Abstract
Objective
To assess the relationship between vitamin D status and diabetic retinopathy
Methods
A clinic-based, cross-sectional study was conducted at Emory University. A total of 221 subjects were classified into five groups based on diabetes status and retinopathy findings: no diabetes or ocular disease (n = 47), no diabetes with ocular disease (n = 51), diabetes with no background diabetic retinopathy (No BDR; n = 41), nonproliferative diabetic retinopathy (NPDR; n = 40), and proliferative diabetic retinopathy (PDR; n = 42). Key exclusion criteria included type 1 diabetes and those taking > 1000 IU vitamin D daily. Subjects underwent dilated fundoscopic examination and were tested for hemoglobin A1c, serum creatinine, and 25-hydroxy-vitamin D (25(OH)D) between December 2009 and March 2010.
Results
Between the groups, there was no statistical difference in age, race, sex, or multivitamin use. Diabetic subjects had lower 25(OH)D levels than non-diabetic subjects (22.9 ng/ml versus 30.3 ng/ml, p<0.001). The mean 25(OH)D levels were as follows: No diabetes or ocular disease = 31.9 ng/ml, No diabetes with ocular disease = 28.8 ng/ml, No BDR = 24.3 ng/ml, NPDR = 23.6 ng/ml, PDR = 21.1 ng/ml. Univariate analysis of the 25(OH)D levels demonstrated statistical significance between the study groups, race, body mass index, multivitamin use, hemoglobin A1c, serum creatinine, and estimated glomerular filtration rate. In a multivariate linear model with all potential confounders, only multivitamin use remained significant (p<0.001).
Conclusions
This study suggests that diabetic subjects, especially those with PDR, have lower 25(OH)D levels than those without diabetes.
Diabetes mellitus continues to be a tremendous health burden in America. In 2007, the prevalence of diabetes was estimated to be 23.6 million people, or 7.8% of the population.1 The number of people diagnosed with diabetes is expected to increase to 48.3 million people by the year 2050.2 Diabetes is also the leading cause of new blindness in patients 20 to 74 years of age.1 While it has been well established that intensive blood glucose control can lower the risk of microvascular complications from diabetes, the pathophysiology of retinopathy progression is not completely understood.3-5
Vitamin D is essential for a vast number of physiologic processes and vitamin D insufficiency has reached pandemic proportions, with more than half the world’s population at risk.6 Vitamin D insufficiency has been implicated in the development of diabetes and also correlated with an elevated risk of cardiovascular disease, cancer, and mortality.6-8 Additionally, vitamin D insufficiency has been associated with neurologic conditions, such as multiple sclerosis and Parkinson’s disease.9-10
Vitamin D may play a role in the pathogenesis of diabetic retinopathy through its effects on the immune system and on angiogenesis. Vitamin D exerts an anti-inflammatory effect by decreasing the proliferation of lymphocytes, natural killer cells, and several pro-inflammatory cytokines.11 Additionally, it has been shown that the active metabolite of vitamin D, calcitriol, is a potent inhibitor of retinal neovascularization in a mouse oxygen-induced ischemic retinopathy model.12 Given these associations, we sought to determine the relationship between vitamin D insufficiency and diabetic retinopathy.
Methods
The Emory University School of Medicine Institutional Review Board approved this study and all work was conducted in accordance with the Health Insurance Portability and Accountability Act regulations. This research followed the tenets of the Declaration of Helsinki. A clinic-based cross sectional study was designed at the Emory Eye Center. All patients were enrolled between December 16, 2009 and March 21, 2010 to minimize seasonal bias. All patients who were seen in the retina, glaucoma, cornea, and comprehensive ophthalmology clinics during the enrollment period were considered potential study subjects. These patients were screened by the study investigators to determine their diabetes status, age, race, and sex. Patients underwent routine ophthalmic examination, including dilated fundoscopy, and were asked to participate in the study if they were able to be matched into a study group based on their demographics. Attempts were made to keep the study groups equally matched according to age (within 10 years), race, and sex. For example, if a 50 year-old Caucasian male was enrolled in the proliferative diabetic retinopathy (PDR) group, we tried to match a Caucasian male between 45 and 55 years of age in each of the other four groups. Towards the end of the enrollment period, subjects were enrolled even if they did not have a corresponding match in the other groups. Study subjects provided informed consent prior to participation in the study, and once enrolled, completed a medical history questionnaire and had their blood tested for hemoglobin A1c, serum creatinine, and 25-hydroxy vitamin D (25(OH)D).
A total of 221 subjects were divided into five distinct groups based on their diabetes status and retinopathy findings. The first group consisted of subjects without diabetes or any ocular disease. Subjects in the second group also lacked diabetes but had some other form of ocular disease, such as uveitis or macular degeneration. The no background diabetic retinopathy (No BDR) group consisted of subjects with type 2 diabetes but no evidence of diabetic retinopathy, such as microaneurysms, cotton-wool spots, intraretinal hemorrhages, or macular edema. Subjects in the nonproliferative diabetic retinopathy (NPDR) group had evidence of retinopathy, such as microaneurysms, cotton-wool spots, intraretinal hemorrhages, or macular edema, but no evidence of retinal or iris neovascularization. The PDR group consisted of subjects with neovascularization on the optic disc, retina, or iris, with or without vitreous hemorrhage or prior panretinal photocoagulation. When the diabetic retinopathy was asymmetric, the subject was assigned to the group corresponding to the eye with the worse retinopathy findings.
Subjects were excluded if they had type 1 diabetes, were younger than 18 or older than 90 years of age. Subjects were also excluded if they were taking vitamin D analogues, multivitamins containing more than 1000 IU of vitamin D per day, or any medications that could alter vitamin D metabolism, such as rifampin, phenytoin, or phenobarbital. Subjects with prior diseases that suggested baseline alterations in vitamin D and calcium metabolism, such as hyperparathyroidism or hypoparathyroidism, or recent nephrolithiasis were also excluded. Patients who were cognitively impaired or unable to provide written informed consent were excluded as well.
Data collected in the medical history questionnaire included demographic variables, such as age, sex, height, weight, and race (self-assigned as white, black, or Asian). The presence of hypertension or macrovascular disease was also recorded. Subjects were considered to have macrovascular disease if they had ever been diagnosed with a myocardial infarction, cerebrovascular accident, if they had undergone coronary artery bypass grafting surgery or a cardiac stenting procedure. Additionally, the duration of diabetes, which was defined as the interval between their diagnosis of type 2 diabetes and the time of their enrollment into this study, and insulin usage was recorded for each diabetic patient. Subjects were also asked to report their usage of daily multivitamins. A phone interview was subsequently performed with those individuals who were taking multivitamins to ascertain the exact dosage of vitamin D contained in their multivitamin.
Demographic variables were categorized as following: age was grouped by decades of life (18 to 39 years, 40 to 49 years, 50 to 59 years, 60 to 69 years, 70 to 90 years); and body-mass index (BMI) was grouped as well (less than 25.0 kg/m2, 25.1 to 29.9 kg/m2, 30.0 to 34.9 kg/m2, and greater than 35 kg/m2). For those subjects with diabetes, duration of diabetes was categorized into groups (less than 10 years, 10.1 to 14.9 years, 15.0 to 19.9 years, 20.0 to 24.9 years, and greater than 25 years). Laboratory variables were grouped as following: hemoglobin A1c (less than 6.0, 6.0 to 6.9, 7.0 to 7.9, 8.0 to 8.9, 9.0 to 9.9, greater than 10.0), and serum creatinine (less than 1.0 mg/dl, 1.0 to 1.49 mg/dl, 1.50 to 1.99 mg/dl, and greater than 2.0 mg/dl). The estimated glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease formula. The results for estimated GFR were grouped according to the chronic kidney disease staging criteria (greater than 90 ml/min/1.73m2, 60 to 89 ml/min/1.73m2, 30 to 59 ml/min/1.73m2, 15 to 29 ml/min/1.73m2, and less than 15 ml/min/1.73m2).
For the laboratory testing, the serum creatinine was assayed via high performance liquid chromatography, which had been standardized using isotope dilution mass spectroscopy. Hemoglobin A1c levels were also measured using high performance liquid chromatography. The 25(OH)D levels were measured from serum samples via an automated competitive immunoassay detected by chemiluminescence (IDS iSYS assay; Scottsdale, AZ). The interassay coefficients of variation for the IDS iSYS assay at 6.5 ng/ml, 27.3 ng/ml, and 64.9 ng/ml were 15.9%, 12.4%, and 9.8%, respectively. The intra-assay coefficients of variation at 10.9 ng/ml, 40.4 ng/ml, and 69.2 ng/ml were 3.5%, 5.0%, and 3.4%, respectively. The Emory University laboratory is certified by the Clinical Laboratory Improvement Amendments (CLIA), and also participates in Vitamin D External Quality Assessment Scheme (DEQAS), which is an international organization that aims to ensure the analytical reliability of 25(OH)D assays.
Statistical Considerations
The means and percentages for patient characteristics were compared across study groups using one-way analysis of variance (ANOVA) and the chi-square test. Unadjusted comparisons of mean 25(OH)D were made across levels of patient characteristics using one-way ANOVA. Two-way ANOVA was done to compare mean 25(OH)D levels across study groups while adjusting for multivitamin use using least squares means. Tukey’s multiple comparison procedure was used to test pair-wise comparisons of mean 25(OH)D between levels of the patient characteristics in the adjusted and unadjusted analyses.
Vitamin D was also analyzed as a dichotomous variable with patients being labeled as vitamin D sufficient (25(OH)D ≥ 30.0 ng/ml) or vitamin D insufficient (25(OH)D < 30.0 ng/ml).13 The percentages of subjects with vitamin D insufficiency were compared across the various patient characteristics using the chi-square test.
A multiple linear regression model was used to assess the statistical significance of the 25(OH)D levels amongst the study groups and those using multivitamins. A second model was used to determine the significance of all of the patient characteristics found to be significant in the univariate analyses. Statistical calculations were performed using SAS and statistical significance was set as a two sided p-value of 0.05. The mean and standard deviation are denoted as mean ± standard deviation.
In the design phase of the study, the power of the study was calculated based on comparing the mean 25(OH)D level between two groups using the independent group’s t test. The two-sided probability of a Type I error was set to 0.005 (0.05/10) to provide a Bonferroni adjustment to account for the 10 pair-wise comparisons that could be made among the five groups. Based on the work of Pepper et al and personal experience, the standard deviation for the 25(OH)D levels was assumed to be 5.0 ng/ml.14 With 40 patients per group, the difference between the means of two groups that could be detected with 80% power was 4.2 ng/ml.
Results
Subject characteristics
Table 1 shows the univariate analysis across the study groups. There was no statistical difference between the study groups with regards to age, race, sex, or multivitamin use. However, there was a statistically significant difference in the study groups with regards to BMI (p = 0.003), hemoglobin A1c (p < 0.001), hypertension (p < 0.0001) and macrovascular disease (p = 0.002). Amongst those with diabetes, there was also a statistically significant difference in the duration of diabetes (p < 0.001), and insulin usage (p < 0.001).
Table 1.
Clinical characteristics of the five study groups.
| No Diabetes or Ocular Disease (n = 47) |
No Diabetes with Ocular Disease (n = 51) |
No BDR Group (n = 41) |
NPDR Group (n = 40) |
PDR Group (n = 42) |
p value | |
|---|---|---|---|---|---|---|
| Age (years) | 62.0 ± 11.6 | 59.8 ± 13.3 | 62.4 ± 11.3 | 68.3 ± 10.0 | 59.8 ± 12.0 | 0.797 |
| Race: Black | 25 (53%) | 23 (45%) | 21 (51%) | 22 (55%) | 22 (52%) | 0.977 |
| Sex: Male | 25 (53%) | 23 (45%) | 21 (51%) | 21 (53%) | 21(50%) | 0.938 |
|
Body Mass Index
(kg/m2) |
27.9 ± 6.1 | 28.7 ± 8.0 | 31.7 ± 9.8 | 31.2 ± 6.7 | 33.1 ± 6.7 | 0.003 |
|
Multivitamin
Usage |
26 (55%) | 22 (43%) | 20 (49%) | 19 (48%) | 15 (36%) | 0.439 |
|
Mean Daily Dose
Vitamin D (IU) |
312.8 ± 341.8 |
207.8 ± 284.8 |
200 ± 265.5 |
282.5 ± 371.3 |
228.6 ± 354.3 |
0.700 |
|
Duration of
Diabetes (years)* |
- | - | 7.4 ± 7.8 | 18.9 ± 11.1 | 22.0 ± 10.5 | < 0.001 |
| Hemoglobin A1c | 5.8 ± 0.5 | 5.8 ± 0.3 | 7.5 ± 2.0 | 7.4 ± 1.2 | 8.1 ± 1.9 | < 0.001 |
| Insulin Usage* | - | - | 10 (24%) | 29 (73%) | 31 (74%) | < 0.001 |
|
Macrovascular
Disease |
9 (19%) | 4 (8%) | 6 (15%) | 13 (33%) | 19 (45%) | 0.002 |
| Hypertension | 32 (68%) | 23 (45%) | 27 (66%) | 38 (95%) | 39 (93%) | < 0.001 |
|
Serum Creatinine
(mg/dL) |
0.95 ± 0.02 | 1.02 ± 0.87 | 0.92 ± 0.28 | 1.89 ± 1.87 | 2.38 ± 2.70 | < 0.001 |
|
Estimated GFR†
(mL/min/1.73m2) |
80.98 ± 18.70 | 84.75 ± 26.43 | 89.30 ± 30.07 |
59.00 ± 31.88 |
48.62 ± 29.61 |
< 0.001 |
IU: international units;
Only those patients with diabetes were included in this analysis
Estimated glomerular filtration rate as calculated by the MDRD formula
Vitamin D analysis
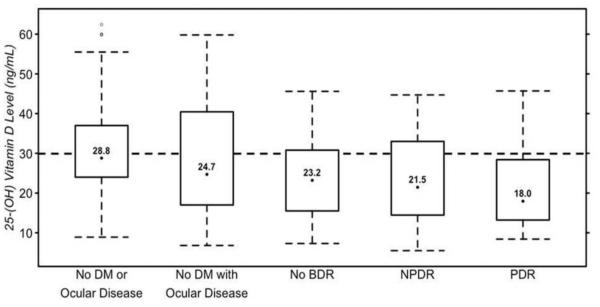
Overall, subjects with diabetes (n = 123) had lower 25(OH)D levels than those without diabetes (n = 98) (22.9 ± 10.4 ng/ml versus 30.3 ± 13.7 ng/ml respectively, p < 0.001). Additionally, black subjects had lower 25(OH)D levels compared to white subjects (23.7 ng/ml versus 29.2 ng/ml, p = 0.001). Figure 1 shows the 25(OH)D levels for the five study groups. Table 2 shows the univariate analyses of the mean 25(OH)D levels and of those with vitamin D insufficiency. This analysis revealed a statistically significant difference in mean 25(OH)D levels according to the study groups (p < 0.001), race (p = 0.033), BMI (p = 0.010), multivitamin use (p < 0.001), hemoglobin A1c (p = 0.003), serum creatinine (p = 0.030), and estimated GFR (p = 0.047). When analyzing vitamin D as a dichotomous variable, it was found that approximately two-thirds (65.2%) of the 221 study subjects were vitamin D insufficient. There was a significant difference in the percentage of patients with vitamin D insufficiency amongst the study groups (p = 0.048). The univariate analysis also showed subjects who were taking multivitamins were less likely to experience vitamin D insufficiency (p < 0.001).
Figure 1.
25(OH)D levels for the five study groups. The upper limit of each box represents the 75th percentile while the lower limit represents the 25th percentile. The dotted lines represent the 95th and 5th percentile, and the numerical value is the median for each group.
Table 2.
The analysis of the mean 25(OH)D levels and of those with vitamin D insufficiency.
| Mean 25(OH)D Level |
p value | Vitamin D Insufficiency (n = 144) |
p value | |
|---|---|---|---|---|
|
| ||||
| Study Group | < 0.001 | 0.048 | ||
| No DM or Ocular Disease (n= 47) | 31.9 ± 12.9 | 26 (55%) | ||
| No DM with Ocular Disease (n= 51) | 28.8 ± 14.3 | 28 (55%) | ||
| No BDR Group (n= 41) | 24.3 ± 10.3 | 28 (68%) | ||
| NPDR Group (n= 40) | 23.6 ± 10.3 | 28 (70%) | ||
| PDR Group (n= 42 ) | 21.1 ± 10.5 | 34 (81%) | ||
|
| ||||
| Age (years) | 0.306 | 0.241 | ||
| Younger than 40 (n= 7) | 29.4 ± 12.1 | 4 (57%) | ||
| 40 to 49 (n= 30) | 22.1 ± 13.0 | 24 (80%) | ||
| 50 to 59 (n= 57) | 25.5 ± 12.0 | 40 (70%) | ||
| 60 to 69 (n= 65) | 26.9 ± 11.8 | 40 (62%) | ||
| Older than 70 (n= 62) | 27.6 ± 13.3 | 36 (58%) | ||
|
| ||||
| Race | 0.033 | 0.048 | ||
| Black (n= 113) | 23.7 ± 12.5 | 81 (72%) | ||
| White (n= 102) | 29.2 ± 11.7 | 58 (57%) | ||
| Asian (n= 6) | 22.0 ± 14.3 | 5 (83%) | ||
|
| ||||
| Sex | 0.639 | 0.450 | ||
| Male (n= 111) | 25.7 ± 12.0 | 75 (68%) | ||
| Female (n= 110) | 26.6 ± 13.0 | 69 (63%) | ||
|
| ||||
| Body Mass Index (kg/m2) | 0.010 | 0.045 | ||
| Less than 25.0 (n= 48) | 30.1 ± 14.4 | 25 (52%) | ||
| 25.1 to 29.9 (n= 84) | 27.0 ± 11.9 | 52 (62%) | ||
| 30.0 to 34.9 (n= 43) | 25.0 ± 11.7 | 32 (74%) | ||
| Greater than 35.0 (n= 46) | 21.8 ± 10.8 | 35 (76%) | ||
|
| ||||
| Daily Multivitamin Usage | < 0.001 | <0.001 | ||
| Yes (n= 102) | 31.1 ± 11.1 | 45 (44%) | ||
| No (n= 119) | 22.0 ± 12.1 | 99 (83%) | ||
|
| ||||
| Duration of Diabetes (years)* | 0.463 | 0.656 | ||
| Less than 10.0 (n= 42) | 23.2± 9.8 | 31 (74%) | ||
| 10.0 to 14.9 (n= 15) | 26.5 ± 7.6 | 11 (73%) | ||
| 15.0 to 19.9 (n= 14) | 19.5 ± 10.0 | 12 (86%) | ||
| 20.0 to 24.9 (n= 18) | 23.5 ± 11.4 | 11 (61%) | ||
| Greater than 25 (n=3 4) | 22.2 ± 11.6 | 25 (28%) | ||
|
| ||||
| Hemoglobin A1c | 0.003 | 0.094 | ||
| Less than 6.0 (n= 78) | 30.3 ± 13.6 | 43 (55%) | ||
| 6.0 to 6.9 (n= 66) | 26.1 ± 11.9 | 43 (65%) | ||
| 7.0 to 7.9 (n= 39) | 21.7 ± 9.5 | 32 (82%) | ||
| 8.0 to 8.9 (n= 17) | 20.8 ± 11.4 | 13 (76%) | ||
| 9.0 to 9.9 (n= 13) | 23.7 ±11.5 | 8 (62%) | ||
| Greater than 10.0 (n= 8) | 24.0 ± 12.2 | 5 (63%) | ||
|
| ||||
| Insulin Usage* | 0.387 | 0.748 | ||
| Yes (n= 70) | 22.2 ± 10.5 | 52 (74%) | ||
| No (n= 53) | 23.9 ± 10.2 | 38 (72%) | ||
|
| ||||
| Macrovascular Disease | 0.162 | 0.207 | ||
| Yes (n= 51) | 24.0 ± 10.2 | 37 (73%) | ||
| No (n=170) | 26.8 ± 13.0 | 107 (63%) | ||
|
| ||||
| Hypertension | 0.753 | 0.414 | ||
| Yes (n= 159) | 26.4 ± 13.0 | 101 (64%) | ||
| No (n= 62) | 25.8 ± 11.1 | 43 (69%) | ||
|
| ||||
| Serum Creatinine (mg/dL) | 0.030 | 0.024 | ||
| Less than 1.0 (n= 112) | 27.2 ± 13.1 | 71 (63%) | ||
| 1.0 to 1.49 (n= 69) | 26.5 ± 12.3 | 43 (62%) | ||
| 1.50 to 1.99 (n= 16) | 28.0 ± 12.6 | 8 (50%) | ||
| Greater than 2.0 (n= 24) | 19.1 ± 7.5 | 22 (92%) | ||
|
| ||||
| Estimated GFR | 0.047 | 0.050 | ||
| Less than 15 (n= 8) | 20.5 ± 7.6 | 7 (88%) | ||
| 15 to 29 (n= 13) | 18.6 ± 7.8 | 12 (92%) | ||
| 30 to 59 (n= 47) | 26.9 ± 12.3 | 26 (55%) | ||
| 60 to 89 (n= 95) | 28.1 ± 12.8 | 26 (58%) | ||
| Greater than 90 (n= 58) | 24.9 ± 12.7 | 41 (71%) | ||
Only those patients with diabetes were included in this analysis
Because the univariate analysis of the mean 25(OH)D levels showed a significant difference between 25(OH)D levels amongst the study groups (p < 0.001), pair-wise comparisons were done to determine which groups were significantly different. There was a significant difference between the group with no diabetes or ocular disease and each of the three diabetic groups (No BDR group, NPDR group, and PDR group). There was also a significant difference between the no diabetes with ocular disease group and the PDR group. Because multivitamin use appeared to affect the 25(OH)D levels in the univariate analysis, a model was then fit that included both study group and multivitamin use. Both variables were found to be statistically significant (study group, p = 0.003; multivitamin use, p < 0.001). Therefore, the pair-wise comparisons among study groups were adjusted for multivitamin use (Table 3). The differences between the groups were the same in the adjusted pair-wise comparisons as the unadjusted pair-wise comparisons.
Table 3.
The pair-wise analysis of the differences in mean 25(OH)D levels between the study groups when controlling for multivitamin use
| Group Comparison | Difference between mean 25(OH)D levels (ng/ml) |
95% Confidence Limits |
p-value | |
|---|---|---|---|---|
|
No DM or Ocular Disease vs.
No DM with Ocular Disease |
2.06 | −4.18 | 8.30 | 0.893 |
| No DM or Ocular Disease vs. No BDR | 7.07 | 0.50 | 13.65 | 0.028 |
| No DM or Ocular Disease vs. NPDR | 7.65 | 1.03 | 14.27 | 0.015 |
| No DM or Ocular Disease vs. PDR | 9.15 | 2.57 | 15.73 | 0.002 |
| No DM with Ocular Disease vs. No BDR | 5.01 | −1.44 | 11.46 | 0.208 |
| No DM with Ocular Disease vs. NPDR | 5.59 | −0.91 | 12.08 | 0.129 |
| No DM with Ocular Disease vs. PDR | 7.09 | 0.68 | 13.50 | 0.022 |
| No BDR vs. NPDR | 0.57 | −6.26 | 7.41 | 0.999 |
| No BDR vs. PDR | 2.08 | −4.69 | 8.85 | 0.916 |
| NPDR vs. PDR | 1.51 | −5.30 | 8.31 | 0.974 |
No BDR: no background diabetic retinopathy; NPDR: nonproliferative diabetic retinopathy; PDR: proliferative diabetic retinopathy
A multivariate linear regression model was created to compare all potential confounders (study group, BMI, hemoglobin A1c, serum creatinine, estimated GFR, and multivitamin use). Only multivitamin use remained statistically significant (p < 0.001).
Discussion
There were two purposes of this cross-sectional study. The first was to assess the relationship between vitamin D status and diabetic retinopathy and the second was to establish baseline data from which a larger prospective clinical study could be designed. It was found that patients with type 2 diabetes, particularly those with PDR, had lower vitamin D levels than those without diabetes. Moreover, there was a higher percentage of subjects with vitamin D insufficiency in the diabetic retinopathy groups. Multivitamin usage had a significant impact on the vitamin D levels of the study subjects.
There is evidence to suggest that vitamin D may play a role in the pathogenesis of diabetic retinopathy through its effects on the immune system. Inflammatory cytokines, such as TNF-α, TNF-β, IL-6, and plasminogen activator inhibitor-1 are upregulated in patients with type 2 diabetes, and it has been shown that vitamin D decreases the production of several pro-inflammatory cytokines, such as IL-2, IL-6, IL-8, IL-12, and TNF-α.11 Vitamin D also exerts an anti-inflammatory effect by decreasing the proliferation of helper T-cells, cytotoxic T-cells and natural killer cells.11 A recent study found that vitamin D deficiency was associated with vascular endothelial dysfunction in middle aged and elderly adults. The authors concluded that this dysfunction was related to increased vascular endothelial cell expression of the pro-inflammatory transcription factor, nuclear factor ĸB.15
Vitamin D may also contribute to diabetic retinopathy via angiogenesis mechanisms. Albert and colleagues have shown that the active metabolite of vitamin D, calcitriol, was a potent inhibitor of retinal neovascularization in vivo.12 This study also found that calcitriol inhibits retinal endothelial cell capillary morphogenesis in vitro.12 Furthermore, calcitriol downregulates hypoxia-inducible factor-1 (HIF-1) transcriptional activity, as well as HIF-1 target genes, such as vascular endothelial growth factor (VEGF).16 As several of the complications in diabetic retinopathy, such as macular edema and neovascularization, are driven by VEGF production17-19, vitamin D could exert its positive effect via calcitriol mediated VEGF reduction.
Vitamin D may also play a protective role through its effects on glycemic control and hypertension, both significant risk factors for the development and progression of diabetic retinopathy.3-5,20 There is evidence to suggest that vitamin D plays a role in normal insulin secretion in response to glucose.11,21,22 Additionally, vitamin D and calcium citrate supplementation in patients with impaired fasting glucose leads to a reduction in insulin resistance and an attenuation of the rise in fasting glucose levels.23 Vitamin D deficiency has also been implicated in the development of hypertension as several studies have shown an inverse association between vitamin D levels and blood pressure.24,25 A large prospective cohort study found that vitamin D deficiency was associated with 6.1-fold and 2.7-fold increased relative risk of developing hypertension in men and women, respectively.26 Interestingly, the current study did not show an association between hypertension and vitamin D status. It is possible that the sample size of this study was not sufficient to detect such an association. Nonetheless, it is possible that treating vitamin D insufficiency may lead to an improvement in blood sugar and blood pressure control, which could ultimately slow the progression of retinopathy.
This is the largest study to date that has been designed to assess the relationship between vitamin D status and retinopathy in subjects with type 2 diabetes mellitus. The only other study designed to evaluate this relationship consisted of 66 diabetic patients and 20 normal controls.27 Concurrent with this study, mean serum 25(OH)D levels were higher in nondiabetic patients than those with diabetes (60.6 versus 31.0 nmol/L, p<0.001). However, differences in demographic variables, such as race or BMI were not addressed, and multivitamin use was not reported. Obesity has been shown to affect vitamin D levels, possibly through vitamin D sequestration in fat deposits.28 The current study found a statistically significant association between vitamin D concentration and BMI (p = 0.010), but this association fell out of significance in the multivariate model. It is possible that with a larger subset of patients, this would have remained a significant factor. Furthermore, because multivitamin use can influence vitamin D levels, it will be important to account for this in future studies. In order to improve our understanding of vitamin D deficiency and diabetic retinopathy, a larger prospective study will be needed. The data derived from this study points to a potential relationship between vitamin D deficiency and proliferative retinopathy and can be used in the design of larger studies. Certainly any study examining the relationship between vitamin D and retinopathy prospectively will take years to examine.
There are several limitations to the current study. First, the cross sectional design of this study limits the ability to assess causality. For example, it is not possible to determine, from this study, if the vitamin D insufficiency leads to diabetic retinopathy or if diabetic retinopathy leads to vitamin D insufficiency. Second, only one time point was recorded for the subjects in this study. It would be valuable to follow these patients with serial fundoscopic examinations and blood testing. Third, there is possible selection bias as this is not a study of consecutive patients seen at our institution. While dietary intake and outdoor exposure data were not collected, these limitations would not be expected to have a large effect on the results. Heaney and colleagues have shown that the amount of daily vitamin D obtained from dietary sources have small effects on serum 25(OH)D levels.29 Additionally, effects from sunlight exposure were minimized in this study as all subjects were enrolled over a three month period in winter. Finally, the overall mean standard deviation of 25(OH)D in this study was 11.9 ng/ml. Using this data, the sample size only provided the opportunity to detect a 9.4 ng/ml difference between two groups. This limited our ability to detect smaller differences between the groups, but can be used to calculate the sample sizes for future studies.
Conclusion
In conclusion, this study showed that subjects with type 2 diabetes mellitus, especially those with PDR, had lower vitamin D levels and were more likely to be vitamin D insufficient than patients without diabetes. The use of multivitamins was also somewhat protective against vitamin D insufficiency. While this is the largest study to date designed to assess this relationship, larger studies are needed to confirm these findings. Additionally, studies are needed to assess whether the treatment of vitamin D insufficiency will slow the progression of diabetic retinopathy.
Acknowledgements
Support: Supported in part by a grant to Emory University Eye Center from the Research to Prevent Blindness, Inc., and through a departmental grant from the National Eye Institute, EY06360. The sponsors had no role in the design or conduct of this research.
Footnotes
Financial Disclosures: The authors have no financial or proprietary interest in any product mentioned herein.
References
- 1.Centers for Disease Control and Prevention National diabetes fact sheet: general information and national estimates on diabetes in the United States. 2007 http://www.cdc.gov/diabetes/pubs/factsheet07.htm. Accessed June 6, 2010.
- 2.Narayan KM, Boyle JP, Geiss LS, et al. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006;29:2114–2116. doi: 10.2337/dc06-1136. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Moss SE. Relationship of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med. 1996;124:90–96. doi: 10.7326/0003-4819-124-1_part_2-199601011-00003. [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 5.The ACCORD Study Group and ACCORD Eye Study Group Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–44. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362. doi: 10.1093/ajcn/79.3.362. 371. [DOI] [PubMed] [Google Scholar]
- 7.Baz-Hecht M, Goldfine AB. The impact of vitamin D deficiency on diabetes and cardiovascular risk. Curr Opin Endocrin Diab Obes. 2010;17:113–119. doi: 10.1097/MED.0b013e3283372859. [DOI] [PubMed] [Google Scholar]
- 8.Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci. 2009;338(1):40–44. doi: 10.1097/MAJ.0b013e3181aaee91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson S, Jr, Taylor B, Blizzard L, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68:193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]
- 10.Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;65:1348–52. doi: 10.1001/archneur.65.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10:185–197. doi: 10.1111/j.1463-1326.2007.00710.x. [DOI] [PubMed] [Google Scholar]
- 12.Albert DM, Scheef EA, Wang S, et al. Calcitriol is a potent inhibitor of retinal neovascularization. Invest Ophthalmol Vis Sci. 2007;48:2327–2334. doi: 10.1167/iovs.06-1210. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff-Ferrari HA, Giovanucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 14.Pepper KJ, Judd SE, Nanes MS, Tangpricha V. Evaluation of vitamin D repletion regimens to correct vitamin D status in adults. Endocr Pract. 2009;15:95–103. doi: 10.4158/EP.15.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–9. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Shoshan M, Amir S, Dang DT, et al. 1A,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007;6:1433–1439. doi: 10.1158/1535-7163.MCT-06-0677. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–2305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakabayashi Y, Usui Y, Okunuki Y, et al. Correlation of vascular endothelial growth factor with chemokines in the vitreous in diabetic retinopathy. Retina. 2010;30:339–344. doi: 10.1097/IAE.0b013e3181bd2f44. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Wang G, Wang Y. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2009;148:883–889. doi: 10.1016/j.ajo.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 20.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 21.Gedik A, Akalin S. Effects of vitamin D deficiency and repletion on insulin and glucagon sectretion in man. Diabetologia. 1986;29:142–145. doi: 10.1007/BF02427083. [DOI] [PubMed] [Google Scholar]
- 22.Chiu KC, Chu A, Go VLW, et al. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 23.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 24.Kristal-Boneh E, Froom P, Harari G, Ribak J. Association of calcitriol and blood pressure in normotensive men. Hypertension. 1997;30:1289–1294. doi: 10.1161/01.hyp.30.5.1289. [DOI] [PubMed] [Google Scholar]
- 25.Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008 Jan;87(1):136–41. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- 26.Forman JP, Giovannucci E, Homes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 27.Aksoy H, Akcay F, Kurtul N, Baykal O, Avci B. Serum 1,25 dihydroxy vitamin D (1,25(OH)2D3), 25 hydroxy vitamin D (25(OH)D) and parathormone levels in diabetic retinopathy. Clin Biochem. 2000;33:47–51. doi: 10.1016/s0009-9120(99)00085-5. [DOI] [PubMed] [Google Scholar]
- 28.Vashi PG, Lammersfeld CA, Braun DP, Gupta D. Serum 25-hydroxyvitamin D is inversely associated with body mass index in cancer. Nutr J. 2011;10:51. doi: 10.1186/1475-2891-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]