Abstract
Agricultural workers have high rates of airway and skeletal health disease. Studies recently demonstrated that inhaled agricultural organic dust extract (ODE) induced-airway injury is associated with bone deterioration in an animal model. However, the effect of age in governing these responses to organic dusts is unclear, but might be important in future approaches. Young (7–9 weeks) and older (12–14 months) male C57BL/6 mice received intranasal (i.n.) inhalation exposure to ODE from swine confinement facilities once or daily for 3 weeks. Acute ODE-induced neutrophil influx and cytokine and chemokine (TNF-α, IL-6, CXCL1, CXCL2) airway production was reduced in older compared to young mice. Repetitive ODE treatment, however, increased lymphocyte recruitment and alveolar compartment histopathologic inflammatory changes in older mice. Whole lung cell infiltrate analysis revealed that young, but not older mice, repetitively treated with ODE demonstrated an elevated CD4:CD8 lymphocyte response. Acute inhalant ODE exposure resulted in a 4-fold and 1.5-fold rise in blood neutrophils in young and older mice, respectively. Serum IL-6 and CXCL1 levels were elevated in young and older mice i.n. exposed once to ODE, with increased CXCL1 levels in younger compared to older mice. Although older mice displayed reduced bone measurements compared to younger mice, younger rodents demonstrated ODE-induced decrease in bone mineral density, bone volume and bone micro-architecture quality as determined by CT analysis. Collectively, age impacts the airway injury, and systemic inflammatory and bone loss response to inhalant ODE, suggesting an altered and enhanced immunologic response in younger as compared to older counterparts.
Keywords: Age, Mouse, Organic Dust, Airway, Lung, Bone, Systemic, Inflammation, Injury
INTRODUCTION
Agricultural work exposures increase the risk of several adverse health effects (Alavanja and Bonner, 2012; Rinsky et al, 2013; Calvert, et al. 2013; Hawley et al, 2015). Rhinosinusitis, non-allergic asthma, chronic bronchitis, and chronic obstructive pulmonary disease (COPD) as well as lung function decline over time are respiratory disorders amongst individuals persons exposed to agriculture-related organic dusts (Viegas et al, 2013; Wells, et al. 2014; May, et al. 2012; Hawley et al, 2015). In addition, farmers suffer from systemic illnesses such as musculoskeletal diseases (approximately 90% lifetime prevalence), with agricultural work representing one of the highest rates of fracture risk in an occupational setting (Osborne, et al. 2012a; 2012b; Leon, et al. 2011). Dusad et al. (2013) demonstrated in an animal model that lung injury induced by inhaled agricultural organic dust extract (ODE) exposure results in significant reduced bone mineralization and increased deterioration. This is important because osteoporosis and increased fracture risk represent debilitating systemic features of chronic respiratory diseases, and bone loss may be present in these respiratory illnesses independent of established osteoporosis risk factors such as low body mass index, gender, sedentary life-style, cigarette smoking, nutritional status, and medications (Jung, et al. 2014; Graat-Verboom, et al. 2012). Whereas pattern recognition receptors, including Toll-like receptor (TLR2, TLR4, TLR9)/MyD88 and scavenger receptor A/CD204 significantly contribute to airway inflammatory responses (Bauer, et al. 2013; Poole, et al. 2011; 2014; Bailey, et al. 2008), other potential characteristics might govern host responses. Age is likely an important host factor that plays a role in mediating the risk of developing of organic dust-induced airway inflammatory disease and subsequent adverse bone consequences.
The age range of exposure to agricultural environments is wide and variable as exposure may commence in early childhood and continue throughout adulthood. Merchant and colleagues (2005) found that the highest risk of asthma in a rural cohort of children (mean age of approximately 10 years) was among farm children who lived on farms that raised swine (44%–56% prevalence). Zejda and colleagues (1993) demonstrated that a relative excess of respiratory symptoms and reduced lung function variables was higher among swine producers aged 26–35 years, which may have reflected more intense occupational exposure. Approximately 30% of America’s farms are now operated by individuals older than 65 years and it is recognized that older farmers have high risk of musculoskeletal conditions and injuries (Tonelli, et al. 2014; Reed, et al. 2012) and COPD (Bailey, et al. 2007). To date, animal models investigating the pathogenesis underlying agriculture dust-induced airway inflammatory disease focused on relatively young aged mice, approximately 8–12 weeks, which would correspond to young adult years in humans.
Therefore, this study is based on the hypothesis that age represents an important risk factor in the susceptibility to the airway inflammatory and systemic bone consequences induced by inhaled swine confinement facility organic dust extract (ODE) exposures. To test this hypothesis airway inflammatory and systemic biomarkers, and bone health consequences to inhalant ODE exposure were compared between 2 ages of mice, 7–9 weeks and 12–14 months, representing a young human (age teens/20s years) and middle aged human (~age 50 years).
METHODS
Organic dust extract
Aqueous organic dust extracts (ODE) were prepared from dust collected from horizontal surfaces (approximately 3 feet above floor) from swine confinement feeding operations in the local area utilizing previously described methods (Poole, et al. 2014). Settled surface dust (1 g) was placed into sterile Hank’s Balanced Salt Solution (10 ml; Sigma, St. Louis, MO), incubated at room temperature for one hr, centrifuged at 2000g for 20 min. The final supernatant was filter sterilized (0.22 μm), a process that also removes coarse. The aqueous ODE stock solution (i.e. “100%”) was diluted to a final concentration (vol/vol) of 12.5% in sterile phosphate buffered saline (PBS; pH: 7.4; diluent), a concentration that elicits optimal experimental outcomes in C57BL/6 mice and is well tolerated (Poole, et al. 2009). The stock ODE solution contains roughly 4 mg/ml of total protein as measured by spectrophotometry (NanoDrop Technologies, Wilmington, DE). Endotoxin concentrations are routinely measured throughout experimental studies, and the stock ODE endotoxin concentrations ranged from 160 EU/ml to 400 EU/ml as determined using the limulus amebocyte lysate assay according to manufacturer’s instruction (Sigma). Muramic acid levels, a molecular component of bacterial cell wall peptidoglycans, were determined by mass spectrometry (Poole, et al. 2010) and were 70 ng/mg.
Animals
All animal procedures are in accordance with NIH guidelines for the use of rodents and were approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center. Male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Two age populations were studied: 1) young mice were defined as 7–9 weeks of age weighing (mean) 23.96 ± (standard deviation) 0.52 g, and 2) older mice were defined as 12–14 months of age weighing 32.71 ± 0.18 g. The young C57BL/6 mice are representative of the age and strain of mice utilized in all of our prior ODE exposure animal studies (Poole, et al. 2009; 2011; Dusad, et al. 2013; Bauer, et al. 2013).
Exposure animal model
Using our established intranasal (i.n.) inhalation exposure animal model (Poole, et al. 2009), mice were exposed to 50 μl sterile saline (phosphate buffered solution; PBS) or 12.5% ODE after receiving sedation with isoflurane. Mice were exposed once (acute, 1x exposure) or daily for 3 weeks with weekends excluded (repetitive exposure). No mice exhibited respiratory distress or weight loss throughout the treatment period.
Bronchoalveolar lavage fluid cell and cytokine/chemokine analysis
Bronchoalveolar lavage (BAL) was performed using 3 × 1 ml PBS, and total cell number recovered from pooled lavages was enumerated and differential cell counts determined from cytospun-prepared slides (Cytopro Cytocentrifuge, Wescore Inc, Logan, UT) stained with DiffQuick (Siemens, Newark, DE). Cell counts determined the differential ratio of cell types in 200 cells per slide per mouse. TNF-α, IL-6, and murine neutrophil chemoattractants (keratinocyte chemoattractant/CXCL1 and macrophage inflammatory protein-2/CXCL2) were quantitated from the cell-free supernatant of the first lavage by ELISA kits (R&D Systems) with thresholds of detection of 10.9, 7.8, 15.6, and 7.8 pg/ml, respectively.
Histopathology
After whole lung lavage, lungs were harvested, inflated with 1 ml 10% formalin, and hung under a pressure of 20 cmH2O for 1 day while submerged in 10% formalin for optimal preservation of lung parenchymal architecture as previously described (Poole, et al. 2014). Fixed lung tissues were standardly processed and embedded in paraffin, and sections (4–5 μM) cut and stained with hematoxylin and eosin. Each slide was reviewed in entirety, using (2x, 4x, and 10x objectives; Nikon Eclipse Model E600 microscope, Nikon, Tokyo, Japan) and semi-quantitatively assessed for the degree and distribution of lung inflammation utilizing a previously published scoring system by a lung pathologist blinded to the treatment conditions (Poole, et al. 2009). The scoring system is a Likert scale (0–3) with higher values representing increased histopathologic inflammation.
Whole lung cell phenotyping
In separate studies, after whole lung lavage, cells were isolated from whole lungs and stained with monoclonal antibodies (BD Biosciences, San Jose, CA) to determine distribution of lung macrophages, lymphocytes, and neutrophils, as previously described (Poole, et al. 2012a; 2012b; 2014) The right ventricle was infused with sterile PBS to remove blood from the pulmonary vasculature. Next, lung cells were dissociated by an automated dissociation procedure using a MAC Dissociator instrument (Miltenyi Biotech, Auburn, CA) according to manufacturer’s instructions. The resulting solution was passed through a nylon mesh (40 μM; Thermo Fisher Scientific Waltham, MA) to remove any large fragments. Red blood cells were lysed using 0.85% (w/v) ammonium chloride treatment. Following centrifugation, the remaining cells were re-suspended in PBS. A LIVE/DEAD Fixable Violet Dead Cell Stain kit (Life Technologies, Carlsbad, CA) was used to assess cell viabilities, and no marked differences in cell viability noted between saline and ODE-treated groups or between young and older mice (data not shown). Lung cells from each animal were then incubated with anti-CD16/32 (Fc Block, BD Biosciences) to minimize non-specific antibody staining, and then stained with monoclonal antibodies (mAb) directed against CD45, CD11c, CD11b, Ly-6G, CD3, CD4, and CD8. Parallel cell preparations were treated with appropriate isotype control mAb. Compensation was performed with antibody capture beads (BD Biosciences) stained separately with each individual mAb used in test samples. Details and depiction of the gating strategy have been previously published (Poole, et al. 2012a; 2012b; 2014). The % of respective populations of CD11c+ lung macrophages, Ly6G+ neutrophils, and CD3+CD4+ and CD3+CD8+ lymphocytes were determined from CD45+ lung leukocytes after excluding debris and dead cells which standardizes data to CD45+ leukocytes across multiple animals and experiments.
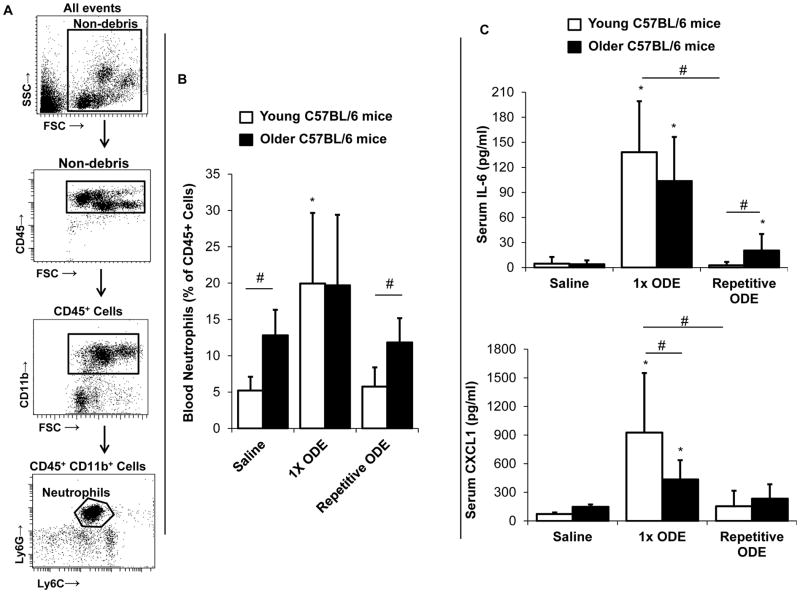
Blood neutrophil and serum analysis
Whole blood was collected from mice at the time of euthanasia from the axillary artery. Whole blood from each animal was incubated with mAb directed against CD45, CD11b, Ly6C, and Ly6G. After exclusion of debris, blood neutrophils were defined by forward/side scatter properties and by positive staining for CD45, CD11b, Ly6C, and Ly6G. The % blood neutrophils was calculated as the number of gated neutrophils divided by total number of gated CD45+ leukocytes multiplied by 100. Serum IL-6 and CXCL1 was quantified according to manufacturer’s instruction using a Quantikine enzyme-linked assay kit (R&D Systems).
Bone micro-CT analysis
The right hind limbs from repetitively exposed mice were excised and processed for micro-CT scanning as previously described (Dusad, et al. 2013; 2015). The proximal tibia was scanned using high-resolution micro-CT (Skyscan 1172; Skyscan, Aartselaar, Belgium) with images acquired at a resolution of 6.07 μm. The imaging source was set at 48kV and 187 μA with a 0.5-mm-thick aluminum filter with an exposure time of 620 msec. Scanning was performed at 0.4° intervals, and 6 average frames were obtained for each rotation. NRECON (Skyscan) software was used to reconstruct scanned images, and analysis conducted on stacked reconstructed images using CTAn (Skyscan) software as previously described (Dusad, et al. 2015). The tibial position was corrected using Dataviewer (Skyscan) software to ensure proper orientation along the longitudinal axis and growth plates were identified as the reference point. Analysis started at 75 slices distal to reference point and mineralized cartilage was excluded from analysis. Final analysis was conducted on a volume of interest of 1.82 mm distance (300 × 6.07 μm; 300 slides), for which an interpolated region of interest was manually drawn to exclude the cortical shell. CT-Vox and CT-Vol software (Skyscan) were used to construct two- or three-dimensional (2D/3D) images.
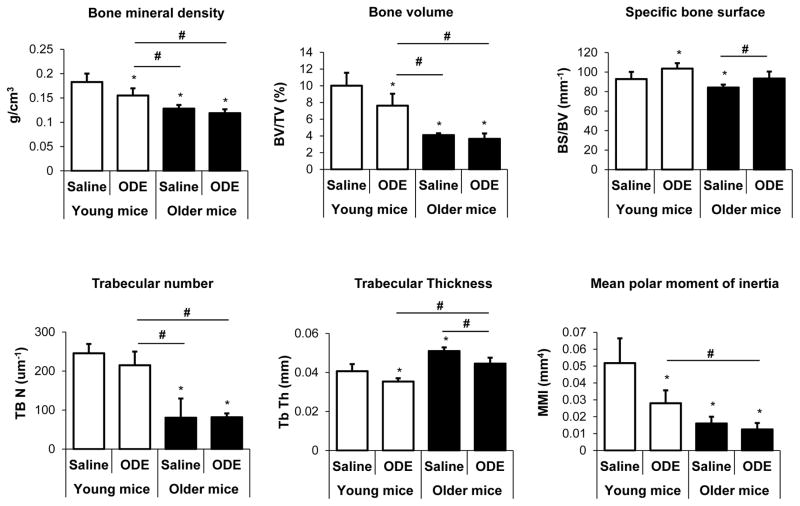
The following 3D parameters were measured for the trabecular bone in the proximal tibial metaphysis: bone mineral density (g/mm3), % bone volume (bone volume to tissue volume ratio, BV/TV, %), specific bone surface (bone surface to bone volume ratio, BS/BV, mm−1), trabecular thickness (Tb Th, mm), trabecular number (TB N, mm−1) and polar moment of inertia (MMI, mm4). Methods to calculate these bone parameters are described on the Skyscan website (www.skyscan.be) as bone mineral density, % bone volume, trabecular thickness, and trabecular number decrease with quantitative bone loss, whereas bone surface area increases with bone deterioration. Polar moment of inertia defines the geometric index of bone strength to resist torsion (Bellido, et al. 2010), with lower values suggestive of lower bone quality.
Statistical methods
Data are presented as mean and standard deviation (SD). Statistical significance was assessed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc analysis when group differences were significant (p<0.05) and a two-tailed Mann-Whitney test, where appropriate (inflammatory score of histopathology). GraphPad (Version 5.02, La Jolla, CA) software was used. Significance was accepted at p values < 0.05.
RESULTS
Airway inflammatory cellular influx following ODE treatment in young and older mice
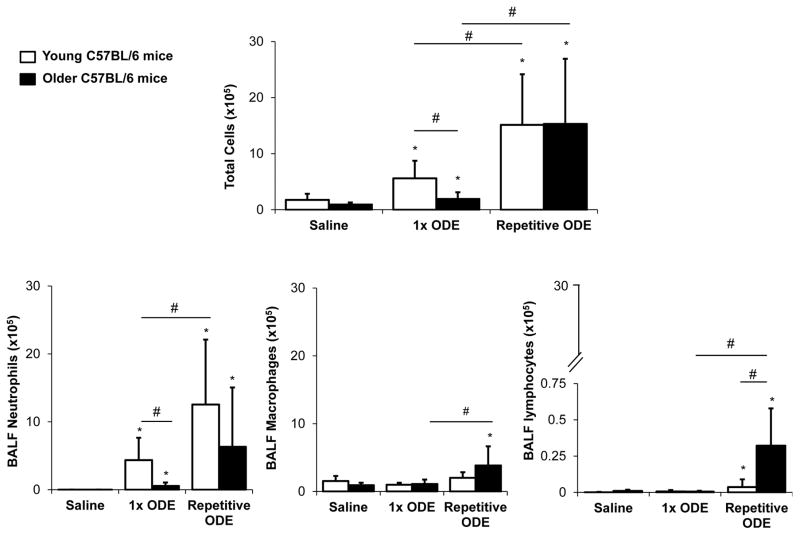
In these studies, influx of inflammatory cells in the bronchoalveolar lavage fluid (BALF) was compared between young (7–9 week) and older (12–14 month) C57BL/6 mice in simultaneous experiments. ODE treatment induced a significant increase in total cells, predominantly neutrophils, following acute and repetitive ODE exposure in young mice (Figure 1A). As compared to saline control, ODE exposure also induced a neutrophil response following acute and repetitive treatment in older mice; however, older mice demonstrated a less robust neutrophil influx response following acute exposure compared to younger animals (Figure 1B). Moreover, there was an enhanced lymphocyte response in older compared to younger mice following repetitive exposures (Figure 1D). There was also a significant rise in ODE-induced macrophages in older mice as compared to saline treatment following repetitive treatments (Figure 1C). These data demonstrate that older C57BL/6 mice display a diminished neutrophil response to ODE treatment acutely, but with repetitive exposures an enhanced lymphocyte and macrophage response was noted.
Figure 1. Airway inflammatory cellular influx following organic dust extract (ODE) exposure in young and older mice.
Young (7–9 week) and older (12–14 month) C57BL/6 mice were treated with ODE once (1x) or daily for 3 weeks (repetitive) using i.n. inhalation method. Five hours following final exposure, mice were euthanized and bronchoalveolar lavage fluid (BALF) was collected. Mean with standard deviation bars of total cells, neutrophils, macrophages, and lymphocytes from each treatment group is shown (n=9–12 mice/group from a minimum of 2 independent studies). Statistically significant (*p<0.05) vs. saline. Lines between groups denote statistical significance (#p<0.05).
Airway inflammatory cytokine/chemokine response to ODE treatment
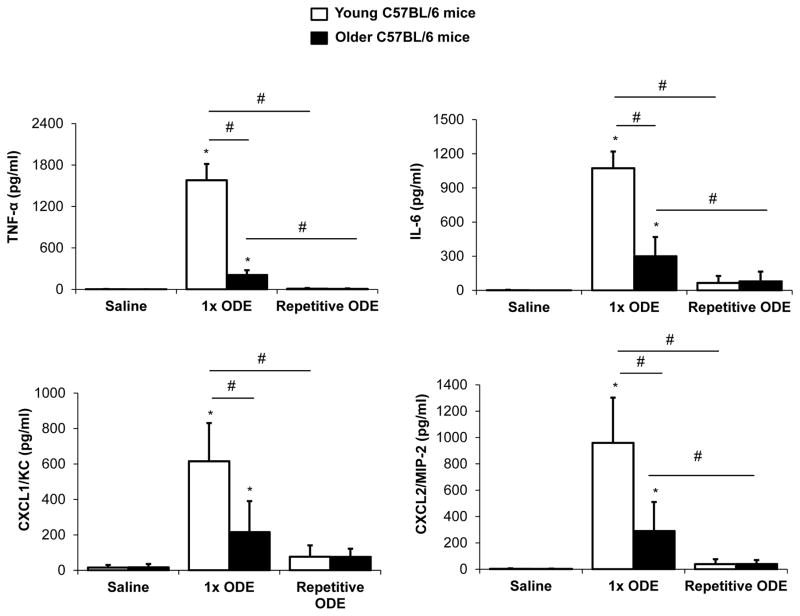
ODE-induced TNF-α, IL-6, CXCL1, and CXCL2 release was increased following acute ODE treatment in young and older mice, but the response was significantly attenuated in older compared to younger rodents for all mediators (Figure 2). There was no marked difference in these mediators between young and old mice following repetitive ODE challenges (Figure 2), and our findings are consistent with the adaptation response previously reported (Poole, et al. 2009). These cytokine/chemokine responses are consistent with the ODE-induced cellular influx response, demonstrating a diminished mediator response to acute ODE treatment in older mice.
Figure 2. Organic dust extract (ODE)-induced airway cytokine/chemokine response in young and older mice.
Young (7–9 week) and older (12–14 month) C57BL/6 mice were treated with ODE once (1x) or daily for 3 weeks (repetitive) using i.n. inhalation method. Five hours following final exposure, mice were euthanized and bronchoalveolar lavage fluid (BALF) was collected. Mean levels with standard deviation bars of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and murine neutrophil chemoattractants CXCL1 and CXCL2 are shown (n=5 mice/group, repeated twice with representative sample shown). Statistically significant (*p<0.05) vs. saline. Lines between groups denotes statistical significance (#p<0.05).). Statistically significant (*p<0.05) vs. saline. Lines between groups denotes statistical significance (#p<0.05).
Lung histopathology following repetitive ODE exposures
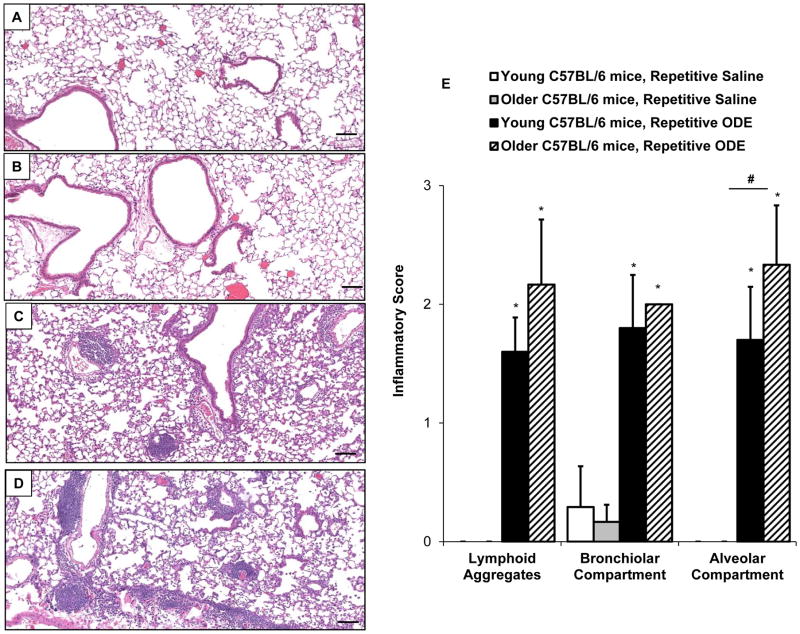
Repetitive, daily exposure to ODE for 3 weeks results in increased histopathologic inflammatory pulmonary changes including development of parenchymal lymphoid aggregates and inflammatory cell recruitment in both bronchiolar and alveolar compartment space in mice (Poole, et al. 2009). In the current study, there were notable increases in inflammatory cells as evidenced by lung histopathology induced by repetitive ODE treatment in both young (Figure 3C) and older mice (Figure 3D). Overall inflammatory histopathology consequences were elevated to a greater extent in older (Figure 3D) compared to young mice (Figure 3C). By semi-quantitative inflammatory scoring by the pathologist blinded to treatment conditions (Figure 3E), there were significant differences in alveolar compartment inflammatory score with older mice indicating enhanced inflammatory changes. However, no significant differences between younger and older mice following repetitive ODE were found in the degree and distribution of lymphoid aggregates or inflammation within the bronchiolar compartment.
Figure 3. Lung histopathology following repetitive ODE exposure in young and older mice.
Young (7–9 week) and older (12–14 month) C57BL/6 mice were i.n. inhalant treated with ODE or saline daily for 3 weeks. A representative 4–5-μm thick section (H&E stained) of one animal per treatment group (n=3–5 mice/group) is shown (10x magnification). A, Young mice + saline; B, Older mice + saline; C, Young mice + ODE; D, Older mice + ODE. E, Mean with standard deviation bars of semi-quantitative distribution of inflammatory scores of lung lymphoid aggregates, bronchiolar inflammation and alveolar inflammation are shown. Statistically significant denoted by asterisks (*p<0.05) vs. saline; hatchmark (#p<0.05) denotes difference between young/older mice. Line scale represents 100 μm.
ODE-induced lung tissue cellular infiltrates in young versus older mice
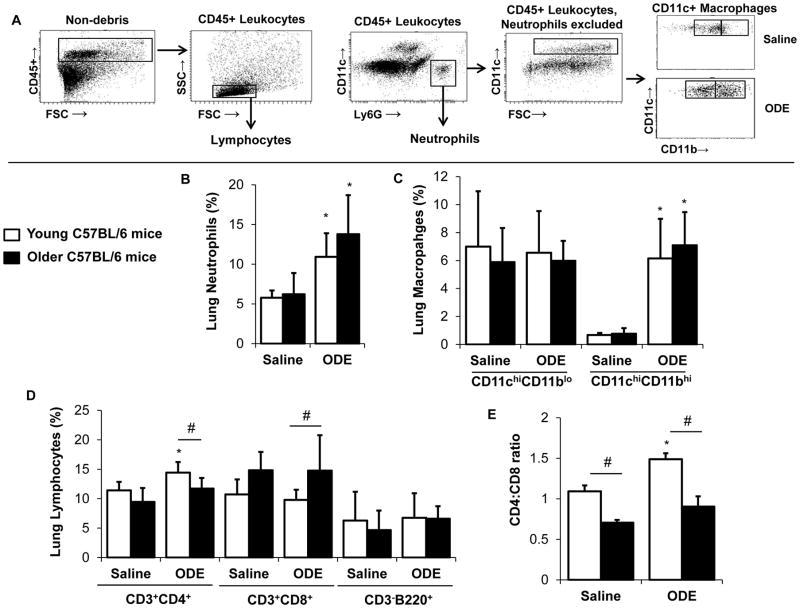
Due to enhanced histopathologic inflammatory changes observed in the older mice, the ODE-mediated inflammatory cell infiltrate was further delineated in older compared to young mice. Following exposure, whole lung cells were dissociated and analyzed by FACS analysis as described in the Methods. The overall gating strategy is depicted in Figure 4A and the distribution of neutrophils, macrophages, lymphocytes is shown as % lung CD45+ leukocytes (Figure 4B–D). Lung neutrophil influx was increased in both young and older mice following repetitive ODE treatment compared to respective saline treatment with no marked difference based upon age (Figure 4B). There was no significant difference in the distribution of alveolar macrophages (CD11chi/CD11blo macrophages) with saline/ODE or young/older mice, but repetitive ODE treatment resulted in elevated levels of lung exudative CD11chi/CD11bhi macrophages (Figure 4C). Similar to young mice, older mice also demonstrated increased influx of exudative macrophages following repetitive ODE treatment (Figure 4C). Data demonstrated that repetitive ODE treatment elevates CD4+ T lymphocytes as opposed to CD8+ T lymphocytes (Figure 4D), which is consistent with prior reports (Poole, et al. 2012b). The older mice, however, had a predominant CD8+ T lymphocyte distribution as opposed to CD4+ T lymphocytes with saline and ODE treatment, which is highlighted in Figure 4E showing the CD4:CD8 ratio between treatment groups.
Figure 4. The effect of age on repetitive organic dust extract (ODE) induced inflammatory lung cell infiltrates.
Young (7–9 week) and older (12–14 month) C57BL/6 mice were i.n. inhalant treated with ODE or saline daily for 3 weeks whereupon mice were euthanized, lavage fluid removed, pulmonary vasculature perfused, and lung cells dissociated and analyzed by flow cytometry. A, Depiction of gating strategy, which is detailed in the Methods section. Distribution of lung Ly6G+ neutrophils (B); alveolar CD11chiCD11blo and exudative CD11chi CD11bhi macrophages (C); and CD3+CD4+and CD3+CD8+ T lymphocytes and CD3−B220+ B lymphocytes (D) are shown as mean percentage with standard deviation bars of CD45+ leukocytes. E, Ratio of CD4:CD8 cells demonstrating that CD4 lymphocytes predominant the T cell response in young mice and CD8 lymphocytes predominant in older mice. N=5 mice/group. Statistically significant (*p<0.05) vs. respective saline. #p<0.05 denotes statistical significance between young/older mice.
Systemic inflammatory response to acute and repetitive inhaled ODE
The systemic effects of chronic lung diseases are increasingly recognized to contribute to overall morbidity and mortality, and it was postulated that age would be an important factor in enhancing the susceptibility of systemic effects induced by i.n. ODE inhalation. In this first set of studies, blood neutrophils were assessed by FACS analysis with gating strategy depicted in Figure 5A. At baseline, older mice had a higher level of blood neutrophils compared to younger animals. A one-time inhaled ODE exposure resulted in a robust, acute increase (approximate 4-fold rise) in blood neutrophils in young compared to no marked change in older mice (Figure 5B). Following repetitive ODE treatments, there was a tolerance/adaptation-like response whereby blood neutrophils returned to baseline (saline-treatment) levels in both young and old rodents (Figure 5B).
Figure 5. Systemic blood neutrophil and serum IL-6 and CXCL1 response following intranasal ODE treatment in young and older mice.
Young (7–9 week) and older (12–14 month) C57BL/6 mice were i.n. treated with saline or ODE once (1x) or daily for 3 weeks (repetitive). Five hours following final exposure, mice were euthanized and blood was collected. A, Gating strategy for blood neutrophils by flow cytometry is depicted and detailed in Methods section. B, Percentage of blood neutrophils calculated by dividing the number of neutrophil events by respective CD45+ events and multiplying by 100 for each mouse with bar graph showing mean with standard deviation (SD) bar (N=9–12 mice/group). C, Bar graphs depict means with SD of serum IL-6 and CXCL1 protein levels (N=6–8 mice/group). Asterisks denote statistical significance (*p<0.05) vs. respective saline. Lines between groups denotes statistical significance (#p<0.05).
Serum levels of IL-6 and CXCL1 increased following a one-time treatment with ODE in both young and older mice (Figure 5C). Younger mice demonstrated an elevated serum CXCL1 response with acute ODE treatment compared to older animals (Figure 5C). Consistent with the systemic neutrophil response, there was an adaptation response with serum IL-6 and CXCL1 levels decreasing with repetitive ODE exposure. However, serum IL-6 levels remained significantly increased in older mice repetitively exposed to ODE as compared to young animals.
Age effects on inhaled ODE-induced systemic bone deterioration
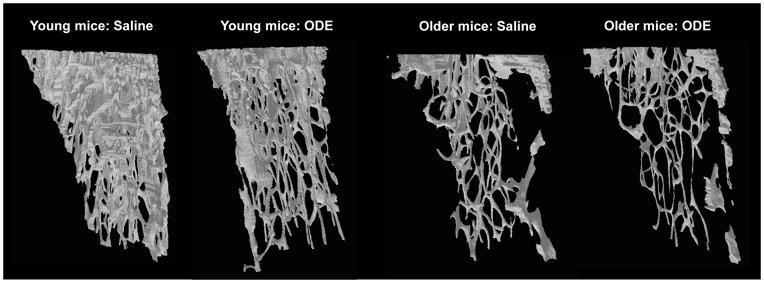
Whereas it was postulated that older age would increase the adverse bone effects to inhalant ODE treatment, data showed that young mice were more susceptible to ODE (Figure 6 and 7). Age alone resulted in greater bone deterioration effects than ODE treatments (Figure 6 and 7) with older mice, demonstrating negligible bone effects related to this exposure. Figure 6 shows a representative 3D reconstructed image of the ROI of the tibia from each treatment group. This ROI was utilized to quantify differences in specific bone parameters between exposure groups (Figure 7). Young mice exposed to ODE displayed decreased bone mineral density, % bone volume, and trabecular thickness and increased specific bone surface. In comparison, saline control treated older mice were found to have profound changes in bone parameters (i.e. bone mineral density, % bone volume, and trabecular number) compared to saline control treated young aged animals (Figure 7). In the older mice, inhalant ODE treatment did affect specific bone surface and trabecular thickness (Figure 7). Finally, the mean polar moment of inertia, which represents overall bone strength to resist torsion, was significantly reduced in young mice treated with ODE and in older rodents treated with saline or ODE compared to young controls treated with saline (Figure 7).
Figure 6. Three-dimensional reconstructed images of region of interest of proximal tibia of young and older mice treated repetitively with inhalant ODE.
Young (7–9 week) and older (12–14 month) C57BL/6 mice were i.n. treated daily with saline or ODE for 3 weeks. A representative 3D reconstructed images from region of interest of proximal tibia from one mouse per treatment group (5 mice/group). Note the substantial loss of trabecular bone in the young mice treated with ODE. Also note that age only greatly reduces trabecular bone in the older mice treated with saline or ODE.
Figure 7. Age effects on the systemic bone deterioration response to repetitive ODE inhalation exposure by micro-CT analysis.
Young (7–9 week) and older (12–14 month) C57BL/6 mice were i.n. treated daily with saline or ODE for 3 weeks whereupon trabecular bone of proximal tibia was subjected to micro-CT analysis. Changes in bone quality and bone quantity were consistently observed in ODE-treated young mice as compared to saline. Age alone significantly affected bone measurements in older mice. Bar graphs are means with standard deviation (N=5 mice/group). Asterisks denote statistical significance (*p<0.05) vs. young saline treated mice. Lines between groups denotes statistical significance (#p<0.05).
DISCUSSION
Age is an important host factor in airway and systemic response to acute and repetitive i.n. inhalation of ODE in C57BL/6 mice. This study found that acute ODE-induced airway inflammatory response was blunted in older mice, yet older mice displayed evidence of increased lymphocyte influx and alveolar compartment inflammation on histopathology following repetitive ODE treatments. The acute systemic inflammatory response to ODE was also numerically reduced in older compared to young mice. However, the older animals had higher blood neutrophils at baseline. Young mice were more susceptible to systemic bone consequences following repetitive ODE challenges, whereas older rodents had significant bone deterioration that was attributable to age alone. This is the first animal study, to our knowledge, to account for age in the investigations of inhaled agricultural ODE on lung and bone health. Our findings might have implications for the farming industry whereby the age of the target study population and adverse health consequences are important. Namely, addressing age in future studies might lead to improved understanding of pathogenesis induced by organic dust that might allow for developing more effective preventative and interventional therapeutic and/or outreach strategies.
Aging is a complex phenomenon that affects innate and adaptive immune responses resulting in an overall decline in immunity (Grubeck-Loebenstein and Wick. 2002). A striking finding in our study was the blunted neutrophil response and airway inflammatory cytokine/chemokine release to acute inhalant ODE challenge in older mice. Neutrophil migration into the lung was also shown to be impaired in aged mice and rats (e.g. aged 18–24 months) following infectious challenges such as Francisella tularenesis, Pseudomonas aeruginosa, influenza A (Mares, et al. 2010; Wen, et al. 2014; Chen, et al. 2014; Birmingham, et al. 2014). This impairment might be attributed to a delay in the kinetics of neutrophil responses. Mares, et al (2010) demonstrated that neutrophils appeared 2 days earlier in young compared to older mice infected with Francisella; yet neutrophil chemoattractant production was not delayed. Other studies found that following intratracheal (IT) lipopolysaccharide (LPS) administration, the degree of neutrophil influx in older mice (aged 15 month) did not surpass younger animals (aged 11 week) until 72 hr post-treatment (Ito, et al. 2007). LPS treatment also resulted in trends toward reduced CXCL1 and CXCL2 response in older aged mice up to 24 hr post challenge, but chemokine levels persisted longer and were higher in older rodents at 72 hr post-treatment (Ito, et al. 2007).
It is possible that age might modulate other properties or mediators that affect neutrophil influx that were not investigated in this current study. Von Essen, et al. (1994) reported that agricultural ODE elicited direct neutrophil chemotactic activity. Chen, et al (2014) demonstrated that migration properties of neutrophils are aberrant in aging and characterized by reduced chemotaxis yet preserved chemokinesis in neutrophils isolated from healthy older subjects. Subsequently data showed that the ODE-induced neutrophil infiltration persisted following repetitive exposures despite a decrease in CXCL1 and CXCL2 in both young and older animals, which is consistent with reported adaptation response (Poole, et al. 2009). This observation underscores the fact that factors such as neutrophil adherence and survival factors might be important considerations for future investigations.
Despite the blunted airway inflammatory responses to acute ODE treatment, there was evidence that older mice displayed elevated airway lymphocyte influx and increased alveolar compartment inflammation as evidenced by histopathology following repetitive treatments. Data suggest that this as a dysregulated immune response in aged mice to appropriately respond and resolve repetitive ODE challenges, consistent with our prior observations of diminished ciliary motility/clearance parameters with normal aging (Bailey, et al. 2014). Young mice demonstrated an increased CD4:CD8 response that was not found in older mice following repetitive ODE exposure (Figure 4E). It has been well recognized that impaired cellular immunity in the elderly is marked by a decline in T cell repertoire diversity (Grubeck-Loebenstein and Wick. 2002; Lanzer, et al. 2014). In aging-related COPD lung research, there is an age-dependent decline in naïve T cells with oligoclonal expansion of CD8+ T cells (Sharma, et al. 2009). It has been postulated that a rise in CD8+ T regulatory cells inhibits antigen-specific CD4+ T cells, which leads to a decline in adaptive response (Sharma, et al. 2009). Our ongoing data collections, however, are unable to confirm an increase in T regulatory cells or lung IL-10 responses in aged mice following ODE treatments (data not shown).
Lung injury induced by inhalant environmental toxins serves as a potential risk factor for systemic disease. Data demonstrated for the first time that acute ODE-treatment markedly increases (by 4-fold) blood neutrophils and elevated levels of serum IL-6 and CXCL1 in young mice (Figure 5). Older mice displayed a reduced magnitude of blood neutrophil response (approximately 1.5 fold, non-significant increase) to acute ODE treatment; however, blood neutrophils were higher in older mice at baseline, which might have impacted their ability to mount a further response. It is known that neutrophil production rises as a normal part of the aging process in the mouse (Boggs, et al. 1986). Systemic neutrophil, IL-6, and CXCL1 responses fell with repetitive ODE treatment, which is entirely consistent with the adaptation response. Of note, serum IL-6 levels were higher in older mice with repetitive ODE, which might be important to systemic disease outcomes in the aging because IL-6 has been linked to cardiovascular disease and osteoporosis (Ferrari, et al. 2013; Thiolat, et al. 2014; Sin and Macnee. 2013).
Whereas it was postulated that older mice might be more susceptible to inhaled ODE-induced adverse bone consequences, evidence indicated that younger mice were more susceptible. In these C57BL/6 mice, age appeared to exert a profound influence on measurements reflecting bone deterioration, which is consistent with other aging bone studies (Pietschmann, et al. 2007; Lopas, et al. 2014; Ferguson, et al. 2003). Based upon the high prevalence of musculoskeletal disease and fracture risk in the farming population (Osborne, et al. 2012a; 2012b), our findings underscore that skeletal health considerations in agricultural workers may need to be addressed in younger adult populations as opposed to waiting until elder years. Data suggest that future longitudinal studies investigate bone mineral density of agricultural workers, particularly younger workers with high intensity exposure. Vitamin D supplementation is an intervention that was shown to reduce inhalant ODE-induced bone loss in young aged mice, and thus, might be readily applied to agricultural workers (Dusad, et al. 2015).
There are potential limitations to our study. Airway and systemic bone consequences were evaluated in only two age groups of mice, and inclusion of more age groups might further delineate ODE-mediated effects across the normal aging spectrum. The C57BL/6 murine strain was utilized to study lung and skeletal health consequences to be consistent with agriculture-related organic dust and skeletal health studies. However, the C57BL/6 murine strains preferentially produce more Th1-cytokines and favor pro-inflammatory macrophage responses (Tacchini-Cottier, et al. 2012). Thus, confirmation of these findings in different murine strains will be informative.
In summary, this study demonstrates that age is an important factor in the host response to ODE treatments with older mice demonstrating a blunted airway and systemic inflammatory response acutely, but enhanced evidence of lung inflammatory changes by histopathology with repetitive exposures. Moreover, the younger aged mice were more susceptible to ODE-induced bone loss. Whereas the traditional farmer is aging, there remain high numbers of young workers in the modern swine production industry. These studies suggest that age is an important host variable to consider in animal and human studies to understand the pathogenesis of agriculture-related airway and systemic associated diseases.
Acknowledgments
Declaration of all sources of funding for the research reported in the manuscript: Study supported by grants from the National Institute of Environmental Health Sciences (R01: ES019325 to JAP), National Institute of Alcohol Abuse and Alcoholism (K08AA019503 to KLB); National Institute of Occupational Safety Health (U54OH010162-01 to JAP and TAW and R01OH008539-01 to DJR). This work was supported in part by the Central States Center for Agricultural Safety and Health (CS-CASH).
The authors wish to thank Xiaoyan Wang, Jane DeVasure, Christopher Bauer, Angela Gleason, and Jackie Pavlik for technical assistance and Lisa Chudomelka for manuscript preparation assistance. We also thank the UNMC Tissue Science Facility for assistance with lung tissue processing, sectioning, H&E staining, and assistance with digital microscopy images prepared for the manuscript.
Abbreviations
- BALF
Bronchoalveolar lavage fluid
- COPD
Chronic obstructive pulmonary disease
- CXCL1
Keratinocyte chemoattractant
- CXCL2
Macrophage inflammatory protein-2
- IL
Interleukin
- i.n
intranasal
- ODE
Swine confinement facility organic dust extract
- ROI
Region of interest
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor-alpha
References
- Alavanja MC, Bonner MR. Occupational pesticide exposures and cancer risk: A review. J Toxicol Environ Health B. 2012;15:238–263. doi: 10.1080/10937404.2012.632358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KL, Bonasera SJ, Wilderdyke M, Hanisch BW, Pavlik JA, DeVasure J, Robinson JE, Sisson JH, Wyatt TA. Aging causes a slowing in ciliary beat frequency, mediated by PKCepsilon. Am J Physiol Lung Cell Mol Physiol. 2014;306:L584–L589. doi: 10.1152/ajplung.00175.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KL, Meza JL, Smith LM, Von Essen SG, Romberger DJ. Agricultural exposures in patients with COPD in health systems serving rural areas. J Agromed. 2007;12:71–76. doi: 10.1300/J096v12n02_10. [DOI] [PubMed] [Google Scholar]
- Bailey KL, Poole JA, Mathisen TL, Wyatt TA, Von Essen SG, Romberger DJ. Toll-like receptor 2 is upregulated by hog confinement dust in an IL-6-dependent manner in the airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1049–L1054. doi: 10.1152/ajplung.00526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C, Kielian T, Wyatt TA, Romberger DJ, West WW, Gleason AM, Poole JA. Myeloid differentiation factor 88-dependent signaling is critical for acute organic dust-induced airway inflammation in mice. Am J Respir Cell Mol Biol. 2013;48:781–789. doi: 10.1165/rcmb.2012-0479OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido M, Lugo L, Roman-Blas JA, Castaneda S, Caeiro JR, Dapia S, Calvo E, Largo R, Herrero-Beaumont G. Subchondral bone microstructural damage by increased remodelling aggravates experimental osteoarthritis preceded by osteoporosis. Arthritis Res Ther. 2010;12:R152. doi: 10.1186/ar3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham JM, Gillespie VL, Srivastava K, Li XM, Busse PJ. Influenza A infection enhances antigen-induced airway inflammation and hyperresponsiveness in young but not aged mice. Clin Exp Allergy. 2014;44:1188–1199. doi: 10.1111/cea.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs D, Patrene K, Steinberg H. Aging and hematopoiesis. VI. neutrophilia and other leukocyte changes in aged mice. Exp Hematol. 1986;14:372–379. [PubMed] [Google Scholar]
- Calvert GM, Luckhaupt SE, Sussell A, Dahlhamer JM, Ward BW. The prevalence of selected potentially hazardous workplace exposures in the US: Findings from the 2010 National Health Interview Survey. Am J Ind Med. 2013;56:635–646. doi: 10.1002/ajim.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MM, Palmer JL, Plackett TP, Deburghgraeve CR, Kovacs EJ. Age-related differences in the neutrophil response to pulmonary pseudomonas infection. Exp Gerontol. 2014;54:42–46. doi: 10.1016/j.exger.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusad A, Thiele GM, Klassen LW, Gleason AM, Bauer C, Mikuls TR, Duryee MJ, West WW, Romberger DJ, Poole JA. Organic dust, lipopolysaccharide, and peptidoglycan inhalant exposures result in bone loss/disease. Am J Respir Cell Mol Biol. 2013;49:829–836. doi: 10.1165/rcmb.2013-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusad A, Thiele GM, Klassen LW, Wang D, Duryee MJ, Mikuls TR, Staab EB, Wyatt TA, West WW, Reynolds SJ, Romberger DJ, Poole JA. Vitamin D supplementation protects against bone loss following inhalant organic dust and lipopolysaccharide exposures in mice. Immunol Res. 2015;62:46–59. doi: 10.1007/s12026-015-8634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone. 2003;33:387–398. doi: 10.1016/s8756-3282(03)00199-6. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Tanni SE, Caram LM, Correa C, Correa CR, Godoy I. Three-year follow-up of interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respir Res. 2013;14:24-9921-14-24. doi: 10.1186/1465-9921-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graat-Verboom L, Smeenk FW, van den Borne BE, Spruit MA, Donkers-van Rossum AB, Aarts RP, Wouters EF. Risk factors for osteoporosis in caucasian patients with moderate chronic obstructive pulmonary disease: A case control study. Bone. 2012;50:1234–1239. doi: 10.1016/j.bone.2012.02.638. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243–284. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- Hawley B, Schaeffer J, Poole JA, Dooley GP, Reynolds S, Volckens J. Differential response of human nasal and bronchial epithelial cells upon exposure to size-fractionated dairy dust. J Toxicol Environ Health A. 2015;78:583–594. doi: 10.1080/15287394.2015.1015699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Betsuyaku T, Nasuhara Y, Nishimura M. Lipopolysaccharide-induced neutrophilic inflammation in the lungs differs with age. Exp Lung Res. 2007;33:375–384. doi: 10.1080/01902140701634843. [DOI] [PubMed] [Google Scholar]
- Jung JW, Kang HR, Kim JY, Lee SH, Kim SS, Cho SH. Are asthmatic patients prone to bone loss? Ann Allergy Asthma Immunol. 2014;112:426–431. doi: 10.1016/j.anai.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Lanzer KG, Johnson LL, Woodland DL, Blackman MA. Impact of ageing on the response and repertoire of influenza virus-specific CD4 T cells. Immun Ageing. 2014;11:9. doi: 10.1186/1742-4933-11-9. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon ME, Beane Freeman LE, Douwes J, Hoppin JA, Kromhout H, Lebailly P, Nordby KC, Schenker M, Schuz J, Waring SC, Alavanja MC, Annesi-Maesano I, Baldi I, Dalvie MA, Ferro G, Fervers B, Langseth H, London L, Lynch CF, McLaughlin J, Merchant JA, Pahwa P, Sigsgaard T, Stayner L, Wesseling C, Yoo KY, Zahm SH, Straif K, Blair A. AGRICOH: A consortium of agricultural cohorts. Int J Environ Res Public Health. 2011;8:1341–1357. doi: 10.3390/ijerph8051341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopas LA, Belkin NS, Mutyaba PL, Gray CF, Hankenson KD, Ahn J. Fractures in geriatric mice show decreased callus expansion and bone volume. Clin Orthop Relat Res. 2014;472:3523–3532. doi: 10.1007/s11999-014-3829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares CA, Ojeda SS, Li Q, Morris EG, Coalson JJ, Teale JM. Aged mice display an altered pulmonary host response to Francisella tularensis live vaccine strain (LVS) infections. Exp Gerontol. 2010;45:91–96. doi: 10.1016/j.exger.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S, Romberger DJ, Poole JA. Respiratory health effects of large animal farming environments. J Toxicol Environ Health B. 2012;15:524–541. doi: 10.1080/10937404.2012.744288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant JA, Naleway AL, Svendsen ER, Kelly KM, Burmeister LF, Stromquist AM, Taylor CD, Thorne PS, Reynolds SJ, Sanderson WT, Chrischilles EA. Asthma and farm exposures in a cohort of rural Iowa children. Environ Health Persp. 2005;113:350–356. doi: 10.1289/ehp.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne A, Blake C, Fullen BM, Meredith D, Phelan J, McNamara J, Cunningham C. Prevalence of musculoskeletal disorders among farmers: A systematic review. Am J Ind Med. 2012a;55:143–158. doi: 10.1002/ajim.21033. [DOI] [PubMed] [Google Scholar]
- Osborne A, Blake C, Fullen BM, Meredith D, Phelan J, McNamara J, Cunningham C. Risk factors for musculoskeletal disorders among farm owners and farm workers: A systematic review. Am J Ind Med. 2012b;55:376–389. doi: 10.1002/ajim.22001. [DOI] [PubMed] [Google Scholar]
- Pietschmann P, Skalicky M, Kneissel M, Rauner M, Hofbauer G, Stupphann D, Viidik A. Bone structure and metabolism in a rodent model of male senile osteoporosis. Exp Gerontol. 2007;42:1099–1108. doi: 10.1016/j.exger.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Poole JA, Anderson L, Gleason AM, West WW, Romberger DJ, Wyatt TA. Pattern recognition scavenger receptor A/CD204 regulates airway inflammatory homeostasis following organic dust extract exposures. J Immunotoxicol. 2014;12:64–73. doi: 10.3109/1547691X.2014.882449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, Mehaffy J, Reynolds SJ. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A. 2010;73:684–700. doi: 10.1080/15287390903578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Gleason AM, Bauer C, West WW, Alexis N, Reynolds SJ, Romberger DJ, Kielian T. Alphabeta T cells and a mixed Th1/Th17 response are important in organic dust-induced airway disease. Ann Allergy Asthma Immunol. 2012a;109:266–273. doi: 10.1016/j.anai.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Gleason AM, Bauer C, West WW, Alexis N, van Rooijen N, Reynolds SJ, Romberger DJ, Kielian TL. CD11c+/CD11b+ cells are critical for organic dust-elicited murine lung inflammation. Am J Respir Cell Mol Biol. 2012b;47:652–659. doi: 10.1165/rcmb.2012-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Wyatt TA, Kielian T, Oldenburg P, Gleason AM, Bauer A, Golden G, West WW, Sisson JH, Romberger DJ. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am J Respir Cell Mol Biol. 2011;45:711–719. doi: 10.1165/rcmb.2010-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1085–L1095. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DB, Rayens MK, Conley CK, Westneat S, Adkins SM. Farm elders define health as the ability to work. Workplace Health Saf. 2012;60:345–351. doi: 10.1177/216507991206000804. [DOI] [PubMed] [Google Scholar]
- Rinsky JL, Hoppin JA, Blair A, He K, Beane Freeman LE, Chen H. Agricultural exposures and stroke mortality in the Agricultural Health Study. J Toxicol Environ Health A. 2013;76:798–814. doi: 10.1080/15287394.2013.819308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Hanania NA, Shim YM. The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:573–580. doi: 10.1513/pats.200904-022RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin DD, Macnee W. Chronic obstructive pulmonary disease and cardiovascular diseases: A “vulnerable” relationship. Am J Respir Crit Care Med. 2013;187:2–4. doi: 10.1164/rccm.201210-1953ED. [DOI] [PubMed] [Google Scholar]
- Tacchini-Cottier F, Weinkopff T, Launois P. Does T helper differentiation correlate with resistance or susceptibility to infection with L. major? some insights from the murine model. Front Immunol. 2012;3:32. doi: 10.3389/fimmu.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiolat A, Semerano L, Pers YM, Biton J, Lemeiter D, Portales P, Quentin J, Jorgensen C, Decker P, Boissier MC, Louis-Plence P, Bessis N. Interleukin-6 receptor blockade enhances CD39+ regulatory T cell development in rheumatoid arthritis and in experimental arthritis. Arthritis Rheumatol. 2014;66:273–283. doi: 10.1002/art.38246. [DOI] [PubMed] [Google Scholar]
- Tonelli S, Culp K, Donham K. Work-related musculoskeletal disorders in senior farmers: Safety and health considerations. Workplace Health Saf. 2014;62:333–341. doi: 10.1177/216507991406200804. [DOI] [PubMed] [Google Scholar]
- Viegas S, Mateus V, Almeida-Silva M, Carolino E, Viegas C. Occupational exposure to particulate matter and respiratory symptoms in Portuguese swine barn workers. J Toxicol Environ Health A. 2013;76:1007–1014. doi: 10.1080/15287394.2013.831720. [DOI] [PubMed] [Google Scholar]
- Von Essen SG, O’Neill DP, Robbins RA, Rennard SI. Neutrophil chemotaxis to extracts of grain plant components. Am J Ind Med. 1994;25:85–88. doi: 10.1002/ajim.4700250122. [DOI] [PubMed] [Google Scholar]
- Wells AD, Poole JA, Romberger DJ. Influence of farming exposure on the development of asthma and asthma-like symptoms. Int Immunopharmacol. 2014;23:356–363. doi: 10.1016/j.intimp.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Li CM, Gu L, Yin SJ, Li W, Yang R. Aging reduces the expression of lung CINC and MCP-1 mRNA in a P. aeruginosa rat model of infection. Inflammation. 2014;37:933–941. doi: 10.1007/s10753-014-9813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zejda JE, Hurst TS, Rhodes CS, Barber EM, McDuffie HH, Dosman JA. Respiratory health of swine producers. Focus on young workers. Chest. 1993;103:702–709. doi: 10.1378/chest.103.3.702. [DOI] [PubMed] [Google Scholar]