thalamus, hippocampus, and amygdala). To standardize these volumetric measures for head size we divided volumes by total intracranial volume (TBV + cerebral spinal fluid), yielding a percentage tissue volume. Cerebral infarcts were identified by trained radiographers as defects in the brain parenchyma with associated hyperintensity on T2 and fluid-attenuated inversion recovery images with at least a maximal diameter of 4mm. There were no size criteria for infarcts in the cerebellum and brain stem or infarcts with cortical involvement.
Assessment of Demographics and Vascular Measurements
To assess the potential independent value of brain volume to predict death we adjusted for the following covariates that might explain the association of brain tissue volume to mortality: educational level (college or university vs lower education), alcohol use (never, former, current), and smoking history (never, former, current) assessed by questionnaire; body mass index (measured in kg/m2); total serum cholesterol and high-sensitive C-reactive protein all assayed using standard protocols; prevalent coronary heart disease (self-reported doctor’s diagnosis of coronary artery disease or coronary artery bypass surgery or angioplasty or angina pectoris on the Rose Angina Questionnaire, or evidence on electrocardiography of possible or probable myocardial infarction); diabetes mellitus (self-reported doctor’s diagnosis of diabetes, use of glucose-modifying medication, or a fasting blood glucose of >7 mmol/L); hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, or self-reported doctor’s diagnosis of hypertension, or using antihypertensive medication); and computed tomography-detected coronary artery calcification sum of four coronary artery scores, quantified according to the Agatston method (8). We also adjusted the analyses for MRI manifestations of cerebral vascular disease (infarcts and WM lesion load). Cognitive function was adjusted for with the standardized Digit Symbol Substitution Test score.
Endpoint Definition
Complete mortality follow-up for this analysis went through to May 2011; adjudicated cause-specific data is available to January 2009. Fact and cause of death was obtained from Statistics Iceland, which classified cause of death based on a nosologist review of medical and death records. The main endpoints in this study were deaths from all causes, and CV and non-CV causes separately. CV-related death included International Classification of Disease-10th revision codes I10-I25, I42-I52, I61, I63-I74 (hypertensive disease, myocardial infarction, coronary artery disease, ischemic and hemorrhagic stroke, and aneurysms) (9), Non-CV deaths included International Classification of Disease-10th revised codes for neoplasms, airway disease, diseases of the nervous system other than cerebrovascular diseases, disease of the digestive system, disease of the genitourinary system, mental and behavioral disorders, and other causes.
Analytic Sample
Of the 5,764 AGES—RS participants, we excluded subjects with dementia (n = 319), adjudicated mild cognitive impairment (MCI) (n = 550) or stroke (n = 401 of which 48 also had dementia and 58 had MCI), or missing and incomplete data on cognitive status or stroke (n = 336). From the remaining 4,264 participants, 194 had contraindications for MRI scanning, 219 refused the MRI scan, 180 had MRI scans which could not be segmented, 39 only participated in a home visit, and 89 were excluded due to missing covariate data. Therefore, 3,543 nondemented, stroke-free participants with complete data were available for the analysis in this study. This included 647 all-cause deaths; of these 321 deaths had cause of death information with 129 attributed to CV disease and 192 to other causes, as above.
Compared to the 3,543 subjects with complete data, the 2221 who were excluded (including stroke, dementia, and MCI cases) for this analysis were significantly older, had a higher body mass index, scored lower on Digit Symbol Substitution Test, were more likely to have diabetes mellitus, be a current smoker, and have a history of coronary heart disease. Of the 2,221, the 1,071 with MRI data had lower volumes of total brain, WM, total GM, cortical GM, and subcortical GM. WM lesion volume and number of infarcts were higher than the final analytical sample (Supplementary Table 1).
Statistical Analysis
Nondeceased and deceased participants were compared on demographic characteristics, vascular risk factors, and MRI brain measures using regression analysis corrected for age and sex. To obtain comparable hazard ratios (HRs), we created Z-scores for the total intracranial volume standardized—total brain, WM, total GM, cortical GM, and subcortical GM. Cox proportional hazard regression was used to estimate the association of brain tissue volume to all-cause, CV, and non-CV mortality, using age as a timescale (10). For reasons described in detail in Supplementary Table 5 we chose to use age as the timescale instead of time followed up. The age timeline models are more conservative, but more importantly they result in unbiased estimates of the association between brain volume and mortality. This approach does adjust for age as the comparisons of those who died is made to those who survived to the same age, and not to those followed up the same amount of time, which has no intrinsic clinical or biologic meaning, To define the timescale we used left truncation at the age at MRI assessment and right censoring at the age of death or age at end of follow-up, which was May 2011 for all-cause mortality and January 2009 for cause-specific mortality.
Initially, we included age-adjusted models only, reported as Model 1, for the different mortality endpoints. In Model 2, we adjusted for sex, education, and Digit Symbol Substitution Test score. Additionally, in Model 3 we added to Model 2, indicators of vascular risk factors, disease, and MRI brain markers (smoking, alcohol use, total serum cholesterol, high-sensitive C-reactive protein, body mass index, coronary artery calcification, history of coronary heart disease, hypertension, diabetes mellitus, and number of cerebral infarcts). We tested the Cox models for possible violations of the proportional hazards assumption and found none. Significance testing was two sided and based on a 5% probability level. The analysis was done with IBM SPSS Statistics version 20.
Results
Characteristics of the Study Population
The study population was mean age 75.6 years (range 66–95 years old), and followed for mean time of 7.2 (0–10; SD: 1.65) years. Compared with those who did not die in the follow-up (n = 2,896), those who died (n = 647) were older, more often male, had more CV risk factors and disease, performed more poorly on a test of cognitive function, and had lower brain tissue volumes (Table 1).
Table 1.
Baseline Clinical and MRI Characteristics of the Study Population by Vital Status: Age Gene/Environment Susceptibility—Reykjavik Study
| Characteristics, Mean (SD) or n (%) | Combined | Deceased (n = 647) | Nondeceased (n = 2,896) | p Value* |
|---|---|---|---|---|
| Age, y | 75.5 (5.2) | 78.8 (5.8) | 74.8 (4.8) | <.0001 |
| Gender, male | 1,433 (40.5) | 321 (49.6) | 1,112 (38.4) | <.0001 |
| Higher education | 1,056 (29.8) | 212 (32.8) | 844 (29.1) | .36 |
| Current smoker | 412 (11.6) | 97 (15.0) | 315 (10.9) | <.0001 |
| BMI, kg/m2 | 27.0 (4.3) | 26.5 (4.7) | 27.1 (4.3) | .36 |
| Total cholesterol, mmol/L | 5.7 (1.2) | 5.5 (1.2) | 5.7 (1.1) | .03 |
| C-reactive protein, mg/L | 3.6 (6.0) | 4.7 (8.3) | 3.3 (5.4) | <.0001 |
| Systolic blood pressure, mmHg | 141.8 (20.2) | 142.9 (21.7) | 141.6 (19.8) | .22 |
| Diastolic blood pressure, mmHg | 73.9 (9.6) | 72.9 (10.0) | 74.1 (9.5) | .19 |
| Hypertension | 2,793 (78.8) | 547 (84.5) | 2,246 (77.6) | .06 |
| Diabetes | 386 (10.9) | 90 (13.9) | 296 (10.2) | .02 |
| History of coronary heart disease | 661 (18.7) | 168 (26.0) | 493 (17.0) | .0008 |
| Current alcohol drinkers | 2,411 (68.1) | 412 (63.7) | 1,999 (69.0) | .12 |
| DSST standardized score | 0.3 (0.9) | −0.03 (0.8) | 0.4 (0.9) | <.0001 |
| Coronary calcium, Agatston score | 637.2 (995.7) | 1,033.5 (1352.4) | 548.6 (872.8) | <.0001 |
| MRI brain findings, mean (SD) | ||||
| Total brain volume†, % | 72.6 (3.8) | 70.4 (3.8) | 73.1 (3.6) | <.0001 |
| White matter volume†, % | 25.9 (1.8) | 25.0 (1.8) | 26.0 (1.8) | <.0001 |
| GM volume†, % | 45.5 (3.2) | 43.7 (3.4) | 45.9 (3.0) | <.0001 |
| Cortical GM†, % | 34.6 (2.5) | 33.1 (2.7) | 34.9 (2.4) | <.0001 |
| Subcortical GM†, % | 3.1 (0.2) | 3.0 (0.3) | 3.1 (0.2) | <.0001 |
| Infarcts number ≥2 | 313 (14.1) | 117 (18.1) | 305 (10.5) | .03 |
| WML†, % | 1.2 (1.2) | 1.6 (1.5) | 1.1 (1.2) | <.0001 |
Notes: BMI = body mass index; DSST = Digit Symbol Substitution Test; GM = gray matter; MRI = magnetic resonance imaging; WML = white matter lesions.
*Regression analyses corrected for age and gender.
†% of total intracranial volume.
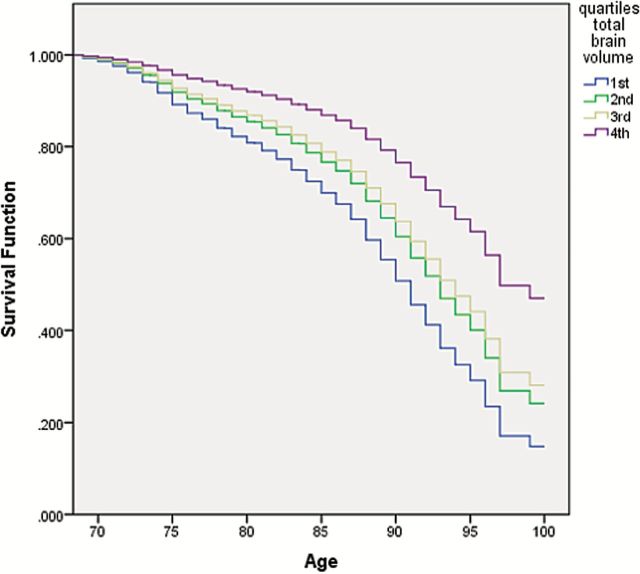
Figure 1 represents the unadjusted survival distribution of the population by age and quartile of TBV. From this figure, we see for those in the highest quartile of TBV, more than 90% survived to age 80 and more than 85% survived to age 85; in contrast, for those in the lowest quartile of TBV, about 80% survived to age 80, and only just less than 70% survived to age 85 years.
Figure 1.
Distribution of survival based on age and total brain volume in the Age Gene/Environment Susceptibility—Reykjavik Study. The figure represents the distribution of survival at any given age by quartiles of total brain volume.
Brain Volume and Mortality Risk
Age-adjusted TBV was significantly associated with lower risk for mortality (HR, 95% confidence intervals = 0.66, 0.61–0.72); thus, for every 1 SD of greater brain tissue (see Table 1 for SD), the risk for mortality is lower—in the case of TBV, by 34%. Adjusted for additional demographic variables, greater TBV was significantly associated with a lower risk for mortality (0.70, 0.64–0.76). This estimate of higher volume and lower risk was consistent in all the tissue subgroups except subcortical GM (Table 2, Model 2): WM (0.83, 0.76–0.89), total GM (0.73, 0.67–0.79), cortical GM (0.73, 0.65–0.81), and subcortical GM (0.94, 0.84–1.05). Adding the vascular risk factors, disease, and MRI markers analyses modestly attenuated the associations of mortality to TBV, total GM, total WM, and cortical GM tissue volumes but the associations remained significant (Table 2, Model 3).
Table 2.
Brain Volume-Associated All-cause Mortality Risk, Age-Adjusted (Model 1), Adjusted for Additional Demographics (Model 2) and Vascular Disease (Model 3): Age Gene/Environment Susceptibility—Reykjavik Study
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | |
| Total brain volume | 0.66 (0.61–0.72) | <.001 | 0.70 (0.64–0.76) | <.001 | 0.71 (0.65–0.78) | <.001 |
| Total WM volume | 0.81 (0.75–0.88) | <.001 | 0.83 (0.76–0.89) | <.001 | 0.85 (0.78–0.93) | <.001 |
| Total GM volume | 0.70 (0.64–0.75) | <.001 | 0.73 (0.67–0.79) | <.001 | 0.74 (0.68–0.81) | <.001 |
| Cortical GM volume | 0.70 (0.63–0.77) | <.001 | 0.73 (0.65–0.81) | <.001 | 0.78 (0.70–0.87) | <.001 |
| Subcortical GM volume | 0.92 (0.82–1.03) | <.15 | 0.94 (0.84–1.05) | .26 | 0.90 (0.81–1.01) | .07 |
Notes: Model 1: adjusted for age. Model 2: adjusted for age, sex, education, DSST. Model 3: adjusted for Model 2 covariates + indicators of vascular risk factors, disease, and MRI brain markers (smoking, alcohol use, total serum cholesterol, hsCRP, BMI, CAC, history of coronary heart disease, hypertension, DM, and number of cerebral infarcts). BMI = body mass index; CAC = coronary artery calcification; CI = confidence interval; DM = diabetes mellitus; DSST = Digit Symbol Substitution Test; GM = gray matter; hsCRP = high-sensitive C-reactive protein; MRI = magnetic resonance imaging; WM = white matter.
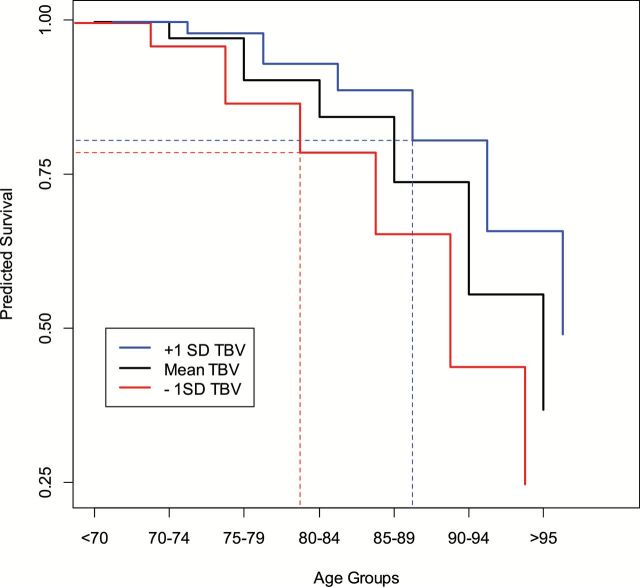
For illustration, Figure 2 represents the predicted survival by age group and differences in TBV, based on the fully adjusted model (Table 2, Model 3). For each age group, the predicted survival is higher for greater TBV compared with smaller TBV, indicative that brain volume is an independent predictor of mortality. In addition, the figure illustrates comparable survival between older individuals with larger TBV compared with younger individuals with smaller TBV, further suggestive of the benefits conferred by higher brain volume with respect to mortality.
Figure 2.
Predicted survival by age group and total brain volume in the Age Gene/Environment Susceptibility—Reykjavik Study. Predicted survival is based on a fully adjusted model of all-cause mortality (Table 2, Model 3). For each age group, greater total brain volume (TBV) represents a higher predicted survival over 7-y follow-up. In addition, the figure indicates comparable predicted survival in older subjects with greater TBV with respect to subjects in the next youngest age group with smaller TBV, as represented by the dashed lines.
Although the results reflect hazard estimates based on 1 SD units, HRs (95% confidence interval) associated with a fixed percentage difference in brain volume can be calculated. For example, 1 SD in cortical GM equals 2.5% (Table 1); therefore, 5% greater cortical GM, relative to the adjusted population average, is the equivalent of 2 SD of cortical GM. Using the coefficient and standard error estimates that correspond with a 1 SD change from the risk model for cortical GM (Supplementary Table 2, Model 2) we find the HR (95% confidence interval), for 5% greater cortical GM is 0.53, (0.42–0.65), using a formula given in Supplementary Table 2.
Cause-Specific Mortality and Brain Volume
For greater cortical GM tissue, the risk for non-CV mortality (0.76, 0.63–0.92) and for CV mortality (0.79, 0.62–1.00) decreased. In general, there was no difference in the association of CV compared with non-CV causes of mortality (Tables 3 and 4) to the different measures of brain volume.
Table 3.
Brain Volume-Associated Cause-Specific Mortality Risk: Cardiovascular (CV) Causes, Age-Adjusted (Model 1), Adjusted for Additional Demographics (Model 2) and Vascular Disease (Model 3): Age Gene/Environment Susceptibility—Reykjavik Study
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | |
| Total brain volume | 0.66 (0.56–0.78) | <.001 | 0.69 (0.57–0.83) | <.001 | 0.70 (0.57–0.87) | <.001 |
| Total WM volume | 0.83 (0.70–0.99) | .04 | 0.86 (0.72–1.03) | .10 | 0.94 (0.76–1.15) | .54 |
| Total GM volume | 0.69 (0.58–0.82) | <.001 | 0.70 (0.58–0.85) | <.001 | 0.72 (0.58–0.89) | <.01 |
| Cortical GM volume | 0.71 (0.56–0.90) | <.01 | 0.74 (0.58–0.94) | .01 | 0.79 (0.62–1.00) | .05 |
| Subcortical GM volume | 0.85 (0.66–1.09) | .20 | 0.85 (0.67–1.10) | .22 | 0.82 (0.65–1.03) | .09 |
Notes: Model 1: adjusted for age. Model 2: adjusted for age, sex, education, DSST. Model 3: adjusted for Model 2 covariates + indicators of vascular risk factors, disease, and MRI brain markers (smoking, alcohol use, total serum cholesterol, hsCRP, BMI, CAC, history of coronary heart disease, hypertension, DM, and number of cerebral infarcts). BMI = body mass index; CAC = coronary artery calcification; CI = confidence interval; DM = diabetes mellitus; DSST = Digit Symbol Substitution Test; GM = gray matter; hsCRP = high-sensitive C-reactive protein; MRI = magnetic resonance imaging; WM = white matter.
Table 4.
Brain Volume-Associated Cause-Specific Mortality Risk: Noncardiovascular (non-CV) Causes, Age-Adjusted (Model 1), Adjusted for Additional Demographics (Model 2) and Vascular Disease (Model 3): Age Gene/Environment Susceptibility—Reykjavik Study
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | |
| Total brain volume | 0.65 (0.57–0.75) | <.001 | 0.71 (0.60–0.84) | <.001 | 0.74 (0.62–0.87) | <.001 |
| Total WM volume | 0.82 (0.71–0.94) | <.01 | 0.83 (0.72–0.96) | .01 | 0.87 (0.74–1.03) | .10 |
| Total GM volume | 0.68 (0.58–0.79) | <.001 | 0.73 (0.61–0.86) | <.001 | 0.76 (0.64–0.90) | <.01 |
| Cortical GM volume | 0.66 (0.55–0.79) | <.001 | 0.69 (0.57–0.84) | <.001 | 0.76 (0.63–0.92) | <.01 |
| Subcortical GM volume | 1.03 (0.85–1.25) | .76 | 1.07 (0.88–1.31) | .51 | 1.02 (0.84–1.25) | .81 |
Notes: Model 1: adjusted for age. Model 2: adjusted for age, sex, education, DSST. Model 3: adjusted for Model 2 covariates + indicators of vascular risk factors, disease, and MRI brain markers (smoking, alcohol use, total serum cholesterol, hsCRP, BMI, CAC, history of coronary heart disease, hypertension, DM, and number of cerebral infarcts). BMI = body mass index; CAC = coronary artery calcification; CI = confidence interval; DM = diabetes mellitus; DSST = Digit Symbol Substitution Test; GM = gray matter; hsCRP = high-sensitive C-reactive protein; MRI = magnetic resonance imaging; WM = white matter.
Discussion
In this large population-based cohort of older persons, we found an inverse association between global brain volume and mortality risk. Total, GM, WM, and cortical GM were associated with a decreased risk of mortality; volumes of subcortical GM structure (the basal ganglia, thalamus, hippocampus, and amygdala), were not statistically associated with mortality. The observed decreased mortality risk was independent of dementia and CV risk factors and disease.
Prior studies have shown imaging markers of brain atrophy-predicted mortality in cohorts of older persons (1–4). However, these studies were limited to the oldest old (3) or included stroke and dementia cases, which are at high risk for death, or did not examine cause-specific deaths.
Advanced age is associated with widespread changes in the brain that ends in neurodegeneration, damage to and loss of the vasculature, and alterations in hormonal, metabolic, and neurotransmitter systems (11,12), all of which result in altered patterns of cognitive and physical functional activity (13,14). Brain volume loss relative to intracranial volume (atrophy) is generally a marker of pathology in the aging brain and here we show it is significantly associated with mortality within a 7-year period. Consistent with studies of the relation between brain atrophy and CV disease, such as hypertension or diabetes mellitus (15–17), lower brain volume was associated with an increased mortality due to CV disease. There is far less information on the association of brain atrophy to non-CV diseases (18). This is the first study to show in a community sample, a similar risk for mortality due to non-CV disease.
Because different regions of the brain atrophy at different rates (19–21) we investigated whether specific brain regions/tissue type differentially predicted mortality. We found no differences between GM and WM but did find a difference between cortical GM and subcortical GM. It is important to note, the subcortical region has a smaller volume and measured with less precision than larger structures. However, the HR was closer to 1 than the HRs for the other structures. Because the subcortical GM structures are central to several neurodegenerative diseases, our exclusions of participants with MCI and dementia likely reduced the range of volume in that region of interest area. In addition, aside from neurodegenerative pathology, subcortical GM structures may be more regulated by genetic factors than environmental factors (22–24), or, because it regulates important functions, more “protected” against atrophy.
The brain, obviously, has important regulating functions essential for survival, such as for autonomic functioning, maintaining energy balance, metabolic and electrolyte homeostasis, as well as mood and behavior. As such global and local atrophy, whether caused by neurodegeneration, vascular disease, or other pathologic processes, changes the ability of an individual to maintain the balance needed to function normally. In the study and care of older individuals integrated measures of function, such as physical performance tests, frailty metrics, and omnibus tests of cognition, are frequently used to evaluate, beyond chronologic age, an individual’s general health and risk for morbidity and mortality (25). Our finding of similar associations of GM and WM volume to CV and non-CV–related deaths suggests global brain volume alone, measured in a short MRI sequence, may be an integrated measure of mortality risk.
Strengths of the current study include the large well-described cohort, adjustment for multiple factors associated with mortality, quantitatively processed high-quality brain MRIs, and a nosologist evaluation of cause of death. A limitation of the current study is exclusion of subjects who did not receive a MRI scan, were missing data as the result of MRI postprocessing, had incomplete or missing information on cognitive status or stroke, or were missing covariates. Subjects excluded from the analysis were older, less healthy for a number of health parameters, and more likely to die. Those excluded subjects with MRI measures had lower total brain, WM, GM brain volumes. Moreover, those who did not participate in AGES—RS, based on individuals who were still alive from the original RS, were similarly older, less healthy, and at greater risk for mortality. There is no reason to suggest an opposite association between lower brain volume and mortality between subjects included or not from the analysis. However, as with any cohort, it is possible still that the risk estimates in the study are not entirely representative of brain volume-related mortality risk in community-dwelling older individuals with no dementia or stroke.
In conclusion, in a large dementia and stoke-free community-dwelling cohort of older persons lower brain volumes of WM and GM independently predicted mortality. Further studies of brain atrophy in major disease states such as cancer and lung disease may contribute to patient management, including identifying individuals at higher risk for death. Further investigation into the clinical utility of using brain volume as a tool for patient management is necessary. Its advantage over currently used metrics for geriatric evaluation is the possibility that it is a more integrated measure of health.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by NIH contract N01-AG-12100, the NIH/NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), the Althingi (the Icelandic Parliament), and the University of Iceland Research Fund.
Supplementary Material
References
- 1. Ikram MA, Vernooij MW, Vrooman HA, Hofman A, Breteler MM. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging. 2009;30:450–456. [DOI] [PubMed] [Google Scholar]
- 2. Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. [DOI] [PubMed] [Google Scholar]
- 3. Olesen PJ, Guo X, Gustafson D, et al. A population-based study on the influence of brain atrophy on 20-year survival after age 85. Neurology. 2011;76:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Staff RT, Murray AD, Ahearn T, et al. Brain volume and survival from age 78 to 85: the contribution of Alzheimer-type magnetic resonance imaging findings. J Am Geriatr Soc. 2010;58:688–695. [DOI] [PubMed] [Google Scholar]
- 5. Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qiu C, Cotch MF, Sigurdsson S, et al. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology. 2010;75:2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sigurdsson S, Aspelund T, Forsberg L, et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik study. Neuroimage. 2012;59:3862–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 9. WHO. International Classification of Diseases, Tenth Revision (ICD-10). Geneva, Switzerland: WHO. [Google Scholar]
- 10. Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. [DOI] [PubMed] [Google Scholar]
- 11. Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006;30:791–807. [DOI] [PubMed] [Google Scholar]
- 12. Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, Naumann E. Resting cerebral blood flow, attention, and aging. Brain Res. 2009;1267:77–88. [DOI] [PubMed] [Google Scholar]
- 13. Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. [DOI] [PubMed] [Google Scholar]
- 14. Grady CL, Yu H, Alain C. Age-related differences in brain activity underlying working memory for spatial and nonspatial auditory information. Cereb Cortex. 2008;18:189–199. [DOI] [PubMed] [Google Scholar]
- 15. Longstreth WT, Jr, Arnold AM, Manolio TA, et al. Clinical correlates of ventricular and sulcal size on cranial magnetic resonance imaging of 3,301 elderly people. The Cardiovascular Health Study. Collaborative Research Group. Neuroepidemiology. 2000;19:30–42. [DOI] [PubMed] [Google Scholar]
- 16. Schmidt R, Launer LJ, Nilsson LG, et al. ; CASCADE Consortium. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes. 2004;53:687–692. [DOI] [PubMed] [Google Scholar]
- 17. Strassburger TL, Lee HC, Daly EM, et al. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997;28:1410–1417. [DOI] [PubMed] [Google Scholar]
- 18. Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121:4287–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 20. DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 21. Fjell AM, Walhovd KB, Fennema-Notestine C, et al. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29:15223–15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alemany S, Mas A, Goldberg X, et al. Regional gray matter reductions are associated with genetic liability for anxiety and depression: an MRI twin study. J Affect Disord. 2013;149:175–181. [DOI] [PubMed] [Google Scholar]
- 23. de Jong LW, Forsberg LE, Vidal JS, et al. Different susceptibility of medial temporal lobe and basal ganglia atrophy rates to vascular risk factors. Neurobiol Aging. 2014;35:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stephenson-Jones M, Ericsson J, Robertson B, Grillner S. Evolution of the basal ganglia: dual-output pathways conserved throughout vertebrate phylogeny. J Comp Neurol. 2012;520:2957–2973. [DOI] [PubMed] [Google Scholar]
- 25. Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB. A physiologic index of comorbidity: relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.