CONSPECTUS
Today, 1 in 2 males and 1 in 3 females in the US will develop cancer at some point during their lifetimes, and 1 in 4 males and 1 in 5 females in the U.S. will die from the disease. New methods for detection and treatment have dramatically improved cancer care in the United States. However, as improved detection and increasing exposure to carcinogens has led to higher rates of cancer incidence, clinicians and researchers have not balanced that increase with a similar decrease in cancer mortality rates. This mismatch highlights a clear and urgent need for increasingly potent and selective methods with which to detect and treat cancers at their earliest stages.
Nanotechnology, the use of materials with structural features ranging from 1 to 100 nm in size, has dramatically altered the design, use, and delivery of cancer diagnostic and therapeutic agents. The unique and newly discovered properties of these structures can enhance the specificities with which biomedical agents are delivered, complementing their efficacy or diminishing unintended side effects. Gold (and silver) nanotechnologies afford a particularly unique set of physiological and optical properties which can be leveraged in applications ranging from in vitro/vivo therapeutics and drug delivery to imaging and diagnostics, surgical guidance, and treatment monitoring.
Nanoscale diagnostic and therapeutic agents have been in use since the development of micellar nanocarriers and polymer-drug nanoconjugates in the mid-1950s, liposomes by Bangham and Watkins the mid-1960s, and the introduction of polymeric nanoparticles by Langer and Folkman in 1976. Since then, nanoscale constructs such as dendrimers, protein nanoconjugates, and inorganic nanoparticles have been developed for the systemic delivery of agents to specific disease sites. Today, more than 20 FDA-approved diagnostic or therapeutic nanotechnologies are in clinical use with roughly 250 others are in clinical development. The global market for nano-enabled medical technologies is expected to grow to $70-160 billion by 2015, rivaling the current market share of biologics worldwide.
In this Account, we explore the emerging applications of noble metal nanotechnologies in cancer diagnostics and therapeutics carried out by our group and by others. Many of the novel biomedical properties associated with gold and silver nanoparticles arise from confinement effects: i) the confinement of photons within the particle which can lead to dramatic electromagnetic scattering and absorption (useful in sensing and heating applications, respectively); ii) the confinement of molecules around the nanoparticle (useful in drug delivery); and iii) the cellular/subcellular confinement of particles within malignant cells (such as selective, nuclear-targeted cytotoxic DNA damage by gold nanoparticles). We then describe how these confinement effects relate to specific aspects of diagnosis and treatment such as: i) laser photothermal therapy, optical scattering microscopy, and spectroscopic detection, ii) drug targeting and delivery, and iii) the ability of these structures to act as intrinsic therapeutic agents which can selectively perturb/inhibit cellular functions such as division. We intend to provide the reader with a unique physical and chemical perspective on both the design and application of these technologies in cancer diagnostics and therapeutics. We also suggest a framework for approaching future research in the field.
Graphical Abstract
I. Photon Confinement: Imaging Probes, Photothermal Therapy, and Spectroscopic Detection
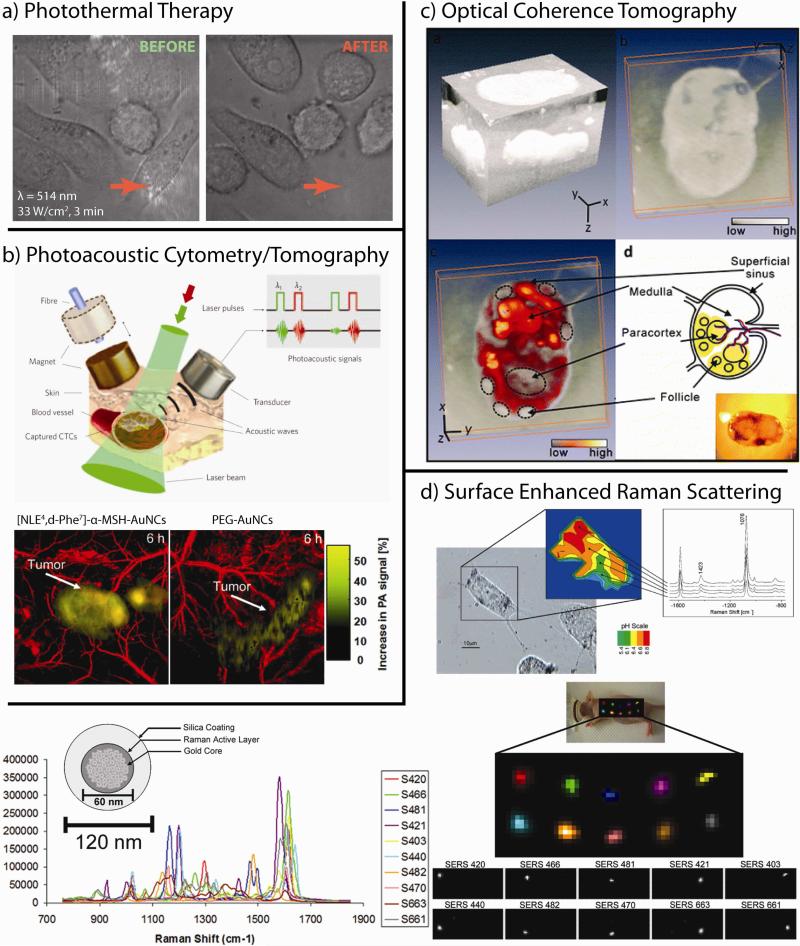
Gold nanoparticles are particularly attractive platforms for targeted diagnostics and therapeutics due to their unique optical and electronic properties. These structures can be conjugated with upwards of 1014 ligands per cm2 (102 – 103 times higher than that typically attainable with liposomal or polymeric nanoparticles, respectively)1 and the selective accumulation of gold nanoparticles at solid tumors can facilitate highly efficient photothermal ablation via non-invasive near-infrared (NIR) laser exposure2-6 (Figure 1a), high-Z enhanced X-ray computed tomography/radiotherapy,7,8 non-invasive photoacoustic imaging/cytometry6,9,10 (Figure 1b), contrast-enhanced optical coherence tomographic imaging11 (OCT, Figure 1c), and the electromagnetic enhancement in non-invasive spectroscopic biomarker detection schemes both in vitro12 and in vivo13 (Figure 1d).
Figure 1. Properties of gold nanotechnologies that can be used to enhance therapeutic treatment and diagnostic imaging of cancer.
(a) Photothermal therapy: gold nanoparticles can serve as contrast agents for the selective laser photothermal ablation of tumor cells. Arrow indicates laser focus. Image obtained in collaboration with Prof. X. Huang (U of Memphis) and Prof. C.K. Payne (Georgia Tech). (b) Photoacoustic cytometry/tomography: pulsed laser excitation of cells/tissues labeled with gold nanoparticles can be used to detect or sequester circulating tumor cells (CTCs, upper panel) or to non-invasively image/diagnose/stage tumors and guide surgical procedures (lower panel). (c) Optical coherence tomography (OCT): the backscattering and photothermal properties of gold nanotechnologies can be used to enhance OCT contrast for monitoring disease metastasis to the lymphatic system. (d) Surface enhanced Raman scattering (SERS): Electromagnetic near-field enhancements generated by gold nanoparticles can improve non-invasive in vitro (upper panel) and in vivo (lower panel) spectral cancer diagnostics. Adapted with permission from (b) Refs. 9,10, (c) Ref. 11, and (d) Refs. 13,14. Copyright (b) 2009 Macmillan Publishers Ltd: Nature Nanotechnology and 2010 American Chemical Society, (c) 2011 American Chemical Society, and (d) 2007 American Chemical Society and 2009 National Academy of Sciences.
Gold nanoparticles are also highly useful probes for microscopic imaging-based applications. For example, the resonant optical scattering intensity from a single 80 nm gold nanoparticle15 is equivalent to the emission intensity of 500,000 of the most efficient Alexa Fluor® dyes or 2,000 of the most efficient Qdot® 800 quantum dots.16 While gold nanoparticles have been used for decades as labels in immunohistochemical and electron microscopic analysis of tissue sections,17 Sokolov et al. first demonstrated in 2003 that the resonant scattering from gold nanoparticles could be used to image subcellular cancer biomarkers (EGFR) in vitro using confocal reflectance microscopy of immunolabeled gold nanoparticles (ca. 12 nm core diameter) for potential cancer diagnostics, staging, and treatment monitoring. Our group18,19 has shown that simpler, less expensive dark-field optics can also be used to obtain high-contrast scattering images from immunolabeled gold nanoparticles in vitro, in this case in true-color, to achieve the identification and selective (photothermal) labeling of malignant cells (Figure 2a). Currently, the method is in wide use for both the imaging and spectroscopy of nanoparticles and their labeling of living cells and tissues.20,21
Figure 2. In vitro applications of targeted gold nanotechnologies in diagnostic imaging and photothermal therapy.
(a) High optical scattering contrast from malignant cells selectively labeled with antibody-conjugated gold nanospheres and nanorods (anti-epidermal growth factor receptor, anti-EGFR). (b) Specific-labeling by these particles significantly lowers the laser power exposure threshold required to kill malignant, but not normal, cells. Adapted with permission from Ref. 19. Copyright 2006 American Chemical Society.
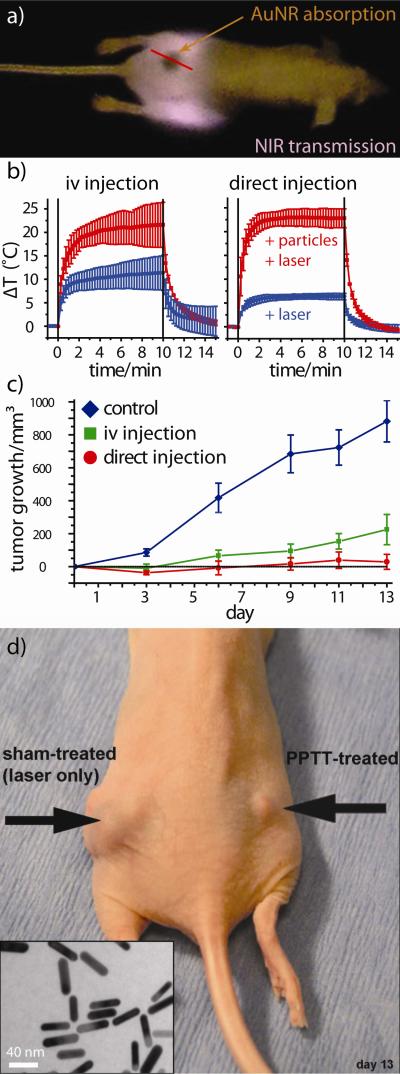
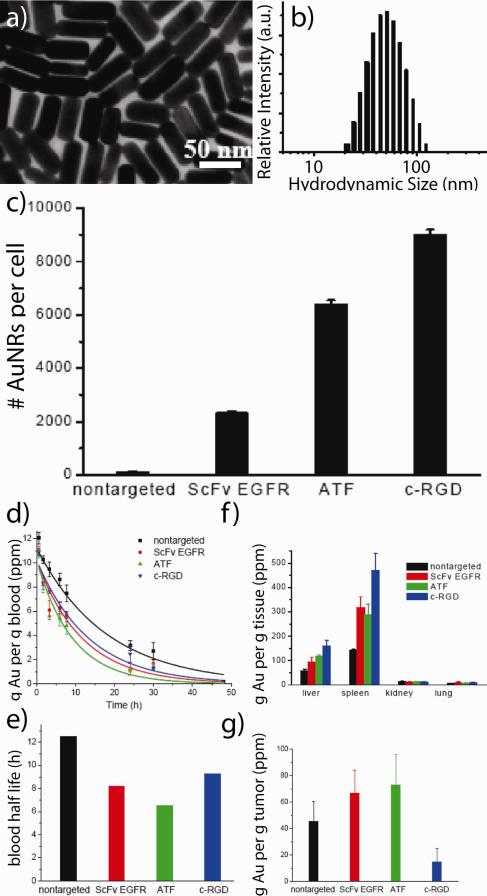
While mild hyperthermic cancer treatments have been in clinical use since the early 1980s,22 laser phothermal therapy (or ablation) using plasmonic nanoparticles was first demonstrated in 2003 by locally injecting so-called gold nanoshells (silica-gold core-shell nanoparticles) directly into the tumor interstitium and later by the passive accumulation of systemically delivered nanoshells.23 Our group2 was the first to show that increasingly efficient3 gold nanorods could be used as contrast agents for in vitro and in vivo near-infrared (NIR) laser photothermal therapy to achieve selective tumor cell ablation and resorption/remission in vivo (Figure 3). In the latter, NIR-absorbing PEGylated gold nanorods were systemically or intratumorally administered in mice bearing head and neck tumor models (squamous cell carcinoma). Subsequent exposure of the nanoparticle-loaded tumors for just 10 min was found to result in upwards of 20 °C temperature increases at the tumor center, with minimal damage to surrounding tissues. Complete tumor resorption was observed in more than 50% of the group directly administered gold nanorods and 25% of those systemically administered at two weeks post-laser treatment.2 Subsequent studies by our group24 exploring active targeting of these nanorods by i) a single-chain variable fragment (ScFv) peptide that recognizes the epidermal growth factor receptor (EGFR), ii) an amino terminal fragment (ATF) peptide that recognizes the urokinase plasminogen activator receptor (uPAR), or iii) a cyclic RGD peptide that recognizes the avb3 integrin receptor were also performed (hydrodynamic diameter 68-81 nm, zeta potential −5 to −25 mV). As expected, active targeting significantly improved the cellular accumulation of these nanoconjugates in vitro (A549 lung cancer cells). The blood half life of PEGylated gold nanorods was reduced by 25 - 48% upon co-conjugation with these active targeting ligands and the tumor accumulation (24 h) of ATF- and ScFV EGFR-targeted gold nanorods was enhanced ca. 67 and 46% relative to passively targeted PEGylated gold nanorods administered in mice models (Figure 4). Surprisingly, the tumor accumulation of cyclic RGD-targeted gold nanorods was significantly diminished (ca. −57 % relative to PEGylated gold nanorods), suggesting that laser photothermal therapy using this specific formulation may be best suited to intratumoral injection schemes. The use of gold nanocage25 and hollow gold nanoparticles26 as contrast agents in preclinical laser photothermal cancer therapy have also been subsequently explored by Xia and Li, respectively, and human pilot studies investigating the treatment of refractory and/or recurrent head and neck tumors using plasmonic laser photothermal therapy are currently ongoing.27
Figure 3. The first report demonstrating the use of gold nanorods as contrast agents for laser photothermal cancer therapy.
(a) Near-infrared (NIR) transmission image of tumor-bearing mice (rear flank) showing gold-nanorod contrast agents specifically accumulated at the tumor site (dark, highly absorbing region). (b) Thermal transient measurements from the tumor center during NIR laser exposure of gold nanorod-loaded tumors treated by intravenous (left) and intratumoral (right) administration. High thermal contrast is observed between particle-treated mice (red curves) and controls treated by laser only (blue). (c) Tumor regression following intravenous (green) and intratumoral (red) laser photothermal treatment as compared to untreated controls (blue). No detectable disease was observed at day 13 in >50% of the interstitially-treated group and 25% of the intravenously-treated group. (d) Dramatic tumor growth remission/resorption even in a weakly responding mouse treated by intratumoral nanorod injection (electron microscopy, inset) and laser photothermal therapy versus sham (laser alone) treatment. Adapted with permission from Ref. 2. Copyright 2008 Elsevier Science B.V..
Figure 4. Effects of active targeting on the in vitro uptake and in vivo pharmacokinetics/biodistribution of anticancer gold nanorods.
(a) Electron micrographs of near-infrared absorbing colloidal gold nanorods and (b) their corresponding distribution of hydrodynamic diameters. (c) Enhanced in vitro binding/uptake of actively targeted gold nanorods as determined by mass spectrometry (ICP). In vitro accumulation of single-chain variable fragment (ScFv), peptide fragment (ATF), and cyclic cell-penetrating peptide (c-RGD) targeted nanorods was significantly enhanced. In contrast, in vivo (d) pharmacokinetics, (e) blood half-lives, (f) biodistribution, and (g) tumor-specific accumulation was only marginally enhanced. Adapted with permission from Ref. 24. Copyright 2010 American Chemical Society.
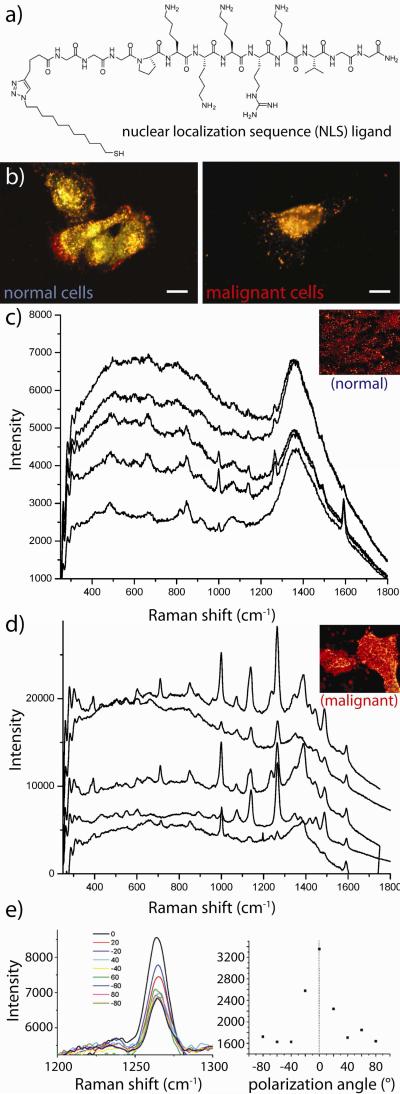
The unique photophysical properties of gold nanostructures can be further leveraged to enhance a number of spectroscopically-relevant processes28-32 with applications in biodiagnostics and treatment monitoring. These processes include two-photon luminescence (TPL) imaging,33 metal enhanced fluorescence (MEF), optical coherence tomography (OCT),11 photoacoustic tomography/cytometry,6,9,10 surface enhanced infrared absorption (SEIRA), and most notably, surface enhanced Raman scattering (SERS).34 In an early study demonstrating the utility of SERS for in vitro diagnostics, our group35 showed that conjugation of gold nanorods with a nuclear localization sequence (NLS) peptide greatly promotes their uptake by both malignant and non-malignant cells (Figure 5a-b). High resolution Raman microscopy found that spectral responses from the cells exhibited increasing contributions from peptides, nucleic acids, DNA backbone vibrations, chromatin, and histone structures upon transfection. Moreover, malignant cells were also found to selectively exhibit increasing contributions from adenine nucleobases and a characteristic band at 398 cm−1, both of which may serve as potential diagnostic cancer markers. Prior research by our group36 has also indicated that cell-surface labeling can be useful in identifying malignant and premalignant cells based on their characteristic overexpression of epidermal growth factor receptor (EGFR, Figure 5c-e). Here, SERS from malignant and non-malignant cells was interrogated following labeling with polystyrene-coated gold nanorods electrostatically conjugated with EGFR antibodies. Optical dark-field scattering microscopy and high-resolution microabsorption spectroscopy found that labeling of the malignant cells was roughly two-fold greater than the non-malignant cell line. Raman analysis found that 90% of the malignant cells exhibited detectible SERS response while only 20% of the non-malignant cells exhibited detectible SERS. Moreover, due to the high density and orientation of these nanorods about the surfaces of the malignant cells, SERS signal was detected in a polarization-dependent manner, serving as another potential diagnostic marker for cancer. Natan, Gambhir, and Nie have also explored the in vivo use of gold nanoparticles decorated with so-called Raman reporter molecules for non-invasive imaging diagnostics,37 surgical guidance,38 and multiplexed in vivo labeling application.39
Figure 5. Applications of targeted gold nanotechnologies for spectral cancer diagnostics.
(a) Structure of a synthetic nuclear-localization sequence (NLS) peptide derivative which (b) promotes cellular uptake and cancer-selective perinuclear localization of gold nanorod bioconjugates. (c) Optical spectroscopy of immunolabeled gold nanorods (anti-EGFR) showing low surface enhanced Raman scattering (SERS) from normal cells and a high degree of cancer-specific spectral features caused by the high density of SERS-enhancing particles labeling malignant cells. (e) High-density orientation of the nanorods about the malignant cell surface leads to polarized Raman scattering intensity from the vibrations of molecules on the nanorod surfaces. Adapted with permission from (a-b) Ref. 35 and (c-e) Ref. 36. Copyright 2007 American Chemical Society.
II. Molecular Confinement: Drug Delivery
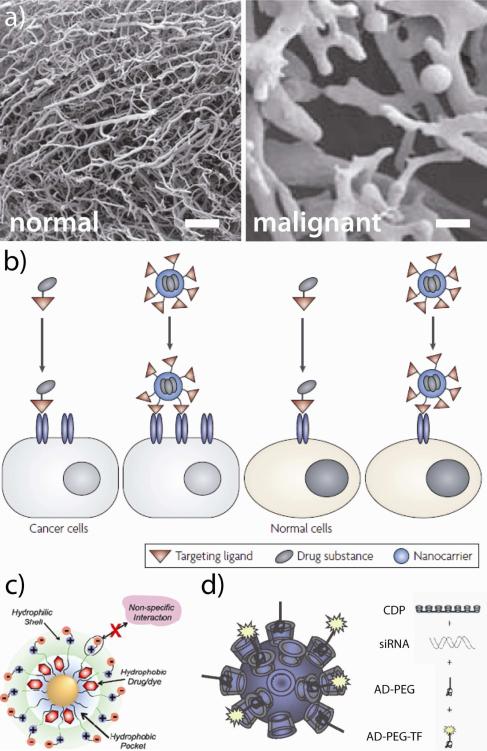
Gold nanoparticles exhibit a range of properties which make them particularly amenable to systemic delivery applications. Due to their relatively large size, these constructs can preferentially accumulate at tumor sites due to the enhanced permeability and retention (EPR) effect40,41 (Figure 6a) and because of the high density of atoms on their surfaces, their receptor binding affinity can easily tuned over several orders of magnitude.42 Because multivalent nanoconjugates are large enough to occupy multiple, adjacent cellular binding sites,43 enhanced avidity can greatly improve the selectivity of their targeting, uptake, and/or delivery (Figure 6b). These platforms protect and facilitate the delivery of physiologically unstable diagnostic/therapeutic agents or those which exhibit poor intracellular penetration (e.g. hydrophobic chemotherapeutics (Figure 6c),44 enzymatically-degradable siRNA (Figure 6d),45 etc.).
Figure 6. Properties of nanoscale technologies that can be leveraged to enhance the diagnosis and treatment of solid tumors.
(a) The EPR effect: Polymer cast replicas of blood vessels in normal (left) and malignant tissues (right) illustrate why high molecular weight compounds (i.e. nanoparticles) can preferentially accumulate at tumor sites supplied by disordered vasculatures. (b) Multivalent avidity: unlike monovalent ligands, nanoscale constructs can simultaneously bind multiple adjacent receptors to augment uptake/selectivity. (c-d) Enhanced stability: 70% of new active pharmaceutical ingredients fail in development because of poor solubility;46 nanoscale carriers can enhance the circulatory half lives, biodistribution, and intracellular penetration of water-insoluble chemotherapeutics and proteins/nucleic acids (e.g. siRNA) which are susceptible to enzymatic degradation and subject to poor intracellular penetration rates. Scale bars represent 100 μm. Adapted with permission from (a) Ref. 41, (b) Ref. 47, (c) 44, and (d) Ref. 45. Copyright (a) 2009 Macmillan Publishers Ltd: British Journal of Cancer, (c) 2008 Macmillan Publishers Ltd: Nature Reviews Drug Discovery, (c) 2009 American Chemical Society, and (d) 2010 National Academy of Sciences.
One of the earliest reports involving the use of gold nanoparticles in drug delivery applications came in 1954, when Root et al. investigated the clinical use of colloidal Au198 particles for radiotherapy treatment of liver cancer and leukemia, both with minimal success.48 In 2001, Paciotti and Tamarkin began research studying the use of gold nanoparticles as a platform for the delivery of tumor necrosis factor α (TNFα) to solid tumor models in mice.49,50 Subsequent Phase I clinical trials found that the PEGylated 27 nm diameter gold nanoparticles were well tolerated in patients and able to safely deliver TNFα at dosages 3-fold higher than the previously reported maximum tolerable dose for the lone drug with no serious adverse side effects; Phase II trials are currently pending.51
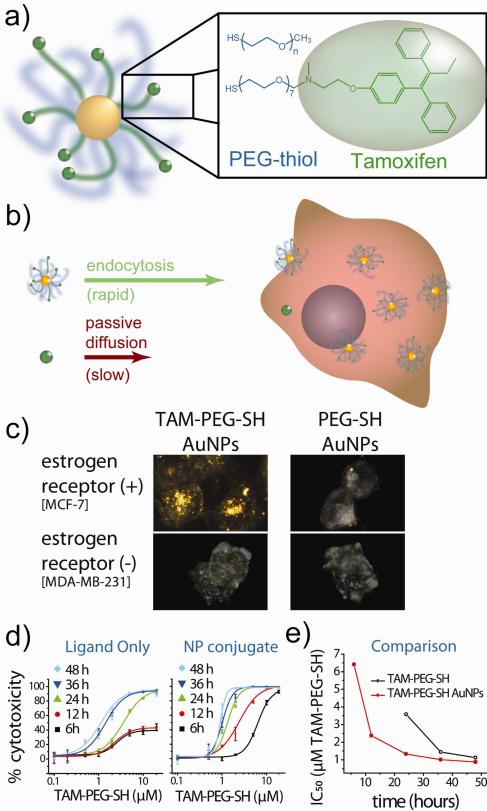
Our group3-5,52 is currently developing technologies for the treatment of hormone-dependent malignancies such as breast and prostate cancer using gold nanostructures which incorporate derivatives of small molecule receptor antagonists that can serve as combined targeting and therapeutic agents. 60-70% of breast cancers express estrogen receptor53 and 80-90% of prostate cancers express androgen receptor.54 Classically, these proteins function as intracellular gene transcription factors, that is, hormones passively diffuse across the cell membrane, engage the receptor, and the two translocate to the nucleus where they bind DNA and transcribe proteins associated with malignant growth and cancer progression. Over the past 15 years, researchers have become increasingly aware that these hormone receptors are also expressed in large quantities on the plasma membrane following their post-translational modification.55 Because these membrane hormone receptors still maintain binding affinity for their endogenous hormones and antibodies, these receptors can serve as targets for tissue-selective drug delivery to breast, prostate, and other hormone-dependent cancers. Or lab has shown that a derivative of the breast cancer treatment drug tamoxifen can be used to selectively deliver our PEGylated gold nanoparticle platform to breast cancer cells in a receptor- and ligand-dependent manner, and can do so with accompanying potency at concentrations more than 10,000-fold higher than the lone drug (Figure 7).52 Moreover, we've shown that in vitro laser photothermal therapy can be used to selectively induce phototoxicity of these drug conjugates to breast cancer cells at otherwise sub-lethal concentrations,5 providing opportunities for subsequent radiotherapy enhancement, treatment monitoring by contrast-enhanced x-ray CT, photoacoustic imaging, phase-contrast OCT, and/or non-invasive SERS analysis. We are currently further exploring the applications of this technology in treating estrogen-dependent malignancies and also investigating an increasingly selective and potent antiandrogen nanoparticle construct for the treatment of hormone-insensitive prostate cancers (which comprise nearly all prostate cancers 2-3 years following initial endocrine treatments).56 Mirkin,57 Rotello,58 and Xia59 have also extensively explored the use of gold nanotechnologies in drug delivery applications for the treatment of solid tumors; interested readers are directed to Dreaden et al. for further reading.3,4,6
Figure 7. Antiestrogen gold nanoparticles for hormone receptor-targeted breast cancer treatment strategies.
(a) Illustration of 25 nm gold nanospheres conjugated with mixed self-assembled monolayers (SAMs) of a high molecular weight polymer stabilizer and a polymer-linked estrogen receptor antagonist, tamoxifen (TAM). Here, TAM acts as a combined targeting and therapeutic moiety which binds membrane- and cytoplasmic-estrogen receptor, inducing apoptosis. (b) High-density gold nanoparticle ligation dramatically accelerates drug influx rates via endocytosis of (104 TAM molecules per particle) versus passive diffusion of the free drug. (c) Optical dark-field scattering microscopy shows nanoparticle uptake into breast cancer cells in a receptor- and ligand-dependent manner. (d) Time-dependent dose-response kinetics show >104 enhanced drug potency from the targeted nanoparticle construct with up to 2.7-fold enhanced potency per equivalent tamoxifen molecule. Adapted with permission from Refs. 3,52. Copyright 2009 American Chemical Society and 2011 Royal Society of Chemistry.
IIIA. Cellular Confinement: Intrinsic Pharmacodynamic Properties of Nanoparticles
Like other nanoscale materials, gold nanoparticles are capable of perturbing normal physiological functions depending on their location within the cell and the extent with which they interact with various biomolecules (Table 1).60 For example, Fe3O4 nanoparticles can interfere with electronic and/or ion transport processes, CeO2 nanoparticles can induce protein fibrillation, and dendrimers can cause damage to cell membranes (vide infra). Our group21,61 is exploring the possibility of exploiting such deleterious cellular interactions to selectively treat malignant cells (which are increasingly susceptible to damage). Gold nanoparticles have been shown capable of modifying protein charge, size, and hydrophobicity,60 as well as thermal stability.62 These particles have also demonstrated preferential binding to single-stranded DNA versus double-stranded DNA (which can interfere with cell division), G2/M cell cycle arrest for radiotherapy enhancement,63 and direct association with the heparin-binding domain of VEGF-165 to inhibit its ability to associate with cell surface receptors necessary for cancer-related angiogenesis.64
Table 1. Deleterious nano-bio interactions which can be leveraged in targeted therapeutic strategies for cancer.
Adapted with permission from Ref. 60. Copyright 2009 Macmillan Publishers Ltd: Nature Materials.
| Nanoparticle | Nano-Bio Interaction |
|---|---|
| TiO2 | ROS production mediated by electron-hole-pairs Glutathione depletion and toxic oxidative stress as a result of photoactivity and redox properties Nanoparticle-mediated cell membrane disruption lead to cell death; protein fibrillation |
| ZnO | ROS production Dissolution and release of toxic cations Lysosomal damage Inflammation |
| Ag | Dissolution and Ag+ release inhibits respiratory enzymes and ATP production ROS production Disruption of membrane integrity and transport processes |
| Au nanospheres & nanorods | Disruption of protein conformation |
| CdSe | Dissolution and release of toxic Cd and Se ions |
| SiO2 | ROS production by surface defects and impurities Protein unfolding Membrane disruption |
| Fe2O3 | ROS production and oxidative stress Liberation of toxic Fe2+ Disturbance of the electronic and/or ion transport activity in the cell membrane |
| CeO2 | Protein aggregation and fibrillation |
| Carbon nanotubes (multiwalled) | Frustrated phagocytosis causes chronic tissue inflammation and DNA oxidative injury |
| Carbon nanotubes (single-walled & multiwalled) | Generation of ROS due to the metal impurities trapped inside CNTs Pro-inflammatory effects due to oxidant injury; Granulomatous inflammation due to hydrophobic CNT aggregation Interstitial pulmonary fibrosis due to fibroblast-mediated collagen production; ROS production (spontaneous or photoactivated) Hydrophobic surface increases aggregation but promotes intramembranous localization |
| Fullerenes | ROS production (spontaneous or photoactivated) Hydrophobic surface increases aggregation but promotes intramembranous localization |
| Cationic nanospheres & dendrimers | Membrane damage, thinning and leakage Damage to the acidifying endosomal compartment by the proton sponge effect that allows entry into the cytosol |
| Co/Ni ferrite nanoparticles, magnetic metallic nanoparticles | Liberation of toxic cations |
| Al2O3 | ROS production Pro-inflammatory response |
| Cu/CuO | DNA damage and oxidative stress |
| MoO3 | Membrane disruption |
IIIB. Subcellular Confinement: Selectively Localizing Nanoparticles at Cancer Cell Nuclei
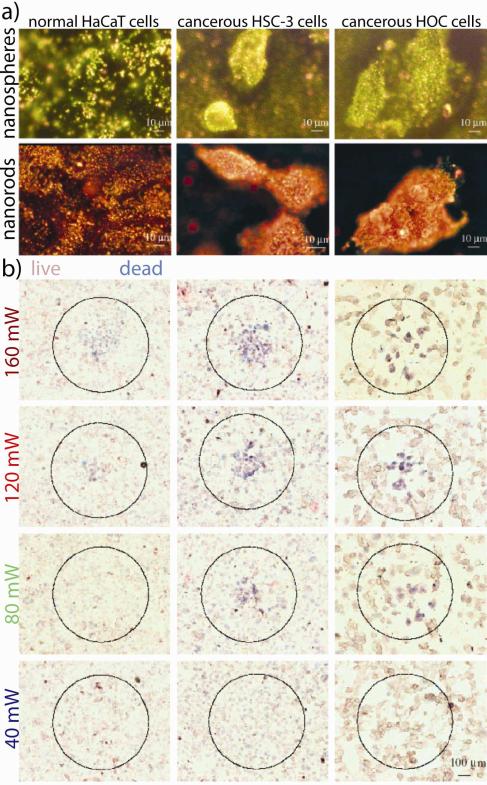
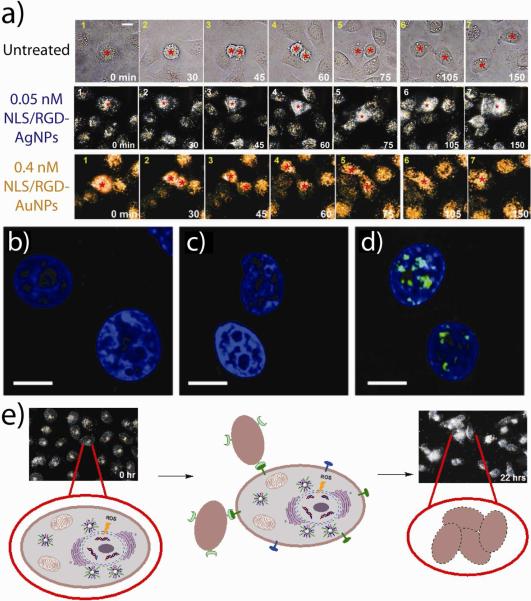
We hypothesized that by using peptides to direct the accumulation of gold nanoparticles (and other nanomaterials) at discrete intracellular locations, that we can selectively perturb malignant cell growth and associated disease progression. In a recent investigation using real-time, live-cell, dark-field scattering videography (which also makes use of the photon confinement properties of nanoscale noble metal nanoparticles), our group21 showed that spherical gold nanoparticles decorated with cell-penetrating RGD and nuclear localization sequence (NLS) peptides can impair cell division and induce cytokinesis arrest in a concentration-dependent manner (with no such effects observed for nanoparticles conjugated with either peptide alone) (Figure 9, Supporting Video S1). G2/M cell cycle arrest, binucleate cell formation, apoptosis, and histone cleavage was observed in malignant cells treated with the RGD/NLS gold nanoparticles, while the cell cycle of non-malignant cells was not significantly affected at therapeutically-relevant concentrations (0.4 nM). In a more recent, but related, study investigating cellular effects from RGD/NLS silver nanoparticles, our group61 observed that apoptotic induction in malignant cells by these particles led to the attraction of neighboring cells and their subsequent engulfment by these cells (Figure 9, Supporting Video S2). We also found that increases in particle concentration beyond this apoptotic threshold (0.05 nM) further increased cell death, also believed to be attributable to oxidative stress, but decreased cell clustering which we concluded occurred due to rapid apoptotic induction and a loss of capacity for intercellular signaling.61
Figure 9. Intrinsic pharmacological properties of gold and silver nanoparticles can be leveraged to selectively treat malignant cells.
(a) Optical dark-field scattering videography shows that silver (middle panel) and gold (lower panel) nanoparticles targeted with cell-penetrating RGD and nuclear localization sequence (NLS) peptides can selectively impair cell division and induce cytokinesis arrest in malignant cell lines. Confocal immunofluorescence microscopy of (b) control and (c) RGD-targeted gold nanoparticle-treated carcinoma cell nuclei (blue) showed no indication of DNA damage (green) while (d) combined RGD/NLS-targeted gold nanoparticle-treated carcinoma cells showed substantial DNA double strand breaks. (e) Schematic diagram of the proposed sequence of events leading to cellular clustering and non-professional phagocytosis in cancer cells treated with RGD/NLS-targeted silver nanoparticles which generate reactive oxygen species (ROS) that causes DNA damage, programmed cell death, and intercellular signal transduction. (a) Adapted with permission from (a-d) Ref. 21 and (a, e) Ref. 61 Copyright 2010-2011 American Chemical Society.
V. Conclusions
In summary, we have shown that gold nanoparticles can serve as highly multifunctional platforms for the targeted diagnosis and treatment of solid tumors. These structures exhibit a particularly unique combination of physical, chemical, optical, and electronic properties distinct from all other biomedical nanotechnologies and provide a multimodal platform with which i) to image and diagnose cancers with increasingly sensitivity and selectivity, ii) to preferentially deliver therapeutic agents and electromagnetic radiation to malignant cells with increasing potency, and iii) to selectively target, sensitize and exert intrinsic pharmacodynamic effects on malignant cells. The studies discussed herein attempt, in particular, shed light on newly emerging applications of gold nanotechnologies in anticancer diagnostics and therapeutics and emphasize some of the novel aspects of their photophysical properties, pharmacodynamic profiles, and fundamental biophysical interactions.
VI. Challenges and Outlook
Tremendous strides have been made over the past decade in the preclinical and clinical development of gold nanotechnologies for the treatment of solid tumors. Going forward, several challenges will need to be addressed in order to achieve wide acceptance of these platforms for both local and systemic delivery. The lack of long term studies investigating potential chronic inflammatory response65 and increasingly efficient hepatobiliary excretion of these constructs is a significant bottleneck in development pipeline of gold nanotechnologies and should be made a priority. Investigations applying these highly flexible technologies in personalized medicine (perhaps biopsy-specific nanoconjugate formulation) is an attractive, but underrepresented area of research. High-throughput synthesis, formulation, and screening of biomedical gold nanotechnologies is yet another area lacking significant attention. Longer-term studies exploring potential mutagenicity or effects on reproductive or cardiovascular heath have yet to be widely investigated. Alternative strategies for gold surface linkage and biocompatible polymer functionalization are also warranted. Increasing efficacy may be observed from studies involving multiple or follow-up treatment regimens. Those in the field will also need to effectively speak to the common misconception that gold nanotechnologies are prohibitively expensive (for example, the gold content present in a full-course dosage of TNFα-gold nanoparticles recently administered in a Phase I clinical trial51 currently costs less than 12¢ per patient; the quantity of gold salt used to produce the nanorods administered in our recent photothermal therapy studies66 currently costs less than 23¢ per mouse).67 Although these technologies exhibit a unprecedented degree of multifunctionality, few studies simultaneously address these to investigate potential synergy between, for example, i) combined gold nanoparticle drug delivery and enhanced radiation or photothermal therapy, ii) whole body Raman diagnostics and subsequent intraoperative guidance, or iii) enhanced diagnostic CT contrast and posttreatment monitoring for metastasic spread by photoacoustic cytometry and OCT. Increasingly ambitious funding proposals will be needed to explore such areas. We also wish to emphasize that gold nanoparticles cannot be simply thought of as “inert cores” for drug delivery, but that these structures present a tremendous amount of value-added for multimodal diagnostics and treatment strategies ranging from combined optical, CT, Raman, OCT, and, photoacoustic imaging probes, to contrast agents for photothermal therapy and photoacoustic cytometry, to long-circulating, high affinity/avidity, high payload-bearing drug carriers, to targeted cytotoxic chemotherapeutics.
The clinical translation of liposomal nanotechnologies for cancer treatment took nearly 30 years since its first description68 as a potential drug carrier (Doxil® FDA approval for ovarian cancer in 1999). The clinical development of therapeutic monoclonal antibodies also took nearly as long since the seminal work69 of Köhler, Milstein, and Jerne in 1975 (Avastin® FDA approval for in 2004). We and our colleagues in the field of biomedical gold nanotechnologies, initially developmed60 in 2001, are prepared for the challenges ahead and are excited and enthusiastic for tremendous research strides soon to come.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge generous support from the U.S. National Institutes of Health (1U01CA151802-01) and the US National Science Foundation's Department of Materials Research (0906822), proofreading assistance from J.A. Bordley and T. McSweeney, and support from the Julius Brown Chair Professorship in Chemistry and Biochemistry.
Biography
Erik C. Dreaden received his PhD from the School of Chemistry and Biochemistry at Georgia Institute of Technology in 2012. His work at the Laser Dynamics Laboratory (LDL) emphasized fundamental and applied nanotechnology and focused on plasmonics and nanoscale cancer therapeutics. This work aimed, in part, to leverage the unique physical, chemical, and electronic properties of gold nanoparticles to develop technological platforms for the increasingly selective and efficacious treatment of solid tumors. Another thrust of Erik's work aimed to better understand how the unique photophysical properties of gold nanostructures interface with one another and with other electronic systems such as solid-state semiconductors and photosynthetic proteins, and further, to exploit these novel interactions in order to realize enhanced performance in photovoltaic and spectroscopic applications. Erik is currently a postdoctoral researcher at the David H. Koch Institute for Integrative Cancer Research at MIT, working with Prof. Paula T. Hammond on the development of colloidal LbL nanotechnologies for synergistic RNA interference and cancer chemotherapy.
Mostafa A. El-Sayed is Regents’ Professor and Julius Brown Chair in the School of Chemistry and Biochemistry at Georgia Institute of Technology. He obtained his PhD from Florida State University in 1959 with Michael Kasha, and after postdoctoral fellowships at Harvard, Yale, and Cal Tech, he joined the faculty at UCLA in 1961 and Georgia Tech later in 1994. He is currently an elected member of the US National Academy of Science, an elected fellow of the American Academy of Arts and Sciences, former editor-in-chief of the Journal of Physical Chemistry, recipient of the Ahmed Zewail prize in molecular sciences, the ACS Irving Langmuir Prize in Chemical Physics, the Glenn T. Seaborg Medal, and the US National Medal of Science. El-Sayed's lab is consistently ranked among the top chemical research labs of the past decade, #4 worldwide in 2009 by Times Higher Education. His current research interests include the fundamental optical and electronic properties of nanomaterials and their applications in nanocatalysis, nanomedicine, photovoltaics, and photobiology.
Footnotes
SUPPORTING INFORMATION
Live-cell, dark-field plasmonic scattering videography of HSC-3 squamous cell carcinoma cells incubated with 0.4 nM gold and 0.05 nM silver nanoparticles conjugated with cell-penetrating (RGD) and nuclear-targeting (NLS) peptides. Both nanoparticles induced cellular apoptosis; however, gold nanoparticles were shown to induce cytokinesis arrest (Video S1) while silver nanoparticles were shown to induce cell clustering and engulfment by neighboring cells (Video S2). This information is available free of charge via the Internet at http://pubs.acs.org/.
REFERENCES
- 1.For example, while gold nanoparticle surfacesa can be routinely functionalized with active ligands (i.e. not stabilizers) at densities approximately 1.0 × 106 molecules/ m2, those typically attainable using more conventional liposomesb and PLGA (poly(lactic-co-glycolic acid)) nanoparticlesc are 2 to 3 orders of magnitude less, 1.2 × 104 and 4.4 × 103 molecules/ m2, respectively, allowing for augmented targeting affinity, avidity, and multifunctionality from gold nanostrucures, as well as drug loading densities comperable to those attainable using nanoencapsulation methods. Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. 2nd ed. John Wiley & Sons, Inc.; New York: 2001. Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8786–8791. doi: 10.1073/pnas.151247498.Park J, Mattessich T, Jay SM, Agawu A, Saltzman WM, Fahmy TM. Enhancement of surface ligand display on PLGA nanoparticles with amphiphilic ligand conjugates. J. Control. Release. 2011;156:109–115. doi: 10.1016/j.jconrel.2011.06.025.
- 2.Dickerson EB, Dreaden EC, Huang XH, El-Sayed IH, Chu HH, Pushpanketh S, McDonald JF, El-Sayed MA. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269:57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dreaden EC, Mackey MA, Huang X, Kang B, El-Sayed MA. Beating cancer in multiple ways using nanogold. Chem. Soc. Rev. 2011;40:3391–3404. doi: 10.1039/c0cs00180e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012;41:2740–2779. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oyelere AK, El-Sayed MA, Dreaden EC. Targeted Cellular Delivery of Nanoparticles. 2010 U.S. patent 12/890,519 pending.
- 6.Dreaden EC, El-Sayed MA, El-Sayed IH. Nanotechnology and Nanostructures Applied to Head and Neck Cancer. In: Preedy VR, Srirajaskanthan R, editors. Nanomedicine and Cancer. Science Publishers; Enfield, NH: 2011. [Google Scholar]
- 7.von Maltzahn G, Park J-H, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Computationally Guided Photothermal Tumor Therapy Using Long-Circulating Gold Nanorod Antennas. Cancer Res. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hainfeld JF, Dilmanian FA, Zhong Z, Slatkin DN, Kalef-Ezra JA, Smilowitz HM. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010;55:3045. doi: 10.1088/0031-9155/55/11/004. [DOI] [PubMed] [Google Scholar]
- 9.Kim C, Cho EC, Chen J, Song KH, Au L, Favazza C, Zhang Q, Cobley CM, Gao F, Xia Y, Wang LV. In Vivo Molecular Photoacoustic Tomography of Melanomas Targeted by Bioconjugated Gold Nanocages. ACS Nano. 2010;4:4559–4564. doi: 10.1021/nn100736c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanzha EI, Shashkov EV, Kelly T, Kim J-W, Yang L, Zharov VP. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat. Nanotechnol. 2009;4:855–860. doi: 10.1038/nnano.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung Y, Reif R, Zeng Y, Wang RK. Three-Dimensional High-Resolution Imaging of Gold Nanorods Uptake in Sentinel Lymph Nodes. Nano Lett. 2011;11:2938–2943. doi: 10.1021/nl2014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallaoro A, Braun GB, Moskovits M. Quantitative ratiometric discrimination between noncancerous and cancerous prostate cells based on neuropilin-1 overexpression. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16559–16564. doi: 10.1073/pnas.1109490108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zavaleta CL, Smith BR, Walton I, Doering W, Davis G, Shojaei B, Natan MJ, Gambhir SS. Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13511–13516. doi: 10.1073/pnas.0813327106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kneipp J, Kneipp H, Wittig B, Kneipp K. One- and Two-Photon Excited Optical pH Probing for Cells Using Surface-Enhanced Raman and Hyper-Raman Nanosensors. Nano Lett. 2007;7:2819–2823. doi: 10.1021/nl071418z. [DOI] [PubMed] [Google Scholar]
- 15.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J. Phys. Chem. B. 2006;110:7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 16.Data calculated from the Mie cross section of an 80 nm gold nanosphere in water (λ=549/549 nm, εext~7.69×1010, QY=0.41) as compared to Alexa Fluor® 488 in pH 7 aqueous buffer (λ=495/519 nm, εabsa~7.3×104, QYb=0.92 and Qdot® 800 (λex=350 nm, εabsa~15.8×106, QYc assumed 1). a http://www.invitrogen.com/site/us/en/home/References/Molecular-Probes-The-Handbook/Technical-Notes-and-Product-Highlights/The-Alexa-Fluor-Dye-Series.html retrieved 24 October, 2011. b http://www.invitrogen.com/site/us/en/home/References/Molecular-Probes-The-Handbook/tables/Fluorescence-quantum-yields-and-lifetimes-for-Alexa-Fluor-dyes.html retrieved 24 October, 2011. c http://www.invitrogen.com/site/us/en/home/References/Molecular-Probes-The-Handbook/tables/Extinction-Coefficients-of-Qdot-Streptavidin-Conjugates-at-Common-Wavelengths.html retrieved 24 October, 2011.
- 17.Holgate CS, Jackson P, Cowen PN, Bird CC. Immunogold-silver staining: new method of immunostaining with enhanced sensitivity. J. Histochem. Cytochem. 1983;31:938–44. doi: 10.1177/31.7.6189883. [DOI] [PubMed] [Google Scholar]
- 18.El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 19.Huang XH, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 20.Qian W, Huang X, Kang B, El-Sayed MA. Dark-field light scattering imaging of living cancer cell component from birth through division using bioconjugated gold nanoprobes. J. Biomed. Opt. 2010;15:046025/1–9. doi: 10.1117/1.3477179. [DOI] [PubMed] [Google Scholar]
- 21.Kang B, Mackey MA, El-Sayed MA. Nuclear Targeting of Gold Nanoparticles in Cancer Cells Induces DNA Damage, Causing Cytokinesis Arrest and Apoptosis. J. Am. Chem. Soc. 2010;132:1517–1519. doi: 10.1021/ja9102698. [DOI] [PubMed] [Google Scholar]
- 22.Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487–497. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 23.Lal S, Clare SE, Halas NJ. Nanoshell-Enabled Photothermal Cancer Therapy: Impending Clinical Impact. Acc. Chem. Res. 2008;41:1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Peng X, Wang Y, Shin DM, El-Sayed MA, Nie S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano. 2010;4:5887–96. doi: 10.1021/nn102055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Glaus C, Laforest R, Zhang Q, Yang M, Gidding M, Welch MJ, Xia Y. Gold nanocages as photothermal transducers for cancer treatment. Small. 2010;6:811–7. doi: 10.1002/smll.200902216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, Li C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin. Cancer Res. 2009;15:876–86. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanospectra Biosciences, Inc . ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2000. [2010 Oct 24]. Pilot Study of AuroLase(tm) Therapy in Refractory and/or Recurrent Tumors of the Head and Neck. Available from: http://clinicaltrials.gov/ct2/show/NCT00848042: NCT00848042. [Google Scholar]
- 28.Neretina S, Dreaden EC, Qian W, El-Sayed MA, Hughes RA, Preston JS, Mascher P. The Dependence of the Plasmon Field Induced Nonradiative Electronic Relaxation Mechanisms on the Gold Shell Thickness in Vertically Aligned CdTe-Au Core-Shell Nanorods. Nano Lett. 2009;9:3772–3779. doi: 10.1021/nl901960w. [DOI] [PubMed] [Google Scholar]
- 29.Neretina S, Qian W, Dreaden EC, El-Sayed MA, Hughes RA, Preston JS, Mascher P. Plasmon field effects on the nonradiative relaxation of hot electrons in an electronically quantized system: CdTe-Au core-shell nanowires. Nano Lett. 2008;8:2410–2418. doi: 10.1021/nl801303g. [DOI] [PubMed] [Google Scholar]
- 30.Neretina S, Qian W, Dreaden EC, El-Sayed MA, Hughes RA, Preston JS, Mascher P. Exciton Lifetime Tuning by Changing the Plasmon Field Orientation with Respect to the Exciton Transition Moment Direction: CdTe-Au Core-Shell Nanorods. Nano Lett. 2009;9:1242–1248. doi: 10.1021/nl900183m. [DOI] [PubMed] [Google Scholar]
- 31.Yen C-W, Hayden SC, Dreaden EC, Szymanski P, El-Sayed MA. Tailoring Plasmonic and Electrostatic Field Effects To Maximize Solar Energy Conversion by Bacteriorhodopsin, the Other Natural Photosynthetic System. Nano Lett. 2011;11:3821–3826. doi: 10.1021/nl2018959. [DOI] [PubMed] [Google Scholar]
- 32.Dreaden EC, Neretina S, Qian W, El-Sayed MA, Hughes RA, Preston JS, Mascher P. Plasmonic Enhancement of Nonradiative Charge Carrier Relaxation and Proposed Effects from Enhanced Radiative Electronic Processes in Semiconductor−Gold Core−Shell Nanorod Arrays. J. Phys. Chem. C. 2011;115:5578–5583. [Google Scholar]
- 33.Wang H, Huff TB, Zweifel DA, He W, Low PS, Wei A, Cheng J-X. In vitro and in vivo two-photon luminescence imaging of single gold nanorods. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15752–15756. doi: 10.1073/pnas.0504892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dreaden EC, Near RD, Abdallah T, Talaat MH, El-Sayed MA. Multimodal plasmon coupling in low symmetry gold nanoparticle pairs detected in surface-enhanced Raman scattering. Appl. Phys. Lett. 2011;98:183115–3. [Google Scholar]
- 35.Oyelere AK, Chen B, Huang X, El-Sayed IH, El-Sayed MA. Peptide conjugated gold nanorods for nuclear targeting. Bioconjugate Chem. 2007;18:1490–1497. doi: 10.1021/bc070132i. [DOI] [PubMed] [Google Scholar]
- 36.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer Cells Assemble and Align Gold Nanorods Conjugated to Antibodies to Produce Highly Enhanced, Sharp and Polarized Surface Raman Spectra: A Potential Cancer Diagnostic Marker. Nano Lett. 2007;7:1591–1597. doi: 10.1021/nl070472c. [DOI] [PubMed] [Google Scholar]
- 37.Qian X, Peng X-H, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotech. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 38.Mohs AM, Mancini MC, Singhal S, Provenzale JM, Leyland-Jones B, Wang MD, Nie S. Hand-held Spectroscopic Device for In Vivo and Intraoperative Tumor Detection: Contrast Enhancement, Detection Sensitivity, and Tissue Penetration. Analytical Chem. 2010;82:9058–9065. doi: 10.1021/ac102058k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zavaleta CL, Smith BR, Walton I, Doering W, Davis G, Shojaei B, Natan MJ, Gambhir SS. Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13511–13516. doi: 10.1073/pnas.0813327106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 41.Silván U, Arlucea J, Andrade R, Diez-Torre A, Silio M, Konerding MA, Arechaga J. Angiogenesis and vascular network of teratocarcinoma from embryonic stem cell transplant into seminiferous tubules. Br. J. Cancer. 2009;101:64–70. doi: 10.1038/sj.bjc.6605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tassa C, Duffner JL, Lewis TA, Weissleder R, Schreiber SL, Koehler AN, Shaw SY. Binding Affinity and Kinetic Analysis of Targeted Small Molecule-Modified Nanoparticles. Bioconjugate Chem. 2009;21:14–19. doi: 10.1021/bc900438a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 44.Kim CK, Ghosh P, Pagliuca C, Zhu Z-J, Menichetti S, Rotello VM. Entrapment of Hydrophobic Drugs in Nanoparticle Monolayers with Efficient Release into Cancer Cells. J. Am. Chem. Soc. 2009;131:1360–1361. doi: 10.1021/ja808137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yarnell A. Nanoparticles Boost Drug Solubility. Chem. Eng. News. 2012;90:30–31. [Google Scholar]
- 47.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 48.Root SW, Andrews GA, Kniseley RM, Tyor MP. The distribution and radiation effects of intravenously administered colloidal Au198 in man. Cancer. 1954;7:856–866. doi: 10.1002/1097-0142(195409)7:5<856::aid-cncr2820070506>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 49.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11:169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 50.Paciotti GF, Myer LD, Kim TH, Wang S, Alexander HR, Weinreich D, Tamarkin L. Colloidal gold: A novel colloidal nanoparticle vector for tumor-directed drug delivery. Clin. Cancer Res. 2001;7:3673S–3674S. [Google Scholar]
- 51.Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Gannon WE, Walker M, Seidel GD, Yuldasheva N, Tamarkin L. Phase I and Pharmacokinetic Studies of CYT-6091, a Novel PEGylated Colloidal Gold-rhTNF Nanomedicine. Clin. Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dreaden EC, Mwakwari SC, Sodji QH, Oyelere AK, El-Sayed MA. Tamoxifen-Poly(ethylene glycol)-Thiol Gold Nanoparticle Conjugates: Enhanced Potency and Selective Delivery for Breast Cancer Treatment. Bioconjugate Chem. 2009;20:2247–2253. doi: 10.1021/bc9002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osborne CK. Tamoxifen in the Treatment of Breast Cancer. New Engl. J. Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 54.Heinlein CA, Chang C. Androgen Receptor in Prostate Cancer. Endocr. Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 55.Pedram A, Razandi M, Sainson RCA, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J. Biol. Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 56.Attar RM, Takimoto CH, Gottardis MM. Castration-Resistant Prostate Cancer: Locking Up the Molecular Escape Routes. Clin. Cancer Res. 2009;15:3251–3255. doi: 10.1158/1078-0432.CCR-08-1171. [DOI] [PubMed] [Google Scholar]
- 57.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 59.Xia Y, Li W, Cobley CM, Chen J, Xia X, Zhang Q, Yang M, Cho EC, Brown PK. Gold Nanocages: From Synthesis to Theranostic Applications. Acc. Chem. Res. 2011;44:914–924. doi: 10.1021/ar200061q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 61.Austin LA, Kang B, Yen C-W, El-Sayed MA. Plasmonic Imaging of Human Oral Cancer Cell Communities during Programmed Cell Death by Nuclear-Targeting Silver Nanoparticles. J. Am. Chem. Soc. 2011;133:17594–17597. doi: 10.1021/ja207807t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laera S, Ceccone G, Rossi F, Gilliland D, Hussain R, Siligardi G, Calzolai L. Measuring Protein Structure and Stability of Protein–Nanoparticle Systems with Synchrotron Radiation Circular Dichroism. Nano Lett. 2011 doi: 10.1021/nl202909s. [DOI] [PubMed] [Google Scholar]
- 63.Wilson R, et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology. 2009;20:375101. doi: 10.1088/0957-4484/20/37/375101. [DOI] [PubMed] [Google Scholar]
- 64.Bhattacharya R, Mukherjee P, Xiong Z, Atala A, Soker S, Mukhopadhyay D. Gold nanoparticles inhibit VEGF165-induced proliferation of HUVEC cells. Nano Lett. 2004;4:2479–2481. [Google Scholar]
- 65.Cho W-S, Cho M, Jeong J, Choi M, Cho H-Y, Han BS, Kim SH, Kim HO, Lim YT, Chung BH, Jeong J. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2009;236:16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 66.Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269:57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Estimation for humans based on the Nov 29, 2011 price of gold ($1713.40 USD per 1 troy oz = 31.1034768 g) obtained from http://www.marketwatch.com/investing/future/GOLD and the maximum full-couse 1.2 mg/m2 dosage administered in Libutti et al. (doi 10.1158/1078-0432.CCR-10-0978) for an average adult cancer patient (1.79a m2). Estimation for mice models based on the Nov 29, 2011 price of gold chloride trihydrate ($96.90 USD) obtained from http://www.sigmaaldrich.com, the systemic dosage of gold nanorods administerd in Dickerson. (100 L of OD800=120, doi 10.1016/j.canlet.2008.04.026), and a conservative synthetic yield of 5.078 L of OD800=1 per gram of gold salt. Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The Average Body Surface Area of Adult Cancer Patients in the UK: A Multicentre Retrospective Study. PLoS ONE. 2010;5:e8933. doi: 10.1371/journal.pone.0008933.
- 68.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 69.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.