Abstract
Prior research has suggested benefits of aerobic physical activity (PA) on cognition and brain volumes in HIV uninfected (HIV−) individuals, however, few studies have explored the relationships between PA and brain integrity (cognition and structural brain volumes) in HIV-infected (HIV +) individuals. Seventy HIV + individuals underwent neuropsychological testing, structural neuroimaging, laboratory tests, and completed a PA questionnaire, recalling participation in walking, running, and jogging activities over the last year. A PA engagement score of weekly metabolic equivalent (MET) hr of activity was calculated using a compendium of PAs. HIV + individuals were classified as physically active (any energy expended above resting expenditure, n = 22) or sedentary (n = 48). Comparisons of neuropsychological performance, grouped by executive and motor domains, and brain volumes were completed between groups. Physically active and sedentary HIV + individuals had similar demographic and laboratory values, but the active group had higher education (14.0 vs. 12.6 years, p = .034). Physically active HIV + individuals performed better on executive (p = .040, unadjusted; p = .043, adjusted) but not motor function (p = .17). In addition, among the physically active group the amount of physical activity (METs) positively correlated with executive (Pearson’s r = 0.45, p = 0.035) but not motor (r = 0.21; p = .35) performance. In adjusted analyses the physically active HIV + individuals had larger putamen volumes (p = .019). A positive relationship exists between PA and brain integrity in HIV + individuals. Results from the present study emphasize the importance to conduct longitudinal interventional investigation to determine if PA improves brain integrity in HIV + individuals.
Keywords: HIV, Exercise, Neuropsychology performance, Brain volume, Magnetic resonance imaging (MRI), Putamen
INTRODUCTION
An estimated 1.5 million individuals are infected with HIV in the United States (US CDC, 2011). While highly active antiretroviral therapy (HAART) has resulted in decreased morbidity and mortality among HIV infected (HIV +) individuals (Valcour, 2013), cognitive impairments persist. However, prior clinical trials using adjunctive therapies to reduce cognitive impairment have had limited success (McGuire, Barrett, Vezina, Spitsin, & Douglas, 2014). Since HIV-associated neurocognitive disorders (HAND) are still prevalent (as high as 50%) (Heaton et al., 2010; Simioni et al., 2010), research on alternative therapies and/or healthy lifestyle factors (e.g., exercise) on HIV-related dysfunction are necessary as HIV + individuals continue to live longer.
Regular engagement in aerobic physical activity (PA) has been shown to improve cognitive function and brain health in normal aging populations (Chan, Yan, & Payne, 2013; Chapman et al., 2013; Voss et al., 2010). Specifically, previous work has revealed that PA has neuroprotective effects as reported by delays in cognitive decline, improved neuropsychological performance (NP), and reduced atrophy in the hippocampus and prefrontal cortex (Chapman et al., 2013; Erickson, Leckie, & Weinstein, 2014). The benefits of PA on brain integrity (cognitive performance and brain structure) have been extensively investigated in a variety of neurodegenerative conditions (e.g., Alzheimer’s and Huntington’s disease) (Cruickshank et al., 2015; Honea et al., 2009; Okonkwo et al., 2014), but not HIV.
Observed improvements in brain integrity due to PA may occur by direct and/or indirect pathways (Cade et al., 2008; Fazeli et al., 2014; Lee et al., 2011; Phillips et al., 1997). PA can directly affect molecular targets in the brain through up-regulation of growth factors (Rojas Vega, Knicker, Hollmann, Bloch, & Struder, 2010; Voss et al., 2010; Whiteman et al., 2014). These changes within the brain can lead to increases in neuronal survival and neurogenesis. PA can also have indirect effects on general health by improving physical fitness (cardiorespiratory capacity, muscle strength, and muscle mass) and attenuate atherosclerosis. These changes can lead to improved insulin control and decreased inflammation (Rojas Vega et al., 2010; Whiteman et al., 2014). Altogether, a healthy lifestyle with regular PA could provide benefits to HIV + individuals where immune systems are known to be chronically compromised (Friis-Moller et al., 2010; Nogueira Pinto, 2005).
To date, most studies of PA in HIV + individuals have focused on changes in body morphology and metabolism (Cade et al., 2013, 2008; Yarasheski et al., 2012, 2011). Only a few have investigated relationships between PA and cognition (Cade et al., 2013; Dufour et al., 2013; Fazeli et al., 2014; Gomes-Neto, Conceicao, Oliveira Carvalho, & Brites, 2013). Two studies reported a positive correlation between PA and cognition in HIV + individuals, however, PA was defined as any activity that increased heart rate over the previous 72 hr (Dufour et al., 2013; Fazeli et al., 2014). An additional study found a positive correlation between maximal oxygen uptake (VO2 MAX) on a treadmill test and cognitive performance in HIV + individuals (Mapstone et al., 2013). These studies suggest that a physically active lifestyle is beneficial; however, PA measurements have typically not measured sustained engagement in exercise.
In the present study we used neuroimaging and neuropsychological measures to determine if a physically active lifestyle provides benefit to brain integrity in HIV + individuals. We used a validated quantitative exercise history questionnaire to calculate weekly metabolic equivalent (MET) hr of PA and measured brain integrity (cognition and brain volumetrics) within a cohort of physically active and sedentary HIV + individuals. We hypothesized that physically active HIV + individuals would exhibit better cognitive performance, compared to sedentary individuals. In addition, we hypothesized larger brain volumes among physically active HIV + individuals compared to sedentary HIV + individuals.
METHODS
Participants
Seventy HIV + individuals were recruited from the Infectious Disease Clinic at Washington University in St. Louis (WUSTL). All participants provided informed consent using forms approved by the WUSTL Institutional Review Board. Participants were excluded if they reported a history of head injury with loss of consciousness >30 min, major psychiatric disorders, opportunistic CNS infections, or contraindications for MRI scanning.
Neuropsychological Performance Testing
All participants were administered a brief neuropsychological (NP) battery (including Trail Making Tests A and B (TMT-A and TMT-B) (Reitan, 1958), Hopkins Verbal Learning Test-Revised (HVLT-R) (Benedict, Schretlen, Groninger, & Brandt, 1998; Brandt & Benedict, 2001), Digit-Symbol Modalities Test (DSMT) (Wechsler, 1997), letter fluency (FAS) (Spreen & Benton, 1963), verb fluency (VF) (Piatt et al., 1999), and Grooved Pegboard non-dominant (GPn) (Matthews & Klove, 1964) to assess cognitive domains commonly affected by HIV (Baker et al., 2014; Overton et al., 2011; Tozzi et al., 2009). Performance on each neuropsychological test was converted to a standardized score (Z-score) based on published normative data with adjustments applied for age and where available, ethnicity, education, and/or sex (Au et al., 2004; Gladsjo et al., 1999; Norman et al., 2011). Standardized cognitive scores were grouped into two primary domains for analyses based on previous studies: executive function (NPZe) was defined by HVLT-R learning efficiency, TMT-B, and FAS, and verb fluency while motor function (NPZm) was comprised of the DSMT, TMT-A, and GPn. NPZe and NPZm scores were derived based on prior studies in HIV− and HIV + individuals (Duff, Schoenberg, Scott, & Adams, 2005; Parsons, Rogers, Hall, & Robertson, 2007; Vanderploeg, Schinka, & Retzlaff, 1994; Woods et al., 2005). Individuals were not screened for HAND in the current study.
Substance Use
Lifetime substance use was evaluated using the Risk Assessment Battery (RAB). The RAB is a self-administered questionnaire designed for substance-abusing populations to assess HIV risk behavior (Metzger, Nalvaline, & Woody, 2001). In the present study, we used a modified version of the RAB to determine the percentage of individuals in our sample that have engaged in substance use (alcohol, crack/cocaine, methamphetamines, opiates) in their lifetime. Additionally, current tobacco use was determined by self-report.
Physical Activity QuantiFIcation
A self-reported exercise questionnaire was used to quantify PA over the past year. Aerobic activity (running, walking, and jogging) history from the previous year including frequency and duration of exercise workouts was obtained from a standard questionnaire (Bowles, FitzGerald, Morrow, Jackson, & Blair, 2004). Responses from questions were used to determine standardized MET values based on a compendium of various PAs (Bowles et al., 2004; Bugg & Head, 2011; Liang et al., 2010; Pizzie et al., 2014; Head, Singh, & Bugg, 2012; Berchicci, Lucci, Perri, Spinelli, & De Russo, 2014). Specifically, the MET-hr/week was calculated by multiplying the exertion rate (METs) for the PA by time engaged in the PA as a weekly average per year. For example, an individual who reported walking (a 4.0 MET activity) for 0.5 hr twice a week (2 × a week), 6 months of the year would have a yearly average of 2.0 MET-hr/week of exercise for the year (Ainsworth et al., 2000; Bowles et al., 2004). Participants were split into two groups—physically active (n = 22) and sedentary (n = 48)—based on self-reported history of engagement in PA. Individuals who did not report any exercise in the past year were classified as sedentary (MET-hr <1). Physically active HIV + individuals were defined as expending energy above resting energy expenditure and had a reported weekly MET-hr ≥ 1 (Park et al., 2014). The questionnaire was designed to accommodate both increased intensities (METs) and durations such that both athletes and casual walkers were accurately assessed.
Neuroimaging
All participants had neuroimaging performed on a 3 Tesla Siemens Tim Trio whole body MR scanner (Siemens AG, Erlangen, Germany) with a 12-channel transmit/receive head coil. Sagittal-oriented structural images were acquired using a T1-weighted three-dimensional magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence [time of repetition (TR) = 2400 ms, echo time (TE) = 3.16 ms, inversion time (TI) = 1000 ms, flip angle = 8°, 162 slices, and voxel size = 1 × 1 × 1 mm3].
Quantification of regional volumes was obtained using the FreeSurfer software suite (v5.1) (Martinos Center, Harvard University, Boston, MA; http://surfer.nmr.mgh.harvard.edu). Briefly, this freely available automated software program transformed MPRAGE images of an individual into a template space with the skull stripped and the brain segmented into white matter, gray matter, and ventricles. The brain was further parcellated into subcortical and cortical regions of interest (ROIs) using a surface deformation onto a common atlas (Dale, Fischl, & Sereno, 1999; Desikan et al., 2006; Fischl, Sereno, & Dale, 1999). We analyzed brain regions commonly affected by HIV including the caudate, putamen, hippocampus, total gray matter, and total white matter (Ances, Ortega, Vaida, Heaps, & Paul, 2012). All brain regions were normalized to the intracranial volume across the entire cohort to account for potential variations in participant head size (Free et al., 1995).
Statistical Analyses
Demographic and clinical characteristics [age, education, recent and nadir CD4 count, HIV viral load, duration of infection, sex, and intracranial volume (ICV)] were compared between the two groups using the Wilcoxon rank-sum test for continuous variables, and Fisher’s exact test for binary and categorical variables.
The neuropsychological composite scores for executive (NPZe) and motor (NPZm) domains were compared between the physically active and sedentary groups in unadjusted and adjusted analyses. The unadjusted analyses used the independent samples t-test with pooled variance. The adjusted analyses corrected for the effect of confounders using multiple linear regression. The possible confounders considered were: recent and nadir CD4, viral load (log10-transformed and detectable/undetectable, with 20 copies/mL limit of detection), duration of infection, HAART, and body mass index (BMI). Since NPZe and NPZm composite scores had been already adjusted for age, sex, race, and education, these variables were not considered as confounders in the adjusted analyses. The model building strategy for the adjusted analyses was as follows: the starting multiple regression model for each outcome included the exercise group indicator and all covariates that were significant at p < .20 level in single-predictor analyses for that outcome. Then stepwise backward model selection was used, with a p <.20 threshold for inclusion in the final model (Vittinghoff, Glidden, Shiboski, & McCulloch, 2012). The association between exercise and NPZe and NPZm scores in the physically active group was examined using Pearson’s correlation.
The brain volumetrics for each of the five regions of interest were compared between the two groups using a similar approach as for neuropsychological outcomes, in unadjusted analyses, and adjusting for confounders using backward model selection with a threshold of inclusion of p <.20. The candidates for inclusion in the multivariable model were those mentioned above, in addition to age, sex, race, and education.
The relationship between each of the five regional brain volumes and each of the two composite NP scores (NPZe, NPZm) for the two groups combined was assessed using the Pearson correlation coefficient, including 95% confidence intervals. The test of no correlation was conducted both without controlling for multiple comparisons and with a Benjamini-Hochberg correction for 10 comparisons (Benjamini and Hochberg, 1995). The normality of the outcome variables was confirmed with Shapiro-Wilk tests (all p >.1) (Shapiro & Wilk, 1965).
RESULTS
The physically active (n = 22) and sedentary (n = 48) HIV + groups were similar with respect to demographic (age, sex, and ethnicity) and HIV-related variables (nadir and recent CD4 cell count, viral load, HAART, and duration of infection). The physically active group had higher education overall (14.0 years vs. 12.6 years; p-value = .034; Table 1). Additionally, both groups had similar reported current smoking status and lifetime drug use (Table 1). Most HIV + individuals were on a stable HAART regimen (96%) and 80% of the individuals overall had undetectable plasma HIV viral loads (<20 virus copies/mL).
Table 1.
Demographic and clinical characteristics for physically active and sedentary HIV + individuals
| HIV + Physically Active (n = 22) | HIV + Sedentary (n = 48) | p-value | |
|---|---|---|---|
| Age, years, mean (SD) | 44.1 (17.2) | 40.5 (15.6) | 0.34 |
| Sex, (%) | 0.18 | ||
| Male | 23 | 42 | |
| Female | 77 | 58 | |
| Race, (%) | 1.00 | ||
| African American | 73 | 75 | |
| White | 27 | 25 | |
| Education, years, mean (SD) | 14.0 (2.7) | 12.6 (2.6) | 0.034 |
| Nadir CD4, cells/μL, mean (SD) | 207 (185) | 275 (202) | 0.23 |
| Current CD4, cells/μL, mean (SD) | 575 (334) | 614 (253) | 0.60 |
| Current plasma log10 HIV VL, copies/mL, mean (SD) | 1.50 (0.52) | 1.59 (0.83) | 0.78 |
| HIV viral load >20 copies/mL, (%) | 24 | 19 | 0.75 |
| Duration of HIV infection, months, mean (SD) | 144.5 (113.3) | 100.5 (71.2) | 0.24 |
| Anti-retroviral Regimen, (%) | 0.92 | ||
| NRTI + NNRTI | 9 (41) | 21 (44) | |
| NRTI + PI | 6 (27) | 14 (29) | |
| NRTI + INST | 4 (18) | 7(15) | |
| Other HAART | 3 (14) | 4 (8) | |
| None | 0 (0) | 2 (4) | |
| Body mass index, kg/m2, mean (SD) | 26.9 (5.9) | 28.5 (7.5) | 0.47 |
| Intracranial volume, cc, mean (SD) | 1,393 (287) | 1,296 (210) | 0.18 |
| Lifetime substance use (%) | |||
| Alcohol | 43 | 42 | 1.00 |
| Opiates | 0 | 2 | 1.00 |
| Crack/cocaine | 14 | 19 | 1.00 |
| Marijuana | 52 | 56 | 1.00 |
| Methamphetamine | 0 | 2 | 1.00 |
| Tobacco | 50 | 54 | 0.80 |
Note. Comparisons used Wilcoxon rank-sum test for continuous variables, and Fisher’s exact test for binary and categorical variables. SD = standard deviation, VL = viral load, NRTI = nucleoside reverse transcriptase inhibitor, NNRTI = non-nucleotide reverse transcriptase inhibitor, PI = protease inhibitor, INSTI = integrase strand transfer inhibitor; HAART = highly active antiretroviral therapy.
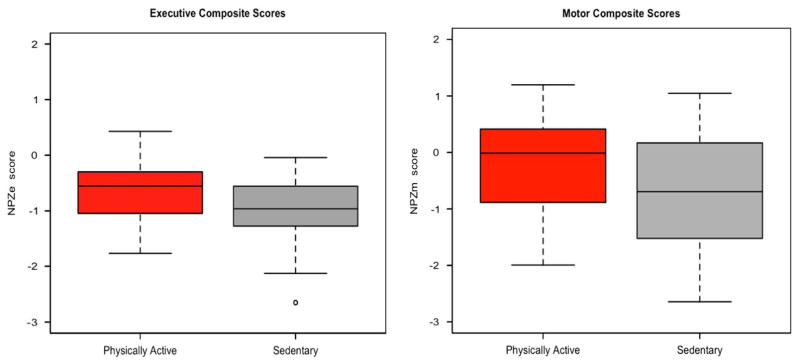
NPZ composite scores for both the executive and motor domains were higher in the physically active group compared to the sedentary HIV + group. The difference was significant for the NPZe scores, in both unadjusted and adjusted analyses (Table 2 and Figure 1). Specifically, mean NPZe scores were −0.65 and −0.96 for physically active and sedentary groups, respectively (t[68] = 2.09; p = .040). The adjusted analysis using a stepwise multiple regression model, adjusting for log10 viral load (VL), revealed an effect of physical activity on NPZe (exercise mean effect = + 0.29; p = .043, Table 2). While the physically active group had higher mean NPZm scores than the sedentary group (−0.33 and −0.83, respectively), this difference was not significant (t[68] = 1.40; p = .17) (Figure 1). No adjusted analysis was necessary for comparison of NPZm, as all univariate predictors were above the inclusion cut-off (p >.20).
Table 2.
Neuropsychological composite scores for executive (NPZe) and motor (NPZm) domains comparison between the physically active and sedentary HIV + groups in unadjusted and adjusted analyses
| HIV + PA group (n = 22) | HIV + sedentary (n = 48) | Unadjusted difference PA-sedentary | Adjusted difference PA-sedentary1 | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean (SD) | Mean (SD) | Mean (95% CI) | p-Value | Mean (95% CI) | p-Value | |
| NPZe | −0.65 (0.57) | −0.96 (0.56) | 0.30 (0.01, 0.59) | .040* | 0.29 (0.01, 0.57) | .04* |
| NPZm | −0.33 (1.15) | −0.83 (1.46) | 0.50 (0.33, 0.83) | .17 | 0.50 (0.33, 0.83) | .17 |
Note. Adjusted analyses control for confounders significant at p >0.20 level in backward model selection. Final model for NPZe adjusted for log10 viral load. Final model for NPZm did not adjust for covariates, since none of them met the p >0.20 threshold of significance.
Statistically significant correlation.
PA = physically active; SD = standard deviation; CI = confidence interval.
Fig. 1.
Effects of aerobic exercise on neuropsychology performance in HIV + individuals. Neuropsychological composite Z-scores for the executive (NPZe, left panel) and motor domains (NPZm, right panel), by physical activity groups. The mean Z-scores for executive function performance were higher in physically active compared to sedentary HIV + individuals (p = .040 unadjusted). However, within the motor tests the sedentary and physically active groups had similar performance (p = .17, unadjusted).
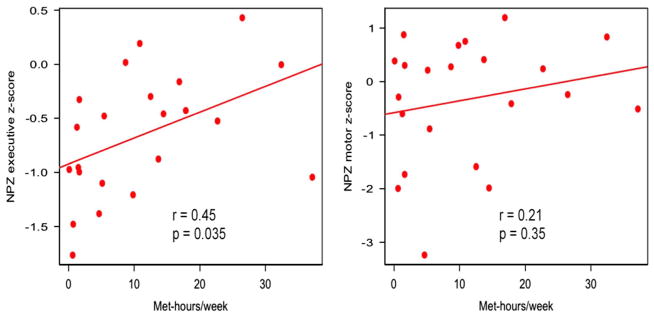
We further examined the association between the continuous measure of exercise engagement (MET hr/week) and NPZe or NPZm in the physically active group. The total amount of weekly exercise activity in METs was significantly positively correlated with NPZe (Pearson’s r = 0.45, [95% confidence interval {CI}, 0.04, 0.73], p = .035), but not NPZm (r = 0.21, 95% CI [−0.23, 0.58], p = .35) (Figure 2).
Fig. 2.
Relationship between neuropsychological performance and aerobic exercise within physically active HIV + individuals. Scatterplots and linear regression slopes for NPZ by weekly Met-hr/week for the physically active cohort. (Left) NPZe scores were significantly positively correlated to amount of physical activity (PA) (p = .035). (Right) NPZm scores were not correlated to PA. Adjusted R-values are shown for both figures.
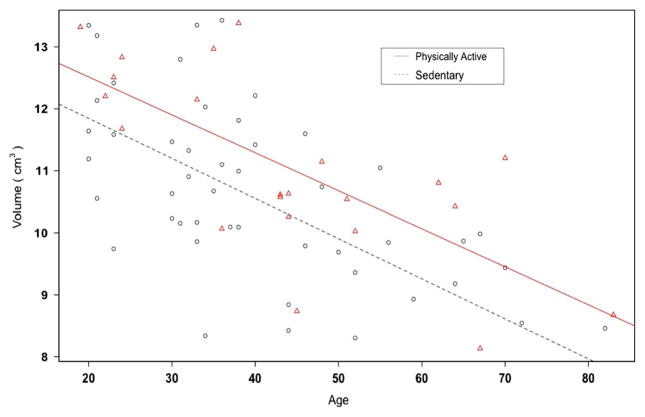
We found no volume differences between the exercise groups in the five brain regions of interest examined using independent sample t-tests (Table 3). However, there was a statistically significant difference between the two groups in the putamen in the adjusted analyses, but not for the other four regions. The putamen volume was significantly larger in the physically active group, by 0.86 cm3 (cc) 95% CI (0.15 cc, 1.57 cc), p = .019 (Table 3). In the adjusted model, that also included age, sex, and nadir CD4, older age was significantly correlated with smaller putamen volumes (p < .001) (Figure 3). For both physically active and sedentary HIV + individuals, brain volume decreased with age at similar rates (p-value for age-by-PA interaction = 0.41), but physically active HIV + individuals had larger putamen volumes at any age. The estimated additive effect of exercise was equivalent to 14.1 fewer years of age, such that a 30-year-old sedentary HIV + individual was equivalent to a 44-year-old physically active HIV + individual.
Table 3.
Comparison of the five brain regions between the physically active and sedentary HIV + groups, in unadjusted and adjusted analyses
| Brain volume, in cc | HIV + PA group (n = 22) | HIV + sedentary (n = 48) | Unadjusted difference PA-sedentary | Adjusted difference PA-sedentary1 | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean (SD) | Mean (SD) | Mean (95% CI) | p-Value | Mean (95% CI) | p-Value | |
| Total Gray Matter | 608.6 (57.0) | 595.4 (60.0) | 13.2 (−17.2, 43.5) | .39 | 18.8 (−6.9, 44.5) | .15 |
| Total White Matter | 461.4 (60.0) | 446.2 (52.8) | 15.2 (−13.1, 43.5) | .29 | 18.3 (−9.5, 46.0) | .19 |
| Caudate | 7.12 (1.13) | 6.98 (1.25) | 0.14 (−0.48, 0.76) | .66 | 0.35 (−0.24, 0.95) | .24 |
| Putamen | 11.04 (1.48) | 10.52 (1.70) | 0.52 (−0.32, 1.36) | .22 | 0.86 (0.15, 1.57) | .02* |
| Hippocampus | 8.00 (0.93) | 7.69 (0.97) | 0.31 (−0.19, 0.80) | .22 | 0.28 (−0.21, 0.77) | .25 |
Note. Adjusted analyses control for confounders significant at 0.20 level in backward model selection. All brain volumes are normalized for intra-cranial volume. Final adjusted (multivariable) model is controlling for the following covariates. Total gray matter: age, sex, education. Total white matter: age, sex, education. Caudate: age, nadir CD4, log10 viral load. Putamen: age, sex, nadir CD4. Hippocampus: age, education. For all five brain regions, age had a significant negative association with the brain volume.
Statistically significant correlation.
PA, physically active; SD, standard deviation; CI, confidence interval
Fig. 3.
Physically active individuals have a larger putamen than sedentary individuals across the age-span. Reduced physical activity (p = .043) and older age (p <.001) are independently associated with smaller putamen volumes. Physically active individuals: triangles, solid line; sedentary individuals: circles, dashed line.
We examined the correlation of the brain volumes for the five brain regions with the composite neuropsychological scores (NPZe and NPZm). NPZe performance was modestly positively correlated to total gray matter (r = 0.30; p = .011), caudate (r = .28; p = .016), and putamen (r = 0.28; p = .018) volumes. Additionally, NPZm performance was associated with larger volumes of total gray matter (r = 0.32; p = .007), total white matter (r = 0.31; p = .008), and hippocampus (r = 0.26; p = .028). All these associations remained statistically significant after correction for multiple comparisons keeping the false discovery rate level at 0.05 (Benjamini and Hochberg, 1995).
DISCUSSION
In the present study, results revealed that physically active HIV + individuals had better executive function than sedentary HIV + individuals. Additionally, results revealed that NPZe was positively associated with MET values of exercise. Physically active HIV + individuals additionally had larger putamen volumes compared to sedentary HIV + individuals. Lastly, executive and motor performance were associated with brain volumetrics across both groups.
Studies in seronegative individuals have demonstrated that PA benefits executive function and task switching (Barnes, Yaffe, Satariano, & Tager, 2003). However, relatively few studies have investigated these relationships, with most using only acute measures of current activity (Dufour et al., 2013; Fazeli et al., 2014; Mapstone et al., 2013). We captured PA values for aerobic activities over the past year with a concentration on running, walking, and jogging (Ainsworth et al., 2000; Bowles et al., 2004). Our questionnaire was different than other studies that have typically used the international PA questionnaire (IPAQ), which focuses on activity over the past week. Intensive long-term exercise has been shown to lead to sustain benefits in neuropsychological performance in HIV− individuals (Dregan & Gulliford, 2013).
Results of the present study that that physically active HIV + individuals may have better executive function than sedentary individuals, specifically since MET-hr/week was positively correlated to NPZe scores. These findings are similar to previous studies showing healthy lifestyles and PA was associated with greater working memory and cognitive benefits (Dufour et al., 2013; Fazeli et al., 2014). However, the physically active HIV + individuals did not perform significantly better on NPZm tests. The lack of relationship between PA and NPZm scores is similar to results observed in HIV- literature where exercise interventions improved executive function and working memory, but not motor performance (Barnes et al., 2003; Chapman et al., 2013). Larger studies are still necessary to determine if PA attenuates cognitive decline by domain. Most importantly, NPZe was significantly positively correlated with amount of PA (Figure 2), thus larger gains and stronger effect sizes may be possible for those who increase the intensity or duration of aerobic PA.
Our neuroimaging findings indicate that physically active HIV + individuals may have larger brain volumes in the putamen, a critical region affected by HIV (Ances et al., 2012; Pfefferbaum et al., 2012; Thompson et al., 2005). Results are consistent with previous studies that have exhibited the benefits of exercise on brain volumes in HIV-individuals (Chapman et al., 2013; Law, Barnett, Yau, & Gray, 2014). In particular, observed changes in putamen volume due to PA may be beneficial regardless of age for HIV + individuals (Figure 3). These effects were not driven by BMI or duration of HIV infection, since these variables were not significantly contributing to the final adjusted model. However, since the effect of PA was limited to a single region, larger more comprehensive studies are needed to investigate the subtle effects of PA on brain integrity.
The volumetric results appear to fall outside of previous literature regarding correlations between the putamen and cognitive domains. Specifically, studies have defined the caudate as a region associated with executive function (Bonelli & Cummings, 2007; Paul et al., 2008), however, we observed that larger putamen volume and better NPZe were seen in physically active HIV + individuals. This result is supported by recent structural and functional MRI studies showing that anterior striatum (both caudate and putamen) areas receive projections from frontal cortex and are involved in task switching (Thames et al., 2012; Walhovd et al., 2015). Additionally, HIV infection is associated with greater atrophy in the anterior caudate and putamen (Becker et al., 2011). Our volumetric methods only quantified total ROI volume, therefore, we cannot confirm whether PA ameliorated the anterior putamen. However, our NPZe results suggest a small protective effect on executive function may be associated with anterior striatum (Tziortzi et al., 2014). Future studies mapping the morphology or functional changes occurring at the ROI level may resolve this ambiguity in a larger cohort.
Conclusions regarding the impact of PA on brain integrity should be tempered by several limitations of the present study. The calculated exercise measures result from a self-reported questionnaire and required individuals to recall details about their workout schedules during the past year. This may create a potential bias for athletic individuals with fixed exercise schedules and limit the ability for individuals that do not exercise in a consistent manner to recall the amount of exercise completed. Furthermore, due to a relatively small sample size, we were unable to test for differences between rigorous and low intensity exercise regimens. Future studies should perform physical function tests in addition to observational questionnaires to differentiate the effect and determine the amount of exercise necessary to have positive effects on brain integrity. Finally, it is important to note that the associations between PA and brain structure do not define a causal relationship, as it could also be argued that individuals with larger brain structures are more likely to be healthy and physically active. Nevertheless, well-controlled longitudinal studies examining the potential of PA to improve brain function among chronically infected HIV patients represents an important area of future work.
In summary, this cross-sectional study found modest beneficial effects of aerobic PA on brain structure and executive function in a HIV + cohort. As the prevalence of HAND is likely to increase with age, it is important to find adjunctive therapeutic strategies to HAART. This study provides impetus to pursue larger longitudinal clinical trials to evaluate causality and the neuroprotective effects of exercise for HIV + individuals.
Table 4.
Correlation of brain volumes for five regions of interest with neuropsychological composite scores for executive (NPZe) and motor (NPZm) domains in 70 HIV + individuals (the two groups combined)
| NPZe (executive)
|
NPZm (motor)
|
|||
|---|---|---|---|---|
| r (95% CI) | p-Value | r (95% CI) | p-Value | |
| Total gray matter | 0.30 (0.07, 0.50) | 0.0109*§ | 0.32 (0.09, 0.52) | 0.0068*§ |
| Total white matter | 0.22 (−0.01, 0.43) | 0.065 | 0.31 (0.08, 0.51) | 0.0082*§ |
| Caudate | 0.29 (0.06, 0.49) | 0.0155*§ | 0.11 (−0.13, 0.33) | 0.38 |
| Putamen | 0.28 (0.05, 0.49) | 0.0178*§ | 0.18 (−0.06, 0.40) | 0.14 |
| Hippocampus | 0.20 (−0.03, 0.42) | 0.094 | 0.26 (0.03, 0.47) | 0.0279*§ |
Note. Pearson correlation coefficient r (and 95% confidence intervals) and uncorrected p-values of the test of no correlation are given.
Statistically significant correlation (uncorrected).
Statistically significant correlation after a Benjamini-Hochberg correction for multiple testing (all 10 tests)
Acknowledgments
FUNDING
The authors thank Jodi Heaps-Woodruff, PhD., Denise Head, PhD, Elizabeth Westerhaus, MA, and Gina Rhee for their assistance. This work was supported by the National Institutes of Health (NIH) (B.M.A., grant numbers R01NR12657, R01NR012907, R01NR014449, and R21MH0999979), Grossman Chancellor’s Fellowship (MO), the National Science Foundation (M.O., grant number IGERT 0548890), the Washington University School of Medicine Institute of Clinical and Translational Sciences (B.M.A., grant number UL1 TR000448), and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant (P30 CA091842).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Conflicts, or Potential Conflicts of Interest: The authors do not have a commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding). Drs. Ortega and Baker contributed equally to this work
References
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, Leon AS. Compendium of Physical Activities: An update of activity codes and MET intensities. Medicine and Science in Sports and Exercise. 2000;32(Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. Journal of Acquired Immune Deficiency Syndrome. 2012;59(5):469–477. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au R, Seshadri S, Wolf PA, Elias M, Elias P, Sullivan L, D’Agostino RB. New norms for a new generation: Cognitive performance in the framingham offspring cohort. Experimental Aging Research. 2004;30(4):333–358. doi: 10.1080/03610730490484380. [DOI] [PubMed] [Google Scholar]
- Baker LM, Paul RH, Heaps JM, Westerhaus E, Chang JY, Williams S, Ances BM. Impact of human immunodeficiency virus on neurocognition and risky behaviors in young adults. Journal of Neurovirology. 2014;20(5):466–473. doi: 10.1007/s13365-014-0264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. Journal of the American Geriatric Society. 2003;51(4):459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Multicenter ACS. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging and Behavior. 2011;5(2):77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test - Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Berchicci M, Lucci G, Perri RL, Spinelli D, Di Russo F. Benefits of physical exercise on basic visuomotor functions across age. Frontiers in Aging Neuroscience. 2014;6:48. doi: 10.3389/fnagi.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues in Clinical Neuroscience. 2007;9(2):141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles HR, FitzGerald SJ, Morrow JR, Jr, Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. American Journal of Epidemiology. 2004;160(3):279–286. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RH. Hopkins Verbal Learning test - Revised professional manual. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiology of Aging. 2011;32(3):506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade WT, Overton ET, Mondy K, de las Fuentes L, Davila-Roman VG, Waggoner AD, Yarasheski KE. Relationships among HIV infection, metabolic risk factors, and left ventricular structure and function. AIDS Research and Human Retroviruses. 2013;29(8):1151–1160. doi: 10.1089/AID.2012.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade WT, Reeds DN, Lassa-Claxton S, Davila-Roman VG, Waggoner AD, Powderly WG, Yarasheski KE. Post-exercise heart rate recovery in HIV-positive individuals on highly active antiretroviral therapy. Early indicator of cardiovascular disease? HIV Medicine. 2008;9(2):96–100. doi: 10.1111/j.1468-1293.2007.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JS, Yan JH, Payne VG. The impact of obesity and exercise on cognitive aging. Frontiers in Aging Neuroscience. 2013;5:97. doi: 10.3389/fnagi.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, Defina LF, Keebler MW, Didehbani N, Lu H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Frontiers in Aging Neuroscience. 2013;5:75. doi: 10.3389/fnagi.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank TM, Thompson JA, Dominguez DJ, Reyes AP, Bynevelt M, Georgiou-Karistianis N, Ziman MR. The effect of multidisciplinary rehabilitation on brain structure and cognition in Huntington’s disease: An exploratory study. Brain and Behavior. 2015;5(2):e00312. doi: 10.1002/brb3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dregan A, Gulliford MC. Leisure-time physical activity over the life course and cognitive functioning in late mid-adult years: A cohort-based investigation. Psychological Medicine. 2013;43(11):2447–2458. doi: 10.1017/S0033291713000305. [DOI] [PubMed] [Google Scholar]
- Duff K, Schoenberg MR, Scott JG, Adams RL. The relationship between executive functioning and verbal and visual learning and memory. Archives of Clinical Neuropsychology. 2005;20(1):111–122. doi: 10.1016/j.acn.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Dufour CA, Marquine MJ, Fazeli PL, Henry BL, Ellis RJ, Grant I, Group H. Physical exercise is associated with less neurocognitive impairment among HIV-infected adults. Journal of Neurovirology. 2013;19(5):410–417. doi: 10.1007/s13365-013-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiology of Aging. 2014;35(Suppl 2):S20–S28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PL, Woods SP, Heaton RK, Umlauf A, Gouaux B, Rosario D, Group H. An active lifestyle is associated with better neurocognitive functioning in adults living with HIV infection. Journal of Neurovirology. 2014;20(3):233–242. doi: 10.1007/s13365-014-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Free SL, Bergin PS, Fish DR, Cook MJ, Shorvon SD, Stevens JM. Methods for normalization of hippocampal volumes measured with MR. AJNR: American Journal of Neuroradiology. 1995;16(4):637–643. [PMC free article] [PubMed] [Google Scholar]
- Friis-Moller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S DAD Study Group. Predicting the risk of cardiovascular disease in HIV-infected patients: The data collection on adverse effects of anti-HIV drugs study. European Journal of Cardiovascular Prevention and Rehabilitation. 2010;17(5):491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999;6(2):147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Gomes-Neto M, Conceicao CS, Oliveira Carvalho V, Brites C. A systematic review of the effects of different types of therapeutic exercise on physiologic and functional measurements in patients with HIV/AIDS. Clinics (Sao Paulo) 2013;68(8):1157–1167. doi: 10.6061/clinics/2013(08)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Singh T, Bugg JM. The moderating role of exercise on stress-related effects on the hippocampus and memory in later adulthood. Neuropsychology. 2012;26(2):133. doi: 10.1037/a0027108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Charter Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2009;23(3):188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law LL, Barnett F, Yau MK, Gray MA. Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: A systematic review. Ageing Reseach Reviews. 2014;15:61–75. doi: 10.1016/j.arr.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Lee MH, Wang T, Jang MH, Steiner J, Haughey N, Ming GL, Venkatesan A. Rescue of adult hippocampal neurogenesis in a mouse model of HIV neurologic disease. Neurobiology of Disease. 2011;41(3):678–687. doi: 10.1016/j.nbd.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Head D. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Annals of Neurology. 2010;68(3):311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapstone M, Hilton TN, Yang H, Guido JJ, Luque AE, Hall WJ, Shah K. Poor aerobic fitness may contribute to cognitive decline in HIV-infected older adults. Aging and Disease. 2013;4(6):311–319. doi: 10.14336/AD.2013.0400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C, Klove N. Instruction manual for the adult neuropsychology test battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- McGuire JL, Barrett JS, Vezina HE, Spitsin S, Douglas SD. Adjuvant therapies for HIV-associated neurocognitive disorders. Annals of Clinical and Translational Neurology. 2014;1(11):938–952. doi: 10.1002/acn3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DS, Nalvaline HA, Woody GE. Encyclopedia of drugs, alcohol and addictive behavior. Farmington Mills, MI: Macmillan Reference USA; 2001. Assessment of substance abuse: HIV risk assessment battery. [Google Scholar]
- Nogueira Pinto A. AIDS/HIV infection and cerebrovascular disease. Seminars in Cerebrovascular Diseases and Stroke. 2005;5:40–46. [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D, Jr, Cysique L, Ake C HNRC Group. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. Journal of Clinical and Expimental Neuropsychology. 2011;33(7):793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Sager MA. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology. 2014;83(19):1753–1760. doi: 10.1212/WNL.0000000000000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton ET, Kauwe JS, Paul R, Tashima K, Tate DF, Patel P, Clifford DB. Performances on the CogState and standard neuropsychological batteries among HIV patients without dementia. AIDS and Behavior. 2011;15(8):1902–1909. doi: 10.1007/s10461-011-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Miyashita M, Takahashi M, Kawanishi N, Hayashida H, Kim HS, Nakamura Y. Low-volume walking program improves cardiovascular-related health in older adults. Journal of Sports Science & Medicine. 2014;13(3):624–631. [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Rogers S, Hall C, Robertson K. Motor based assessment of neurocognitive functioning in resource-limited international settings. Journal of Clinical and Expimental Neuropsychology. 2007;29(1):59–66. doi: 10.1080/13803390500488538. [DOI] [PubMed] [Google Scholar]
- Paul RH, Ernst T, Brickman AM, Yiannoutsos CT, Tate DF, Cohen RA H.I.V. MRS Consortium. Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. Journal of the International Neuropsychological Society. 2008;14(5):725–733. doi: 10.1017/S1355617708080910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, Sullivan EV. Regional brain structural dysmorphology in human immunodeficiency virus infection: Effects of acquired immune deficiency syndrome, alcoholism, and age. Biological Psychiatry. 2012;72(5):361–370. doi: 10.1016/j.biopsych.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips EJ, Ottaway CA, Freedman J, Kardish M, Li J, Singer W, Fong IW. The effect of exercise on lymphocyte redistribution and leucocyte function in asymptomatic HIV-infected subjects. Brain, Behavior, and Immunity. 1997;11(3):217–227. doi: 10.1006/brbi.1997.0494. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Troster AI. Action (verb naming) fluency as an executive function measure: Convergent and divergent evidence of validity. Neuropsychologica. 1999;37(13):1499–1503. doi: 10.1016/s0028-3932(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Pizzie R, Hindman H, Roe CM, Head D, Grant E, Morris JC, Hassenstab JJ. Physical activity and cognitive trajectories in cognitively normal adults: The adult children study. Alzheimer Disease and Associated Disorders. 2014;28(1):50–57. doi: 10.1097/WAD.0b013e31829628d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Rojas Vega S, Knicker A, Hollmann W, Bloch W, Struder HK. Effect of resistance exercise on serum levels of growth factors in humans. Hormone and Metabolic Research. 2010;42(13):982–986. doi: 10.1055/s-0030-1267950. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance test for normality. Biometrika. 1965;52(3–4):591–611. [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Du Pasquier RA. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Simulation of mental deficiency on a visual memory test. American Journal of Mental Deficiency. 1963;67:909–913. [PubMed] [Google Scholar]
- Thames AD, Foley JM, Wright MJ, Panos SE, Ettenhofer M, Ramezani A, Hinkin CH. Basal ganglia structures differentially contribute to verbal fluency: Evidence from Human Immunodeficiency Virus (HIV)-infected adults. Neuropsychologia. 2012;50(3):390–395. doi: 10.1016/j.neuropsychologia.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4 + T lymphocyte decline. Proceedings of the Nationall Academy of Sciences of the United States of America. 2005;102(43):15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Salvatori MF, Vlassi C, Liuzzi G, Giancola ML, Antinori A. Changes in cognition during antiretroviral therapy: Comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. Journal of Acquired Immune Deficiency Syndromes. 2009;52(1):56–63. doi: 10.1097/qai.0b013e3181af83d6. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Haber SN, Searle GE, Tsoumpas C, Long CJ, Shotbolt P, Gunn RN. Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cerebral Cortex. 2014;24(5):1165–1177. doi: 10.1093/cercor/bhs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG. HIV, aging, and cognition: Emerging issues. Topics in Antiviral Medicine. 2013;21(3):119–123. [PMC free article] [PubMed] [Google Scholar]
- Vanderploeg RD, Schinka JA, Retzlaff P. Relationships between measures of auditory verbal learning and executive functioning. Journal of Clinical and Experimental Neuropsychology. 1994;16(2):243–252. doi: 10.1080/01688639408402635. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression methods in biostatistics. New York: Springer-Verlag; 2012. [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Kramer AF. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Frontiers in Aging Neuroscience. 2010;2:32. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Tamnes CK, Bjornerud A, Due-Tonnessen P, Holland D, Dale AM, Fjell AM. Maturation of cortico-subcortical structural networks-segregation and overlap of medial temporal and frontostriatal systems in development. Cerebral Cortex. 2015;25(7):1835–1841. doi: 10.1093/cercor/bht424. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale® - Third Edition (WAIS®-III) San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]
- Whiteman AS, Young DE, He X, Chen TC, Wagenaar RC, Stern CE, Schon K. Interaction between serum BDNF and aerobic fitness predicts recognition memory in healthy young adults. Behavioral Brain Research. 2014;259:302–312. doi: 10.1016/j.bbr.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Dawson MS, Morgan EE, Carey CL, Heaton RK H.I.V. Neurobehavioral Research Center (HNRC) Group. Construct validity of Hopkins Verbal Learning Test-Revised component process measures in an HIV-1 sample. Archives of Clinical Neuropsychology. 2005;20(8):1061–1071. doi: 10.1016/j.acn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Laciny E, Overton ET, Reeds DN, Harrod M, Baldwin S, Davila-Roman VG. 18FDG PET-CT imaging detects arterial inflammation and early atherosclerosis in HIV-infected adults with cardiovascular disease risk factors. Journal of Inflammation. 2012;9(1):26. doi: 10.1186/1476-9255-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarasheski KE, Scherzer R, Kotler DP, Dobs AS, Tien PC, Lewis CE Metabolic Change in H. I. V. I. Age-related skeletal muscle decline is similar in HIV-infected and uninfected individuals. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2011;66(3):332–340. doi: 10.1093/gerona/glq228. [DOI] [PMC free article] [PubMed] [Google Scholar]