Summary
Antibodies that neutralize diverse strains of HIV-1 develop in ~20% of HIV-1-infected individuals, and isolation and structural characterization of these antibodies is revealing how they recognize the envelope glycoprotein spike. Broadly reactive neutralizing antibodies utilize just a few sites of spike vulnerability and converge on select modes of recognition. These antibodies have unusual features: uncommonly long complementarity-determining loops, extensive somatic mutation, or both. Recent advances in deep sequencing of antibody-gene transcripts are providing genetic records of the development of neutralizing antibodies. These records inform an understanding of the naïve B cell repertoire, of somatic mutation, and of the resulting antibody features that are critical to effective HIV-1 neutralization; based on these, we propose an ontogeny and structure-based system of antibody classification. The human immune system is capable of developing antibodies that broadly neutralize HIV-1 – and an increasingly detailed view is accumulating for how effective immunity against HIV-1 can be generated.
Graphical abstract

Introduction
Infection by the human immunodeficiency virus type 1 (HIV-1) elicits a robust antibody response to both the surface unit (gp120) and the transmembrane unit (gp41) of the envelope glycoprotein (Env) as well as to conformationally dependent epitopes formed by the trimeric Env spike. However, these Env-directed responses consist predominantly of non-neutralizing or strain-specific antibodies (reviewed in Pantophlet and Burton, 2006; Mascola and Montefiori, 2010). The nature of this humoral immune response is partially explained by the structural definition of the HIV-1 envelope glycoprotein (Env) spike (Fig. 1), which reveals numerous mechanisms of humoral evasion including sequence-variable loops, extensive glycosylation, and conformational masking of vulnerable epitopes (Kwong et al., 2002; Starcich et al., 1986; Wyatt et al., 1998) reviewed in (Burton et al., 2005; Kong and Sattentau, 2012; Pantophlet and Burton, 2006; Verkoczy et al., 2011; Wyatt and Sodroski, 1998). These work in concert to inhibit the induction of neutralizing antibodies to conserved Env regions and to impede the recognition of the viral spike by otherwise potentially protective antibodies. Augmented by the overall genetic variability of the viral Env, these mechanisms also provide avenues for viral escape from the neutralizing antibody response. Indeed, longitudinal studies of HIV-1 infection show viral evolution to outstrip the adaptive capabilities of the antibody-mediated immune response (Albert et al., 1990; Gray et al., 2007; Pilgrim et al., 1997; Richman et al., 2003; Rong et al., 2009; Sagar et al., 2006; Wei et al., 2003).
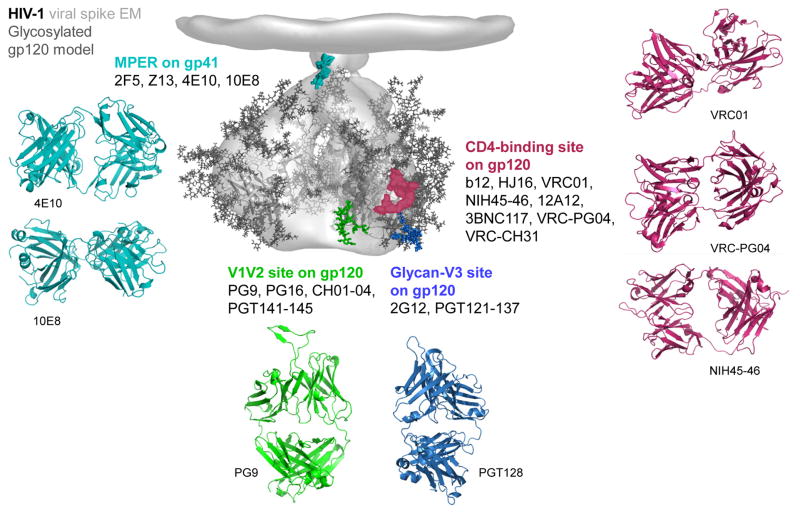
Figure 1. HIV-1 spike and its recognition by neutralizing antibodies.
The ~20 Å cryo-electron tomogram of the HIV-1 BaL isolate viral spike (Liu et al., 2008) is shown in grey, fitted with three copies of HIV-1 gp120 core in the CD4-bound conformation (Pancera et al., 2010a), with modeled glycans, and with modeled sites of Env vulnerability colored: red (CD4-binding site), green (glycan N160 in V1/V2), blue (glycan N332 at base of V3) and cyan (MPER of gp41). Effective mAbs are shown that recognize each site (see main text for fuller descriptions and references). A movie of the viral spike and of recognizing antibodies can be viewed.
This rather bleak view of the humoral immune response to HIV-1 dominated the first 20 or so years of HIV-1 research, punctuated by the isolation and characterization of a few – less than ideal – cross-reactive neutralizing monoclonal antibodies (mAbs) such as b12, 2F5, 4E10 and 2G12 (Burton et al., 1994; Muster et al., 1994; Stiegler et al., 2001; Trkola et al., 1996) as well as by occasional reports of broadly neutralizing sera elicited in select HIV-1-infected donors (Binley et al., 2004; Mascola et al., 1994; Pilgrim et al., 1997). The development of panels of diverse HIV-1 isolates and of highly reproducible neutralization assays – capable of accurately quantifying the breadth and potency of HIV-1 neutralization from sera and mAbs (Binley et al., 2004; Blish et al., 2007; Li et al., 2005; Mascola et al., 2005; Seaman et al., 2010; Simek et al., 2009)– allowed cohorts of sera to be evaluated for their ability to neutralize HIV-1. Starting in ~2004, several groups of investigators began to report the identification of sera that could neutralize genetically diverse strains of HIV-1 (Binley et al., 2008; Binley et al., 2004; Li et al., 2007; Piantadosi et al., 2009; Simek et al., 2009; Wu et al., 2006), with some sera neutralizing the majority of HIV-1 isolates tested (Binley et al., 2008; Doria-Rose et al., 2010; Li et al., 2007; Simek et al., 2009; Tomaras et al., 2011). Longitudinal studies demonstrated that cross-reactive neutralizing antibodies generally arose after 2 to 4 years of HIV-1 infection (Gray et al., 2011; Mikell et al., 2011; Moore et al., 2011) and analyses of such sera provided initial insights into the viral epitopes targeted by neutralization antibodies. A number of techniques, including affinity purification of serum antibodies and neutralization assays with epitope-specific mutant Env-pseudoviruses, were used to demonstrate that broadly reactive neutralizing sera contained antibodies to specific regions of the viral Env, including the CD4-binding site of gp120, glycan-containing regions on the surface of gp120, and the membrane-proximal external region of gp41 (MPER) (Binley et al., 2008; Gray et al., 2009a; Li et al., 2007; Li et al., 2009; Tomaras et al., 2011; Walker et al., 2010). Together, these studies provided proof-of-concept that the immune system can generate potent neutralizing antibodies against vulnerable regions of the HIV-1 Env.
Difficulty with defining the specific antibodies responsible for the observed serum neutralization delayed further insight into the manner by which the humoral immune system effectively neutralized HIV-1. In the absence of neutralizing mAbs that recapitulated serum neutralization, it was unclear if serum neutralization resulted from polyclonal mixtures of antibodies that provided breadth by their cumulative or synergistic activity, or if serum neutralization resulted from a more limited subset of antibodies targeting conserved neutralization epitopes (Binley et al., 2008; Gray et al., 2007; Li et al., 2007; Li et al., 2009; Rong et al., 2009; Sather et al., 2009; Scheid et al., 2009; Stamatatos et al., 2009; Tomaras et al., 2011; Walker et al., 2010).
Do individual antibodies exist that are capable of neutralizing diverse strains of HIV-1? The successful identification of the potent and broadly reactive mAbs PG9 and PG16 in 2009 provided a resounding “yes” to this question. These antibodies were directed against the variable loop regions 1 and 2 (V1V2) of the HIV-1 gp120 envelope glycoprotein and were able to neutralize 70–80% of viruses tested, often at concentrations less than 1 ug/ml (Walker et al., 2009). Over the ensuing years, dozens of additional broadly reactive neutralizing mAbs have been identified, and sequences for thousands more identified (Corti et al., 2010; Scheid et al., 2011; Walker et al., 2011; Wu et al., 2010; Wu et al., 2011b; Zhou et al., 2010). These HIV-1-neutralizing antibodies arise late in the course of HIV-1 infection and do not appear to provide substantial clinical benefit to the infected individuals in whom they occur; however, when present prior to viral exposure, neutralizing antibodies are able to prevent HIV-1 infection in non-human primate models of infection (reviewed in Pantophlet and Burton, 2006; Mascola and Montefiori, 2010). Thus, the generation of neutralizing antibodies in humans by active or passive immunization may protect against HIV-1 infection. Here we review the identification of broadly neutralizing antibodies, the epitopes on the HIV-1 spike that they target, the molecular tricks they use to achieve their remarkable breadth, and the B cell ontogenies of their generation and maturation.
Identification of human antibodies capable of broadly neutralizing HIV-1
In the early 1990s, four human antibodies were identified that neutralized diverse primary strains of HIV-1 (reviewed in (Burton et al., 2005; Pantophlet and Burton, 2006). One of these, mAb b12 was isolated from a phage-display library by Barbas, Burton, and colleagues (Burton et al., 1994), and three others (2G12, 2F5, and 4E10) were isolated by Trokla, Katinger, and colleagues (Muster et al., 1994; Stiegler et al., 2001; Trkola et al., 1996). While providing glimpses of the potential of the human immune system to effectively neutralize HIV-1, each of these four mAbs was less than ideal. The gp41 mAbs 2F5 and 4E10 demonstrated auto-reactive properties (Alam et al., 2007; Haynes et al., 2005), mAb 2G12 bound to glycans on the surface of the viral spike using an unusual recognition involving heavy- and light-chain swapping of domains (Calarese et al., 2003), and mAb b12 recognized the CD4-binding site of gp120 via a heavy chain-only interaction (Zhou et al., 2007). In addition, mAbs b12, 2G12, and 2F5 displayed less than 50% neutralization breadth (Binley et al., 2004; Walker et al., 2009; Wu et al., 2010) and mAb 4E10 displayed modest potency (Binley et al., 2004; Zwick et al., 2001).
Although a number of groups expended substantial effort to identify additional broadly neutralizing human antibodies, several factors complicated the discovery of such mAbs. These included the small fraction of B cells that secrete neutralizing antibodies (< 1% of HIV-1-specific B cells), the inefficiency of traditional EBV-transformation methods to create B cell lines, the inability to identify and isolate B cells making neutralizing antibodies, and the lack of high throughput methods to culture and screen large numbers of B cells for antibody secretion. Several methodological advances, however, created a framework for the eventual isolation of numerous neutralizing HIV-1 mAbs. Wardermann, Nussenzweig and colleagues described the PCR amplification of heavy and light chain-antibody regions from single B cells, which allowed recovery and expression of antigen-specific mAbs (Tiller et al., 2008 Wardemann et al., 2003). Poignard and Burton, working with colleagues at Theraclone, used a high-throughput microculture method to stimulate small numbers of B cells (2–6 per well) to secrete IgG suitable for direct neutralization screening. Antibodies from positive wells could then be recovered by PCR amplification of heavy and light chains as noted above, and this led to the identification of the potent neutralizing mAbs PG9 and PG16. Notably, these two mAbs bound poorly to recombinant gp120, and thus would not have been identified without the direct assessment of B cell supernatants for HIV-1 neutralization (Walker et al., 2009).
The initial isolation of broadly reactive mAbs PG9 and PG16 was rapidly followed by additional successes. In early 2010, Weiss, Lanzavecchia, and colleagues used improved EBV immortalization and culture methodologies to identify memory B cells making HIV-1-neutralizing mAbs (Corti et al., 2010). Among the 58 HIV-1-specific mAbs identified, the most broadly reactive was mAb HJ16 which was directed to the CD4-binding site of gp120 and could neutralize about 40% of HIV-1 strains. Also in 2010, Mascola, Nabel and colleagues reported the use of an engineered protein probe to identify and sort epitope-specific B cells (Wu et al., 2010). Starting with the atomic-level structure of core gp120 (Kwong et al., 1998) and Rosetta-computational tools (Kuhlman et al., 2003), they designed the glycoprotein probe to be stabilized in the CD4-bound conformation (Zhou et al., 2007) and to have all surfaces altered, except for the outer domain-contact site for the CD4 receptor (Wu et al., 2010). This structure-based probe, termed resurfaced stabilized core 3 (RSC3), was used to fish out select memory B cells (~1 per 1000 memory B cells) that bound tightly to the probe and not to a control probe with a mutation in the CD4-binding site. Single-cell PCR amplification of heavy and light chain sequences led to the recovery of two distinct broadly neutralizing mAbs: VRC01 (~90% breadth) and VRC03 (~50% breadth).
Early 2011 brought additional successes. Bonsignori, Haynes and colleagues used a B cell culture and neutralization-screening approach to isolate four clonally related V1V2-directed mAbs (CH01-04) that could neutralize up to 50% of HIV-1 strains (Bonsignori et al., 2011). Burton, Poignard and colleagues again used direct neutralization screening of B cell supernatants, but this time choosing to analyze the four most potent sera from a cohort (Protocol G) of 1800 HIV-1-infected donors (Simek et al., 2009). From each donor, a set of clonally related glycan-dependent neutralizing antibodies was recovered (PGT121-123, PGT125-131, PGT135-137, and PGT141-145) (Walker et al., 2011). Scheid, Nussenzweig and colleagues reported the development of new primers that more efficiently amplified highly affinity-matured gene products, and used gp140 (Scheid et al., 2009) and a modified gp120 core (Dey et al., 2009; Zhou et al., 2007) as probes to fish out several hundred unique antibodies from four HIV-1-infected donors. A number of these antibodies were both potent and broadly neutralizing (~90% breadth) including NIH45-56, 3BNC117, and 12A12 (Scheid et al., 2011). Kwong, Mascola and colleagues used the RSC3-bait strategy on two additional donors to identify several potent neutralizing mAbs including VRC-PG04 and VRC-CH31, each with ~80% breadth (Wu et al., 2011b). In addition to the recovery of mAbs from antigen-specific B cells, deep sequencing of expressed heavy and light chain B cell transcripts (the “antibodyome”) was carried out on two donors, and this identified several thousand sequences of clonal variants and related lineages.
Reports of additional broadly neutralizing HIV-1 antibodies continue to roll in. New antibodies to the CD4-binding site (VRC13 and VRC16) (Wu et al., 2012), and to the MPER of gp41 (10E8) (Huang et al., 2012) have been recently reported. mAb 10E8 is of particular interest because it recapitulates the extraordinary breadth of mAb 4E10, but is 5- to 10-fold more potent and lacks detectable reactivity with self antigens. Virtually all of these new antibodies are being identified by the two strategies described above: direct neutralization screening of B cell supernatants or probe-based B cell isolation, both of which recover heavy and light chain sequences from individual B cells. Deep sequencing of antibody-gene transcripts – which determines many thousands of sequences of the antibodyome and then attempt to identify sequences of functional importance – offers a different strategy. Starting with the sequence of a known neutralizing antibody, sequences of clonal relatives and of related antibody lineages can be identified using parameters such as sequence homology, germline divergence, phylogenetic relationships, or additional bioinformatics signatures (Wu et al., 2011b; Zhu et al., 2012). This is reviewed in more detail below. It seems likely that the current approaches of genetic recovery of antibodies from sorted or cultured B cells and deep sequencing of antibodyomes, or combinations of the two, will continue to yield additional broadly neutralizing antibodies against HIV-1.
Four HIV-1 sites-of-vulnerability to neutralizing antibody
The HIV-1-viral spike has a number of regions common to diverse isolates where antibodies might bind and effectively neutralize. These include the sites on gp120 involved in receptor binding (for the primary receptor CD4 or for the co-receptors, CCR5 or CXCR4), exposed variable regions such as V1V2 and variable region 3 (V3), and sites on gp41 such as its conserved helices and MPER (Starcich et al., 1986; Wyatt and Sodroski, 1998). Whereas these sites are highly accessible on some laboratory-adapted strains of HIV-1 (Mascola et al., 1996; Wrin et al., 1995), antibody access to neutralization epitopes on primary virus isolates appears to be limited by the extensive glycosylation and overall structural configuration of the trimeric viral spike (Chen et al., 2009; Decker et al., 2005; Kwong et al., 2002) and reviewed in (Burton et al., 2005; Kong and Sattentau, 2012; Pantophlet and Burton, 2006).
The epitopes of the most broadly reactive mAbs –those antibodies capable of neutralizing over 80% of HIV-1 isolates– offer a means to identify sites of broad vulnerability. Overall only four such sites have been identified (Fig. 1), three on gp120 and one on gp41. In addition to recognition by at least one effective antibody, these sites are also targeted by a number of antibodies with less breadth, with epitopes that overlap the site of broad vulnerability.
The CD4-binding site on gp120 is recognized by a number of broadly neutralizing mAbs. These include VRC01, NIH45-46, 12A12, 3BNC117, VRC-PG04 and VRC-CH31, which neutralize 80% to 90% of viruses (Fig 1) (Scheid et al., 2011; Wu et al., 2010; Wu et al., 2011b; Zhou et al., 2010). Numerous additional antibodies have been isolated that neutralize with somewhat less potency and breadth including: b12, HJ16, VRC03, 1B2530 and 8ANC131 among others (Burton et al., 1994; Corti et al., 2010; Scheid et al., 2009; Scheid et al., 2011; Wu et al., 2010). The CD4-binding site of the HIV-1 Env is functionally conserved (Kwong et al., 1998; Wyatt and Sodroski, 1998), though the precise extent of its vulnerability to antibody recognition is still under investigation (Falkowska et al., 2012; Wu et al., 2009). Several liganded-crystal structures reveal that VRC01 and related mAbs partially mimic the CD4 interaction with gp120 and focus recognition on the site of initial gp120 attachment to CD4 (Diskin et al., 2011; Wu et al., 2011b; Zhou et al., 2010). Recognition of the CD4 receptor by the HIV-1 Env involves considerable conformational changes in Env (Kwong et al., 1998; Myszka et al., 2000; Pancera et al., 2010a), and – in the absence of a structure of the entire viral spike, which has not yet been determined at atomic-level resolution– the extent that the CD4-binding site is assembled on primary isolates prior to encounter with cell-surface CD4 is unclear. Interestingly, several of the most potent CD4-binding site mAbs have been shown to induce a conformational change similar to that induced by soluble CD4 upon binding to recombinant gp120. However, unlike sCD4 these mAbs do not appear to induce this change on the native viral spike (Falkowska et al., 2012; Scheid et al., 2011; Wu et al., 2010; Wu et al., 2011b). The outer domain region of gp120 that first contacts CD4 has been proposed as a “site-of-vulnerability” by Kwong and colleagues (Chen et al., 2009; Zhou et al., 2007), and recognition of this site, which accounts for about 60% of the entire contact of CD4, correlates well with neutralization breadth (Zhou et al., 2010). Bjorkman and colleagues, however, found the potency of mAb NIH45-46 to be enhanced with a Gly54Trp mutation, which makes contact with the gp120-bridging sheet region in a manner analogous to a critical phenylalanine of CD4 (Diskin et al., 2011). In addition, Weiss and colleagues isolated a near pan-reactive llama antibody named J3 that also appears to recognize regions outside the outer domain contact of CD4 (McCoy et al., 2012). Lastly, CD4 contacts bridging sheet and other regions of gp120, not just the outer domain, and shows substantial breadth (Kwong et al., 1998; Wu et al., 2010). Thus, a precise definition of the boundary of this site-of-vulnerability may require an understanding of the ability of antibody to induce spike-conformational change, not only a definition of Env-surface contacted.
A V1V2 site forms a second site of spike vulnerability to neutralizing antibody. Broadly reactive neutralizing V1V2-directed mAbs include PG9, PG16 and PGT145, and those with somewhat less potency or breadth: CH01-04 and PGT141-144. Initial identification of a neutralization site involving residue 160 in the V1V2 region of gp120 involved an extraordinary potent antibody (mAb 2909), which recognized rare HIV-1 isolates with a lysine at residue 160 (Gorny et al., 2005; Wu et al., 2011a). The 2909 antibody appeared to be exclusively quaternary structure-specific; i.e. it only recognized the assembled viral spike, not individual subunits. The more broadly neutralizing V1V2-directed antibodies also share a preference for the assembled viral spike, though some of them, such as PG9, do recognize select gp120s in the monomeric context. Interestingly, these antibodies display some dependence on the V3 regions of the assembled viral spike (Walker et al., 2009), but in the monomeric gp120 context this dependence disappears, and recognition occurs with specific V1V2 glycans in addition to V1V2-peptide contacts. Structural determination of antibody PG9 with a scaffolded-V1V2 revealed a site of recognition involving two glycans and a strand (McLellan et al., 2011) (Fig. 1). A Man5 glycan appeared to be required at residue N160. Analysis of resistant isolates indicates that the recognized strand (“strand C” of V1V2), is a primary determinant of resistance to all broadly neutralizing antibodies that bind V1V2 (Doria-Rose et al., 2012a). Overall, this region appears to be susceptible to considerable conformational change. V1V2-deleted viruses are generally functionally competent for entry, but not for immune evasion, and it thus appears that the conservation of a glycan at residue N160 is related to immune evasion.
A conserved glycan-V3 site on gp120 forms a third site of spike vulnerability. Antibodies to this conserved glycan cluster, which is located on the outer domain of gp120 proximal to the V3 region (Fig. 1), include effective neutralizers PGT121 and PGT128, as well as those with somewhat less potency or breadth: 2G12, PGT122-123, PGT125-127, PGT129-131, and PGT135-137. Initial discovery of this glycan site-of-vulnerability involved inferences from mutational analysis, modeling, and a number of structures of mAb 2G12 in complex with various N-linked glycan (Calarese et al., 2003; Sanders et al., 2002; Scanlan et al., 2002). However the 2G12 mAb has limited potency and breadth of reactivity and contains an unusual domain-swapped structure (Calarese et al., 2003). The finding that three of the most potent neutralizing serum from protocol G contained glycan N332-dependent antibodies was unexpected and raised interest in this region. The structure of mAb PGT128 in complex with an outer domain region of gp120 showed recognition of two high mannose sugars (attached to residues N301 and N332) as well as a few residues of the V3 (Pejchal et al., 2011). Mutational analysis as well as heavy- and light-chain complementation indicate that these glycan-V3-directed antibodies have diverse modes of recognition, some recognizing a bit of V3 protein as well as N-linked glycans attached to residues N301 and N332 (e.g. PGT128) and others recognizing only glycans (e.g. 2G12, which appears to recognize the mannose branches of N-linked glycans at N295, N332 and N395) (Calarese et al., 2003; Doores et al., 2010; Walker et al., 2011). So while all of the glycan-V3-directed broadly neutralizing antibodies appear to recognize glycans N301 or N332, antibodies from different donors appear to recognize specific features of the surrounding surface in diverse ways. Thus mAbs PGT128, PGT135 and 2G12 all recognize distinct epitopes; it’s just that there is some overlap in the involvement of glycan at N332. Like the V1V2 site, the conservation of an N-linked glycan at N332 appears to be related to immune evasion.
The MPER on gp41 forms a fourth site of spike vulnerability to neutralizing antibody. mAb 4E10 and the recently described more potent 10E8 are the two most broadly reactive antibodies to the MPER of gp41. Other neutralizing mAbs with somewhat less breadth include 2F5, Z13e1, m66, and CAP206-CH12 (Morris et al., 2011; Muster et al., 1994; Zhu et al., 2011; Zwick et al., 2001). Of all the sites recognized by effective neutralizers, the MPER is the only one on gp41 (Fig. 1). Like the CD4-binding site, the conservation of the MPER appears to be related to the role of gp41 in viral entry (Buzon et al., 2010; Weissenhorn et al., 1997). Five tryptophans and other highly conserved hydrophobic residues are contained in the MPER, and substitution of these with polar or charged residues leads to severe impairment of viral entry (Salzwedel and Berger, 2000). Structural definition of antibodies 4E10 or 10E8 with MPER (Cardoso et al., 2005; Huang et al., 2012) reveals the MPER to fold into an α-helix (residues 672-683), capped on one end by an Asn, Thr, or Ser at residue 671 and terminated on the other end by the transmembrane-spanning region, which is predicted to start at residue 684. The structure of a late fusion intermediate (Buzon et al., 2010) indicates that the residues recognized by 4E10 and 10E8 reside on the outside of the post-fusion coiled-coil (Chan et al., 1997; Weissenhorn et al., 1997). Of note, the 2F5 and Z13e1 antibodies recognize non-helical conformations of the MPER (Ofek et al., 2004; Pejchal et al., 2009), substantially different from that of the post-fusion structure. Overall the MPER is thought to undergo significant conformational changes during entry, and may –in pre- or intermediate-conformations– interact directly with the viral membrane (Kim et al., 2011; Ofek et al., 2004; Song et al., 2009; Sun et al., 2008).
Convergence in recognition by effective mAb neutralizers
Recognition of complex antigens by the humoral immune system is generally accomplished by a diverse multiplicity of recognition modes and of recognized surfaces. For example, structural characterization of HIV-1 V3-directed antibodies shows diverse recognition (Bell et al., 2008; Stanfield et al., 2006). By contrast, potent antibodies that target the CD4-binding site, isolated from half a dozen different donors, all utilize the same mode of heavy-chain mimicry of the CD4 interaction with gp120, and all recognize the same outer domain contact site of CD4 (Diskin et al., 2011; Wu et al., 2011b; Zhou et al., 2010) (Fig. 1). Structures of antibodies that target the CD4-binding site, but display lower breadth such as antibodies b12, F105, and b13 (approximately 40%, 10%, and 10% breadth, respectively), show different modes of recognition and contact different regions of the CD4-binding site (Chen et al., 2009; Zhou et al., 2007). The likely explanation – that only a very few modes of antibody recognition allow for broad recognition of the CD4-binding site – indicate that while the human immune system can recognize this site, it is pushed to extraordinary limits to do so.
Antibodies 4E10 and 10E8, which both show remarkable near pan-neutralization, converge in their recognition of very similar gp41 residues (Cardoso et al., 2005; Huang et al., 2012; Ofek et al., 2004) (Fig. 1). Their modes of antibody recognition, however, differ considerably, as do the genetic origins of their heavy and light chains. Unlike 4E10 (Haynes et al., 2005), the 10E8 mAb achieves broad recognition without the property of reactivity with self antigens. It is currently unclear if the different properties of 4E10 and 10E8 relative to lipid binding are related to the different modes of recognition, or represent different degrees of the intrinsic properties that are coupled to MPER recognition. Nonetheless, the diversity antibody recognition at the MPER suggests that the human immune system can follow a number of different pathways to generate near pan-neutralization at the MPER.
Antibodies PG9 and PGT128, meanwhile, recognize N-linked glycans on different regions of the HIV-1 Env, but converge in mode of recognition (McLellan et al., 2011; Pejchal et al., 2011) (Fig. 1). All of the V1V2-directed broadly neutralizing antibodies identified to date appear to have extended anionic heavy chain 3rd-complementarity-determining regions (CDR H3s) (McLellan et al., 2011; Pancera et al., 2010b; Pejchal et al., 2010; Walker et al., 2011; Walker et al., 2009), though differences in CDR H3 length, tyrosine sulfation, and a general lack of heavy- and light-chain complementation (Bonsignori et al., 2011; McLellan et al., 2011; Walker et al., 2011; Walker et al., 2009) indicate that there may be substantial differences in their exact mode of recognition. Meanwhile, the glycan-V3-directed broadly neutralizing antibodies also show diverse V-gene origins, CDR H3s, and differential response to substitutions of residues around N332 (Pejchal et al., 2011; Walker et al., 2011), suggesting a diversity of ways by which the human immune can recognize N-linked glycan. Nonetheless, the convergence in mode of recognition between PG9 and PGT128 –both utilizing an extended CDR H3 to reach a bit of protein surface, while heavy and light chain CDRs each recognize an N-linked glycan– suggest a limited number of modes of glycan recognition, which are compatible with very broad reactivity. It will be interesting to see if other glycan-dependent effective neutralizers also use this mode of recognition: Does the highly effective PGT121, for example, like PG9 and PGT128, also utilize two glycans and a bit of Env-protein surface to affect its broad recognition? If so, then a single mechanism of recognition would be employed by all of the highly effective glycan-reactive HIV-1 neutralizers.
Broadly reactive neutralizing antibodies: unusual features
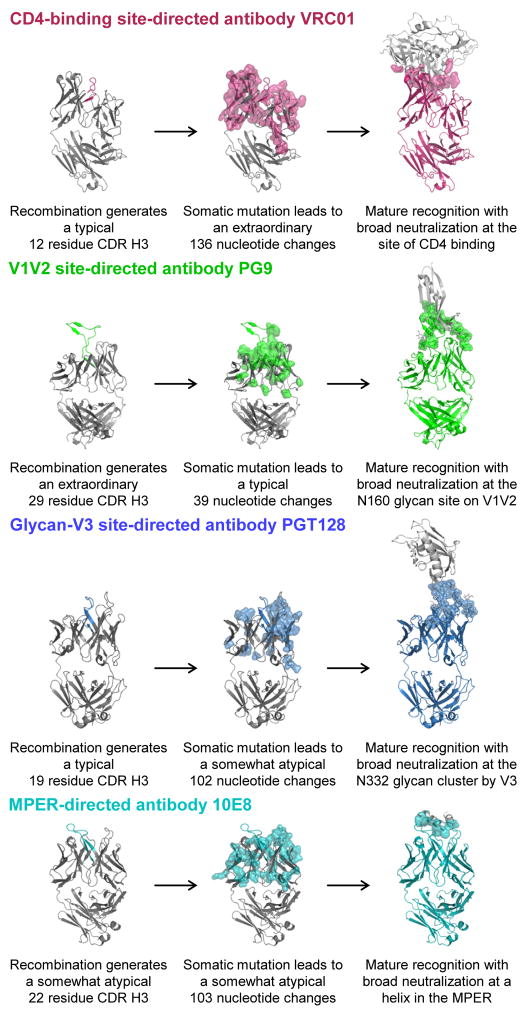
All of the effective neutralizers against HIV-1 characterized to date have one or more unusual features. Although the humoral response during the course of HIV-1 infection consists of many highly affinity-matured antibodies (Breden et al., 2011; Scheid et al., 2009), the CD4-binding site-directed antibodies display extraordinary maturation, reaching nucleotide somatic mutation frequencies of 32% and 20% in heavy and kappa chain V genes (VH and VK), respectively (represented by antibody VRC01 in Fig. 2). This contrasts to the mutation rate of influenza antibodies during a secondary immune response, which rarely exceed 10% (Moody et al., 2011; Wrammert et al., 2008). These maturation changes appear necessary to evolve from the weak gp120 recognition of unmutated germline ancestors to the high affinity gp120 recognition of the mature neutralizing antibodies (Xiao et al., 2009; Zhou et al., 2010). In addition, the heavy chains of the most broadly reactive CD4-binding site mAbs originate from a restricted set of VH genes (VH1-2 or VH1-46) (Scheid et al., 2011; Wu et al., 2010; Wu et al., 2011b), likely due to the crucial nature of contact sites encoded by these genes (West et al., 2012; Wu et al., 2011b; Zhou et al., 2010). The V1V2-directed effective neutralizers show extraordinarily long CDR H3s of 24-32 residues, by Kabat-sequence definition of CDR (Kabat et al., 1991), which appear to be required to penetrate the glycan shield at the V1V2 site of vulnerability (McLellan et al., 2011; Pejchal et al., 2010) (representative PG9 antibody shown in Fig. 2). Their more moderate affinity maturation frequencies (11–18% for VH and 9–16% for VK or VL) and varied heavy and light chain origin genes (Bonsignori et al., 2011; Walker et al., 2011; Walker et al., 2009) indicate that once an appropriate CDR H3s is formed, recognition is compatible with a number of heavy and light chain pairs. The glycan-V3-directed effective neutralizers have CDR H3s of more moderate length (18–24 residues), but display a moderate to high degree of affinity maturation (15–23% for VH and 9–24% for VK or VL), often involving insertions or deletions (Walker et al., 2011) (represented by the PGT128 antibody in Fig. 2). The higher degree of affinity maturation may be required to generate the tighter glycan binding observed with these antibodies relative to the V1V2-directed ones. Finally, the MPER-directed effective neutralizers show moderately long CDR H3s (17-22 residues) and moderate to high degrees of affinity maturation (13–21% for VH and 6–14% for VK or VL) (represented in Fig. 2 by the 10E8 antibody). The extensive CDR H3 recognition provides a rationale for the longer CDR H3s, and affinity maturation in this case may be needed to enhance CDR H3 recognition, perhaps through addition of hydrophobic amino acids with affinity to membrane (Ofek et al., 2010). Elicitation of effective neutralizers is likely to require an understanding of how the immune system generates unusual features such as extensive affinity maturation and extended CDR H3 loops.
Figure 2. B cell ontogenies of broadly neutralizing antibodies.
Effective neutralizing human antibodies have unusual features, a result of unusual recombination, somatic mutation, or both. CD4-binding site antibodies, represented here by VRC01. A number of effective neutralizers use mimicry of CD4 by their heavy chain to affect near-pan-neutralization. All of these derive from similar VH genes, VH1-2 or VH1-46, but otherwise display a variety of CDR H3 lengths and Vκ or Vλ partners. Deep sequencing and systems-level bioinformatics allow specific CDR H3 lineages to be traced to identify thousands of clonal variants. For VRC01, only the more highly affinity matured antibodies are capable of neutralizing HIV-1. V1V2-directed antibodies, represented here by PG9. All V1V2-directed broadly neutralizing antibodies identified to date display unusually long CDR H3s. These are likely required to penetrate the glycan shield to reach conserved features of Env protein surface. For PG9, recombination that generates a 29-amino acid CDR H3 appears to occur as an early event, with somatic mutation fine-tuning glycan recognition. Glycan-V3-directed antibodies, represented here by PGT128. Antibodies directed towards this site are highly diverse. Some, such as PGT128 have CDR H3s that are not so unusual, but display high degrees of somatic mutation. MPER-directed antibodies, represented here by 10E8. These gp41-directed neutralizers are quite diverse, with some such as 2F5, Z13 and 4E10 requiring significant degrees of β-strand interactions, while 10E8 interacts entirely through its CDR loops. Despite this diversity, all show moderately long CDR H3s and unusual degrees of somatic mutation.
Genetic records and B cell ontogenies
In principle, the memory B cell compartment retains a genetic record of the antibody response, including both its generation and maturation (Glanville et al., 2009; Lerner et al., 1991; Tian et al., 2008). This record can be inferred, or even partially reconstructed, by the isolation of antigen-specific B cells from various clonal lineages and by studying variants of a common clonal lineage (Bonsignori et al., 2011; Haynes et al., 2012; Kashyap et al., 2008; Moody et al., 2011; Scheid et al., 2009; Wrammert et al., 2008). In the case of the HIV-1 broadly neutralizing antibodies, immense interest has focused on understanding their unmutated ancestors and maturation history, as each effective neutralizer provides a potential template for vaccination strategies to elicit a similar antibody response (Burton et al., 2005; Haynes et al., 2012; Kong and Sattentau, 2012; Moir et al., 2011; Verkoczy et al., 2011). This is viewed as particularly important for HIV-1, because current vaccine immunogens do not induce potent and broadly reactive neutralizing antibodies (reviewed in (Mascola and Montefiori, 2010; Pantophlet and Burton, 2006)). Deep sequencing of B cell transcripts has the potential to permit an in depth analysis of large sets of related antibody sequences, thus addressing questions about initial V(D)J recombination, CDR H3 length, and affinity maturation (see for example, (Briney et al., 2012b; Wu et al. 2011b)). Despite this interest, the technical challenges required to read the genetic record of B cell development are substantial, and– while under investigation by a number of groups – published B cell ontogenies are in most cases highly incomplete.
Little is currently known about the exact B cell-maturation pathways for most effective HIV-1-neutralizing antibodies. The two most broadly reactive MPER-directed mAbs 4E10 and 10E8 have moderately long CDR H3s (18 and 20 residues, respectively) and a high degree of somatic mutations (13% and 21% for VH, 6% and 14% for VK/VL, respectively) (Haynes et al., 2012; Huang et al., 2012; Zwick et al., 2001) (Fig. 2)). In terms of clonal variants, 4E10 was isolated as a single clone (Stiegler et al., 2001; Zwick et al., 2001), and 10E8 was isolated along with a single closely related relative (Huang et al., 2012), so we are unable to infer the exact B cell-maturation pathway of these antibodies. The most effective glycan-V3-directed neutralizing antibodies PGT121 and PGT128 have moderately long CDR H3s (24 and 19 residues, respectively) and a high degree of somatic mutations (17% and 19% for VH, 18% and 9% for VL, respectively) (Walker et al., 2011) (Fig. 2). A handful of mature clonal variants have been isolated, two for PGT121 (PGT122 and PGT123) and five for PGT128 (PGT125-131). Even within a clonal B cell family, these antibodies show substantial divergence in somatic mutations and in glycan-binding specificity. Thus, the lack of early variants from these two clonal B cell families make it difficult to infer the unmutated ancestor (and CDR H3) or the evolutionary pathway of affinity maturation. The same is true for the most effective V1V2-directed antibodies, PG9 and PGT145, which show uncommonly long CDR H3s (28 and 31 residues, respectively) and a moderate to high degree of somatic mutations (13% and 18% for VH, 6% and 16% for VL/VK, respectively) (Walker et al., 2011; Walker et al., 2009) (Fig. 2). In terms of clonal variants, again only a limited number of mature variants have been obtained, one for PG9 (the PG16 antibody) and four for PGT145 (PGT141-144). Heavy and light chain complementation, swapping of CDR H3s, and V-gene reversion of PG9 and PG16 (Pancera et al., 2010b) as well as deep sequencing of naïve templates (Briney et al., 2012a) suggest an initial rare recombination to produce a long CDR H3, with weak neutralization. The process of B cell-somatic mutation then greatly enhances recognition, increasing both breadth and potency of neutralization. Though informative, the extremely limited sampling of the B cell lineage hinders insight into the initial recombination event that appears to be the primary barrier to generating these antibodies.
Among the most effective CD4-binding site-directed antibodies, however, there are larger data sets available by which to infer B cell lineages. Unlike the V1V2- or V3-glcan-directed mAbs, these mAbs have common CDR H3 lengths (ranging from 10–16 residues). They do however have unusually high degrees of somatic mutation (often exceeding 25% in VH and 15% in VK) (Scheid et al., 2011; Wu et al., 2010; Wu et al., 2011b) (Fig. 2). With these antibodies, substantially greater sampling of clonal variants has occurred. For example, Scheid, Nussenzweig and colleagues used probe-based B cell sorting to identify numerous clonal relatives of the broadly reactive mAbs 3BNC117 and 12A12 (Scheid et al., 2011). To augment the information attained by isolation of tens or even hundreds of isolated B cells, deep sequencing technologies can generate hundreds of thousands of sequence reads across the full spectrum of antibody-gene families (Boyd et al., 2010; Prabakaran et al., 2012; Rothberg and Leamon, 2008; Tian et al., 2008; Wu et al., 2011b). Currently, 454 pyrosequencing is the only technology with the ability to obtain reads of over 400 bases, thus producing sequences containing the full antibody V(D)J variable regions (Rothberg and Leamon, 2008). By using the 454 platform to sequence B cell antibody transcripts, Kwong, Mascola and colleagues analyzed the antibody repertoire of the donor from whom the CD4-binding site mAb VRC-PG04 was isolated. Among ~120,000 VH1 family sequences, they identified several thousand sequences that were members of the VRC-PG04 clonal family. These sequences contained a remarkable record of the antibody-B cell lineage, ranging from VH sequences with a single somatic mutation to fully mature sequences with over 100 nucleotide somatic mutations (Wu et al., 2011b). Importantly, the less mutated sequences allowed a clear definition of the heavy chain unmutated ancestor of VRC-PG04, and this may be valuable in assessing vaccine immunogens (Haynes et al., 2012; Pancera et al., 2010b; Xiao et al., 2009). The wealth of clonal variants that can be recovered by current antibody isolation technologies, coupled with detailed structural analysis and to antibodyomics analysis of B cell lineages and developmental intermediates, provides substantial new insights into the B cell ontogeny of effective CD4-binding site-directed neutralizers, such as VRC01.
As noted above, the heavy chain of VRC01 and other CD4-binding site antibodies that mimic CD4 derive from the VH1-2 or the closely related VH1-46 gene. Based on single nucleotide polymorphism analysis that distinguishes VH1-2 alleles, an estimated 95% of humans appear to have the required VH1-2 alleles to encode VRC01-like antibodies (West et al., 2012), though the full IgG-locus sequencing needed to determine the frequency of human IgG-heavy and -light chain gene alleles is not yet complete. Interestingly, diverse JH-genes and CDR H3 sequences appear to be compatible with the heavy chain sequences that make up the set of VH1-2 neutralizing antibodies (Scheid et al., 2011; West et al., 2012; Wu et al., 2011b; Zhou et al., 2010). For VRC01-like light chains, diverse VK genes appear to be compatible, though there is a requirement for a 5 amino acid CDR L3 (Scheid et al., 2011; West et al., 2012; Wu et al., 2011b; Zhou et al., 2010). Although only ~1% of human CDR L3s have only 5 amino acids (West et al., 2012), substantial numbers of recombinants are likely produced in the estimated billion new B cells that adult humans generate each day. A far more serious barrier to the elicitation of VRC01-like antibodies appears to the poor affinity between the unmutated ancestor of VRC01 and typical variants of HIV-1 gp120 (Wu et al., 2011b; Zhou et al., 2010). The extraordinary degree of somatic mutation required to develop naïve B cell recombinants into effective neutralizers also appears to be a serious impediment (Schneid et al., 2011; Wu et al., 2011b).
Development of broadly neutralizing HIV-1 immunity
B cell ontogenies inferred from analysis of mAbs and from deep sequencing provide substantial insight into the immune processes by which unusual features of broadly neutralizing HIV-1 antibodies are generated. These features, uncommonly long CDR H3s or extraordinary degrees of affinity maturation, appear to follow different paths, which are specific to the antibody specificity and its mode of recognition (Fig. 2). Whereas ~20% of HIV-1-infected individuals generate broadly neutralizing immunity, analysis of the most potent sera indicates that serum neutralization is often conferred by only one or two dominant antibody specificities (Binley et al., 2008; Bonsignori et al., 2012; Tomaras et al., 2011; Walker et al., 2010). It should be noted, however, that the humoral immune system generates populations of B cells expressing diverse antibodies, not just a single mAb. Even highly related antibodies can have substantial differences in HIV-1 recognition – as observed explicitly through deep sequencing, bioinformatics analysis, and functional characterization of the PGT135-137-related antibodies (Zhu et al., 2012) Thus, the vaunted diversity of HIV-1 is opposed by populations of B cells expressing diverse antibodies; and whereas individual mAbs can show substantial breadths of neutralization, the polyclonal serum response is often substantially more effective than any single antibody at neutralizing HIV-1.
Ontogeny and structure-based classification
The B cell ontogenies and structures of broadly neutralizing HIV-1 antibodies and their recognized epitopes reviewed here suggest a basis for classification (Table 1). In such a scheme, structural definition of mode and of recognized site on HIV-1 Env would provide broad organization, while B cell ontogeny would provide details and relative placement, utilizing nomenclature developed by B cell immunologists. Thus, mAbs of a particular B cell-clonal lineage would form a clonal “family”, and families with specified genetic similarities (e.g. related B cell ontogenies such as VRC01-like antibodies from different donors) would define an antibody “class”. Structural modes of recognition, meanwhile, can be thought of as a “type” of broadly neutralizing antibody, with the four HIV-1-sites of Env vulnerability defining antibody “categories”, each of which consists of a cluster of antibody epitopes. A nice feature of such an ontogeny and structure-based classification is that family and class can be named after the first described monoclonal member (or if several mAbs are described at the same time, then the most potent of the initially described antibodies). As the first described member of a monoclonal family is often a touchstone for researchers in the field, this would reduce new nomenclature and retain an association with the original identification. Thus, for mAbs PG9 and PG16, such a naming convention would place PG9 and PG16 in the “PG9-family”, as PG9 and PG16 are clonal relatives, PG9 is more effective than PG16, and both were described at the same time. If a new family of antibodies with a similar B cell ontogeny to PG9 were discovered, these new antibodies would form their own family, but be part of the “PG9-class” of antibodies. The PG9-class, meanwhile, would have “penetrating CDR H3” or “two glycan and a strand”-type of recognition, and fall into the general “V1V2 site on gp120”-category of broadly neutralizing antibodies (Table 1). Similarly, for mAbs VRC01, 3BNC117 and 12A12, each of these antibodies are the most potent members of a clonal B cell family. These three antibodies arose in independent donors, but derive from the same genomic VH-gene (VH1-2) and share specific maturation features, and thus all three would fall into the “VRC01-class”. Their “CD4-mimicry by heavy chain”-type of recognition defines this class of antibodies within the general “CD4-binding site on gp120”-category. Meanwhile, the “8ANC131-class” of antibodies also recognizes the CD4-binding site via heavy chain mimicry of CD4, but derives from the VH1-46 gene rather than VH1-2. We are cognizant that others have described types of antibodies using properties such as binding and neutralization, for example, highly agonistic CD4-binding-site antibodies (HAADs) by Nussenzweig and colleagues (Scheid et al., 2011) and potent VRC01-like antibodies (PVL) by Bjorkman and colleagues (West et al., 2012), and such naming could be used within the classification scheme that we present. We note, however, that the classification scheme proposed here, based on structural and genetic properties, naturally emphasizes similarities in recognition and development, while preserving a connection to the original identification.
Table 1.
Ontogeny and structure-based classification
| Category Epitope cluster on viral spike used by types |
Type Structural mode of recognition used by each class |
Class Families with related ontogenies in separate donors |
Family Clonal lineage |
Monoclonal Antibody |
|---|---|---|---|---|
| CD4-binding site on gp120 | Heavy chain-only | b12-class | b12-family | b12 |
| Not yet determined | HJ16-class | HJ16-family | HJ16 | |
| CD4-mimicry by heavy chain | VRC01-class | VRC01-family | VRC01, VRC02, NIH45-46 | |
| 3BNC117-family | 3BNC60, 3BNC62, 3BNC117 | |||
| 12A12-family | 12A12, 12A21, 12A30 | |||
| VRC-PG04-family | VRC-PG04, VRC-PG04b | |||
| VRC-CH31-family | VRC-CH30-34 | |||
| 8ANC131-class | 8ANC131-family | 8ANC37, 8ANC131, 8ANC134 | ||
| 1B2530-family | 1B2530, 1NC3, 1NC7 | |||
| V1V2 site on gp120 | Penetrating CDR H3 binds two glycans and strand | PG9-class | PG9-family | PG9, PG16 |
| CH01-family | CH01-04 | |||
| Not yet determined | PGT145-class | PGT145-family | PGT141-145 | |
| Glycan-V3 site on gp120 | Glycans and domain swapping | 2G12-class | 2G12-family | 2G12 |
| Not yet determined | PGT121-class | PGT121-family | PGT121-123 | |
| Penetrating CDR H3 binds two glycans and strand | PGT128-class | PGT128-family | PGT125-131 | |
| Not yet determined | PGT135-class | PGT135-family | PGT135-137 | |
| MPER on gp41 | ELDKWAS loop | 2F5-class | 2F5-family | 2F5 |
| Pre-TM helix | 4E10-class | 4E10-family | 4E10 | |
| 10E8-class | 10E8-family | 10E8, 7H6 | ||
| WNWFDITN loop | Z13-class | Z13-family | Z13 |
Discussion
The last few years have seen a renaissance in our understanding of human antibodies that neutralize HIV-1. Much of this has been enabled by technologies that allow for the identification of effective neutralizers from the B cells of HIV-1-infected donors (Scheid et al., 2009; Scheid et al., 2011; Walker et al., 2011; Walker et al., 2009; Wu et al., 2010; Wu et al., 2011b). Atomic-level characterization of antibody-HIV-1 Env interactions has also provided substantial insight into the mechanism of HIV-1 neutralization (Calarese et al., 2003; Cardoso et al., 2005; Diskin et al., 2011; McLellan et al., 2011; Zhou et al., 2010; Zhou et al., 2007). Deep sequencing coupled to systems-level bioinformatics holds the potential to precisely define B cell ontogenies (Wu et al., 2011b; Zhu et al., 2012), though substantial methodological hurdles remain, such as the current lack of pairing of heavy and light chains produced in separate deep sequencing reactions or the 1–2% error rates of 454 pyrosequencing. Nonetheless, the rapid development of genomics technologies is likely to solve these issues within the next few years.
Once an understanding of the B cell ontogeny of effective HIV-1 neutralizers is achieved, it may be possible to utilize this information in immunization strategies (Fig. 3). Because these highly effective neutralizing antibodies occur naturally, an effective vaccine against HIV-1, for example, might consist of merely ‘pushing the right immune system buttons’ with immunogens. VRC01-like antibodies might be generated by designing immunogens with enhanced affinity for the VH1-2 heavy chain and 5-amino acid CDR L3-germline recombinants. The elicitation of PG9-like antibodies might be aided by immunization in the presence of adjuvants that promote the activation of greater numbers of naïve B cells, thus potentially increasing the percentage of uncommonly long CDR H3s required for this mode of HIV-1 recognition. And PGT128- and 10E8-like antibodies might require a bit of both to enhance the elicitation of required CDR H3 length and high degree of affinity maturation. Although it is difficult to predict how revolutions in biological understanding might translate into improved immunological interventions, it seems reasonable to expect that a detailed understanding of the natural generation of effective HIV-1 neutralizers will provide avenues towards elicitation in vaccine settings.
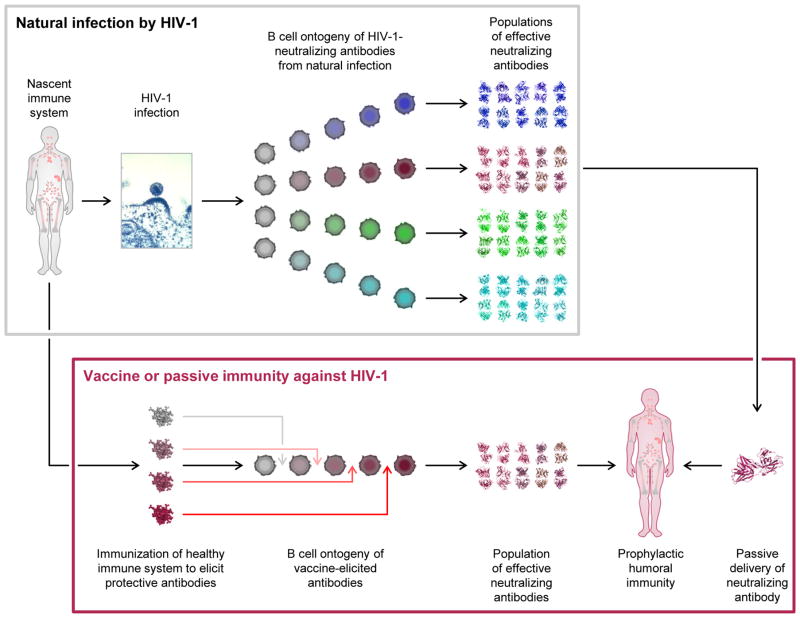
Figure 3. Broadly neutralizing immunity to HIV-1.
Infection by HIV-1 (top panel) generates broadly neutralizing antibodies after two or more years of infection in ~20% of individuals. Deep sequencing of antibody-gene transcripts from memory B cells coupled with bioinformatics analyses of the resulting antibodyomes provides the potential to follow the development of effective neutralizing antibodies during natural infection. HIV-1 neutralizing mAbs can potentially be used in passive modes of protection (far right arrow connecting upper and lower panels), either singly or in combination. Based upon our understanding of the B cell ontogenies of broadly neutralizing antibodies, immunogens can potentially be developed to re-create the elicitation of effective mAb neutralizers (bottom panel).
In addition to serving as vaccine templates, HIV-1-neutralizing mAbs may serve other uses, for example, in passive protection against HIV-1 infection (Fig. 3). As in the case of respiratory syncytial virus infection, where the mAb palivizumab (Synagis) is used to effectively protect neonates from clinical disease, an effective HIV-1 mAb, or combination of antibodies (Bonsignori et al., 2012; Doria-Rose et al., 2012b), might also serve to prevent HIV-1 infection. Indeed, such passive antibody transfer has already been shown to afford protection in animal models of infection (reviewed in Mascola and Montefiori, 2010). Moreover, modalities involving the gene-based expression of antibody from vectors such as AAV show the potential for long term expression of protective titers of neutralizing antibodies (Balazs et al., 2012; Johnson et al., 2009). In summary, a detailed atomic-level knowledge of HIV-1 antibodies and their mechanisms of neutralization, together with an understanding of the genetic pathways for antibody elicitation, are likely to lead to more effective approaches of prophylactic protection from HIV-1. It will be exciting to see how insight into human immunity – based on observation from natural infection – translates into ways to ameliorate, to prevent, or even to cure infection by HIV-1.
Supplementary Material
Acknowledgments
We thank D. Burton, G. Nabel and I. Wilson for discussions, M. Connors for sharing 10E8 results, J. Stuckey for assistance with display items (figures, table and movie), X. Wu for assistance with antibody-sequence analysis, and members of the Structural Biology Section, the Structural Bioinformatics Core, and the Humoral Immunology Section, Vaccine Research Center, for discussions and comments on the manuscript. Support for this work was provided by the Intramural Research Program of the Vaccine Research Center, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, Camacho ZT, Gewirth D, Kelsoe G, Chen P, Haynes BF. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol. 2007;178:4424–4435. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert J, Abrahamsson B, Nagy K, Aurelius E, Gaines H, Nystrom G, Fenyo EM. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. Aids. 1990;4:107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CH, Pantophlet R, Schiefner A, Cavacini LA, Stanfield RL, Burton DR, Wilson IA. Structure of antibody F425-B4e8 in complex with a V3 peptide reveals a new binding mode for HIV-1 neutralization. J Mol Biol. 2008;375:969–978. doi: 10.1016/j.jmb.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Lybarger EA, Crooks ET, Seaman MS, Gray E, Davis KL, Decker JM, Wycuff D, Harris L, Hawkins N, et al. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. Journal of virology. 2008;82:11651–11668. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. Journal of virology. 2004;78:13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blish CA, Nedellec R, Mandaliya K, Mosier DE, Overbaugh J. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. Aids. 2007;21:693–702. doi: 10.1097/QAD.0b013e32805e8727. [DOI] [PubMed] [Google Scholar]
- Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. Journal of virology. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Montefiori DC, Wu X, Chen X, Hwang KK, Tsao CY, Kozink DM, Parks RJ, Tomaras GD, Crump JA, et al. Two Distinct Broadly Neutralizing Antibody Specificities of Different Clonal Lineages in a Single HIV-1-infected Donor: Implications for Vaccine Design. Journal of virology. 2012 doi: 10.1128/JVI.07163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd SD, Gaeta BA, Jackson KJ, Fire AZ, Marshall EL, Merker JD, Maniar JM, Zhang LN, Sahaf B, Jones CD, et al. Individual variation in the germline Ig gene repertoire inferred from variable region gene rearrangements. J Immunol. 2010;184:6986–6992. doi: 10.4049/jimmunol.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breden F, Lepik C, Longo NS, Montero M, Lipsky PE, Scott JK. Comparison of antibody repertoires produced by HIV-1 infection, other chronic and acute infections, and systemic autoimmune disease. PloS one. 2011;6:e16857. doi: 10.1371/journal.pone.0016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briney BS, Willis JR, Crowe JE., Jr Human peripheral blood antibodies with long HCDR3s are established primarily at original recombination using a limited subset of germline genes. PLoS one. 2012a;7:e36750. doi: 10.1371/journal.pone.0036750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briney BS, Willis JR, Hicar MD, Thomas JW, 2nd, Crowe JE., Jr Frequency and genetic characterization of V(DD)J recombinants in the human peripheral blood antibody repertoire. Immunology. 2012b;137:56–64. doi: 10.1111/j.1365-2567.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- Burton DR, Stanfield RL, Wilson IA. Antibody vs. HIV in a clash of evolutionary titans. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14943–14948. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzon V, Natrajan G, Schibli D, Campelo F, Kozlov MM, Weissenhorn W. Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions. PLoS pathogens. 2010;6:e1000880. doi: 10.1371/journal.ppat.1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Chen L, Kwon YD, Zhou T, Wu X, O’Dell S, Cavacini L, Hessell AJ, Pancera M, Tang M, Xu L, et al. Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Science. 2009;326:1123–1127. doi: 10.1126/science.1175868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, Silacci C, Pinna D, Jarrossay D, Balla-Jhagjhoorsingh S, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PloS one. 2010;5:e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, et al. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. The Journal of experimental medicine. 2005;201:1407–1419. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B, Svehla K, Xu L, Wycuff D, Zhou T, Voss G, Phogat A, Chakrabarti BK, Li Y, Shaw G, et al. Structure-based stabilization of HIV-1 gp120 enhances humoral immune responses to the induced co-receptor binding site. PLoS pathogens. 2009;5:e1000445. doi: 10.1371/journal.ppat.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin R, Scheid JF, Marcovecchio PM, West AP, Jr, Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, Bjorkman PJ. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doores KJ, Fulton Z, Huber M, Wilson IA, Burton DR. Antibody 2G12 recognizes di-mannose equivalently in domain- and nondomain-exchanged forms but only binds the HIV-1 glycan shield if domain exchanged. Journal of virology. 2010;84:10690–10699. doi: 10.1128/JVI.01110-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose NA, Georgiev I, O’Dell S, Chuang GY, Staupe RP, McLellan JS, Gorman J, Pancera M, Bonsignori M, Haynes BF, et al. A short segment of the HIV-1 gp120 V1/V2 region is a major determinant of resistance to V1/V2 neutralizing antibodies. Journal of virology. 2012a doi: 10.1128/JVI.00696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose NA, Klein RM, Daniels MG, O’Dell S, Nason M, Lapedes A, Bhattacharya T, Migueles SA, Wyatt RT, Korber BT, et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. Journal of virology. 2010;84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose NA, Louder MK, Yang Z, O’Dell S, Nason M, Schmidt SD, McKee K, Seaman MS, Bailer RT, Mascola JR. HIV-1 neutralization coverage is improved by combining monoclonal antibodies that target independent epitopes. Journal of virology. 2012b;86:3393–3397. doi: 10.1128/JVI.06745-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowska E, Ramos A, Feng Y, Zhou T, Moquin S, Walker LM, Wu X, Seaman MS, Wrin T, Kwong PD, et al. PGV04, an HIV-1 gp120 CD4 binding site antibody, is broad and potent in neutralization but does not induce conformational changes characteristic of CD4. Journal of virology. 2012;86:4394–4403. doi: 10.1128/JVI.06973-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville J, Zhai W, Berka J, Telman D, Huerta G, Mehta GR, Ni I, Mei L, Sundar PD, Day GM, et al. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20216–20221. doi: 10.1073/pnas.0909775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Stamatatos L, Volsky B, Revesz K, Williams C, Wang XH, Cohen S, Staudinger R, Zolla-Pazner S. Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles. Journal of virology. 2005;79:5232–5237. doi: 10.1128/JVI.79.8.5232-5237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. Journal of virology. 2011;85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ES, Madiga MC, Moore PL, Mlisana K, Abdool Karim SS, Binley JM, Shaw GM, Mascola JR, Morris L. Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region. Journal of virology. 2009a;83:11265–11274. doi: 10.1128/JVI.01359-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ES, Moore PL, Choge IA, Decker JM, Bibollet-Ruche F, Li H, Leseka N, Treurnicht F, Mlisana K, Shaw GM, et al. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. Journal of virology. 2007;81:6187–6196. doi: 10.1128/JVI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nature biotechnology. 2012;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. Broad and potent neutralization of HIV-1 by a gp41-specific human antiobody. Nature. 2012 doi: 10.1038/nature11544. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, Yuste E, Desrosiers RC, Clark KR. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nature medicine. 2009;15:901–906. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of Proteins of Immunological Interest. 5. U.S. Department of Health and Human Service, National Institutes of Health; Bethesda MD: 1991. [Google Scholar]
- Kashyap AK, Steel J, Oner AF, Dillon MA, Swale RE, Wall KM, Perry KJ, Faynboym A, Ilhan M, Horowitz M, et al. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5986–5991. doi: 10.1073/pnas.0801367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Sun ZY, Rand KD, Shi X, Song L, Cheng Y, Fahmy AF, Majumdar S, Ofek G, Yang Y, et al. Antibody mechanics on a membrane-bound HIV segment essential for GP41-targeted viral neutralization. Nature structural & molecular biology. 2011;18:1235–1243. doi: 10.1038/nsmb.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Sattentau QJ. Antigenicity and immunogenicity in HIV-1 antibody-based vaccine design. J AIDS Clinic Res. 2012;S8:003. doi: 10.4172/2155-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman B, Dantas G, Ireton GC, Varani G, Stoddard BL, Baker D. Design of a novel globular protein fold with atomic-level accuracy. Science. 2003;302:1364–1368. doi: 10.1126/science.1089427. [DOI] [PubMed] [Google Scholar]
- Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner RA, Barbas CF, 3rd, Kang AS, Burton DR. On the use of combinatorial antibody libraries to clone the “fossil record” of an individual’s immune response. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:9705–9706. doi: 10.1073/pnas.88.21.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. Journal of virology. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK, Wu X, Shaw GM, Connors M, Wyatt RT, Mascola JR. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med. 2007;13:1032–1034. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Svehla K, Louder MK, Wycuff D, Phogat S, Tang M, Migueles SA, Wu X, Phogat A, Shaw GM, et al. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. Journal of virology. 2009;83:1045–1059. doi: 10.1128/JVI.01992-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. Molecular architecture of native HIV-1 gp120 trimers. Nature. 2008;455:109–113. doi: 10.1038/nature07159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, D’Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, Petropoulos CJ, Polonis VR, Sarzotti M, Montefiori DC. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. Journal of virology. 2005;79:10103–10107. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Louwagie J, McCutchan FE, Fischer CL, Hegerich PA, Wagner KF, Fowler AK, McNeil JG, Burke DS. Two antigenically distinct subtypes of human immunodeficiency virus type 1: viral genotype predicts neutralization serotype. J Infect Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- Mascola JR, Snyder SW, Weislow OS, Belay SM, Belshe RB, Schwartz DH, Clements ML, Dolin R, Graham BS, Gorse GJ, et al. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- McCoy LE, Quigley AF, Strokappe NM, Bulmer-Thomas B, Seaman MS, Mortier D, Rutten L, Chander N, Edwards CJ, Ketteler R, et al. Potent and broad neutralization of HIV-1 by a llama antibody elicited by immunization. The Journal of experimental medicine. 2012;209:1091–1103. doi: 10.1084/jem.20112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS pathogens. 2011;7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Malaspina A, Fauci AS. Prospects for an HIV vaccine: leading B cells down the right path. Nature structural & molecular biology. 2011;18:1317–1321. doi: 10.1038/nsmb.2194. [DOI] [PubMed] [Google Scholar]
- Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PloS one. 2011;6:e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL, Gray ES, Sheward D, Madiga M, Ranchobe N, Lai Z, Honnen WJ, Nonyane M, Tumba N, Hermanus T, et al. Potent and broad neutralization of HIV-1 subtype C by plasma antibodies targeting a quaternary epitope including residues in the V2 loop. Journal of virology. 2011;85:3128–3141. doi: 10.1128/JVI.02658-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, Chen B, Parks R, Foulger A, Jaeger F, et al. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PloS one. 2011;6:e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. Journal of virology. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myszka DG, Sweet RW, Hensley P, Brigham-Burke M, Kwong PD, Hendrickson WA, Wyatt R, Sodroski J, Doyle ML. Energetics of the HIV gp120-CD4 binding reaction. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9026–9031. doi: 10.1073/pnas.97.16.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek G, McKee K, Yang Y, Yang ZY, Skinner J, Guenaga FJ, Wyatt R, Zwick MB, Nabel GJ, Mascola JR, Kwong PD. Relationship between antibody 2F5 neutralization of HIV-1 and hydrophobicity of its heavy chain third complementarity-determining region. Journal of virology. 2010;84:2955–2962. doi: 10.1128/JVI.02257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. Journal of virology. 2004;78:10724–10737. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M, Majeed S, Ban YE, Chen L, Huang CC, Kong L, Kwon YD, Stuckey J, Zhou T, Robinson JE, et al. Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility. Proceedings of the National Academy of Sciences of the United States of America. 2010a;107:1166–1171. doi: 10.1073/pnas.0911004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M, McLellan JS, Wu X, Zhu J, Changela A, Schmidt SD, Yang Y, Zhou T, Phogat S, Mascola JR, Kwong PD. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. Journal of virology. 2010b;84:8098–8110. doi: 10.1128/JVI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantophlet R, Burton DR. GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol. 2006;24:739–769. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejchal R, Gach JS, Brunel FM, Cardoso RM, Stanfield RL, Dawson PE, Burton DR, Zwick MB, Wilson IA. A conformational switch in human immunodeficiency virus gp41 revealed by the structures of overlapping epitopes recognized by neutralizing antibodies. Journal of virology. 2009;83:8451–8462. doi: 10.1128/JVI.00685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejchal R, Walker LM, Stanfield RL, Phogat SK, Koff WC, Poignard P, Burton DR, Wilson IA. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11483–11488. doi: 10.1073/pnas.1004600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi A, Panteleeff D, Blish CA, Baeten JM, Jaoko W, McClelland RS, Overbaugh J. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. Journal of virology. 2009;83:10269–10274. doi: 10.1128/JVI.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim AK, Pantaleo G, Cohen OJ, Fink LM, Zhou JY, Zhou JT, Bolognesi DP, Fauci AS, Montefiori DC. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- Prabakaran P, Chen W, Singarayan MG, Stewart CC, Streaker E, Feng Y, Dimitrov DS. Expressed antibody repertoires in human cord blood cells: 454 sequencing and IMGT/HighV-QUEST analysis of germline gene usage, junctional diversity, and somatic mutations. Immunogenetics. 2012;64:337–350. doi: 10.1007/s00251-011-0595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Li B, Lynch RM, Haaland RE, Murphy MK, Mulenga J, Allen SA, Pinter A, Shaw GM, Hunter E, et al. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS pathogens. 2009;5:e1000594. doi: 10.1371/journal.ppat.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg JM, Leamon JH. The development and impact of 454 sequencing. Nat Biotechnol. 2008;26:1117–1124. doi: 10.1038/nbt1485. [DOI] [PubMed] [Google Scholar]
- Sagar M, Wu X, Lee S, Overbaugh J. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. Journal of virology. 2006;80:9586–9598. doi: 10.1128/JVI.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel K, Berger EA. Cooperative subunit interactions within the oligomeric envelope glycoprotein of HIV-1: functional complementation of specific defects in gp120 and gp41. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12794–12799. doi: 10.1073/pnas.230438497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, Venturi M, Schiffner L, Kalyanaraman R, Katinger H, Lloyd KO, Kwong PD, Moore JP. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. Journal of virology. 2002;76:7293–7305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. Journal of virology. 2009;83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1-->2 mannose residues on the outer face of gp120. Journal of virology. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for neutralizing antibody assessment. Journal of virology. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. Journal of virology. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Sun ZY, Coleman KE, Zwick MB, Gach JS, Wang JH, Reinherz EL, Wagner G, Kim M. Broadly neutralizing anti-HIV-1 antibodies disrupt a hinge-related function of gp41 at the membrane interface. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9057–9062. doi: 10.1073/pnas.0901474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- Stanfield RL, Gorny MK, Zolla-Pazner S, Wilson IA. Crystal structures of human immunodeficiency virus type 1 (HIV-1) neutralizing antibody 2219 in complex with three different V3 peptides reveal a new binding mode for HIV-1 cross-reactivity. Journal of virology. 2006;80:6093–6105. doi: 10.1128/JVI.00205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcich BR, Hahn BH, Shaw GM, McNeely PD, Modrow S, Wolf H, Parks ES, Parks WP, Josephs SF, Gallo RC, et al. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986;45:637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- Stiegler G, Kunert R, Purtscher M, Wolbank S, Voglauer R, Steindl F, Katinger H. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2001;17:1757–1765. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- Sun ZY, Oh KJ, Kim M, Yu J, Brusic V, Song L, Qiao Z, Wang JH, Wagner G, Reinherz EL. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity. 2008;28:52–63. doi: 10.1016/j.immuni.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Tian C, Luskin GK, Dischert KM, Higginbotham JN, Shepherd BE, Crowe JE., Jr Immunodominance of the VH1-46 antibody gene segment in the primary repertoire of human rotavirus-specific B cells is reduced in the memory compartment through somatic mutation of nondominant clones. J Immunol. 2008;180:3279–3288. doi: 10.4049/jimmunol.180.5.3279. [DOI] [PubMed] [Google Scholar]
- Tomaras GD, Binley JM, Gray ES, Crooks ET, Osawa K, Moore PL, Tumba N, Tong T, Shen X, Yates NL, et al. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. Journal of virology. 2011;85:11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M, Wainberg MA. Neutralization of multiple HIV-1 isolates from a single subject by autologous sequential sera. J Infect Dis. 1990;162:735–737. doi: 10.1093/infdis/162.3.735. [DOI] [PubMed] [Google Scholar]
- Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. Journal of virology. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Kelsoe G, Moody MA, Haynes BF. Role of immune mechanisms in induction of HIV-1 broadly neutralizing antibodies. Current opinion in immunology. 2011;23:383–390. doi: 10.1016/j.coi.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS pathogens. 2010;6 doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- West AP, Jr, Diskin R, Nussenzweig MC, Bjorkman PJ. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2083–E2090. doi: 10.1073/pnas.1208984109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrin T, Loh TP, Vennari JC, Schuitemaker H, Nunberg JH. Adaptation to persistent growth in the H9 cell line renders a primary isolate of human immunodeficiency virus type 1 sensitive to neutralization by vaccine sera. Journal of virology. 1995;69:39–48. doi: 10.1128/jvi.69.1.39-48.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Changela A, O’Dell S, Schmidt SD, Pancera M, Yang Y, Zhang B, Gorny MK, Phogat S, Robinson JE, et al. Immunotypes of a quaternary site of HIV-1 vulnerability and their recognition by antibodies. Journal of virology. 2011a;85:4578–4585. doi: 10.1128/JVI.02585-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Lynch R, Yang Z, Tran L, Perfetto S, Longo NS, O’Dell S, McKee K, Doria-Rose NA, Connors M, et al. Different gene composition of broadly neutralizing antibodies to the conserved CD4-binding site of HIV-1 envelope. Keystone Symposia: HIV Vaccines (X5); Keystone, Colorado. 2012. p. 361. [Google Scholar]
- Wu X, Parast AB, Richardson BA, Nduati R, John-Stewart G, Mbori-Ngacha D, Rainwater SM, Overbaugh J. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. Journal of virology. 2006;80:835–844. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhou T, O’Dell S, Wyatt RT, Kwong PD, Mascola JR. Mechanism of human immunodeficiency virus type 1 resistance to monoclonal antibody B12 that effectively targets the site of CD4 attachment. Journal of virology. 2009;83:10892–10907. doi: 10.1128/JVI.01142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011b;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- Xiao X, Chen W, Feng Y, Zhu Z, Prabakaran P, Wang Y, Zhang MY, Longo NS, Dimitrov DS. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochemical and biophysical research communications. 2009;390:404–409. doi: 10.1016/j.bbrc.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Do Kwon Y, Scheid J, Shi W, Xu L, et al. Structural Basis for Broad and Potent Neutralization of HIV-1 by Antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Xu L, Dey B, Hessell AJ, Van Ryk D, Xiang SH, Yang X, Zhang MY, Zwick MB, Arthos J, et al. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445:732–737. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, O’Dell S, Ofek G, Pancera M, Wu X, Zhang B, Zhang Z, Mullikin JC, Simek M, et al. Program NCS. Somatic populations of PGT135-137 HIV-1-neutralizing antibodies identified by 454 pyrosequencing and bioinformatics. Frontiers in Virology. 2012 doi: 10.3389/fmicb.2012.00315. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Qin HR, Chen W, Zhao Q, Shen X, Schutte R, Wang Y, Ofek G, Streaker E, Prabakaran P, et al. Cross-reactive HIV-1-neutralizing human monoclonal antibodies identified from a patient with 2F5-like antibodies. Journal of virology. 2011;85:11401–11408. doi: 10.1128/JVI.05312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. Journal of virology. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.